1. Introduction
If healthcare were a country, it would be the 5th biggest carbon emitter in the world [Citation1]. In the UK, the sector accounts for 5% of the national carbon footprint (CF) [Citation2]. Healthcare itself is made up of a complex mix of carbon-producing activities, services and products. Pharmaceuticals are one of the largest inputs, and the NHS has estimated that the manufacture, supply, and use of pharmaceuticals accounts for 25% of the NHS’s total CF [Citation3].
The UK government and the NHS in England have shown international leadership in attempting to tackle the carbon problem of healthcare by setting ambitious targets for balancing their greenhouse gas emissions (positive contribution to total emissions) and greenhouse gas removals (negative contribution to total emissions) in 2019 [Citation4] and 2022 [Citation3], respectively. However, it is clear that to meet these net zero targets, NHS suppliers have an important role to play. Promisingly, many pharmaceutical companies have committed to reaching net zero carbon across their operations (a detail we will return to). However, companies must overcome several significant challenges requiring supportive action from the UK government and the NHS to deliver on these targets.
In this editorial, we focus on five actions that the pharmaceutical industry can take to reduce the CF of medicines. Our recommendations were developed through interviews and a roundtable with environmental experts and industry representatives as part of a project to understand what actions are needed to sustainably reduce the CF of medicines [Citation5].
2. Where do most carbon emissions occur in the supply chain?
Companies can better target interventions to curb those emissions if they know where emissions occur in the supply chain. depicts the main stages in the lifecycle of a pharmaceutical product, from research and development (R&D) to the end of a product’s life, including estimates of the contribution of each stage to the total CF of the product.
Figure 1. Generalised pharmaceutical supply chain diagram with ranges for shares of total carbon footprint. Based on interviews and roundtable with experts from academia and industry.
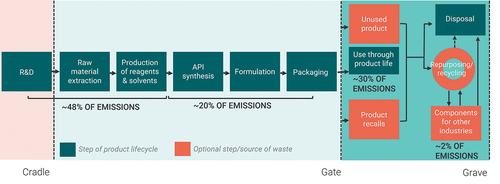
The distribution highlights a few things. Firstly, raw material extraction, an often-forgotten part of the pharmaceutical process, contributes a significant share of the total footprint. It is also a share that sits outside of the direct operations of pharmaceutical companies, but this is one of the sources of emissions that more and more payers are likely to require suppliers to address over the coming decades. Many of us may not realize that, in many cases, our drugs are chemical compounds derived from oil and other carbon-based natural resources, and their processing and refinement to medical-grade use requires substantial energy, overwhelmingly produced through the burning of fossil fuels.
Secondly, the use of the product contributes more to the CF than one may realize and up to 50% in some cases. The carbon produced during the use of a product is particularly marked for products like inhalers and anesthetic gases that include highly potent greenhouse gases. Finally, we also note big ranges in the estimates because the sources of emissions across the supply chain are highly variable from product to product. As a result, those trying to reduce emissions in the pharmaceutical industry as a whole are faced with a moving target.
3. What can be done: recommendations for action
The pharmaceutical industry is complex and highly globalized which makes reducing greenhouse gas emissions difficult to achieve. summarizes the main challenges and recommendations that emerged from our research. This qualitative research consisted of 12 one-hour semi-structured interviews with academic and industry experts conducted over the course of several weeks and an expert roundtable involving seven academic, industry, and policy experts which lasted several hours. The visual summary highlights where our research predicts the biggest impact could be realized for the investment made by the NHS, the UK Government and Industry [Citation5]. Many of the recommendations to the NHS and to the Government are relatively sector nonspecific and therefore have great potential for generating impact across a range of sectors if adopted. We focus, therefore, on pharmaceutical-specific recommendations. Economy-wide interventions may be perceived to be a more efficient way of reducing carbon emissions, but more research is needed to determine their relative efficiency, and the unique constraints facing the pharmaceutical industry mean that sector-specific action will be required.
Figure 2. Recommendations for the NHS, UK Government, and Industry, and alignment with identified challenge areas.
