 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Several therapies for vasomotor symptoms (VMS) due to menopause are available. Treatment preferences and willingness-to-pay for VMS treatment among US women with VMS were evaluated.
Methods
An online survey of women with perimenopausal or postmenopausal VMS was conducted (3/15/21–4/23/21). A discrete choice experiment quantified the impact of 7 treatment attributes on VMS treatment choice: VMS frequency/severity reduction, sleep improvement, risk of breast cancer/cardiovascular events in 6 years, risk of short-term side effects, and out-of-pocket costs. Preference weights (PWs) with 95% confidence intervals (CIs) were estimated and reported.
Results
Among 467 women, 86.5% and 87.8% reported moderate to very severe VMS and sleep problems during the preceding month, respectively. Sleep improvement (PW: 0.843; 95% CI: 0.721, 0.965) and reduction in VMS frequency (PW: 0.658; 95% CI: 0.520, 0.796) and severity (PW: 0.628; 95% CI: 0.500, 0.756) most influenced treatment preference; risk of cardiovascular events (PW: 0.150; 95% CI: 0.069, 0.232) or breast cancer (PW: 0.401; 95% CI: 0.306, 0.496) in 6 years had lesser effect. Willingness-to-pay was an additional $35–$46/month for substantially improved sleep, 80% VMS frequency reduction, and reduction from severe to mild VMS.
Conclusions
Sleep improvement and reductions in VMS frequency/severity were the most important treatment attributes.
Plain Language Summary
Hormone and non-hormone treatments are available to reduce vasomotor symptoms (hot flashes and night sweats) due to menopause. We conducted an online survey of 467 women with moderate to very severe vasomotor symptoms during perimenopause or postmenopause to learn what treatment attributes are most important to women when selecting from among the available therapies and how much women were willing to pay for the attributes. Women were shown 14 cards, each with a side-by-side comparison of 2 treatments with varying descriptions of the following 7 treatment attributes: reduction in frequency of vasomotor symptoms, reduction in severity of vasomotor symptoms, improvement in sleep, risk of breast cancer in 6 years, risk of cardiovascular events in 6 years, risk of short-term side effects, and out-of-pocket costs. Women picked their preferred treatment on each card. Results showed that improvement in sleep was the most important attribute to women, and they were willing to pay an extra $46/month for a treatment that substantially improved sleep. The next most important attributes were reduction in frequency and reduction in severity of vasomotor symptoms. Women were willing to pay $36/month more for a treatment that reduced symptom frequency by 80% compared with one that reduced frequency by 50%, and they were willing to pay $35/month more for treatment that reduced symptoms from severe to mild compared with one that did not reduce symptom severity. These results may help guide development of new treatment options and may help physicians recommend treatments that best fit women’s preferences.
1. Introduction
The menopause transition is characterized by hormonal changes and often accompanied by vasomotor symptoms (VMS), including hot flashes/flushes and/or night sweats [Citation1,Citation2]. Women (and people assigned female at birth) with VMS due to menopause also may experience anxiety, depression, and sleep disturbance [Citation3]. The estimated peak prevalence of VMS in the US during or after the menopausal transition is 60–80% [Citation4–6], with 32–46% of perimenopausal or postmenopausal women experiencing moderate to severe symptoms [Citation5]. Natural menopause occurs at a median age of 51.4 years [Citation7], with women potentially spending more than one third of their lives in postmenopause and experiencing VMS for a median of 7.4 years [Citation3].
According to the North American Menopause Society guidelines, hormone therapy (HT) is considered the most effective treatment of VMS due to menopause and for bothersome VMS in women without contraindications; the benefits of HT are most likely to outweigh the risks for women younger than 60 years or within 10 years of menopause onset [Citation8]. Although HT is the most efficacious treatment available for bothersome VMS due to menopause, not all women are eligible for or interested in taking HT. In fact, less than 10% of postmenopausal women in the US use HT, leaving a substantial unmet need for VMS treatments [Citation9]. Reluctance to use available treatments is attributed to real and perceived concerns regarding the safety of HT and effectiveness of non-HT [Citation10–14].
Symptoms of menopause can be costly for an individual as well as for society overall. US health care costs associated with menopausal symptoms were estimated at $3 billion per year based on data from the 2000–2002 National Medical Expenditure Panel Survey [Citation15]. In a separate analysis, per patient per year direct costs (in US$2011) for women experiencing VMS were $423 higher than for women not experiencing VMS, and indirect costs related to VMS were $326 higher (primarily driven by absenteeism) than for women not experiencing VMS [Citation16]. Reported costs per unit (ie, per pill or per patch) for common brands of oral and transdermal estrogen-only products in 2009 ranged from $47 to $658, and costs for estrogen/progestogen products ranged from $475 to $803, depending on product brand and route of administration [Citation14].
