ABSTRACT
Objectives
To evaluate healthcare costs, resource utilization, associated costs, and lost productivity for colorectal cancer (CRC) screening in an average-risk population
Methods
This retrospective cohort study identified average-risk individuals (50–75 years) with claims in the Optum Research Database for CRC screening test between 1 January 2014 to 31 December 2018. Index date was defined as the first date of a claim for colonoscopy, fecal immunochemical test (FIT), guaiac-based fecal occult blood test (FOBT) or multi-target stool DNA test (mt-sDNA). Screening costs were evaluated with descriptive statistics and multivariable analyses, adjusting for patient characteristics and index screening costs.
Results
In total, 903,831 individuals were identified by test groups: mt-sDNA (n = 29,614), FIT (n = 254,002), guaiac-based FOBT (n = 112,757) and colonoscopy (n = 507,458). Adjusted costs for index screening were, colonoscopy ($3,029), mt-sDNA ($752), FIT ($45), and (FOBT ($153). Adjusted costs across the six months following the index screening were $146 for colonoscopy, $329 for mt-sDNA, $306 for FIT, and $412 for FOBT. Colonoscopy had the highest costs for lost productivity.
Conclusions
Screening colonoscopy had the highest productivity loss and healthcare costs up-front, suggesting potential cost benefits for noninvasive screening modalities. The more frequent screening interval required for FIT and FOBT resulted in a higher yearly cost than colonoscopy or mt-sDNA.
Plain Language Summary
Colorectal cancer (CRC) is a prominent healthcare concern the United States, which accounted for 149,500 new cases and 52,980 deaths in 2021. Screening is effective for diagnosing the condition at earlier more treatable stages, and reducing deaths. However, screening is largely underutilized in part due to perceived cost barriers. This observational study used insurance claims data to calculate healthcare costs, resource use, and lost productivity for CRC screening in an average-risk population aged 50–75 years. A total of 903,831 individuals were identified by test groups: multi-target stool DNA test (mt-sDNA test; 29,614 individuals), fecal immunochemical test (FIT; 254,002 individuals), guaiac-based fecal occult blood test (FOBT; 112,757 individuals) and colonoscopy (507,458 individuals). Adjusted costs for initial screening were $3,029 for colonoscopy, $752 for mt-sDNA, $45 for FIT, and $153 for FOBT. Adjusted colonoscopy-related costs combined across the six months following the initial screening were $146 for the colonoscopy cohort, $329 for mt-sDNA, $306 for FIT, and $412 for FOBT. Colonoscopy had the highest costs for lost productivity. Overall, screening colonoscopy was accompanied by the highest productivity loss and up-front costs, suggesting potential cost benefits for noninvasive screening modalities – mt-sDNA, FIT, and FOBT; however, the more frequent screening interval required by FIT and FOBT resulted in a higher estimated average yearly screening cost.
1. Introduction
Colorectal cancer (CRC) is a leading cause of cancer morbidity and mortality in the United States, with 52,980 deaths and 149,500 new CRC diagnoses projected for 2021 [Citation1]. Increasing diagnoses of CRC at earlier, more treatable stages and reducing death rely on screening, which is effective but underutilized [Citation2]. Substantial direct and indirect costs, which are perceived as potential barriers, are incurred in both initial and follow-up CRC screening [Citation3].
Multiple CRC screening modalities have been endorsed in current practice guidelines, including the American Cancer Society (ACS), the National Comprehensive Cancer Network (NCCN) and the United States Preventative Service Task Force (USPSTF) [Citation4–6]. Among the recommended screening modalities are colonoscopy, fecal immunochemical test (FIT), guaiac-based fecal occult blood test (FOBT), and the multi-target stool DNA test (mt-sDNA; marketed as Cologuard®). The guidelines recommend following up positive results from the other screening modalities with a colonoscopy [Citation4,Citation5].
The costs associated with screening in the period following market availability of mt-sDNA in the US market are largely uncharacterized. An understanding of the cost burden associated with different elements of CRC screening – the initial cost of the procedure, and all costs related to colonoscopy both for the initial screening and after a noninvasive procedure – and the cost impact of these procedures on productivity could aid in the implementation of CRC screening programs.