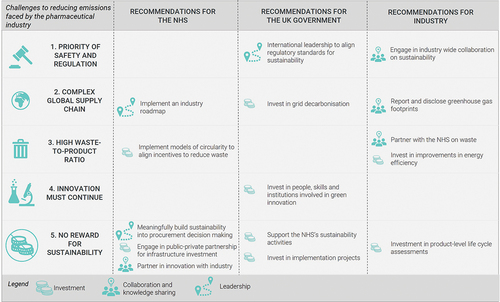
3.1. Two of our recommendations to Industry involve monitoring and evaluation of their carbon emissions (recommendations 1 and 2 in )
Industry should report and publicly disclose their emissions and progress against targets using standardized metrics that allow the rate of progress to be continually and reliably assessed. Further research is needed to investigate how exactly this could be achieved and the implications of any new measurement and reporting standards for companies in terms of compliance costs. In addition, companies should invest in the development of life cycle analysis (LCA) of their products [Citation6] to help the NHS incorporate environmental sustainability into procurement decision-making. As highlighted in the emissions ranges in above, companies will not have a meaningful impact on their emissions unless they know, at the product and company levels, where they are likely to have the biggest impact. A lack of detailed information to support decision-making is a reason for inaction.
3.2. Two of our recommendations to Industry involve partnership (recommendations 3 and 5)
Industry should partner with the NHS to develop collaborative and innovative solutions in areas such as waste management. Engagement should move beyond product-specific, small-scale pilot projects to the deployment of interoperable and scalable circularity models [Citation7–9]. Companies also need to collaborate with each other through appropriate channels to share knowledge and accelerate progress toward industry-wide solutions. Joint industry action is particularly important given the large contribution of emissions that occur outside the company’s operations but which the company is indirectly responsible for producing (scope 3 emissions), such as the emissions embodied in the company’s inputs or emissions generated through consumption of the company’s final products [Citation10]. For example, raw material extraction, as mentioned above, generates up to half of the emissions of a product, and responsible suppliers are used by many companies who can therefore exert joint influence. Where shared problems exist, such as the problem of upstream scope 3 emissions, drug manufacturers will benefit from collaboration to develop a solution that will have much more scale and impact than if one moves alone.
3.3. Finally, we recommend that the industry does the easy, achievable basics well; that is, companies should continue investing in energy efficiency improvements
There are actions that companies can take within existing regulations and reimbursement models to improve the energy efficiency of energy-intensive sites and reduce waste, delivering both a positive private and societal return on investment. Energy efficiency is ‘not sexy,’ as someone we interviewed admitted, and it requires detailed and technical oversight. However, in a context where so many of the activities to reduce carbon are complex to achieve, energy efficiency is a relatively easy win with huge potential for impact- especially in the direct operations that companies are targeting with net zero pledges. For example, an energy efficiency review project at ten manufacturing sites globally to improve energy efficiency generated annual savings of $6.4 million in energy costs (25% reduction), 18600 tonnes of CO2 (22% reduction) and 47 million gallons of water (22% reduction).
4. What can health economics bring to the table?
Part of the challenge to creating change in the pharmaceutical industry is that the current business model does not incentivize companies to invest in environmental sustainability. While there are reputational rewards for companies, the experience of other industries is that this incentive alone will not be enough to generate the required changes. A meaningful environmental incentive will eventually require the reimbursement model of pharmaceuticals to explicitly include environmental dimensions.
To achieve this, we recommend that evidence on the CF of individual medicines is incorporated into health technology assessment (HTA). Environmental changes can directly affect people’s health, and policymakers have broad mandates extending beyond healthcare [Citation11]. Depending on the rate at which future costs and effects are discounted, society may prefer a more environmentally sustainable product to be prioritized over a cheaper one with a larger CF. HTA, or value assessment more broadly, involves decision-makers considering different kinds of factors that affect value. Research into how to incorporate environmental dimensions in HTA is moving, albeit slowly, into the realm of practice.