Research suggests that women have specific preferences when considering VMS treatments, and multiple factors, such as safety and effectiveness of treatment, physician preference, number of symptoms, and how bothersome the symptoms are, could influence the pursuit and selection of treatment [Citation17–19]. A greater understanding of perceptions of VMS due to menopause and available treatments is needed to appreciate the clinical burden of VMS and to inform treatment development. This study aimed to elucidate the treatment attributes that drive treatment preferences for VMS and contribute to marginal willingness-to-pay for these attributes among women with VMS due to menopause. This study also aimed to describe the nature, frequency, and severity of symptoms and current treatments used by this population of women.
2. Methods
2.1. Study design
We conducted an online, cross-sectional survey of perimenopausal and postmenopausal women with VMS (hot flashes and/or night sweats) using a discrete choice experiment (DCE) to quantify the extent to which the relative attributes of a particular treatment may impact choice of treatment. To mitigate the potential for a different impact dependent upon baseline severity level, a minimum baseline severity was required for all participants. The survey included additional questions regarding symptoms and use of treatments for VMS.
2.2. Survey development and conduct
We performed a targeted review of peer-reviewed literature through PubMed and Google Scholar (searches performed on/through 28 February 2020) and also captured product labels to compile an initial list of VMS treatments and their attributes (Table S1; eg, reduction in the frequency of hot flashes, route of administration, out-of-pocket cost) and to obtain a preliminary idea of the associated levels for the DCE. In the literature review, we also identified self-reported VMS symptoms and treatments as well as related assessment tools.
Next, to inform survey development, we conducted 1-hour, one-on-one phone interviews with 20 women. Phone interviews were to include 5 women who were taking HT and 5 who were taking non-HT for VMS due to menopause, 5 who were past users of HT or non-HT, and 5 who never took treatment for VMS; participants in the phone interviews were not eligible to participate in the survey. Interviewers used an interview guide developed based on the literature search findings. The purpose of the interviews was to collect women’s input and opinions on an initial list of treatment attributes and levels of importance developed from the targeted literature review and to solicit suggestions for any additional attributes and levels. The final list included 7 attributes (with 2–3 levels per attribute) selected based on the ranking of importance the women assigned during the interviews (). Key considerations for selecting these attributes and their levels included relevance to women with VMS due to menopause, the potential to influence treatment choice, the degree of correlation with other attributes, and response burden.
Table 1. Attributes and levels included in the DCE.
We developed the online survey based on the information collected from the literature search and phone interviews. It included screening questions to confirm eligibility; questions related to demographics and comorbid conditions; the DCE (described in section 2.3); and questions about respondents’ VMS and treatments. To mitigate potential recall bias, the recall period was restricted to the preceding month when possible (eg, symptoms occurring in the last month).
We next conducted a moderated pretest of the survey via a virtual meeting with 2 eligible women to identify any questions/concerns; based on their responses, we made wording changes to ensure the logic, clarity, and appropriate level of difficulty of the survey questionnaire. To confirm that data quality would meet expectations, the pretest was then followed by a soft launch involving 50 women (10% of the target sample). Based on the soft launch, we made additional revisions to provide further clarification.
The online survey was conducted from March 15 to 23 April 2021, with the full population (target of 500 women). The survey took about 20 minutes to complete, and completers were compensated $6 in the form of panel points for participation. Survey access was limited to those who were invited to participate; responses were collected via an online portal, de-identified, and stored in a secure database.
2.3. DCE
DCE allows for the estimation of the relative importance of different attributes, and trade-offs between these attributes; analysis of the resulting choices is performed to determine the extent to which each attribute or level contributes to overall utility [Citation20]. DCE also allows study investigators to estimate marginal willingness-to-pay for each attribute level by dividing the preference weight (PW) of an attribute level by the PW associated with a $1 out-of-pocket cost per month.
For the DCE portion of the survey, participants were provided with instructions that included detailed explanations of the 7 treatment attributes and associated preference levels, as well as an example DCE choice card pertaining to VMS due to menopause (Table S2). Then VMS treatment attributes and preference levels were presented in the form of 14 choice cards that each described 2 hypothetical treatments (Treatments A and B). Respondents were asked to select the treatment they preferred on each card. If they clicked or hovered over an attribute on a card, they were shown the description of that attribute again. Three blocks of 14 cards each were developed, and respondents were randomly assigned to 1 of the 3 blocks. Within each block, the first 12 cards were presented in random order to avoid potential ordering effect, and the final 2 cards in every block were validation choice cards for the purpose of data quality checking. On the first card, a within-set dominated pairs test was conducted in which 1 treatment profile was designed to have obvious advantages over the other; respondents were expected to choose the more advantageous profile [Citation21]. On the second card, to assess test-retest reliability, a repeated question test was included in which respondents were asked to select treatments from 2 choice cards that had the same treatment profiles; they were expected to make the same choice on both [Citation21]. In addition, if the respondent selected Treatment A or B in 5 consecutive choices during the DCE, this response was called out and the respondents were asked if this answer was intentional or if they wanted to use the ‘back’ button to change any answers, requesting that they be sure to consider all treatment attributes when making a selection. The DCE methodology was used to gauge the treatment preferences of respondents based on the treatment attributes.