The aims of the study were to assess healthcare costs associated with different CRC screening modalities for average-risk patients. Direct costs were calculated for the initial screening, as well as those for subsequent follow-up colonoscopies required within the first six months following the initial screening. Indirect costs were also calculated to examine patients’ lost productivity and wages in those with an index colonoscopy screening or those with follow-up colonoscopies after a positive result from another screening modality.
2. Methods
2.1. Study design
This retrospective cohort study used medical and pharmacy claims data and health plan enrollee information to identify individuals who had at least one CRC screening procedure between 1 January 2014 and 31 December 2018 (study period). The latest complete year of enrollment was used as the year of analysis for each patient. The index date and screening modality (i.e. colonoscopy, FIT, guaiac-based FOBT or mt-sDNA) were defined on the first date of a claim for a CRC screening procedure. Institutional Review Board approval or waiver was not required as the database contained only de-identified information for the study patients. Patient privacy was strictly preserved, and compliance with relevant Health Insurance Portability and Accountability Act (HIPAA) data handling rules was observed throughout.
2.2. Data source
Study data were queried from the Optum Research database (ORD), a nationally representative repository of commercial and Medicare Advantage with Part D (MAPD) medical and pharmacy claims and enrollment information for more than 67 million enrollees across the United States.
At the time of this study, approximately 9% of the commercially enrolled and 17% of the MAPD population were represented in the ORD.
2.3. Study population
2.3.1. Inclusion criteria
Adults aged between 50 and 75 years in the calendar year of the study with continuous health plan enrollment with medical and pharmacy benefits for at least 12 months prior to (baseline period), and at least 12 months after the index date (follow-up period) were included. Data for patients with less than 12 months of follow-up due to death were retained and included in the analysis.
2.3.2. Exclusion criteria
Adults with an elevated risk for CRC were excluded. Elevated risk was indicated by the presence of ≥ 1 medical claim during the baseline period with a diagnosis code in any position on the claim for: adenoma, sessile serrated polyp, and personal history of or current CRC. A diagnostic code for family history of CRC, family history of gastrointestinal cancer, or a diagnostic code for inflammatory bowel disease at any time during the study duration were also indicators of elevated risk, and resulted in exclusion from the study [Citation5].
2.3.3. Screening modality cohorts
Screening modality cohorts were determined in accordance with USPSTF/ACS guidelines. Individuals were assigned to cohorts based on the screening modality received on the index date: colonoscopy, mt-sDNA, FIT, or FOBT. Screening modalities were identified via Current Procedural Terminology (CPT) codes and International Classification of Diseases, ninth revision/tenth revision (ICD-9/ICD-10) procedural codes during the analysis year.
2.4. Outcome measures
2.4.1. Screening-related healthcare costs
Screening-related healthcare costs were calculated separately over two time periods: 1) for the index screening procedure, and 2) for any follow-up colonoscopy procedures with related services occurring within the first six months of the follow-up period (excluding the index screening procedure). Direct costs were attributed to the colonoscopy based on applicable diagnosis and procedure codes (including anesthesia, pathology/laboratory, and prescription fills for bowel preparation products) occurring 14 days prior to and following the colonoscopy procedure, including any inpatient stays or emergency visits. Costs associated with non-colonoscopy screening modalities were captured on the date of service. Screening-related total healthcare costs (medical plus pharmacy costs) were Consumer Price Index (CPI)-adjusted to 2019 US dollars and split into patient-paid and plan-paid amounts [Citation7]. Medical costs included ambulatory (physician office and hospital outpatient), emergency services, inpatient, and other costs (including those for laboratory services. Patient-paid screening costs were calculated as the total patient-paid amount for each screening modality.
2.4.2. Yearly screening cost calculation
An estimated yearly screening cost was calculated to account for the differences in recommended screening intervals for each screening modality. Intervals used for this calculation were: every year for FIT and FOBT, every three years for mt-sDNA, and every 10 years for colonoscopy. Yearly screening costs were calculated by dividing by the recommended screening interval for each modality.
This calculation also accounted for the estimated costs of adverse events (AEs) associated with colonoscopy. A previous study estimated that 4.1% of patients undergoing colonoscopy would incur a gastrointestinal- or cardiovascular-related AE, with a mean cost of $2,914 [Citation8]. These AE estimates were multiplied by the colonoscopy rate (at index or in the six-month follow-up period) for each cohort and the resulting mean AE cost was added to the combined mean cost per screening event, which included both the index screening and follow-up colonoscopy within each cohort.
