ABSTRACT
Introduction
Value-based pricing (VBP) can be a promising tool for optimizing drug prices. However, there is no consensus on the specific value elements and pricing method that should be used for VBP.
Areas covered
We performed a systematic review and narrative synthesis to investigate the value elements and pricing method for VBP. The main inclusion criterion was that value elements, VBP method, and estimated prices for actual drugs were reported. We performed a search in MEDLINE and ICHUSHI Web. Eight articles met the selection criteria. Four studies adopted the cost-effectiveness analysis (CEA) approach and the others used different approaches. The CEA approach included the value elements of productivity, value of hope, real option value, disease severity, insurance value in addition to costs and quality-adjusted life years. The other approaches used efficacy, toxicity, novelty, rarity, research and development costs, prognosis, population health burden, unmet needs, and effectiveness. Each study used individual methods to quantify these broader value elements.
Expert opinion
Both conventional and broader value elements are used for VBP. To allow VBP to be widely applied to various diseases, a simple, versatile method is preferable. Further research is needed to establish VBP method which enables to incorporate broader values.
1. Introduction
In recent years, efforts have been made to reflect the broader value of healthcare items, including benefits to patients, rather than using the conventional approach of focusing on costs commensurate with health outcomes [Citation1]. In 2018, the International Society for Pharmacoeconomics and Outcomes (ISPOR) Special Task Force considered the elements of value in healthcare and identified a series of elements, the so-called ISPOR value flower [Citation2]. The ISPOR value flower was based on a discussion of factors that should be considered in assessing the value of healthcare from the perspective of multiple stakeholders. The frameworks for assessing drugs on the basis of value elements have also been studied. For example, the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), National Comprehensive Cancer Network (NCCN), and Memorial Sloan Kettering Cancer Center (MSKCC) proposed frameworks for drugs in oncology [Citation3–6]. Jommi et al. considered how to implement value-based pricing (VBP) and proposed four VBP processes: identification of value domains, measurement of value, aggregation of measures, and conversion of value into prices [Citation7].
It is desirable that drug pricing systems consider values when determining drug prices. Some developed countries have systems that allow pharmaceutical companies to propose drug prices that reflect values. On the other hand, in Japan it is difficult to reflect values in drug prices because the government has the authority to determine it and the process is too strict. Therefore, a system that is applicable to the Japanese pricing system is considered to be applicable also to other countries.
Drug prices in Japan are determined in accordance only with the cost accounting system, which is based on manufacturing costs, marketing, research and other expenses, or by considering the price of comparable drugs [Citation8], and prices are revised by referring to the actual market prices and market size. These pricing processes do not comprehensively reflect the broader values that drugs can provide to patients and society. Japanese pharmaceutical companies may be reluctant to develop new drugs and the Japanese drug market may be less attractive for international pharmaceutical companies if Japanese pricing rules do not fully appraise broader values when determining prices. In addition, medical care costs in Japan continue to rise because of the advancement of medical treatments, including the emergence of expensive biological and regenerative medical products and the aging of the population, making the optimization of drug costs an urgent issue. However, simply lowering drug prices as a means of reducing medical care costs regardless of drugs’ value may further reduce incentives for research and development on important drugs, resulting in limited access to high quality medical care with high value for patients. Furthermore, medical care costs are borne by patients and insurers in Japan, so a pricing system is likely to be more acceptable if drugs with higher prices are perceived by both parties as having higher value for them, e.g. if an innovative ingredient were to make a relatively high contribution to reducing the burden on informal caregivers or solving unmet needs. In response to these challenges, a pricing method that aggregates broader values in addition to conventional value elements could be a promising tool for optimizing drug prices.
It is crucial to propose and develop VBP methodology that can tackle such issues. However, to date, there is no consensus on the specific value elements and pricing methods that should be used in VBP. In addition, although the ISPOR value flower advocates including a broader range of value elements, there are challenges to its universal use because several elements included in the ISPOR value flower such as value of insurance, severity of disease, value of hope, and value of real option are not clearly defined. All these elements are considered to be important, but a method for quantifying them in monetary terms has not yet been established [Citation2].
It is essential to gain insights into the kind of value elements that are used for VBP, how they can be quantified, and how the price of a drug can be estimated based on them to realize a practical VBP methodology that is applicable to an actual drug pricing system. Therefore, we performed a systematic review to investigate the value elements used in VBP, the method used for quantifying broader values, and the pricing methods used to understand the current status of VBP frameworks that would be suitable for pricing drugs in Japan.
2. Materials and methods
We registered the protocol of this systematic review on the International Prospective Register of Systematic Reviews (PROSPERO: 42022344182) [Citation9] and report this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation10]. The purpose of this systematic review was to review value elements, which were expected to comprise various types of qualitative information, and pricing methods used for VBP. Therefore, the extracted information was synthesized narratively rather than quantitatively. The present review conformed with the PRISMA statement, although some of the items in the PRISMA checklist were not applicable.