2.4. Study population
Interview and survey participants were recruited from an existing nationally representative online panel of US women maintained by a global market research firm (Dynata, Shelton, CT, U.S.A.). Dynata panels comprise people directly invited from various brand loyalty programs, as well as those invited via websites and social media. With more than 5 million profiled healthcare panelists in the United States, survey vendor Dynata invited respondents via e-mail from its existing database of women with menopause to participate in the interviews and an online survey; panelists agreed to be contacted for research opportunities.
Eligible women were aged 40 to 65 years, were (1) perimenopausal with a uterus and at least 1 ovary or (2) naturally postmenopausal with a uterus and at least 1 ovary, or surgically postmenopausal with a hysterectomy with bilateral oophorectomy, and experienced at least 14 hot flashes and/or night sweats (a proxy for the level of severity at baseline) during at least 1 week during the month before they were due to complete the survey. Severity of VMS was self-reported by respondents and was an average rating during the past month. Mild hot flashes and/or night sweats were associated with the sensation of heat without sweating or dampness. Moderate hot flashes and/or night sweats were associated with the sensation of heat and sweating or dampness but the person was able to continue activity. Severe hot flashes and/or night sweats resulted in intense heat and sweating that caused a disruption of activity. Mild sleeping problems caused sleep that was somewhat restless and somewhat refreshing, resulting in fair sleep quality. Moderate sleeping problems often created problems sleeping or staying asleep, resulting in poor sleep quality. Severe sleeping problems almost always caused trouble sleeping or staying asleep, resulting in sleep quality that was very poor. Perimenopausal was defined as cessation of menstruation for 2–12 months or marked change in menstrual cycle regularity and/or flow. Postmenopausal was defined as permanent cessation of menstruation for more than 12 months [Citation1]. Reported treatment history of prescription medications and/or over-the-counter (OTC) medications (HT and non-HT) purchased from a pharmacy or store for hot flashes and/or night sweats was required. Three cohorts were recruited to take the online survey: current users (currently treated with prescription or nonprescription HT or non-HT for a minimum of 30 days), past users (had received HT or non-HT for at least 1 month but stopped treatment at least 3 months ago), and never users (never treated with HT or non-HT). Treatments for VMS are presented in Table S3.
Women were excluded if they had a hysterectomy without oophorectomy or a hysterectomy with a unilateral oophorectomy; an endometrial ablation; current use of a hormonal intrauterine device; VMS induced by medications, such as endocrine therapy or chemotherapy for breast cancer; and current or previous participation in a clinical trial of a VMS therapy in the past 5 years.
2.5. Ethics
The New England Institutional Review Board (IRB) approved the phone interview guide, the preinterview screener, and the study protocol, and confirmed that this survey met criteria for exemption from IRB review. Consent to participate was confirmed at the start of the survey via a check box.
2.6. Study outcomes
The primary endpoints for evaluation were women’s PWs and marginal willingness-to-pay for VMS treatment attribute levels. Secondary endpoints included respondents’ characteristics and self-reported symptoms and treatments. Information collected included sociodemographic characteristics, current characteristics of VMS, current treatment characteristics and patterns, out-of-pocket costs per month for treatments for VMS, time on treatment, and reason for selecting treatment. Women were asked about their satisfaction with treatment they are currently receiving (responses ranged from very dissatisfied to very satisfied). Satisfaction was asked as a general question and was not defined per protocol.
2.7. Sample size
Currently, no standard exists for the determination of sample size for DCEs [Citation22]. However, the commonly used rule of thumb suggests that the sample size required for the main effects experimental design depends on the number of choice tasks (t), the number of alternatives (a), and the largest number of levels for any of the attributes (c) according to the following equation: [Citation23,Citation24]. Alternative methods have also been suggested [Citation24]. Given that the survey included a maximum of 3 levels (c) in each attribute and asked each participant to respond to 12 choice cards (excluding validation choice cards) (t) in pairs of 2 (a), the minimum sample size needed was 63. In addition, to assess treatment use, a targeted sample size was used to ensure that approximately half of the sample comprised current or past users of HT or non-HT (target: 125 current users and 125 past users) and half were never treated with HT or non-HT (target: 250 never users). To capture opinions on different treatments, an ‘opt-out’ option in the choice task was not offered to participants/patients.