2.4.3. Productivity loss (Indirect Costs)
For patient productivity (time) loss, indirect costs for colonoscopy were estimated based on assumptions about the number of hours required for the colonoscopy and included lost time from work, home, or travel time. A colonoscopy was assumed to take approximately eight hours in an office or outpatient setting, and eight hours per admission day for overnight inpatient settings (based on previously published algorithms) [Citation9]. A sensitivity analysis was conducted to reflect overall missed time (including time for colonoscopy preparation) and not just work hours by assigning 12 hours to office or outpatient settings and 24 hours to inpatient settings. The cost associated with productivity loss was calculated as the number of hours lost due to healthcare encounters multiplied by the median wage for all occupations in the United States derived from the Bureau of Labor Statistics (BLS) [Citation10]. To test the sensitivity of the estimates to the wage used, an alternative measure of patient-time cost was created using the federal minimum wage [Citation11].
2.5. Statistical analysis
Descriptive statistics for dichotomous and polychotomous variables were reported as numbers and percentages and means and standard deviations (SD) were reported for continuous variables. Results were stratified by screening cohort; bivariate comparisons of pre-index characteristics and outcomes measures were calculated with a two-sample t-test for continuous measures for pair-wise comparisons, an F-test/ANOVA for continuous measures across all cohorts, and a Pearson chi-square test for binary measures. Cost outcomes on the index (initial screening) date were analyzed using a generalized linear model (GLM) with a gamma distribution and log link to account for the skewed statistical distribution. Direct costs over the six-month follow-up period were analyzed using a two-part model: a logistic regression model to predict the occurrence of any cost, and among those with costs, a GLM with a gamma distribution and log link. A predicted direct cost for the cohorts were calculated based on the combined models. The models controlled for covariates including age group, sex, region, index year, and Charlson comorbidity score to generate cost estimates for each screening modality adjusted for patient characteristics. Alpha was set a priori at 0.05 for statistical significance. While p-values are presented, the large sample sizes resulted in statistical significance for most comparisons, so interpretation based on meaningful or clinical differences was more helpful. All analyses were conducted with SAS statistical software 9.4 (SAS Institute, Cary, NC, U.S.A).
3. Results
3.1. Study population by index screening modality
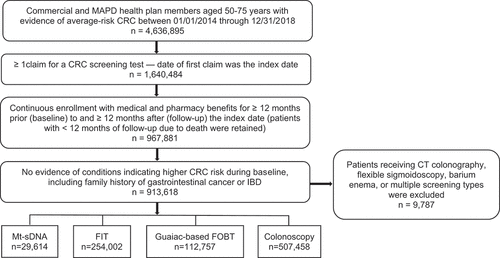
Overall, 4,636,895 health plan enrollees of average CRC risk, aged 50–75 years, were identified, with 35.4% (n = 1,640,484) having ≥1 claim for a CRC screening procedure during the study period. A total of 967,881 patients met the health plan enrollment requirements, and 913,618 had no evidence of CRC risk factors, with a final study population of screening cohorts of 903,831 individuals. As shown in , the screening modality cohorts were mt-sDNA (n = 29,614), FIT (n = 254,002), FOBT (n = 112,757) and colonoscopy (n = 507,458).
3.2. Patient demographics
The mean (SD) age of the overall study sample was 61.4 (7.7) years. The average age of individuals who received a colonoscopy was 60.5 (7.7) years, which was less than the overall sample and for each of the other screening modality cohorts, p < 0.001 (). Females made up a larger proportion of the overall study population (53.9%), and the proportions of females were greater in the mt-sDNA and FIT cohorts than in the colonoscopy cohort, p < 0.001. Overall, 60.8% of the patients had commercial health insurance, and 39.2% had MAPD coverage. Commercial insurance was reported in a larger proportion (66.2%) of patients in the colonoscopy cohort relative to FOBT (60.0%), FIT (53.8%), and mt-sDNA (33.7%) cohorts, p < 0.001. About two-thirds (66.3%) of patients in the mt-sDNA cohort had MAPD coverage compared to 33.8% in the colonoscopy cohort. The largest proportions of patients overall (45.0%) and across all cohorts were from the South, followed by the Midwest (27.4%). Approximately 95% of individuals overall and across all the screening cohorts were in urban settings. Point of Service plans accounted for 47.7% overall, and for 52.0% of the colonoscopy cohort. More than two-thirds of the patients overall, and across the screening cohorts had a baseline Charlson comorbidity score of 0, and about one-quarter overall and across the cohorts had a score of 1–2.