5. How can HTA agencies consider the environmental sustainability of new medicines
Some of the more evolved HTA bodies have started to consider how environmental impacts could be incorporated into guidance. One element of NICE’s strategic pillar 4 is to ‘lead globally on the potential to include environmental impact data in our guidance to reduce the CF of health and care’ [Citation12]. As part of this work, Pinho-Gomes & Yoo et al. [Citation13] conducted a scoping review looking into incorporating environmental and sustainability considerations into HTA as a first step of a broader initiative by NICE to develop their methods and processes in relation to this topic. The authors mention the possibility of requiring pharmaceutical companies to conduct environmental impact assessments alongside clinical trials. They note that this approach would need to be carefully designed to maintain incentives for life-improving and life-extending innovation and that compiling and analyzing data required for a full LCA may not be compatible with current HTA timelines. Greenwood Dufour and colleagues at CADTH [Citation14] discuss introducing a triage process to identify the health technologies for which an environmental assessment would be most useful.
Even if a consensus is reached in the field on how to incorporate environmental impacts into HTA, the question remains as to the implications of this information on pricing and reimbursement decisions. Trade-offs between health economic effects and environmental impacts may need to be made more explicit, which raises countless questions about health sector efficiency as well as health equity.
6. Implications and open questions for the broader inclusion of environmental impacts in HTA
The big unknown is the extent to which we are ready to provide appropriate incentives and resources for pharmaceutical companies to become more environmentally responsible. Many of the people we interviewed stressed that including sustainability in HTA or procurement decision-making means payers have to be willing to pay more for sustainable products. Therefore, incorporating environmental impact assessment in HTA could increase the price of medicines, particularly if governments also want to avoid impacting pharmaceutical innovation. Globally, policymakers are trying to control the rising costs of healthcare with pharmaceutical prices being explicitly targeted through policies such as the drug pricing provisions in the Inflation Reduction Act (IRA) in the US[Citation15].
Including environmental sustainability in reimbursement decision-making may increase prices and, therefore, expenditure in the short term, but it will also have long-term benefits that are hard to foresee and likely difficult to quantify. HTA has the power to make the implicit investment from government explicit to prevent siloed budgets from counteracting each other (the health budget inadvertently rewarding highly emitting products while the business budget throws money at initiatives to encourage industry investment in green projects).
In conclusion, there is no reason why governments, the NHS and pharmaceutical companies cannot take meaningful action to reduce the CF of pharmaceuticals. The transformative change that is needed to avert a climate catastrophe will only happen when all stakeholders work together to redesign the business model to make sustainability the only option.
7. Expert opinion
The evidence to date suggests that pharmaceuticals contribute significantly to carbon footprints of health systems and countries. A number of features of the pharmaceutical industry contribute both to its emission intensity and the difficulty of cutting emissions while maintaining R&D productivity within the sector. Because of these features, coordinated action will be required to reduce emissions, including engagement from payers and policymakers on sector-specific issues. Nevertheless, there are many actions that pharmaceutical companies can take now, unilaterally, but they need to commit more resources to these if they are serious about achieving their net zero targets.
Barriers to progress are threefold: firstly, there is a clash within philosophies of environmental action between top-down, systematic approaches and bottom-up incrementalism, which causes paralysis. Secondly, there is a lack of interdisciplinary collaboration which means those who understand climate science are not working closely with the pharmaceutical sector in large enough numbers. Thirdly, there are weak economic, moral, and reputational incentives for meaningful action to drive progress on either of the first two barriers.
Weaknesses in research fields related to environmental sustainability in pharmaceuticals are cited as significant and a reason to delay action. While often overstated, more research in this field could develop evidence to support progress in two ways: (1) it can support prioritization of resource allocation to address emissions (e.g. should company x invest in plastic-free injectables or low-pressure sterilization methods?) and (2) to develop practical business models and assessment methods that more strongly incentivizes meaningful environmental consideration by pharmaceutical companies and health systems. In this article, we advocate for the value of the second option for progress through research in the development of environmental HTA. More broadly, this would require building a discipline at the intersection of environmental impact and as well as clinical and economic value assessment.
There is reasonable debate about our proposition of the promise of environmental HTA and whether it is a workable, or even an ethical, solution. However, engagement from the health economics community is needed to build environmental factors into the value-based reimbursement model in some form; otherwise, incentives will remain weak. Even very simple research understanding the emissions associated with individual pharmaceutical products through more product-level LCA would support change as evidence of emissions at this level of granularity is currently small. For this discipline to flourish as we suggest is necessary, it needs to involve industry; otherwise, data will be incomplete or imprecise, and it will remain an academic exercise disconnected from the practical steps that are urgently needed.
