1. Introduction
Continued advancements in biomedical research contribute to the discovery and development of new treatments to better our physical and mental health. The latest breakthrough is the introduction of gene therapies into clinical practice [Citation1]. Following the first clinical application of gene transfer in humans in the late 1980s, gene therapy development has taken a flight [Citation2]. In 2022, globally more than 500 ongoing gene therapy clinical trials were reported [Citation3]. Based on development pipelines, it is expected that the European regulatory body will assess 15–20 new products per year in 2025 and the Food and Drug Association (FDA) predicts that by 2025 it will approve 10–20 cell and gene therapies per year [Citation4,Citation5]. With more products reaching advanced development milestones, hopes rise. However, in achieving patient access, Health Technology Assessment (HTA) bodies and payers increasingly express cost and affordability concerns around the incoming gene therapy treatments [Citation6,Citation7]. Although costs and affordability in general are a topic of debate in healthcare, it seems in the context of gene therapies these concerns are voiced louder [Citation8,Citation9]. The perceived concerns and challenges have extensively been described in literature [Citation8–16]. Taking a more European perspective, it can be said that roughly two causes underly these concerns. First the combination of high prices and timing of payment and second lack of long-term evidence, both translating to increased decision-uncertainty.
The curative potential for chronic indications asks for an upfront payment for benefits which are often not (yet) demonstrated at time of decision making [Citation10,Citation13]. Costs which are otherwise spread over multiple years are now (in part) charged at once and upfront. In the short term, these high upfront costs of gene therapies cause high so-called budget impact, risk displacing more valuable health care, or care with more certain benefits, by considerably consume payer balance sheets. In addition, due to the irreversible nature of gene therapies, discontinuation of treatment is not possible. Nor can cost be recouped after treatment failure when alternative payment agreements are not in place [Citation15,Citation17]. This high upfront budget impact is a relative new phenomenon in HTA of pharmaceuticals, but uncertainty itself is not. Reimbursement decisions are often made close to regulatory approval when evidence is immature [Citation18]. Regulatory gene therapy submissions are reportedly based on even less mature evidence (e.g. single arm and/or phase I/II trials) [Citation19]. These evidentiary changes may have been adopted for benefit/risk-assessments but have implications for HTA and reimbursement decisions downstream. However, postponing a decision – which can also be seen as a decision – to gather more evidence to decrease uncertainty is undesirable as it consequently denies all patients access to treatment. And more research does not guarantee decrease in decision uncertainty [Citation20,Citation21]. On the other hand, if portrayed benefits and cost savings are not met, spending on the gene therapies may be wasted. This creates a trade-off between providing (selected) patients early access versus benefits of future patients by postponing approval to establish more evidence.
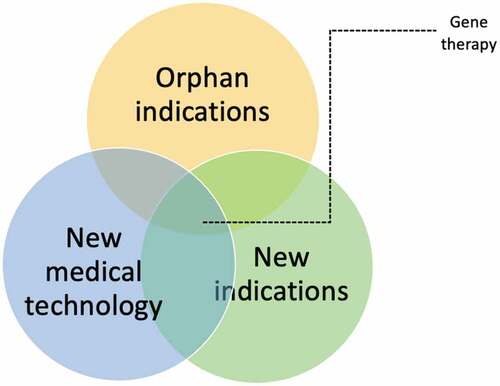
Uncertainty around long-term benefits can in part be linked specifically to the gene therapies themselves for example to uncertain persistence of effect (also known as treatment waning) due to gene shedding and patient selection [Citation22,Citation23]. However, many uncertainties and challenges described in development and HTA of gene therapies arise from more generic – not necessarily gene therapy specific – domains. For example, less robust evidence underlying HTA-decisions caused by small patient samples can be attributed to intended (ultra-)orphan indications. Moreover, gene therapies have the potential to unlock new treatment options and value for indications previously deemed untreatable [Citation24]. Due to this previous untreatable perception, often less has been researched about natural cause of these so-called ‘forgotten or neglected indications’. Therefore, when investigating interventions for these indications, their assessment and development is seen to be accompanied by wider uncertainty around natural history, pathophysiology, patients’ perceptions, and costs of the underlying indication itself. Each individual domain adds to development and decision uncertainty of these gene therapies, see , and further fuels concerns for high budget impact and subsequent opportunity costs.
