ABSTRACT
Objective
To provide an overview of societal burden of osteoarthritis (OA) in the Netherlands.
Methods
Medline (via Ovid) and Embase databases were searched in September 2022 for all publications providing prevalence/incidence, cost or health-related quality of life (HRQoL) data of OA (all sites) in the Netherlands.
Results
Twenty-eight original studies were included in this scoping review; twelve reporting prevalence/incidence data of OA, seven reporting data on the economic burden of OA and twelve reporting HRQoL data of patients with OA. Most of the available data were from Dutch national cohorts. The prevalence of knee OA ranged from 6% to 18% across studies, from 4% to 7% for hip OA and from 12% to 56% for hand OA. OA was shown to be associated with impairment in work participation and long-term requirement of health care utilization, translating into substantial medical costs and societal costs of lost productivity. All studies comparing HRQoL among persons with OA with control persons showed a significantly lower HRQoL in patients with OA after adjustment for age, sex, and various risk factors.
Conclusions
OA is a highly prevalent disease in the Dutch population and is responsible for a significant economic and health burden.
1. Introduction
Osteoarthritis (OA) is a common, degenerative, and debilitating joint disease characterized by pain and functional impairment and is one of the leading causes of global disability [Citation1]. The knee and hip are the two joints that are most frequently affected by this condition. The global prevalence of knee OA is estimated at 3.8% and hip OA at 0.85%, with a higher prevalence in women compared to men [Citation1]. The incidence of OA is rising due to the increase in life expectancy and in the prevalence of obesity [Citation2,Citation3]. OA is associated with significant utilization of healthcare resources, and impairs health-related quality of life (HRQoL) of patients. In a systematic literature review published in 2016 including 28 large sample studies measuring the worldwide economic and/or health burden of OA, Xie et al. [Citation4] reported important impairments in patient’s HRQoL as well as considerable per-patient costs for OA with total annual average direct costs of OA comprised between US$1,442 to US$21,335 and total average indirect costs ranged from US$238 to US$29,900 (all costs adjusted to year 2015). Even though summaries of international data are worthwhile to be informed on the worldwide burden of OA, health-care decisions and resource allocation are usually made at a national level. Therefore, providing country-specific data concerning prevalence, costs and HRQoL of patients suffering from OA is important for national decision-making. For this reason, we aimed to perform a scoping review to identify and summarize relevant data on the prevalence and incidence of OA, on the costs associated with OA, and on the HRQoL of patients suffering from OA in the Netherlands. To our knowledge, no meta-research studies have been made to synthesize the burden of OA in the Netherlands. Reporting country-specific burden of OA is important when estimating the health and budget impact of the disease and when planning to develop health economic models to assess cost-effectiveness of strategies for prevention and various treatment. In addition to providing an overview of the economic and health burden of OA in the Netherlands, the scoping review also intends to identify the current knowledge gaps in this area.
2. Methods
The proposed scoping review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA2020) extension for scoping reviews [Citation5]. The completed PRISMA checklist is available in Appendix 1. A protocol was developed and published in Open Science Framework (https://osf.io/vgx4n/). This scoping review also followed the five-step framework by Arksey and o’Malley [Citation6] and guidance from the Joanna Briggs Institute scoping review methodologies (https://jbi.global/scoping-review-network/resources). Covidence software (i.e. Covidence is a web-based collaboration software platform that streamlines the production of systematic and other literature reviews) was used to manage search results, including deduplication, abstract and title screening, and full-text screening. Data extraction was performed using Microsoft Excel.
2.1. Literature search
A scientific literature search was conducted in Medline (via Ovid) and Embase databases until September 2022 to identify English or Dutch language scientific studies reporting burden of osteoarthritis (excluding studies on degenerative disorders of the spine) that provided original data on prevalence/incidence, cost or HRQoL. A combination of terms of Medical Subject Headings (MeSH) and keywords was used in the search strategy (the complete search strategies for Ovid and Scopus, developed by CB, who is experienced in bibliographic research, are available in the Appendix 2).