Table 1. Demographic and clinical characteristics at baseline by screening modality.
3.3. Direct screening-related healthcare costs
At index, the mean total combined medical plus pharmacy cost was highest in the colonoscopy cohort ($2,452) followed by the mt-sDNA ($493), FOBT ($57) and FIT ($24) cohorts, p < 0.001 (). The mean patient-paid costs were also highest for the colonoscopy cohort ($178), versus $16, $2, and $5 for mt-sDNA, FIT, and FOBT cohorts, respectively. Mean specific services fees incorporated in the cost of colonoscopy were anesthesia ($344), pathology/laboratory ($241), and bowel preparation products ($26). Pharmacy costs were a small part of the total colonoscopy costs (mean [SD] $9 [$26]).
Table 2. Screening-related healthcare costs for index screening.
During the six-month follow-up period, mean total costs for follow-up colonoscopies among the cohorts were lowest for the colonoscopy cohort ($151). Costs for colonoscopies during the follow-up period were also low in the mt-sDNA cohort ($280), the FIT cohort ($285), and the FOBT cohort ($429) as shown in . There were only a small proportion of patients (13.6% of mt-sDNA, 13.9% of FIT, 16.1% of FOBT, and 3.7% of colonoscopy) who had colonoscopy-related costs during the follow-up period, which may explain the low costs. While these proportions are similar to what would be expected based on overall positivity rates [Citation12]; we cannot identify the exact reason the follow-up colonoscopy was performed.
Table 3. Follow-up colonoscopy-related healthcare costs during the six-month follow-up period.
Overall, colonoscopy costs during the six-month follow-up period for the screening cohorts did not outweigh initial testing costs. Mean combined costs of the index screening plus follow-up colonoscopy within each cohort were $773 (mt-sDNA), $309 (FIT), $486 (FOBT), and $2,603 (colonoscopy).
3.3.1. Yearly screening costs
To account for the effect of recommended screening interval on costs, the unadjusted mean cost per screening event (including follow-up colonoscopy) was used as the base ($773 [mt-sDNA], $309 [FIT], $486 [FOBT], and $2,603 [colonoscopy]) (). Combining the rate of follow-up colonoscopy found in our study cohorts with the literature on AE rates and AE costs, the estimated mean colonoscopy-associated AE cost per screening event was $16 (mt-sDNA), $17 (FIT), $19 (FOBT), and $119 (colonoscopy). After adding the estimated AE costs to the base screening event cost, yearly screening costs were $263 (mt-sDNA), $326 (FIT), $505 (FOBT), and $272 (colonoscopy).
Table 4. Total Yearly Screening Cost Including Estimated Colonoscopy Adverse Event (AE) Related Costs.
3.4. Productivity loss (Indirect Costs)
At index, patients in the colonoscopy cohort had mean lost productivity of $373 (BLS) or $105 (minimum wage). Indirect costs for other screening cohorts did not exceed a few dollars, with no lost productivity in the mt-sDNA cohort. During the six-month follow-up period, the amount of productivity lost by patients in the colonoscopy cohort was $8 (BLS) and $2 (minimum wage) while patients in the other cohorts had modest increases: mt-sDNA ($54 [BLS] and $15 [minimum wage]), FIT ($45 [BLS] and $13 [minimum wage]) and FOBT ($49 [BLS] and $14 [minimum wage]) as shown in . Applying the sensitivity analysis increased the mean (SD) estimated productivity loss for index screening to $562 ($357) for the colonoscopy cohort and increased costs by more than 50% for the other cohorts over the six-month follow-up (data not shown). Mean BLS estimates were $86 ($586) for mt-sDNA, $74 ($991) for FIT, and $81 ($553) for FOBT. Similar results were seen for the estimates calculated using minimum wage.
Table 5. Cost of lost productivity (hours) for colonoscopy-related services.