2. Way forward
The product characteristics of gene therapies are different (e.g. live cell and tissues, gene modifying capabilities) from more conventional medicinal products. However, when placing gene therapies in a wider historic context it is also observed that they seem to follow an established pattern of incremental technology development and diffusion [Citation25,Citation26]. What we now consider to be conventional medicinal products (e.g. monoclonal antibodies and proteins) were at some point in time also new and characterized as considerably different [Citation27–29]. However, through combined efforts maturation and dissemination, implementation of these innovations has been achieved. Consequently, we propose to consider gene therapies not as considerably different but considerably new. This may nudge stakeholders involved in their development to view these products not as an isolated medicinal product group but as a an integral part of the evolving healthcare field. To facilitate development of future biomedical innovations and mitigate their development challenges, it could be helpful if stakeholders identify origins of uncertainty, incorporate learnings from past biomedical innovations and permit horizontal (between organizations) and vertical (within an organization) transfer of (novel) policies, methods and frameworks.
To move forward, the non-exhaustive list of challenges highlighted here – as well as others – needs to be addressed. In the long turn, strategies which mitigate risk by reducing the uncertainty around the value of the technology are explored and intend to provide early insights to decrease the chance a new technology is not good value for the healthcare system [Citation30]. To address high upfront budget impact as well reduce uncertainty, use of payment models is suggested. Examples are, but not limited to, installment payments, outcome-based payment scheme’s, coverage with or without evidence generation and risk-sharing agreements. In these payment models part of the risk of paying for a treatment that is not or less effective is partially transferred from the payer to the developer. In theory the aim and use of payment models are widely discussed and accepted. However, their design and implementation in practice are accompanied by many challenges [Citation11,Citation31]. To go from theory to practices it seems piloting payment model agreements, transparency and sharing experiences are key to the design, implementation and assessment of their success. In addition to challenges in HTA discussed here, are also challenges associated with negotiations, reimbursement, contracting and financing of new treatments. From a European context these steps are often undertaken after or in parallel with HTA. However, in the other jurisdictions or multi-payer systems such as the United States, these activities come with specific barriers and stakeholder groups [Citation17,Citation32–34]. Although out of scope of this editorial, addressing all financial challenges – not just in HTA – across the product life cycle is crucial to durably achieve patient access.
3. Early Health Technology Assessment (early HTA)
In the short term, challenges ought to be addressed in within the existing methods and decision frameworks. As described, uncertainty is one of the main issues to be tackled in development, implementation and evaluation of gene therapies, and innovative treatments in general. During development however, focus is often mainly on developmental, clinical, and research steps. Anticipation on future steps, hurdles and opportunities with regard to impact, regulatory approaches and pricing is an essential and often forgotten part of early research. Early HTA research, and especially early economic evaluations, a part of early HTA research, alongside innovation development can decrease uncertainty, improve development decisions and make new developments future proof. This implies that during different technology readiness levels assessment of added values and patient groups, room for improvement analyses (a type of an early economic evaluation aiming to assess the portrayed hypothesis where and how a novel technology can have (most) impact in a care pathway, also referred to as head room analysis) can steer and inform subsequent development steps [Citation35]. This contrasts with the more common described evaluations of added value at the end phase III trials. For instance, an early economic evaluation in which room for improvement is calculated gives insight in parameter uncertainty and is able to steer clinical trial development. But should, off course, be performed before initiation of (later phase) clinical trials. In addition, such early evaluations can (in the case of gene therapies) inform and design payment models to ensure that uncertainty is assessed, ideally decreased, and early dialogue is initiated on added value and pricing. However, it could be assumed that early economic evaluations perhaps in different forms are to some extent conducted in early development by developers. But, perhaps for proprietary reasons not shared or as widely as economic evaluations conducted in later development stages. If this is the case, we encourage sharing insights of these undertakings in different forms such as methods briefs, learnings or best practices. Life cycle HTA, meaning early and continued HTA research in parallel to product development, can be one of the solutions to ensure future proof development, thinking about the last mile at the first step to make innovations but also developers ready for the future.