Additionally, a manual search within the bibliography of relevant papers was also performed to complete the bibliographic search. We also conducted forward references searching of included studies using Web of Science to identify other relevant research that has referenced any article of interest.
2.2. Study selection
Two reviewers (CB and NL) independently screened titles and abstracts of the de-duplicated search output to exclude irrelevant articles. In the second step, the reviewers read the full text of each non-excluded article to determine eligibility for inclusion in this scoping review. Disagreements during both stages were resolved by consensus.
Studies meeting the following criteria were included:
Original published and peer-reviewed studies (cross-sectional, prospective, retrospective, case control, cost studies, baseline data from randomized controlled trials) providing prevalence and/or incidence data, costs data, quality of life data or utilities of patients with OA (any approaches to diagnose OA accepted) at any sites (except spine).
Systematic reviews and/or meta-analyses on the theme:
Population cohorts performed in the Netherlands;
Studies published in English or Dutch language [Citation7].
Non-original studies such as letters, commentary, opinion or protocols were excluded. We also excluded studies that reported costs directly linked to a surgery (i.e. knee replacement) and not to OA itself.
2.3. Data extraction
Data were extracted by the two independent reviewers according to a standardized data extraction form. Disagreements between reviewers were resolved by consensus. The following data were extracted: Authors, year of publication, study design, outcome(s) reported, objective of the study, inclusion/exclusion criteria of the study, name of the cohort study (if available), description of the population, site of OA, ascertainment/approach of diagnosis and main results.
2.4. Synthesis of results
A narrative synthesis of results was presented for each aspect (i.e. prevalence/incidence, costs and HRQoL) for the different OA sites (knee, hip, hand) when possible. For each section, a summary of the evidence is provided to better understand the current state of the art. This summary comprises 1. What is known; 2. What are the weaknesses of knowledge; 3. What is not known. No meta-analysis was undertaken. Consistent with methodology of scoping reviews, no quality assessment of individual studies was performed.
3. Results
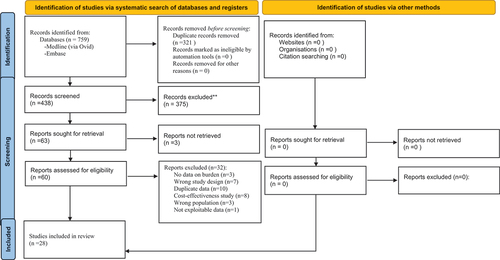
A total of 759 references were identified through the search strategies. After deduplicates, 438 references were screened for eligibility based on their titles/abstracts and 63 of them were further assessed based on their full texts. From those 63 studies, 28 fulfilled our inclusion criteria and were included in this scoping review. The list of excluded studies and their reasons of exclusion is available on our Open Science Framework deposit (https://osf.io/vgx4n/). Flowchart of study selection is available in .
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) flowchart of study selection.
Included studies were published between 1989 and 2022. Only original studies were identified (i.e. no systematic reviews, no meta-analyses). Twelve studies reported prevalence/incidence data of OA in the Netherlands [Citation8–19], seven reported data of economic burden of OA [Citation20–26] and twelve reported HRQoL data of patients with OA [Citation12,Citation20,Citation26–35]. Knee OA, hip OA, hand OA, ankle OA or combinations were investigated throughout these studies.
Nineteen of the 28 studies (68%) of the individual studies reported data from one of the seven main cohorts: the Population-based Netherlands Epidemiology of Obesity (NEO) study, the Hand OSTeoArthritis in Secondary care (HOSTAS) study, the Dutch, population based, musculoskeletal complaints and consequences (DMC3) cohort study, the Cohort Hip and Cohort Knee (CHECK) study, the Epidemiological Preventive Organisation Zoetermeer (EPOZstudy), the Longitudinal Aging Study Amsterdam (LASA) included in the larger European Project on OSteoArthritis (EPOSA) and the Rotterdam study. These different cohorts are described in .