3.5. Multivariable analysis
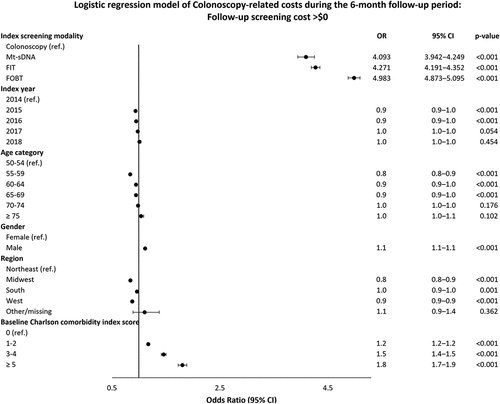
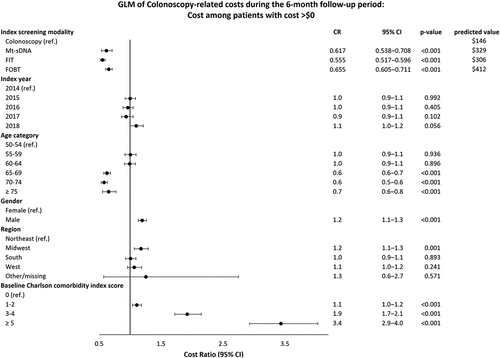
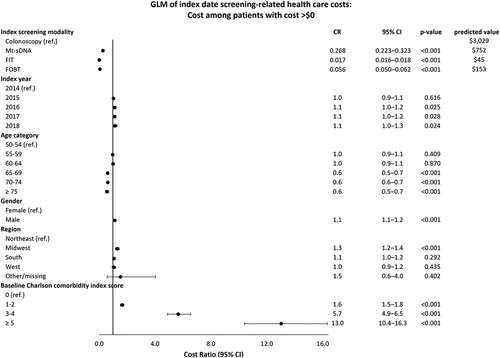
In the logistic regression model, patients with laboratory-based screening modalities had lower odds of having direct costs > $0 compared with patients who received a colonoscopy (OR ranged from 0.021 to 0.035, p < 0.001, ). After adjustment for patient characteristics, direct costs on the index screening date were $3,029 for colonoscopy, $752 for mt-sDNA, $45 for FIT, and $153 for FOBT (). Compared to the colonoscopy cohort, the mt-sDNA, FIT, and FOBT cohorts were at least four times more likely to have direct costs > $0 for follow-up colonoscopies (odds ratio [OR] ranged from 4.1 for mt-sDNA to 5.0 for FOBT) (); however, among patients with follow-up colonoscopy costs > $0, the laboratory-test screening cohorts had direct costs lower than those who received an index colonoscopy (OR = 0.62 for mt-sDNA, 0.56 for FIT, and 0.66 for FOBT) (). The adjusted follow-up colonoscopy direct costs combined across patients with and without costs over the follow-up period were $146 for the colonoscopy cohort, $328 for mt-sDNA, $306 for FIT, and $412 for FOBT ().
Figure 2a. Multivariable model of index date screening-related healthcare costs.
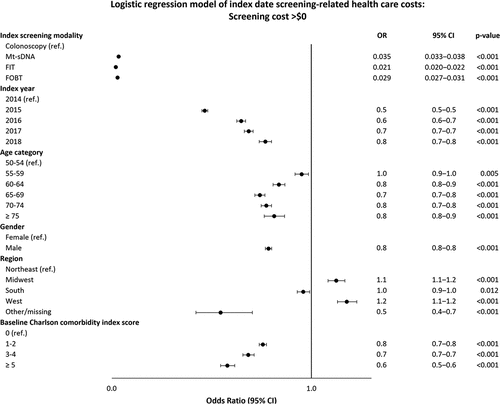
Figure 2b. (Continued).
4. Discussion
The findings of this study provide insights into the costs incurred for patients receiving different screening modalities, the time expended, and productivity lost while participating in colonoscopy procedures.
At index, patients in the colonoscopy cohort incurred the largest screening costs, relative to the other cohorts, not unexpectedly, because of the preparation, laboratory fees, anesthesia, and invasive nature of the procedure. During the six-month follow-up period, as expected, follow-up colonoscopy costs incurred by the colonoscopy cohort were lower than other cohorts; however, among patients who later required additional colonoscopy procedures, costs were higher in the colonoscopy cohort compared with the patients screened with other treatment modalities. It is possible that for the colonoscopy cohort, follow-up colonoscopies may occur due to failed prep for the initial screening or other complicating factors. While any repeat colonoscopies within 14 days of the index procedure would have been included as part of the index episode, colonoscopies scheduled after those 14 days would have been counted as part of the ‘follow-up’ colonoscopy costs. The results of the study are generally consistent with other estimates of costs for different types of colorectal cancer screening but provide more detail about the cost components at a population level [Citation8,Citation13].