4. Expert opinion
As indicated, the product characteristics of gene therapies are different from more conventional medicinal products, however similarities are observed in pathways followed with regard to technology development and diffusion as previous innovations. Consequently, we propose to consider gene therapies not as considerably different but considerably new, which should nudge stakeholders involved seeing these products not as isolated medicinal group but as a spill in an evolving healthcare field. Challenges which should be addressed in the near future are related to describing in detail uncertainty of both outcome measures and costs, use and implementation of payment models and at last implementation and use of early HTA frameworks. Life cycle HTA or parallel HTA research is necessary to ensure future developments comply to both the wishes of the patient and society in terms of health outcomes and sustainable costs.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Hanna E, Toumi M, Dussart C, et al. Funding breakthrough therapies: A systematic review and recommendation. Health Policy. 2018;122:217–229. doi: 10.1016/j.healthpol.2017.11.012
- Rosenberg SA, Aebersold P, Cornetta K, et al. Gene transfer into humans — immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323(9):570–578. doi: 10.1056/NEJM199008303230904
- “U.S. National Library of Medicine“ ClinicalTrials.Gov.
- Gottlieb S Statement from FDA Commissioner Scott Gottlieb, M.D., Marks P. Director of the center for biologics evaluation and research on new policies to advance development of safe and effective cell and gene therapies. Administration, USFD. Montgomery County: (MD), 2019.
- Eder C, Wild C. Technology forecast: advanced therapies in late clinical research, EMA approval or clinical application via hospital exemption. J Mark Access Health Policy. 2019;7(1):1600939. doi: 10.1080/20016689.2019.1600939
- Hollier-Hann G, Cork D, Ralston S, et al. Health technology assessment of gene therapies for inherited genetic disorders in the US and Europe. Value Health. 2018;21:S210. doi: 10.1016/j.jval.2018.04.1421
- Amanda Whittal EI, Hutchings A. Exploring the economics of gene therapy innovation and price. London, UK: Dolon Ltd; 2022. p. 18. https://bit.ly/3AhEoG1
- Goncalves E. Value-based pricing for advanced therapy medicinal products: emerging affordability solutions. Eur J Health Econ. 2021;23(2):155–163. doi: 10.1007/s10198-021-01276-2
- Carr DR, Bradshaw SE. Gene therapies: The challenge of super-high-cost treatments and how to pay for them. Regen Med. 2016;11(4):381–393. doi: 10.2217/rme-2016-0010
- Salzman R, Cook F, Hunt T, et al. Addressing the value of gene therapy and enhancing patient access to transformative treatments. Mol Ther. 2018;26(12):2717–2726. doi: 10.1016/j.ymthe.2018.10.017
- Barlow J, Bens C, Brent T, et al. AMCP partnership forum: designing benefits and payment models for innovative high-investment medications. J Manag Care Spec Pharm. 2019;25:156–162.
- Drummond MF, Neumann PJ, Sullivan SD, et al. Analytic considerations in applying a general economic evaluation reference case to gene therapy. Value Health. 2019;22(6):661–668. doi: 10.1016/j.jval.2019.03.012
- Jørgensen J, Kefalas P. Annuity payments can increase patient access to innovative cell and gene therapies under England’s net budget impact test. J Mark Access Health Policy. 2017;5(1):1355203. doi: 10.1080/20016689.2017.1355203
- Cook K, Forbes SP, Adamski K, et al. Assessing the potential cost-effectiveness of a gene therapy for the treatment of hemophilia a. J Med Econ. 2020;23(5):501–512. doi: 10.1080/13696998.2020.1721508
- Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: An exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–204. doi: 10.3310/hta21070
- Angelis A, Naci H, Hackshaw A. Recalibrating health technology assessment methods for cell and gene therapies. PharmacoEconomics. 2020;38(12):1297–1308. doi: 10.1007/s40273-020-00956-w