Table 1. Description of the 7 national Dutch cohorts identified through the literature search that provided data on prevalence, HRQoL or costs of OA in the Netherlands.
3.1. Prevalence/Incidence data of OA in the Netherlands
Twelve scientific studies reported prevalence data of OA in the Netherlands [Citation8–19] and three of them reported also incidence data of OA in the Netherlands [Citation9,Citation17,Citation19] (). Information on prevalence of OA were obtained mainly from cross-sectional analysis of cohort studies (n = 9). Most of time, studies reported prevalence data for knee [Citation8–14], hip [Citation8,Citation10,Citation11,Citation14,Citation17] and hand OA [Citation8,Citation10,Citation11,Citation13,Citation15,Citation16,Citation19]. Only one study provided data of prevalence of OA in any sites (combined knee, hip and hand OA) [Citation10] and three studies reported prevalence data for different combinations of OA (i.e. knee or hip [Citation19], hip or hand [Citation11], knee and hand [Citation13]) (Appendix 3).
Table 2. Characteristics of 12/28 studies reporting prevalence data for OA in the NL.
Approach to diagnose OA varied across studies. For case definition of hip and knee OA, ICPC codes L-89 or L90 were used [Citation9,Citation17] as well as ACR clinical classification [Citation10–13]. An old study also used the Atlas of Standard Radiographs to identify knee OA based on radiographs [Citation8]. Two studies also used population self-reported OA to establish OA prevalence [Citation11,Citation14]. For hand OA, the ACR clinical classification [Citation10,Citation11,Citation13] was used as well as the radiographic presence of K-L K-L ≥ 2 in two of three joints groups [Citation15,Citation16,Citation19]. One study did not provide information about the criteria used for the assessment of OA [Citation18].
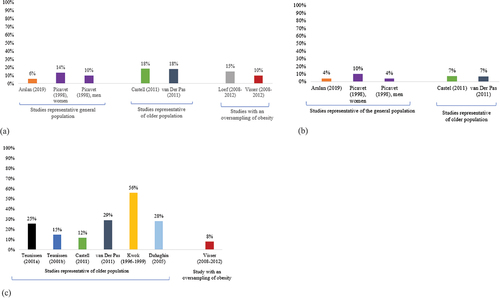
Prevalence of knee OA was reported in 7 out of the 12 studies [Citation8–14]. Another study reported prevalence of hip or knee but did not reported the prevalence of knee OA separately [Citation18]. The oldest prevalence reported was reported in the study of Van Saase in 1989 [Citation8] and the most up-to-date data were reported by Arslan et al. in 2019 [Citation9]. Knee OA prevalence ranged from 2.88% in the study of Arslan et al. [Citation9] to 18.2% in the EPOSA study [Citation10,Citation11].
The study of Arslan et al., including a representative sample of the Dutch population, reported a prevalence of codified knee OA (ICPC code L90) of 2.88% in 2008 and of 6.15% in 2019, highlighting an increase of this prevalence across time. The same authors also reported data on incidence and also showed an increase of incidence with a incidence of 4.88 per 1,000 person year in 2008 versus 6.04 per 1,000 person year in 2019. In 2003, Picavet et al. also provided data over prevalence of knee OA on a sample representative of the adult (≥25 years) population, reporting a prevalence of knee OA in 1998, based on ACR clinical classification criteria, of 10.1% in men and 13.6% in women. Two additional studies reported prevalence of OA within the EPOSA project, which used a subsample of older persons aged 65–85 years [Citation10,Citation11]. Using the ACR clinical classification, a prevalence of 18.2% for knee OA was reported in the study addressing the largest sample [Citation11]. Finally, two studies reported prevalence of knee OA from the NEO study, a population-based cohort including 6,643 adults participants with an oversampling of overweight and obesity. Surprisingly, those two studies, reporting results of the baseline measurement of the NEO study, reported a slightly different prevalence of knee OA (10% [Citation13] versus 15% [Citation11]), despite a similar diagnostic approach and a similar sample size.