2.1. Selection criteria
The selection criteria for identifying eligible studies are shown in . Briefly, the criteria for eligible studies included the following: (1) the drugs were used as interventions; (2) the estimated prices of VBP were reported as outcomes; and (3) the value elements and pricing method used for VBP were described in the article. We defined VBP as the quantification of broader value elements other than conventional cost elements and quality-adjusted life years (QALYs), estimation of the price of drug (including an estimation of the monetary value of drug) that used broader value elements or pricing of drug described as VBP in the article. To assess the quantification of price based on the value of drugs themselves, studies on decision making of reimbursement and payment were excluded. We included studies published from 1 January 2017 to 30 April 2022.We were interested in reviewing recent research triggered by the publication of the ISPOR value flower in 2018 [Citation2], so the search period started in 2017, i.e. one year before the publication, so as not to miss the article and related discussions.
Table 1. Study selection criteria.
2.2. Literature search
To identify potentially relevant studies, we conducted a systematic search in MEDLINE (via the PubMed interface), and ICHUSHI Web (a database for articles written in Japanese) on 26 May 2022, and performed additional hand searches to ensure that relevant articles had not been missed. The detailed search terms and retrieval records are shown in Tables S1 and S2.
First, two reviewers independently reviewed the titles and abstracts of the articles found in the search and excluded records that did not meet the inclusion criteria. The full texts of the screened articles were obtained, and the two reviewers independently assessed whether the studies met the eligibility criteria. Discrepancies between the two reviewers were resolved through discussions with a third reviewer.
2.3. Data extraction
Two independent reviewers extracted the pricing method and value elements used for VBP from eligible studies. We defined value elements as elements that were used for drugs pricing and that may benefit stakeholders such as patients and medical staff, even if the original paper did not refer to them as value elements. For the pricing method, the same reviewers extracted data on the method used to quantify or define value elements in monetary terms and the method used to estimate the drug price. In addition, information was extracted on the population, intervention, and estimated price. Discrepancies between the two reviewers were resolved through discussions with a third reviewer. Our objective was to gain an overview of the method and value elements used for drug pricing rather than to obtain quantitative information, so we narratively summarized the findings and did not perform statistical analyses.
We performed a narrative synthesis of the extracted data but did not perform a quality assessment of the articles because the eligibility criteria allowed inclusion of studies with various designs and bias risk assessment tools would have been difficult to use.
3. Results
3.1. Search results
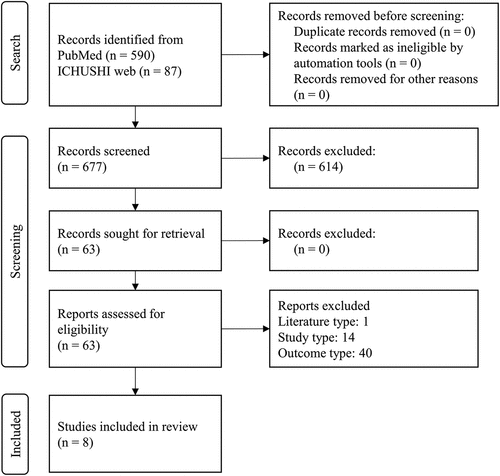
A flow diagram of the literature selection process is shown in . The initial search identified a total of 677 articles, 590 from MEDLINE and 87 from ICHUSHI Web. By reviewing the titles and abstracts, we selected 63 articles for full-text review. Of these 63 articles, 55 were excluded (1 was a review article [Citation11], 14 were not about the study of VBP [Citation12–25], and 40 did not report the outcome of interest [Citation26–65]), and 8 met the selection criteria [Citation66–73]. There were no studies that met the inclusion criteria but were excluded from the narrative synthesis.
3.2. Data extraction
The data extracted from each eligible article are shown in , and additional information on the studies is presented in Supplementary Table S3.
Table 2. Value elements, method used to quantify value elements and pricing method of the included studies.
3.2.1. Background of the eligible studies
The target diseases in the studies described in the eligible articles were as follows: cancer, n = 3 (rare cancer, n = 1 [Citation72]; any cancer, n = 1 [Citation69]; and early-stage Hodgkin’s lymphoma, n = 1 [Citation66]); hemophilia, n = 1 [Citation67]; Alzheimer’s disease, n = 1 [Citation68]; neovascular age-related macular degeneration (nAMD), n = 1 [Citation70]; myocardial infarction, n = 1 [Citation71]; and migraine, n = 1 [Citation73].
The studies were categorized according to the aim of VBP, as follows: perform a cost-effectiveness analysis (CEA) or value quantification of novel therapies (gene therapy, disease-modifying treatment) [Citation67,Citation68,Citation70]; evaluate new forms of use (preventive therapies) [Citation73]; assess previously proposed VBP frameworks [Citation71,Citation72]; quantify the specific value element [Citation69]; and explore the impact on the results of CEA when broader values were considered [Citation66].
3.2.2. Pricing methods
Methods used for estimating drug prices from value elements were summarized for eligible studies except Reed et al. [Citation69], which quantified the specific value elements rather than estimating the drug price. In four studies, drug prices were calculated from cost-effectiveness perspective based on CEA approach using Markov model to estimate utilities (QALYs) and costs [Citation66–68,Citation73]. These four studies used different formulae to determine the price (monetary value). Two studies, Ma et al. [Citation66] and Prados et al. [Citation68], used the concept of net monetary benefit and converted the value of the drug to monetary terms by subtracting the cost from the monetary value of the accumulated benefits. In contrast, the other two studies, Ten Ham et al. [Citation67] and Lipton et al. [Citation73], used the concept that the VBP price is the maximum price at which the drug is considered to be cost-effective from the perspective of a certain willingness to pay (WTP).