One difficulty in the interpretation of cost results is that while they apply to the index screening event plus the follow-up colonoscopy (occurring within the six months following the index screening); they do not account for how often each screening modality is recommended. A more comprehensive cost view would also add in costs of AEs associated with colonoscopies. The estimated yearly screening costs calculated in this study, after accounting for recommended screening interval and AEs, suggest that the overall cost burden is driven by both the direct costs of the procedure and by the screening interval, with the shorter intervals associated with higher mean yearly costs per patient.
Colonoscopies had high indirect (productivity loss) costs due to lost hours for the procedure, ranging from approximately $105 to $373; given a national average wage index of $52,146 in 2018 [Citation14], this could represent a significant burden beyond just the patient-paid component for the test.
These results, while preliminary, have important implications for patients, providers, and payers, who are the key stakeholders in a CRC screening program. Overall, the costs of having an index screening colonoscopy were higher than index screening through noninvasive modalities. Even after accounting for follow-up colonoscopies, the total costs for the index colonoscopy cohort were higher than for cohorts of patients whose index screening was via laboratory-based tests.
4.1. Limitations
The limitations of this study are similar to those in any large claims database studies including limited granularity of clinical data, no CRC screening test results, and the potential for misclassification of high-risk patients as average-risk for CRC, given the diagnosis codes may not indicate the presence of disease (e.g. rule-out diagnosis, incorrect coding). Prior to the availability of a CPT code for mt-sDNA, identification relied on the use of generic FIT CPT codes combined with the name of the laboratory provider. A variable baseline period, consisting of the period in which patients were continuously enrolled prior to the index date, was used to assess prior screening patterns. A variable follow-up with a minimum of six months following the index date was used to assess outcomes including screening patterns. For uniformity, results were adjusted so everyone had six months of follow-up, which could have resulted in missed outcomes, especially for patients with longer follow-up periods. In addition, the varying levels of sensitivity of each screening modality (89% for colonoscopy, 74% for FIT, 50–75% for FOBT, and 93% for mt-sDNA) [Citation15] may impact the follow-up cost estimates. For screening modalities that were less sensitive (i.e. FIT and FOBT), the cost estimates did not account for either missed cancers that resulted in colonoscopies after the initial six months or for significant delays in scheduling follow-up colonoscopies. Costs for productivity loss were only computed for the colonoscopy procedure. In addition, while the costs assumed eight hours for colonoscopies performed in the outpatient/office setting, this underestimates the time commitment from the patient, particularly for preparation in the days prior to the procedure, as well as additional caregiver time on the date of the procedure. The database used for this study includes a combination of commercial and MAPD insurance claims, and may not be generalizable to populations with Medicaid, military insurance, or no insurance; however, the size and scope of the data repository can be used to approximate real-world costs and outcomes for CRC screening.
5. Conclusions
Screening is the most effective measure for detecting pre-symptomatic CRC and reducing CRC-related death; however, economic barriers to CRC screening participation may include direct costs, missed work, and lost wages. Screening colonoscopy had the highest up-front direct costs and productivity loss compared with laboratory-based screening methods. While additional studies are needed, our results suggest a potential cost benefit for noninvasive screening modalities; however, the more frequent screening interval required by FIT and FOBT resulted in a higher estimated average yearly screening cost.
Abbreviations
| AE | = | adverse event |
| ACS | = | American Cancer Society (ACS) |
| BLS | = | Bureau of Labor Statistics |
| CPI | = | Consumer Price Index |
| CPT | = | Current Procedural Terminology |
| CRC | = | colorectal cancer |
| FIT | = | fecal immunochemical test |
| FOBT | = | guaiac-based fecal occult blood test |
| GLM | = | generalized linear model |
| ICD-9/ICD-10 | = | International Classification of Diseases, ninth revision/tenth revision |
| MAPD | = | Medicare Advantage with Part D |
| mt-sDNA | = | multi-target stool DNA test |
| NCCN | = | National Cancer Society (NCCN) |
| ORD | = | Optum Research Database |
| SD | = | standard deviation |
| USPSTF | = | United States Preventative Service Task Force (USPSTF) |
Declaration of interest
N Engel-Nitz is currently employed by Optum and holds stock in UnitedHealth Group and AbbVie. L Miller-Wilson was an employee of Exact Sciences at the time the study was conducted and holds stock in Sanofi and Exact Sciences. L Le is currently employed by Optum and holds stock in UnitedHealth Group. D Fisher was employed by Duke University at the time the study was conducted and is currently employed at Eli Lilly and Company and has served as a consultant for Exact Sciences and Guardant Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval of the version to be published. N Engel-Nitz, L Miller-Wilson and D Fisher were involved in the conception and design of the study and data interpretation. N Engel-Nitz and L Le were involved in the acquisition of data. N Engel-Nitz and L Le were involved in the data analysis.