Prevalence of hip OA was reported in five studies [Citation8,Citation10,Citation11,Citation14,Citation17] conducted between 1978 [Citation8] and 2019 [Citation17]. One additional study reported prevalence of hip or knee but did not report the prevalence of hip OA separately [Citation18]. Range of reported hip OA prevalence across those five studies was 4–7%. As for knee OA, only one study provided prevalence data on a representative sample of the global Dutch population and reported a prevalence of hip OA of 4% [Citation17]. Picavet et al. [Citation14] reported a slightly higher prevalence from a representative adult (≥25 years) Dutch population, with a hip OA prevalence of 10% in women and of 4% in men (about 6.75% when combining both sexes). Finally, two separate manuscripts reported prevalence of hip OA at 7% in the NEO study.
Prevalence of hand OA was reported in 7 studies [Citation8,Citation10,Citation11,Citation13,Citation15,Citation16,Citation19] conducted between 1989 [Citation8] to 2011 [Citation11]. Prevalence data across studies ranged from 11.6% [Citation10] to 56% [Citation15]. However, heterogeneity in terms of diagnostic approach and site of hand OA was noticed. Three studies used the ACR clinical classification for the identification of hand OA and reported, respectively, a prevalence of 29.4% (Van der Pas et al. EPOSA study [Citation11], participants aged 65–85 years), 11.6% (Castell [Citation10], EPOSA study, restricted sample with only 483 participants) and 8% (Visser et al. [Citation13] NEO study, oversampling overweight and obese participants). Using radiographic K-L grades ≥ 2 for the classification of hand OA, Teunissen et al. [Citation19] analyzed baseline data of the Rotterdam study (first 1991 and second 2001 cohorts included) and reported a prevalence of caropometacarpal OA of 25.3% and trapezioscaphoid OA of 14.5%. Authors did not reported directly incidence rates of OA but mentioned that the age-adjusted incidence was generally higher in females compared to males for caropometacarpal OA (4 years OR = 1.59 [95%CI 1.05;2.41] and 12 years OR = 1.59 [95%CI 1.27;2.00]) and trapezioscaphoid OA (4 years OR = 1.76 [95%CI 0.91:3.44] and 12 years OR = 2.09 [95% 1.41;3.09]). Still using data from the Rotterdam study, Dahaghin et al. [Citation16] reported a prevalence of hand OA in 1993 of 28.3% and Kwok et al. [Citation15] further reported a prevalence of hand OA of 56% in the sample 6 years later.
Only one study [Citation10] provided data of prevalence of OA in any sites (combined knee, hip and hand OA). Authors reported a prevalence of people suffering from OA of 26.2% in a population with a mean age of 75 years. Other studies presented prevalence data for different combinations of OA sites; Schellevis et al. [Citation18] reported a prevalence of knee ‘or’ hip OA of 29.3% in older adults (>65 years); Van der Pas et al. [Citation11] reported a prevalence of hip ‘or’ hand OA of 25.9% and Visser et al. [Citation13] reported a prevalence of knee ‘and’ hand OA of 4%.
Table
3.2. Economic burden of OA
Seven studies [Citation20–26] reported the economic burden of OA including (the impact on) work participation (n = 4) with [Citation20–23] or without costs [Citation21,Citation22], healthcare utilization (n = 2) [Citation25,Citation26], costs of healthcare utilization (n = 1) [Citation24] and indirect or productivity costs (n = 3) [Citation20,Citation23,Citation24] caused by OA (). Hand, hip and knee OA were addressed in one, five, and six studies, respectively. Data were obtained from cohort studies (n = 5) [Citation21–25] or baseline data from randomized controlled trials (n = 2) [Citation20,Citation26]. The sample size varied from 117 [Citation30] to 1399 participants [Citation34]. For the classification criteria of OA, ACR clinical criteria as well as the radiographic presence of K-L were used in most studies [Citation21–26]. None of the seven studies presented comparative results between patients with OA and a control group without OA.