Among the studies that did not apply the CEA approach, Berkowitz et al. [Citation70] used a discount cash flow analysis to estimate the discounted present value of a new gene therapy drug capable of treating nAMD with a single dose as the total lifetime cost of existing therapy at a discounted rate; Levaggi et al. [Citation71] discussed the price that would result if a pharmaceutical company attempted to maximize profits according to the payer’s reimbursement policy, based on the assumption that the price of a drug is its utility multiplied by the corresponding monetary term per unit utility; and Lanitis et al. [Citation72] used MSKCC’s Drug Abacus framework to estimate the drug price by performing linear operations with weighted scores of the value elements defined in the framework.
3.2.3. Value elements
The major value elements used in the studies that adopted the CEA approach were as follows: costs, n = 4 [Citation66–68,Citation73]; productivity losses, n = 4 [Citation66–68,Citation73]; QALYs, n = 4 [Citation66–68,Citation73]; value of hope, n = 1 [Citation66]; real option value, n = 1 [Citation66], severity of disease, n = 1 [Citation68]; and insurance value, n = 1 [Citation68]. Here, costs included all conventional cost elements, i.e. both medical and non-medical costs. Burden on informal caregivers was counted as productivity loss because the original articles provided the details of this element as productivity loss of the caregiver. For QALYs, we included the elements listed as quality of life in the original articles.
In the articles that did not adopt the CEA approach, Reed et al. [Citation69] used value of hope; Berkowitz et al. [Citation70], costs; Levaggi et al. [Citation71], effectiveness; and Lanitis et al. [Citation72], efficacy, toxicity, novelty, rarity, research & development (R&D) cost, prognosis, population health burden, and unmet needs.
3.2.4. Method for quantifying broader value elements
We summarized methods used for quantifying broader value elements () except QALYs, costs and productivity loss because their methods were already well established. Ma et al. [Citation66] referred to previous studies and regarded value of hope as the WTP for 1-year survival, whereas Reed et al. used a discrete choice experiment and a logit model to quantify the value of hope for a cancer patient by estimating the monetary value of achieving 10-year survival with 5%, 10%, or 20% probability compared with 0% probability [Citation69]. Ma et al. used mortality data from the Social Security Administration cohort life tables [Citation74] and defined the real option value as the health gains from future new technologies that may become available to patients because the new treatment enables them to live longer [Citation66]. As for insurance value, Prados et al. used actuarial methods and considered both the financial insurance value and the insurance value to unafflicted individuals [Citation68]. The definitions of value elements in the MSKCC Drug Abacus framework used by Lanitis et al. [Citation72] are shown in ; each element was scored and converted to monetary terms by multiplying it by each weight.
4. Discussion
4.1. Value elements
This study was a systematic review of studies on VBP of drugs and evaluated the broader value elements and the respective pricing methods. All four studies that applied the CEA approach considered productivity losses in addition to costs and QALYs; all three elements are considered to be standard value elements in the CEA approach because of their quantifiability. However, the studies differed in whether they included productivity losses and QALYs for caregivers in addition to patients and how they calculated productivity losses. Prados et al. [Citation68] considered caregivers’ QALYs and productivity losses, and Ma et al. [Citation66] also considered the reduction in loss of quality of life by caregivers as spillover health effects on them; in contrast, the other two studies only included patient QALYs and productivity losses [Citation67,Citation73]. For diseases requiring routine care, considering the caregiver burden, e.g. as QALYs and productivity losses, is an established way to include a broader range of values. As for productivity loss, Lipton et al. [Citation73] assumed productivity loss to be 50% for patients with migraine, whereas Ma et al. [Citation66] assumed that 60% of patients with lymphoma return to work and, on the basis of the literature, estimated their lost workdays to be 80 days per year. Other broader value elements value of hope, real option value, disease severity, and insurance value were each considered in only one study.
The ISPOR value flower proposes value elements for assessing the value of healthcare from the perspective of multiple stakeholders [Citation2] and is a possible concept for VBP. However, it contains a variety of values, and the concrete definition and quantification of these values is not necessarily established. As shown in this systematic review, some studies included a number of the value elements proposed in the ISPOR value flower, such as value of hope, real option value, disease severity, and insurance value, but not all of the value elements were considered. Whether or not and how these value elements are considered for VBP may still depend on the disease characteristics [Citation66] and the existence of established and generic quantification methods. Even if the studies incorporated these value elements into drug pricing, they used their own method to do so, and the quantification methods are not yet considered to be versatile enough. Therefore, it remains difficult to incorporate the concept of the ISPOR value flower into VBP.
The studies that used a different framework than the CEA approach to estimate drug prices used broader value elements specific to either the framework [Citation70,Citation71] or the target disease [Citation72] rather than using broader values in addition to costs and QALYs.
Our review shows that it is feasible for pricing to include not only quantitative but also qualitative value elements even though the above indicates that at present, the value elements used and the method for pricing them depend on the disease, approach, and previous studies and that this topic is still in the process of development. Studies that use the CEA approach mainly apply the ISPOR value flower on broader value elements in addition to costs and QALYs that are easy to quantify. Broader values are also considered in non-CEA approaches, such as the MSKCC framework [Citation72]. These studies include both qualitative and quantitative value elements.