Declaration of interest
I Firth was previously employed by the Office of Health Economics, a registered charity and Independent Research Organization, which receives funding from a variety of sources, including the Association of the British Pharmaceutical Industry (ABPI). J Hitch, N Henderson and G Cookson are currently employed by the Office of Health Economics, a registered charity and Independent Research Organization, which receives funding from a variety of sources, including the Association of the British Pharmaceutical Industry (ABPI). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer declares that they are a Visiting Senior Fellow at the Office of Health Economics. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Health Care Without Harm ARUP Health care’s climate footprint: how the health sector contributes to the global crisis and opportunities for action [internet]. Healthcare Without Harm; 2019 [cited 2022 May 23]. Available from: https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_092319.pdf
- Lenzen M, Malik A, Li M, et al. The environmental footprint of health care: a global assessment. Lancet Planet Health. 2020 Jul 1;4(7):e271–9. DOI:10.1016/S2542-5196(20)30121-2
- NHS. Delivering a ‘Net Zero’ National Health Service [Internet]. 2020. [cited 2022 Jan 11]. Available from: https://www.england.nhs.uk/greenernhs/publication/delivering-a-net-zero-national-health-service/
- Department for Business. Energy & industrial strategy and the Rt Hon Chris Skidmore MP. UK becomes first major economy to pass net zero emissions law [Internet]. GOV.UK; 2019; [cited 2023 Feb 21]. Available from: https://www.gov.uk/government/news/uk-becomes-first-major-economy-to-pass-net-zero-emissions-law
- Firth I, Hitch J, Henderson N, et al. Supporting the Era of Green Pharmaceuticals in the UK [Internet]. 2022; [cited 2023 Feb 22]. Available from: https://www.ohe.org/publications/supporting-the-era-of-green-pharmaceuticals-in-the-uk/
- Emara Y, Siegert MW, Lehmann A, et al. Life cycle management in the pharmaceutical industry using an applicable and robust lca-based environmental sustainability assessment approach. In: Benetto E, Gericke K, and Guiton M, editors. Designing sustainable technologies, products and policies: from science to innovation [internet]. Cham: Springer International Publishing; 2018. [cited 2023 Feb 23]. p. 79–88. Available from: DOI:10.1007/978-3-319-66981-6_9
- EFPIA. EFPIA White Paper on Circular Economy [Internet]. 2020. Available from: https://www.efpia.eu/media/554663/circular-economy.pdf
- Ang KL, Saw ET, He W, et al. Sustainability framework for pharmaceutical manufacturing (PM): a review of research landscape and implementation barriers for circular economy transition. J Clean Prod. 2021 Jan 20;280:124264.
- Alshemari A, Breen L, Quinn G, et al. Can we create a Circular Pharmaceutical Supply Chain (CPSC) to reduce medicines waste? Pharmacy. 2020 Dec;8(4):221.
- The Greenhouse Gas Protocol. A corporate accounting and reporting standard: revised edition [Internet]. 2004 [cited 2023 Feb 22]: Available from: https://ghgprotocol.org/corporate-standard
- Marsh K, Ganz ML, Hsu J, et al. Expanding health technology assessments to include effects on the environment. Value Health. 2016 Mar;19(2):249–254.
- NICE. NICE strategy 2021 to 2026: dynamic, collaborative, excellent [Internet]. 2021. Available from: https://www.nice.org.uk/about/who-we-are/corporate-publications/the-nice-strategy-2021-to-2026
- Pinho-Gomes AC, Yoo SH, Allen A, et al. Incorporating environmental and sustainability considerations into health technology assessment and clinical and public health guidelines: a scoping review. Int J Technol Assess Health Care. 2022 Dec 13;38(1):e84. DOI:10.1017/S0266462322003282
- Greenwood Dufour B, Weeks L, De Angelis G, et al. How we might further integrate considerations of environmental impact when assessing the value of health technologies. Int J Environ Res Public Health. 2022 Jan;19(19):12017.
- Adashi EY, O’Mahony DP, Cohen IG. The Inflation Reduction Act: recasting the Medicare Prescription Drug Plans. Am J Preventive Med. Internet. 2023 Feb 25 17; [[cited 2023 Mar 17]]. Available from: https://www.ajpmonline.org/article/S0749-37972300065-X/fulltext