Data sharing statement
The data contained in our database contains proprietary elements owned by Optum and, therefore, cannot be broadly disclosed or made publicly available at this time. The disclosure of this data to third party clients assumes certain data security and privacy protocols are in place and that the third-party client has executed our standard license agreement which includes restrictive covenants governing the use of the data.
Human subjects’ protection
Throughout the process, patient privacy was preserved, and researchers complied strictly with all applicable Health Insurance Portability and Accountability Act data management rules and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Acknowledgments
Authors would like to acknowledge Felix Cao, Lynn Wacha, Priyanka S. Koka, Yiyu Fang, Jaycee L. Karl, and Damon L. Van Voorhis for their assistance with programming and construction of the analytic datasets; Timothy Bancroft and Lee Brekke for consultation on the analyses; and Bernard Tulsi and Deja Scott-Shemon for medical writing assistance.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020 Jan;70(1):7–30. doi:10.3322/caac.21590
- Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. Dec 1 2018;178(12):1645–1658. DOI:10.1001/jamainternmed.2018.4637
- Subramanian S, Tangka FKL, Hoover S, et al. Costs of colorectal cancer screening provision in CDC’s colorectal cancer control program: comparisons of colonoscopy and FOBT/FIT based screening. Eval Program Plann. 2017 Jun;62:73–80. DOI:10.1016/j.evalprogplan.2017.02.007.
- American cancer society guideline for colorectal cancer screening for people at average risk. Accessed at https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html on Dec 1, 2021.
- National Comprehensive Cancer Network. NCCN guidelines colorectal cancer screening, version 1.2018. Accessed at https://www.nccn.org/search-result?indexCatalogue=nccn-search-index&searchQuery=colorectal%20cancer&wordsMode=AllWords Dec 2, 2021.
- USPST F, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: us preventive services task force recommendation statement. JAMA. May 18 2021;325(19):1965–1977. doi:10.1001/jama.2021.6238.
- U.S. Department of Labor, Bureau of Labor Statistics. Consumer price index. medical care. Series ID: CUUR0000SAM. (WA) DC: U.S. Dept. of Labor, Bureau of Labor Statistics. http://data.bls.gov/cgi-bin/surveymost?cu. Accessed Dec 6, 2021.
- Fisher DA, Princic N, Miller-Wilson LA, et al. Costs of colorectal cancer screening with colonoscopy, including post-endoscopy events, among adults with Medicaid insurance. Curr Med Res Opin. 2022 May;38(5):793–801. doi:10.1080/03007995.2022.2049163
- Dong MH, Kalmaz D, Savides TJ. Missed work related to mid-week screening colonoscopy. Dig Dis Sci. 2011 Jul;56(7):2114–2119.
- Occupational Employment and Wage Statistics. US Bureau of Labor Statistics. Accessed at https://www.bls.gov/oes/on Jul 2, 2021.
- US Department of Labor. Wage and hour division. Accessed at https://www.dol.gov/agencies/whd/minimum-wage on Jul 2, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. Jul 10 2014;371(2):187–188. doi:10.1056/NEJMc1405215
- Subramanian S, Tangka FKL, Hoover S, et al. Comparison of program resources required for colonoscopy and fecal screening: findings from 5 years of the colorectal cancer control program. Prev Chronic Dis. Apr 25 2019;16:E50.10.5888/pcd16.180338.
- Social Security Administration. National Average Wage Index. www.ssa.gov/oact/cola/AWI.html. Accessed on Sept 13, 2022.
- NCCN clinical practice guidelines in oncology (NCCN Guidelines): colorectal cancer screening version 2.2021. National Comprehensive Cancer Network. https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf. Accessed Jan 28, 2022.