4.2. Pricing method
Among the eligible studies, as frameworks which estimated drug prices from several broader value elements, four used the CEA approach [Citation66–68,Citation73] and one, the MSKCC framework [Citation72]. Ma et al. [Citation66], Ten Ham et al. [Citation67], and Lipton et al. [Citation73] used the CEA approach to estimate the incremental cost effectiveness ratio (ICER) or net monetary benefit by setting comparators, whereas Prados et al. [Citation68] calculated the monetary benefit by using the CEA approach without comparators to purely accumulate the utility and cost of the target drug. The MSKCC framework [Citation72] also does not use comparators; instead, it aggregates the total value by considering value elements of the drug and converting them to a monetary value. A VBP method for pricing of drug should be applicable to all products even when no comparator is available. In addition to versatility, the introduction of VBP in Japanese drug pricing system requires simplicity because all approved drugs are reimbursed within 60 to 90 days of approval and the system requires prompt application of listing from the drug approval. The Markov model used by Prados et al. [Citation68] can perform precise calculations without comparators, but it is not a simple method because of the complexed development process and the difficulty of collecting information on parameters. The CEA approach is well established, but for the above-mentioned reasons it is not necessarily the best approach for VBP.
Although the calculations in the MSKCC framework are dedicated to a cancer-specific framework and cannot yet be generally applied to other diseases, the MSKCC framework is simple and unique, which sets it apart from other methods. It does not consider costs but rather converts the value into monetary terms purely by considering the potential value elements of the drug itself. Given not only the conceptual differences, but also the difficulty in estimating real-world costs in detail, this method has the potential to evaluate the value of any drug, and if it were to be generalized, it could become a simple, universally applicable method. The probabilistic efficiency frontier is a similar approach in terms of the aggregation of multidimensional benefits [Citation75]. It quantifies the weight of each endpoint by a discrete-choice experiment and estimates net monetary benefit as the maximum reasonable reimbursement price. This approach is potential universal method for VBP, although it covers only clinical endpoints without including broader value elements and has never used in Germany.
Unlike the above studies, the VBP proposed by Levaggi et al. [Citation71] is not a pricing method based on the broad value elements of the drug, but rather a method that calculates the price that maximizes the pharmaceutical company’s profit in a payer-set framework when there is heterogeneity in the patient population for which the drug is indicated. It can be used universally, independent of the disease, and is a conceptual basis for estimating the price of a drug.
We hope that the accumulation of further research will deepen the discussion on the optimal VBP method for pricing of drug.
4.3. Limitations of the study
In this systematic review, only eight eligible articles were identified as we included only studies that attempted to estimate the price of drugs on the basis of values. Although many studies that assessed cost-effectiveness of the drug considering values were reported, they were not included in the review because they assessed the CEA of specific drugs with their pre-defined prices rather than estimating the prices from values. The full-text screening of this review found 16 articles that scored the value of drugs with a value assessment framework without converting the value scores into prices. These studies included ones that performed scoring with existing oncology value frameworks, such as ASCO, ESMO, and NCCN [Citation35,Citation38,Citation42,Citation48,Citation54,Citation56,Citation58–60,Citation62–64], or as part of a multi-criteria decision analysis (MCDA) evaluation [Citation28,Citation44] and those that proposed new scoring frameworks [Citation41,Citation47]. Doyle et al. [Citation47] proposed a concept for pricing but calculated the value of the drug as the sum of the scores of each value element and compared the value with the actual drug price without performing a monetary conversion.
The review revealed that there are challenges not only in the pricing process but also in the quantification of each value. To further understand the methodology of value assessment frameworks, the assessment of values by scoring also needs to be studied. However, even if the value of a drug can be scored by summing the scores of value elements, it remains a challenge to determine how to convert the value into monetary terms, especially if the framework does not include cost elements of existing drugs as a basis for pricing. Phelps et al. [Citation76] introduced a solution for determining the basis for setting budgets in MCDA that contains QALYs as a component with a cutoff for WTP decisions (e.g. $100,000/QALY). Such an approach may also be applicable to VBP.
This study excluded studies on reimbursement and insurer payment because it aimed to identify approaches that could be adapted for use in the Japanese healthcare system. Therefore, the literature on reimbursement and payment decision frameworks was not searched, even though it may contain discussions that could be applicable to pricing.
5. Conclusion
This systematic review investigated value elements, methods for quantifying value elements, and pricing methods for VBP to understand the current status of the VBP framework in drug pricing. Besides cost and QALYs, the studies that adopted the CEA approach included some of the broader values proposed in the ISPOR value flower, such as the value of hope, insurance value, disease severity, and real option value. The studies used individual methods to quantify these values because a uniform quantification method has not yet been established. The CEA approach was the most commonly used pricing method, but other methods based on the disease characteristics and the objective were also proposed. No clear trend was observed for value elements, methods for quantifying value elements, and pricing methods for VBP because of the small number of studies that have considered pricing based on value elements. Research on the implementation of VBP may still be in the development stage. Further research is needed to establish VBP method which enables to incorporate broader values.
6. Expert opinion
Currently drug pricing system in Japan do not necessarily reflect the values provided by drugs and there are discrepancies between their prices and values, which may result in undermining pharmaceutical companies’ willingness to develop and launch new breakthrough drugs and consequently limiting patient access to effective drugs. Therefore, VBP methodology that can tackle such issues is expected and it is essential to gain insights into the kind of value elements that are used for VBP, how they can be quantified, and how the price of a drug can be estimated based on them as a basis of VBP in practice.