Table 3. Characteristics of the 7/28 studies reporting economic data for OA in the Netherlands.
Four studies reported the impact of OA on work participation as ‘natural units’. In both studies of Bieleman et al. [Citation21,Citation22], data were reported on present working status, sick leave and work adaptation; the knee & hip OA study by Hardenberg et al. [Citation23] on sick leave, and the hand OA study by Terpstra et al. [Citation20] on unpaid and paid work restrictions, unpaid work replacement by others, inefficiency at work and sick leave. Two studies by Bieleman et al. [Citation21,Citation22] indicated that the baseline work participation rate (51%) of Dutch people with early hips/knees OA was similar to general Dutch population, but a decrease was captured during 2 years follow-up (46%). In addition, 12% and 14% of workers reported sick leave and work adaptions at baseline, respectively, and a further increase by 6% was captured for work adaptions at 2 years. Subjects who stopped working were on average 4.2 years older than those who continued working. In addition, female sex and lower education level were related to lower participation. Societal factors appear to have had more effect on work participation than health status. Hardenberg et al. [Citation23] reported that knee and hip OA was associated with an average sick leave episode of 186 and 159 calendar days per patient over 3 years (2015–2017), respectively. In the study of Terpstra et al. [Citation20], 45% of patients (with hand OA) who have to perform unpaid work, reported others had to take over such tasks because of hand OA. Absence from paid work (sick leave) in those employed, work restrictions and unproductive hours while at work due to hand OA at work was reported by 7%, 66% and 15% of patients, respectively.
Costs of productivity loss associated with restricted work participation were presented in three studies. Hardenberg et al. [Citation23] reported, using information reported by occupational physicians that an average sick leave episode of knee (186 days) and hip (159 days) OA was associated with €15,550 and €12,482 in costs over 3 years (2015–2017), respectively. These costs are particularly high among male workers and workers with a higher number of weekly working hours. The average annual costs (2015–2017) for the Dutch workforce due to sick leave for knee and hip OA were estimated at €26.9 million and €13.8 million, respectively. Hardenberg et al. [Citation23] also indicated that sick leave (i.e. absenteeism) costs decreased for hip and not for knee OA during 2015–2017. As every OA patient may not visit an occupational physicians, authors acknowledged a potential underestimation of the actual sick leave-related costs in their study. In another study, Hermans et al. [Citation24] estimated the total knee-related productivity costs of conservatively treated symptomatic knee OA patients with paid employment in the Netherlands at €772 per patient per month. Higher pain intensity during activity and performing physically loading work were significantly associated with productivity loss. In a third study [Citation20], the total estimated work-related societal costs (i.e. societal costs of paid labor productivity loss quantifying by lost hours due to paid work absenteeism and presenteeism – in the form of extra hours to catch up with unproductive hours at work caused by OA) per patient with hand OA were estimated at €94 per 2 weeks (€2452 per year).
With regard to health care utilization, Pelle et al. [Citation26] evaluated the short-term effects of use of an app (the app proposes a list of five pre-formulated goals to a healthier lifestyle, based on machine learning techniques fed by data collected in a personal profile, aiming to promote health behaviors, better self-management, and optimal use of non-surgical treatment options). It suggests the number of second line healthcare visits (i.e. orthopedic surgeon, rheumatologist, or physician therapist) increased after OA, visits to physical therapist were most common.