2.8. Statistical analyses
Respondent characteristics collected from the survey were summarized descriptively by study cohort. Continuous variables were summarized using means, standard deviations, medians, interquartile ranges, and ranges, while categorical variables were summarized using counts and proportions. Improbable values entered by respondents were set to missing in the data analyses. Treatment characteristics were summarized for current users by current HT cohort and current non-HT cohort.
We estimated PWs based on the responses to DCE choice cards from the survey. Random parameter logit regression models were used to analyze the impact of treatment attributes on women’s treatment choices, in which the utility that an individual obtains from treatment i is calculated as: Uin = x’iβn + εin where xi is a vector of the attributes of treatment i, βn is a vector of estimated coefficients for individual n, and εin is a random error term reflecting unmeasured factors that can affect the individual n’s utility. Each estimated coefficient is a PW and represents the relative contribution of the attribute level to the utility that an individual assigns to a treatment, and the associated P value indicates whether the treatment attribute has a statistically significant effect on preference relative to the baseline attribute level in the model. The difference between the highest and lowest PWs for each attribute indicates how influential or important that attribute is on choice of treatment for VMS, with higher values indicating an attribute with more importance. Marginal willingness-to-pay was calculated for each treatment attribute level by dividing the PW of the attribute level by the PW associated with $1 out-of-pocket cost per month.
The online surveys did not allow respondents to skip questions; however, an option of ‘not sure’ was provided for some questions (personal/family medical history questions and VMS treatment history questions) but not for the DCE. Responses were coded as missing if ‘not sure’ was selected.
Data analysis was conducted using SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata 16 (StataCorp LLC, College Station, TX).
3. Results
3.1. Participants
Overall, 503 women met eligibility criteria and 467 completed the survey. Participants were categorized into 3 main cohorts based on treatment use (current users, past users, and never users), and current treatment users were categorized further by treatment (HT and non-HT users). A breakdown of all participants included is shown in . Respondents had a mean age of 53.7 years at the time of survey, and 86.3% were White (). Most respondents (80.9%) were postmenopausal at the time of the survey, and the mean time since final menstrual period was 6.8 years ().
Figure 1. Survey respondents.
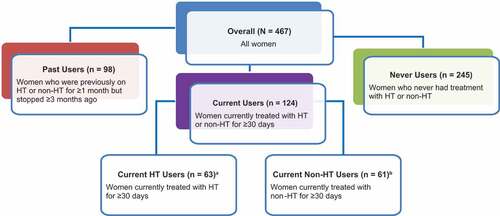
Table 2. Respondent Sociodemographic and Disease characteristics.
3.2. Menopausal symptoms and treatment
On average, respondents experienced 6.0 years of VMS at the time of the survey. Among all women in the study, the median number of VMS experienced in the last week before the survey or the week in the past month during which VMS were most frequent was 19 (). Although all participants had experienced VMS in the past month, respondents also reported a range of other symptoms in the month preceding the survey, with the most common being sleep problems (73.4%), irritability (56.3%), and mood swings (51.6%; ). In the month preceding the survey, 86.5% of respondents reported having moderate to very severe hot flashes and/or night sweats and 87.8% reported having moderate to severe sleep problems.
Table 3. Respondent vasomotor symptoms.
Among current treatment users, respondents using HT were more satisfied, with 65.1% of users reporting they were at least somewhat satisfied, compared with 47.5% of non-HT users (). Moreover, HT users reported more substantial reductions in the frequency of VMS; 39.6% of HT users experienced greater than 70% reductions compared with 8.2% of non-HT users.
Table 4. Treatment characteristics among current treatment users.
3.3. DCE: VMS treatment preferences
shows the PWs for all treatment attribute levels. DCE findings showed that substantial improvement in sleep problems (PW: 0.843; 95% confidence interval [CI]: 0.721, 0.965) followed by substantial reductions in frequency (PW: 0.658; 95% CI: 0.520, 0.796) and severity (PW: 0.628; 95% CI: 0.500, 0.756) of VMS significantly impacted treatment preferences (all P < 0.001; ). Risk of breast cancer (PW: 0.401; 95% CI: 0.306, 0.496) and cardiovascular events (PW: 0.150; 95% CI: 0.069, 0.232) in 6 years, as well as risk of short-term side effects such as weight gain, headache, and nausea (PW for 15% risk: 0.176; 95% CI: 0.065, 0.287) were considered less important than symptom relief (all P≤0.002; ).