We performed a systematic review to investigate value elements, methods for quantifying value elements, and pricing methods for VBP to understand the current status of the VBP framework in drug pricing. Some of the studies that adopted the CEA approach considered broader value elements in the ISPOR value flower and the studies that used a different framework than the CEA approach to estimate drug prices also used broader value elements specific to either the framework or the target disease. Although we observed no clear trend in the value elements used, most studies include both quantitative and qualitative value elements, suggesting that it is feasible for pricing to include not only quantitative but also qualitative value elements. However, quantification method or way for implementation of each value element has not established yet. As for the pricing method, four of the eight eligible articles used the CEA approach, and the others adopted different approaches. Generally, the models were customized for each disease or drug and their purposes, so no clear trend was seen. The reasons for this lack of a clear trend besides the small number of studies may be that the studies focused on different diseases and that the definitions and methods of applying value elements are still being developed.
Considering that VBP will be widely applied to various diseases in the future, a simple and versatile VBP method would be preferable. The CEA approach with Markov model can perform precise calculations, but it is not a simple method because of the complexed development process and the difficulty of collecting information on parameters. In addition, it expresses the final values as cost and QALYs, even though several parameters can be aggregated, which may limit the implementation of broader value elements. The approach is well established, but for these reasons it is not necessarily the best approach for VBP. The framework that performs calculations based on the accumulation of each value, as in the case of the MSKCC framework, is more appropriate than the CEA approach. The advantage of the MSKCC framework is the simple formula it uses to weight and sum each value element for pricing. Furthermore, its value elements consist of the drug features and do not include costs, so the pricing is based purely on the value of drug itself. Although the MSKCC framework currently is applicable only to oncology, it is promising approach that may be suitable for universal adaptation. Doyle et al. [Citation47] also proposed a similar concept for pricing to calculate the value of the drug as the sum of the scores of each value element, though they performed only scoring without pricing in the study. It looks there still remain a challenge to determine how to convert the value into monetary terms in these approaches, especially if the framework does not include cost elements of existing drugs as a basis for pricing. For further development of VBP approaches, more research is required.
Article highlights
We performed a systematic review to investigate the value elements and pricing method for value-based pricing (VBP) using MEDLINE (via the PubMed interface) and ICHUSHI Web (a database for articles written in Japanese). We identified eight articles which met the selection criteria.
Four studies adopted the cost-effectiveness analysis (CEA) approach and the others used different approaches for pricing of drugs. CEA approach included productivity, value of hope, real option value, disease severity, insurance value in addition to costs and quality-adjusted life years as value elements. The other approaches used efficacy, toxicity, novelty, rarity, research and development costs, prognosis, population health burden, unmet needs, and effectiveness. Each study used individual methods to quantify the value elements. Both conventional and broader value elements were used for VBP. The CEA approach was used most often, but there was no consensus on the best approach.
Research on the implementation of VBP may still be in the development stage. Further research is needed to establish VBP method which enables to incorporate broader values.
Declaration of interest
A Takami, M Kato, and H Deguchi are employees of Takeda Pharmaceutical Co. Ltd. The Department of Health Economics and Outcomes Research, Graduate School of Pharmaceutical Sciences, The University of Tokyo, is an endowment department and is supported with an unrestricted grant from Takeda Pharmaceutical Co. Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer is an employee of AbbVie and may hold stocks or stock options in AbbVie. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Ethics statement
Ethical approval was not needed because this article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplemental Material
Download MS Word (48.7 KB)Acknowledgments
The authors thank Tatsuhiro Uenishi and Naotaka Sakashita of Medilead Inc. for the support on performing the systematic review and writing the first draft of the manuscript and Ayako Shoji, PhD, of Healthcare Consulting Inc. and Katsuhiko Iwasaki, PhD, of Medilead Inc. for reviewing the manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2223984
Additional information
Funding
References
- Tsevat J, Moriates C. Value-based health care meets cost-effectiveness analysis. Ann Intern Med. 2018;169(5):329–332. doi:10.7326/M18-0342.
- Lakdawalla DN, Doshi JA, Garrison LP Jr, et al. Defining elements of value in health care—a health economics approach: an ISPOR special task force report [3]. Value In Health. 2018;21(2):131–139. doi:10.1016/j.jval.2017.12.007
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34(24):2925–2934. doi:10.1200/JCO.2016.68.2518
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines) with NCCN Evidence Blocks™. Available from: https://www.nccn.org/guidelines/guidelines-with-evidence-blocks. Accessed Nov 28, 2022.
- Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015;26(8):1547–1573. doi:10.1093/annonc/mdv249
- Memorial Sloan Kettering Cancer Center. Drug abacus methods. Available from: https://www.drugpricinglab.org/tools/drug-abacus/methods. Accessed Nov 28, 2022.
- Jommi C, Armeni P, Costa F, et al. Implementation of value-based pricing for medicines. Clin Ther. 2020;42(1):15–24. doi:10.1016/j.clinthera.2019.11.006
- Pharmaceuticals and Medical Devices Agency – Drug Pricing System in Japan. https://www.pmda.go.jp/files/000243257.pdf [in Japanese]. Accessed Nov 21, 2022
- National Institutes for Health Research PROSPERO: international prospective register of systematic reviews. https://www.crd.york.ac.uk/PROSPERO/. Accessed Nov 21 2022
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
- Walter E. Approaches to capturing value in oncology. Recent Results Cancer Res. 2019;213:85–108.