- Project” MNF. Designing precision financing for cures. In Financing and reimbursement of cures in the US. Initiatives. MNDDP Cambridge, MA: Massachusetts Institute of Technology; 2018. p. 1–3. https://newdigs.tuftsmedicalcenter.org/designing-precision-financing-for-cures/
- Claxton K, Palmer S, Longworth L, et al. A comprehensive algorithm for approval of health technologies with, without, or only in research: the key principles for informing coverage decisions. Value Health. 2016;19(6):885–891. doi: 10.1016/j.jval.2016.03.2003
- ten Ham RMT, Hövels AM, Klungel OH, et al. Development and regulation of gene and cell-based therapies in europe: a quantification and reflection. Trends Pharmacol Sci. 2020;41(2):67–71. doi: 10.1016/j.tips.2019.11.007
- Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear follow-up of AAV5-Hfviii-SQ gene therapy for hemophilia a. N Engl J Med. 2020;382(1):29–40. doi: 10.1056/NEJMoa1908490
- Ozelo MC, Mahlangu J, Pasi KJ, et al. Valoctocogene roxaparvovec gene therapy for hemophilia a. N Engl J Med. 2022;386(11):1013–1025. doi: 10.1056/NEJMoa2113708
- Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr Gene Ther. 2013;13(6):453–468. doi: 10.2174/15665232113136660006
- CfMPfHUC. Guideline on safety and efficacy follow-up and risk management of advanced therapy medicinal products. EMA London: (UK); 2018. https://www.ema.europa.eu/documents/scientific-guideline/guideline-safety-efficacy-follow-risk-management-advanced-therapy-medicinal-products_en.pdf
- Garrison LP, Kamal-Bahl S, Towse A. Toward a broader concept of value: identifying and defining elements for an expanded cost-effectiveness analysis. Value Health. 2017;20(2):213–216. doi: 10.1016/j.jval.2016.12.005
- Gardner J, Webster A, Barry J. Anticipating the clinical adoption of regenerative medicine: Building institutional readiness in the UK. Regen Med. 2018;13(1):29–39. doi: 10.2217/rme-2017-0121
- Gardner J, Faulkner A, Mahalatchimy A, et al. Are there specific translational challenges in regenerative medicine? Lessons from other fields. Regen Med. 2015;10(7):885–895. doi: 10.2217/rme.15.50
- Hopkins MM, Martin PA, Nightingale P, et al. The myth of the biotech revolution: An assessment of technological, clinical and organisational change. Res Policy. 2007;36(4):566–589. doi: 10.1016/j.respol.2007.02.013
- Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharmaceut sci. 2004;93(6):1390–1402. doi: 10.1002/jps.20079
- Shire SJ. Formulation and manufacturability of biologics. Curr Opin Biotechnol. 2009;20(6):708–714. doi: 10.1016/j.copbio.2009.10.006
- Edlin R, Hall P, Wallner K, et al. Sharing risk between payer and provider by leasing health technologies: an affordable and effective reimbursement strategy for innovative technologies? Value Health. 2014;17(4):438–444. doi: 10.1016/j.jval.2014.01.010
- Persson KSC U, Jonsson B. Alternative payment models in haemophilia treatment. Lund:IHE; 2016. https://ihe.se/wp-content/uploads/2016/12/IHE-Report-2016_10_.pdf
- Quinn C, Ciarametaro M, Sils B, et al. Value-based performance arrangements for chronic conditions: an economic simulation of medicaid drug rebate program reforms. Expert Rev Pharmacoecon Outcomes Res. 2023;23(5):535–546. doi: 10.1080/14737167.2023.2193331
- Project” MNF. How can self-insured employers prepare for the portfolio impact of highcost gene therapies coming to market? In Financing and reimbursement of cures in the US. Cambridge, MA:Massachusetts Institute of Technology; 2022. https://newdigs.tuftsmedicalcenter.org/how-can-self-insured-employers-prepare-for-the-portfolio-impact-of-highcost-gene-therapies-coming-to-market/
- Trusheim MR, Cassidy WM, Bach PB. Alternative state-level financing for hepatitis C treatment-the “netflix model”. JAMA. 2018;320(19):1977–1978. doi: 10.1001/jama.2018.15782
- van Nimwegen KJM, Lilford RJ, van der Wilt GJ, et al. Headroom beyond the quality- adjusted life-year: the case of complex pediatric neurology. Int J Technol Assess Health Care. 2017;33(1):5–10. doi: 10.1017/S0266462317000046