Figure 2. Preference weights.
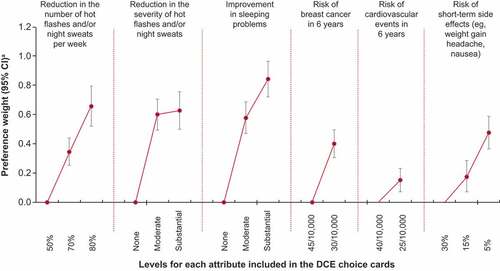
Table 5. Estimated preference weights and marginal willingness-to-pay.
The preference for substantial improvement in sleep compared with no improvement is quantified by the PW difference between these 2 levels, which was 0.843 (0.843 vs 0.000). A value greater than zero means participants prefer a treatment with a given level more than a treatment with the reference level of a particular attribute, with a greater value indicating stronger preference. Similarly, the importance of reducing the frequency of VMS from 50% to 80% per week is quantified by the PW difference between these 2 levels, which was 0.658 (0.658 vs 0.000). In comparison, the importance of an increase in risk of a cardiovascular event in 6 years from 25/10,000 to 40/10,000 was 0.150.
Respondents were willing to pay an additional $46 per month for treatment that substantially improved sleep problems (from severe to mild) compared with a treatment with no effect on sleep and an additional $36 per month for a treatment that reduced the number of VMS per week by 80% compared with a treatment that reduced the number by 50% (). Women were also willing to pay an additional $35 per month for a treatment that reduced the severity of VMS from severe to mild compared with a treatment that did not improve the severity of VMS. Conversely, the least valuable attribute was a reduction in the risk of a cardiovascular event in 6 years from 40/10,000 to 25/10,000. Women were willing to pay an extra $8 per month to reduce their cardiovascular event risk ().
3.3.1. Data validity
Of the 467 respondents, 403 (86.3%) chose the unambiguously better treatment option on the within-set dominated pairs test and 348 (74.5%) selected the same treatment choice on both cards in the repeated question test.
4. Discussion
4.1. Key results/interpretation
Improvement in sleep followed by reductions in the number and severity of hot flashes and/or night sweats significantly impacted treatment choices among women with VMS due to menopause. Sleep problems were among the most burdensome symptoms reported by respondents; among the 73.4% of women who reported experiencing sleep problems, 87.8% experienced moderate to severe problems during the month preceding the survey.
Previous studies also documented a high prevalence of sleep problems among women with VMS due to menopause. Qualitative interviews conducted by English et al in the US and Europe found that the vast majority (93.8%) of respondents stated that their VMS affected sleep, and 75% of the US women stated that sleep disturbance was the most bothersome aspect of VMS [Citation25]. Additionally, frequency and severity of hot flashes have been found to be linearly related to menopause-specific quality of life and sleep; improvements in hot flashes are associated with improved quality of life and better sleep [Citation26].
In the current study, women reported using a variety of HT and non-HT (including OTC treatments) for hot flashes and/or night sweats. Although respondents using HT appeared more highly satisfied with their treatment than non-HT users, a sizable percentage of women were only somewhat satisfied with HTs and non-HTs, indicating room for improvement across treatment types. However, broadly, women’s attitudes on treatment for VMS remain mixed and vary widely. For example, in an online survey of 1464 respondents from a British menopause-focused website, 70% were in favor of HT and 40% believed that the risks of HT had been exaggerated in the media. Conversely, 41% of perimenopausal women reported that they would never use HT, and 77% said they would try alternative therapies before taking HT [Citation27].
The current study is the first to evaluate marginal willingness-to-pay among US women with menopausal symptoms using a survey-based DCE. Women with menopausal symptoms were willing to pay the highest additional amount per month ($46) for a VMS treatment that improved sleep problems from severe to mild compared with a treatment that does not improve sleep problems. Women were willing to pay an additional $36 per month for a treatment that reduced the number of VMS episodes per week by 80% compared with a treatment that reduced the number by 50%. The median out-of-pocket costs, including for prescription and nonprescription medications, were $35 for current HT users and $20 for non-HT users in this study, indicating that women were willing to pay a relatively higher cost for VMS treatments that improve sleep problems.
Safety events had less impact on treatment choice among women in this study. While this study did not specifically assess why safety was valued less in choosing treatment, it is possible that the low risk of long-term adverse events contributed to the reduction of daily symptom burden being favored [Citation28]. Some women report avoiding HT because of a perception that the symptoms of menopause will resolve on their own, followed by the concern about side effects and long-term risks [Citation28].