- Gonçalves E. Value-based pricing for advanced therapy medicinal products: emerging affordability solutions. Eur J Health Econ. 2022;23(2):155–163. doi:10.1007/s10198-021-01276-2.
- Garrison LP, Pezalla E, Towse A, et al. Hemophilia gene therapy value assessment: methodological challenges and recommendations. Value Health. 2021;24(11):1628–1633. doi:10.1016/j.jval.2021.05.008
- Kim DS, Lee G, Cho H, et al. Regenerative medicine in South Korea: bridging the gap between authorization and reimbursement. Front Bioeng Biotechnol. 2021;9:737504. doi:10.3389/fbioe.2021.737504
- Makin C, Neumann P, Peschin S, et al. Modelling the value of innovative treatments for Alzheimer’s disease in the United States. J Med Econ. 2021;24(1):764–769. doi:10.1080/13696998.2021.1927747
- Neumann PJ, Cohen JT, Kim DD, et al. Consideration of value-based pricing for treatments and vaccines is important, even in the COVID-19 pandemic. Health Aff. 2021;40(1):53–61. doi:10.1377/hlthaff.2020.01548
- Lopez MH, Daniel GW, Fiore NC, et al. Paying for value from costly medical technologies: a framework for applying value-based payment reforms. Health Aff. 2020;39(6):1018–1025. doi:10.1377/hlthaff.2019.00771
- Joynt Maddox K, Bleser WK, Crook HL, et al. Advancing value-based models for heart failure: a call to action from the value in healthcare initiative’s value-based models learning collaborative. Circ Cardiovasc Qual Outcomes. 2020;13(5):e006483. doi:10.1161/CIRCOUTCOMES.120.006483
- Dubois RW, Westrich K. As value assessment frameworks evolve, are they finally ready for prime time? Value Health. 2019;22(9):977–980. doi:10.1016/j.jval.2019.06.002.
- Carrera PM. A standard for affordable and sustainable drug prices. Health Aff. 2019;38(7):1229. doi:10.1377/hlthaff.2019.00520.
- Garrison LP, Jackson T, Paul D, et al. Value-based pricing for emerging gene therapies: the economic case for a higher cost-effectiveness threshold. J Manag Care Spec Pharm. 2019;25(7):793–799. doi:10.18553/jmcp.2019.18378
- van Schijndel MA, Caarls PJ, van Wijngaarden JDH, et al. Identifying value-based quality indicators for general hospital psychiatry. Gen Hosp Psychiatry. 2018;55:27–37. doi:10.1016/j.genhosppsych.2018.09.009
- Brixner D, Kaló Z, Maniadakis N, et al. An evidence framework for off-patent pharmaceutical review for health technology assessment in emerging markets. Value Health Reg Issues. 2018;16:9–13. doi:10.1016/j.vhri.2018.01.003
- Garrison LP Jr, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20:213–216. doi: 10.1016/j.jval.2016.12.005
- Nagae T. [DDS and the “in-” of Society] promoting clinical application and commercialization of DDS in response to “in-”. Drug Deliver Sys. 2022;37:63–70. In Japanese.
- Aoki H, Kitano T, Kitagawa D. Disease burden of congenital cytomegalovirus infection in Japan. J Infect Chemother. 2021;27(2):161–164. doi:10.1016/j.jiac.2020.08.018.
- Sugiyama H, Suzuki A. Evaluation of the value of new antimicrobial agents for the introduction of pull-type incentives for the development of new antimicrobial agents in Japan. Jpn J Antibiot. 2021;74:107–115. In Japanese.
- Campolina AG, Estevez-Diz MDP, Abe JM, et al. Multiple Criteria Decision Analysis (MCDA) for evaluating cancer treatments in hospital-based health technology assessment: the Paraconsistent Value Framework. PLoS ONE. 2022;17(5):e0268584. doi:10.1371/journal.pone.0268584
- Gettel CJ, Tinloy B, Nedza SM, et al. The future of value-based emergency care: development of an emergency medicine MIPS value pathway framework. J Am Coll Emerg Physicians Open. 2022;3(2):e126–72. doi:10.1002/emp2.12672
- O’Hara J, Neumann PJ. International haemophilia access strategy council. health technology assessment for gene therapies in haemophilia. Haemophilia. 2022;28 Suppl 2(S2):19–26. doi:10.1111/hae.14413.
- Farghaly MN, Al Dallal SAM, Fasseeh AN, et al. Recommendation for a Pilot MCDA tool to support the value-based purchasing of generic medicines in the UAE. Front Pharmacol. 2021;12:680737. doi:10.3389/fphar.2021.680737
- Jiao B, Garrison LP Jr. The challenge of value-based pricing in combination therapy: the case of trastuzumab and pertuzumab in HER2+ metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):497–504. doi:10.1080/14737167.2021.1896968.
- Shafrin J, Dennen S, Pednekar P, et al. For which diseases do broader value elements matter most? An evaluation across 20 ICER evidence reports. J Manag Care Spec Pharm. 2021;27(5):650–659. doi:10.18553/jmcp.2021.20471
- Casey MJ. Value-based costing of anti-cancer drugs: an ethical perspective grounded in catholic teachings on human dignity and the common good. Issues Law Med. 2021;36(1):44–76.
- Jiang Q, Feng M, Li Y, et al. Choosing PD-1 inhibitors in oncology setting, left or right?-Lessons from value assessment with ASCO-VF and ESMO-MCBS. Front Pharmacol. 2020;11:574511. doi:10.3389/fphar.2020.574511
- Alkhatib NS, Erstad B, Ramos K, et al. Pricing methods in outcome-based contracting: δ3: reference-based pricing. J Med Econ. 2020;23(11):1230–1236. doi:10.1080/13696998.2020.1815027
- Bhandary D, Atreja N, Belletti DA, et al. Translating clinical evidence into value-based payment models: pooled analyses of innovative real-world outcomes agreements for ticagrelor in the United States. Am J Manag Care. 2020;26:S275–86.
- Molto C, Hwang TJ, Borrell M, et al. Clinical benefit and cost of breakthrough cancer drugs approved by the US Food and Drug Administration. Cancer. 2020;126(19):4390–4399. doi:10.1002/cncr.33095
- Vokinger KN, Hwang TJ, Grischott T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol. 2020;21(5):664–670. doi:10.1016/S1470-2045(20)30139-X
- Karmarkar T, Graff JS, Westrich K. Underestimating the value of an intervention: the case for including productivity in value assessments and formulary design. J Manag Care Spec Pharm. 2020;26(5):652–661. doi:10.18553/jmcp.2020.26.5.652.
- Ezeife DA, Dionne F, Fares AF, et al. Value assessment of oncology drugs using a weighted criterion-based approach. Cancer. 2020;126(7):1530–1540. doi:10.1002/cncr.32639
- Wong SE, Everest L, Jiang DM, et al. Application of the ASCO Value Framework and ESMO magnitude of clinical benefit scale to assess the value of abiraterone and enzalutamide in advanced prostate cancer. JCO Oncol Pract. 2020;16(2):e201–10. doi:10.1200/JOP.19.00421
- Angelis A, Linch M, Montibeller G, et al. Multiple criteria decision analysis for HTA across four EU member states: piloting the advance value framework. Soc Sci Med. 2020;246:112595. doi:10.1016/j.socscimed.2019.112595
- Hsu JC, Lin JY, Lin PC, et al. Comprehensive value assessment of drugs using a multi-criteria decision analysis: an example of targeted therapies for metastatic colorectal cancer treatment. PLoS ONE. 2019;14(12):e0225938. doi:10.1371/journal.pone.0225938
- Walker S, Griffin S, Asaria M, et al. Striving for a societal perspective: a framework for economic evaluations when costs and effects fall on multiple sectors and decision makers. Appl Health Econ Health Policy. 2019;17(5):577–590. doi:10.1007/s40258-019-00481-8
- Saunders K, Mably MS, Shull SS, et al. Implementing value assessment in oncology practice: a single-center experience. J Oncol Pharm Pract. 2019;25(4):947–953. doi:10.1177/1078155218815741
- Doyle JJ, Hawryluk E, Niemira J, et al. Evidence-based valuation in oncology: lessons learned from a case study. Ther Innov Regul Sci. 2019;53(3):403–411. doi:10.1177/2168479018786701
- Jiang DM, Chan KKW, Jang RW, et al. Anticancer drugs approved by the food and drug administration for gastrointestinal malignancies: clinical benefit and price considerations. Cancer Med. 2019;8(4):1584–1593. doi:10.1002/cam4.2058
- Angelis A. Evaluating the benefits of new drugs in health technology assessment using multiple criteria decision analysis: a case study on metastatic prostate cancer with the dental and pharmaceuticals benefits agency (TLV) in Sweden. MDM Policy Pract. 2018;3(2):2381468318796218. doi:10.1177/2381468318796218.
- Nugent D, O’Mahony B, Dolan G. International haemophilia access strategy council. value of prophylaxis vs on-demand treatment: application of a value framework in hemophilia. Haemophilia. 2018;24(5):755–765. doi:10.1111/hae.13589.
- Hirst M, Bending MW, Baio G, et al. Cost-effectiveness modeling for neuropathic pain treatments: investigating the relative importance of parameters using an open-source model. J Med Econ. 2018;21(9):930–935. doi:10.1080/13696998.2018.1486845
- Hwang TJ, Kesselheim AS, Gyawali B. Affordability and price increases of new cancer drugs in clinical guidelines, 2007–2016. JNCI Cancer Spectr. 2018;2(2):ky016. doi:10.1093/jncics/pky016.
- Lum K, Bhatti T, Holland S, et al. Diagnosis confirmation model: a value-based pricing model for inpatient novel antibiotics. J Law Med Ethics. 2018;46(S1):66–74. doi:10.1177/1073110518782917
- Lemieux J, Audet S. Value assessment in oncology drugs: funding of drugs for metastatic breast cancer in Canada. Curr Oncol. 2018;25(11):S161–170. doi:10.3747/co.25.3846.