Data validity results of the within-set dominated pairs and repeated question tests were consistent with those reported for these tests in a general analysis of DCE results [Citation21]. Whereas failure rates on these 2 tests in our DCE were 13.7% and 25.5%, respectively, the reported mean (SD) failure rates from a previously published analysis were 18% (20%) for the within-set dominated pairs test based on 21 DCEs and 30% (26%) for the repeated-question test based on 16 DCEs [Citation21].
4.2. Limitations
First, the study sample may not be fully representative of all persons with menopausal symptoms in the United States, which could decrease the generalizability of the results. Women without VMS, those with mild VMS, and women who had undergone hysterectomy without oophorectomy or endometrial ablation or who had a hormonal intrauterine device were not included here and therefore results may not generalize to these populations. Selection bias among those who were willing and able to participate in the survey may result from factors such as digital literacy and access, severity of symptoms, access to health care, or time constraints. Further, non-White women were relatively underrepresented in this study; future research should consider more diverse samples. Second, the included treatment attributes limited the assessment of preferences for VMS treatments, and it is possible that additional treatment attributes not specified in the survey could impact preferences. However, treatment attributes were selected based on the importance rankings provided by women in the interviews to minimize the impact of excluded treatment attributes on the preference results. Also, understanding the included treatment attributes and their levels may have impacted respondent choices. For example, the level of risk of cardiovascular events in 6 years was presented as a proportion, such as 40/10,000 women (0.40%) vs 25/10,000 women (0.25%) on the choice card, which might have been less intuitive. Thus, it is unknown whether respondents considered these factors as less important based on a true understanding of the risk or because of a lack of understanding of the risk. However, a tutorial explaining attributes and levels was presented to respondents before the survey to mitigate potential misunderstanding. Although potential overlap among some attributes exists and can result in biased estimates and double counting, there may also be different underlying mechanisms for the differing attributes that are not fully elucidated. Third, no assessment was made of women’s understanding of menopause in general and how that may have influenced their choices, nor was self-reported treatment history verified. Fourth, the levels of severity of VMS were subjective in nature and not defined for the study participants. Such subjectivity, however, is relatively common in health care research (eg, the use of visual analog scales and Likert scales for assessing pain, treatment satisfaction, or quality of life) and is not unique to this study. Fifth, given that this study relied on self-reported responses, recall bias could exist. The recall period was restricted to symptoms within the last month, when possible, to minimize this bias. Finally, menopausal symptoms may be interdependent (eg, night sweats may impact sleep quality), but we did not attempt to determine whether attributes included in the DCE were related to one another. Additionally, the risk levels used in the study are small and use different denominators. Problem-solving ability and comprehension are worse when the denominators differ (eg, it is easier to compare ‘4 in 1000’ and ‘1 in 1000’ than it is to compare ‘1 in 250’ and ‘1 in 1000’) [Citation29]. During the menopausal transition, all women experience hormonal changes, but not all report VMS; answers to questions may be impacted by social desirability bias [Citation30]. Support from family members and healthcare providers who are fully aware of the expected course of menopause can have a positive impact on women’s experience [Citation31]. The current study assessed a range of treatment options for their reported effects on VMS. However, such a range of options may present a challenge owing to the stronger evidence available for prescription treatments in terms of efficacy in addressing VMS symptoms; therefore, there remains the need for further research to study these treatment categories separately.
5. Conclusions
Among women with menopausal symptoms, improvement in sleep problems followed by reduction in the number and severity of VMS most significantly impacted treatment choices. Similarly, women with VMS are willing to pay the highest additional amount per month for a treatment that improves sleep problems from severe to mild compared with a treatment that does not improve sleep problems. These findings may help inform treatment development and guidance for women with VMS due to menopause.
6. Expert opinion
During the menopause transition, most women (60–80%) experience VMS and up to 50% of women experience moderate to severe VMS. Although HT is most efficacious for bothersome VMS, fewer than 10% of US women in postmenopause use HT. Also, less than half of women in perimenopause reported they would use HT and nearly 80% of women reported they would try alternative therapies before using HT, leaving a substantial unmet need for VMS treatments.
A variety of factors may influence a decision to pursue or prescribe treatment for VMS including safety, efficacy, and effectiveness of treatment, physician preference, and number and severity of symptoms. To better understand treatment preferences of women with VMS due to menopause, we conducted a DCE to assess the treatment attributes that drive treatment preferences for VMS and the marginal willingness-to-pay for these attributes among the women.