- Shah-Manek B, Wong W, Ravelo A, et al. Oncologists’ perceptions of drug affordability using NCCN evidence blocks: results from a national survey. J Manag Care Spec Pharm. 2018;24(6):565–571. doi:10.18553/jmcp.2018.17449
- Saluja R, Arciero VS, Cheng S, et al. Examining trends in cost and clinical benefit of novel anticancer drugs over time. J Oncol Pract. 2018;14(5):e280–94. doi:10.1200/JOP.17.00058
- Ben-Aharon O, Magnezi R, Leshno M, et al. Association of immunotherapy with durable survival as defined by value frameworks for cancer care. JAMA Oncol. 2018;4(3):326–332. doi:10.1001/jamaoncol.2017.4445
- Djatche LM, Goble JA, Chun G, et al. Evaluating oncology value-based frameworks in the U.S. marketplace and challenges in real-world application: a multiple myeloma test case. J Manag Care Spec Pharm. 2018;24(1):39–46. doi:10.18553/jmcp.2018.24.1.39
- Seymour EK, Schiffer CA, de Souza JA. Challenges in the clinical application of the American Society of Clinical Oncology Value Framework: a Medicare Cost-Benefit Analysis in Chronic Lymphocytic Leukemia. J Oncol Pract. 2017;13(12):e1002–11. doi:10.1200/JOP.2017.024778.
- Foote J, Secord AA, Liang M, et al. ASCO value framework highlights the relative value of treatment options in ovarian cancer. J Oncol Pract. 2017;13(12):e1030–9. doi:10.1200/JOP.2017.025106
- Angelis A, Kanavos P. Multiple Criteria Decision Analysis (MCDA) for evaluating new medicines in health technology assessment and beyond: the advance value framework. Soc Sci Med. 2017;188:137–156. doi: 10.1016/j.socscimed.2017.06.024
- Becker DJ, Lin D, Lee S, et al. Exploration of the ASCO and ESMO value frameworks for antineoplastic drugs. J Oncol Pract. 2017;13(7):e653–65. doi:10.1200/JOP.2016.020339
- Shah-Manek B, Galanto JS, Nguyen H, et al. Value Frameworks for the patient-provider interaction: a comparison of the ASCO Value Framework versus NCCN evidence blocks in determining value in oncology. J Manag Care Spec Pharm. 2017;23(6–a Suppl):S13–20. doi:10.18553/jmcp.2017.23.6-a.s13
- Vivot A, Jacot J, Zeitoun JD, et al. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann Oncol. 2017;28(5):1111–1116. doi:10.1093/annonc/mdx053
- Bentley TGK, Cohen JT, Elkin EB, et al. Validity and reliability of value assessment frameworks for new cancer drugs. Value Health. 2017;20(2):200–205. doi:10.1016/j.jval.2016.12.011
- Ma S, Olchanski N, Cohen JT, et al. The impact of broader value elements on cost-effectiveness analysis: two case studies. Value Health. 2022;25(8):1336–1343. doi:10.1016/j.jval.2022.01.025.
- Ten Ham RMT, Walker SM, Soares MO, et al. Modeling benefits, costs, and affordability of a novel gene therapy in hemophilia a. Hemasphere. 2022;6(2):e679. . doi:10.1097/HS9.0000000000000679
- Prados MJ, Liu Y, Jun H, et al. Projecting the long-term societal value of a disease-modifying treatment for Alzheimer’s disease in the United States. Alzheimers Dement. 2022;18(1):142–151. . doi:10.1002/alz.12578
- Reed SD, Yang JC, Gonzalez JM, et al. Quantifying value of hope. Value Health. 2021;24(10):1511–1519. doi:10.1016/j.jval.2021.04.1284
- Berkowitz ST, Patel S. Value of anti–vascular endothelial growth factor gene therapy for neovascular age-related macular degeneration. Ophthalmology Retina. 2021;5(4):357–364. doi:10.1016/j.oret.2020.08.005.
- Levaggi R, Pertile P. Which valued-based price when patients are heterogeneous? Health Econ. 2020;29(8):923–935. doi:10.1002/hec.4033.
- Lanitis T, Ambavane A, Zheng Y, et al. Value assessment of immuno-oncology in the treatment of rare tumors in the era of accelerated conditional approvals. Future Oncol. 2019;15(35):4057–4067. doi:10.2217/fon-2019-0456.
- Lipton RB, Brennan A, Palmer S, et al. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ. 2018;21(7):666–675. doi:10.1080/13696998.2018.1457533.
- Bell FC, Miller ML Life tables for the United States social security area 1900-2100. U.S. Social Security Administration. https://www.ssa.gov/OACT/NOTES/as120/LifeTables_Body.html. Accessed Nov 28, 2022
- Mühlbacher AC, Sadler A. The probabilistic efficiency frontier: a framework for cost-effectiveness analysis in Germany put into practice for hepatitis c treatment options. Value Health. 2017;20(2):266–272. doi:10.1016/j.jval.2016.12.015.
- Phelps CE, Lakdawalla DN, Basu A, et al. Approaches to aggregation and decision making—a health economics approach: an ISPOR special task force report [5]. Value Health. 2018;21(2):146–154. doi:10.1016/j.jval.2017.12.010
- Lakdawalla DN, Phelps CE. Health technology assessment with risk aversion in health. J Health Econ. 2020;72:102346. doi: 10.1016/j.jhealeco.2020.102346
- Brown J, Finkelstein A. Insuring long-term care in the United States. J Econ Perspect. 2011;25(4):119–142. doi:10.1257/jep.25.4.119.