In this study, women with VMS due to menopause reported clear treatment preferences: improvement in sleep problems followed by reduction in the number and severity of VMS most significantly impacted treatment choices. Women reported willingness to pay the highest additional amount per month for a treatment that improves sleep problems from severe to mild impairment compared with a treatment that does not improve sleep problems. Dissemination and use of evidence from this study may impact real-world outcomes in the following ways: (1) development of new treatments, (2) development of treatment algorithms, (3) improvement in patient-clinician conversations about treatment, (4) fulfilling unmet treatment needs for women with VMS due to menopause (ie, well-managed VMS [reduced number of symptoms, reduced severity, and minimal to no sleep disruption]).
Twenty years after the Women’s Health Initiative, a persistent gap in menopause care remains and funding research for women’s health continues to lag [Citation32,Citation33]. With conduct and dissemination of new and ongoing research, and education of clinicians and patients, there is great potential for improved treatment and outcomes for women with VMS due to menopause. Highly effective non-HT with favorable safety profiles is imperative to improve the quality of life of women suffering from moderate to severe VMS. Further research is needed to narrow and close the research gap and address unmet needs for women with VMS due to menopause.
Abbreviations
| DCE | = | discrete choice experiment |
| HT | = | hormone therapy |
| non-HT | = | nonhormonal therapy |
| IRB | = | institutional review board |
| PW | = | preference weight |
| VMS | = | vasomotor symptoms |
Declaration of interest
R Thurston has served as a consultant or on an advisory board for Astellas Pharma, Bayer, Hello Therapeutics, and Happify Health. E Cook and H Yang are employees of Analysis Group, an economic consulting firm that receives consulting fees from Astellas Pharma and other commercial interests. A Shiozawa, D King, R Kristy, and S Mancuso are employees of Astellas Pharma, the study sponsor. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer has coauthored books on cognitive behavior therapy as a management strategy for VMS. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
The authors had access to relevant aggregated study data and other information (such as study protocol, analytic plan and report, validated data tables, and clinical study report) required to understand and report research findings. The authors have been fully involved at all stages of publication and presentation development and agree to be accountable for the published work.
Study design: A Shiozawa, R Kristy
Study investigator: A Shiozawa
Collection and assembly of data: A Shiozawa, E Cook, H Yang, R Kristy
Data analysis: A Shiozawa, E Cook, H Yang, R Kristy
Data interpretation: A Shiozawa, R Thurston, E Cook, H Yang, D King, R Kristy, S Mancuso
Manuscript preparation: All authors
Manuscript review and revisions: All authors
Final approval of manuscript: All authors
Data sharing statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Prior presentations
Data were presented at the Nurse Practitioners in Women’s Health (NPWH) 25th Annual Premier Women’s Healthcare Conference; September 29–2 October 2022; Houston, TX.
Ethics approval and informed consent
The New England Institutional Review Board approved the phone interview guide, the preinterview screener, and the study protocol and confirmed that this survey met criteria for exemption from institutional review board review. Survey responses were collected via an online portal, de-identified, and stored in a secure database.
Consent to participate
Consent to participate was confirmed at the start of the survey via a check box.
Supplemental Material
Download MS Word (61.2 KB)Acknowledgments
Medical writing and editorial support were provided by Lauren Cerruto, Traci Stuve, MA, Tulika Bhushan Bahukhandi, RPh, MS, and LeeAnn Braun, MPH, MEd, of Echelon Brand Communications, LLC, an OPEN Health company, and funded by Astellas Pharma, Inc. Yao Wang, Danni Yang, and Richard Berman of Analysis Group, Inc. (Boston, MA, USA) contributed to the data collection and analysis in this study.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2250916
Additional information
Funding
References
- Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19(4):387–395. doi: 10.1097/gme.0b013e31824d8f40
- Whiteley J, DiBonaventura M, Wagner JS, et al. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health (Larchmt). 2013;22(11):983–990. doi: 10.1089/jwh.2012.3719
- Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. doi: 10.1001/jamainternmed.2014.8063
- Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation. Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936
- Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn ovarian aging study cohort. Menopause. 2014;21(9):924–932. doi: 10.1097/GME.0000000000000196
- Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11(1):32–43. doi: 10.1080/13697130701744696
- Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865
- NAMS. Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24(7):728–753. doi: 10.1097/GME.0000000000000921
- Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999-2010. Obstet Gynecol. 2012;120(3):595–603. doi: 10.1097/AOG.0b013e318265df42
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040
- Arbuckle R, Humphrey L, Abraham L, et al. Qualitative cross-cultural exploration of vaginal bleeding/spotting symptoms and impacts associated with hormone therapy in postmenopausal women to inform the development of new patient-reported measurement tools. Maturitas. 2014;78(3):219–227. doi: 10.1016/j.maturitas.2014.04.019
- Abraham L, Humphrey L, Arbuckle R, et al. Qualitative cross-cultural exploration of breast symptoms and impacts associated with hormonal treatments for menopausal symptoms to inform the development of new patient-reported measurement tools. Maturitas. 2015;80(3):273–281. doi: 10.1016/j.maturitas.2014.11.019
- Ameye L, Antoine C, Paesmans M, et al. Menopausal hormone therapy use in 17 European countries during the last decade. Maturitas. 2014;79(3):287–291. doi: 10.1016/j.maturitas.2014.07.002
- Williams-Frame A, Carpenter JS. Costs of hormonal and nonhormonal prescription medications for hot flashes. Womens Health (Lond Engl). 2009;5(5):497–502. doi: 10.2217/WHE.09.49
- Kjerulff KH, Frick KD, Rhoades JA, et al. The cost of being a woman: a national study of health care utilization and expenditures for female-specific conditions. Womens Health Issues. 2007;17(1):13–21. doi: 10.1016/j.whi.2006.11.004
- Sarrel P, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause. 2015;22(3):260–266. doi: 10.1097/GME.0000000000000320
- Tao M, Teng Y, Shao H, et al. Knowledge, perceptions and information about hormone therapy (HT) among menopausal women: a systematic review and meta-synthesis. PLoS One. 2011;6(9):e24661. doi: 10.1371/journal.pone.0024661
- Clark HD, O’Connor AM, Graham ID, et al. What factors are associated with a woman’s decision to take hormone replacement therapy? Evaluated in the context of a decision aid. Health Expect. 2003;6(2):110–117. doi: 10.1046/j.1369-6513.2003.00216.x
- Constantine GD, Graham S, Clerinx C, et al. Behaviours and attitudes influencing treatment decisions for menopausal symptoms in five European countries. Post Reprod Health. 2016;22(3):112–122. doi: 10.1177/2053369116632439
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. PharmacoEconomics. 2008;26(8):661–677. doi: 10.2165/00019053-200826080-00004
- Johnson FR, Yang JC, Reed SD. The internal validity of discrete choice experiment data: a testing tool for quantitative assessments. Value Health. 2019;22(2):157–160. doi: 10.1016/j.jval.2018.07.876
- Speckemeier C, Krabbe L, Schwenke S, et al. Discrete choice experiment to determine preferences of decision-makers in healthcare for different formats of rapid reviews. Syst Rev. 2021;10(1):121. doi: 10.1186/s13643-021-01647-z
- de Bekker-Grob EW, Donkers B, Jonker MF, et al. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi: 10.1007/s40271-015-0118-z
- Yang JC, Johnson FR, Kilambi V, et al. Sample size and utility-difference precision in discrete-choice experiments: a meta-simulation approach. J Choice Model. 2015;16:50–57. doi: 10.1016/j.jocm.2015.09.001
- English M, Stoykova B, Slota C, et al. Qualitative study: burden of menopause-associated vasomotor symptoms (VMS) and validation of PROMIS sleep disturbance and sleep-related impairment measures for assessment of VMS impact on sleep. J Patient Rep Outcomes. 2021;5(1):37. doi: 10.1186/s41687-021-00289-y
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause. 2016;23(10):1060–1066. doi: 10.1097/GME.0000000000000678
- Cumming GP, Currie H, Morris E, et al. The need to do better – are we still letting our patients down and at what cost? Post Reprod Health. 2015;21(2):56–62. doi: 10.1177/2053369115586122
- Nappi RE, Kroll R, Siddiqui E, et al. Global cross-sectional survey of women with vasomotor symptoms associated with menopause: prevalence and quality of life burden. Menopause. 2021;28(8):875–882. doi: 10.1097/GME.0000000000001793
- Ancker JS, Senathirajah Y, Kukafka R, et al. Design features of graphs in health risk communication: a systematic review. J Am Med Inform Assoc. 2006;13(6):608–618. doi: 10.1197/jamia.M2115
- Arnot M, Emmott EH, Mace R, et al. The relationship between social support, stressful events, and menopause symptoms. PLoS One. 2021;16(1):e0245444. doi: 10.1371/journal.pone.0245444
- Refaei M, Mardanpour S, Masoumi SZ, et al. Women’s experiences in the transition to menopause: a qualitative research. BMC Womens Health. 2022;22(1):53. doi: 10.1186/s12905-022-01633-0
- Trémollieres FA, André G, Letombe B, et al. Persistent gap in menopause care 20 years after the WHI: a population-based study of menopause-related symptoms and their management. Maturitas. 2022;166:58–64. doi: 10.1016/j.maturitas.2022.08.003
- Smith K. The funding gender gap: nature. [13 Jul 2023]. Available from: https://www.nature.com/immersive/d41586-023-01475-2/assets/d41586-023-01475-2.pdf