Hoogeboom et al. [Citation25] included in a study on care utilization comprising analgesic use, supplement use, contact with a General Practitioner (GP), contact with a Health Professional (HP), contact in secondary care, and alternative medicine use and indicated that analgesic use, contact with a GP, and contact with an HP were most frequently used health care by patients with knee and/or hip OA at baseline. Individuals with early symptomatic OA rely in the first 2 years mainly on analgesics, contacting with a GP significantly decreased while supplement use increased during 2 years follow-up. Education, ethnicity and familiarity with care are strongly associated with more health care use, suggesting health care use should be optimized by taking the health needs of patients in OA into consideration and by minimizing the influence of predisposing factors.
For medical costs related to OA, Hermans et al. [Citation24] reported that the total knee-related medical costs of conservatively treated symptomatic knee OA patients with paid employment in the Netherlands were €149 (median €137, IQR €72–198) per patient per month. Visits to primary care professionals physical therapist and general practitioner were the main component of the total medical costs, with an average total costs of €62 (median €31, IQR €9–96) per patient per month. The mean total costs for secondary care (orthopedic surgeon) were €33 (median €24, IQR €24–48) per patient per month. Other medical costs included drug costs, aids and diagnostic imaging costs.
Table
3.3. HRQoL burden of OA in the Netherlands
Twelve studies reporting HRQoL data for Dutch patients with OA were identified in this scoping review among [Citation12,Citation20,Citation26–35] which six were cross-sectional studies [Citation12,Citation20,Citation29,Citation31,Citation33,Citation35], one was a prospective study [Citation34], one was a case control study [Citation32] and four were randomized controlled trial for which only baseline data of participants were used [Citation26,Citation27,Citation29,Citation30] (). Regarding sites of OA, four studies studied only knee OA [Citation12,Citation20,Citation24,Citation30,Citation35]; three studies knee and hip OA [Citation26,Citation33,Citation34]; one ankle OA [Citation32]; another study studied hand OA and hand and knee OA [Citation31] and the remaining three studies included a sample of patients suffering from OA localized in multiple sites (i.e. generalized OA) [Citation27–29].
Table 4. Characteristics of 12/28 studies reporting HRQoL data for OA in the Netherlands.
In most of the studies (7/12, 58.3%), the Short Form-36 (SF-36) questionnaire was used to measure HRQoL of patients [Citation20,Citation27,Citation31–35]. The other tools used were the quality of life domain of the patient reported outcome measure Knee Injury and Osteoarthritis Outcome Score (KOOS) (3/12 studies [Citation12,Citation26,Citation30], 25%) and the Sickness Impact Profil (SIP) Questionnaire (2/12 studies [Citation28,Citation29], 16.7%). Three additional studies (3/12 studies [Citation26,Citation30,Citation33], 25%) used the Euroquol-5 dimension (EQ-5D) questionnaire and provided a utility score.
Half studies presented data of HRQoL only for patients suffering from OA and did not therefore provide a comparison of HRQoL between patients with OA and a control group without OA [Citation26,Citation27,Citation29,Citation30,Citation33,Citation34]. The other half studies presented data of HRQoL for patients with OA and compared those data to a control group, without OA [Citation12,Citation20,Citation28,Citation31,Citation32,Citation35]. In studies including only OA patients, sample size varied from 117 [Citation30] to 979 participants [Citation34]. In studies including both patients with or without OA, sample sizes varied from 191 [Citation32] to 6,643 participants [Citation31].
Four out of the six studies that compared a sample of patients with OA to a sample of control patients without OA highlighted a significant lower HRQoL in patients with OA [Citation12,Citation28,Citation31,Citation32], even after adjustment for age, sex and various risk factors. However, among those studies, Loef et al. reported no significant reduction of MCS of the SF-36 between patients with/without hand or hand&knee OA [Citation31] based on the results of the NEO study. Two additional studies reported lower scores for patients with knee OA but did not reported the results of the statistical tests to estimate the difference between groups [Citation20,Citation35].
Six other studies reported HRQoL for patients with OA without any comparison with a control group [Citation22,Citation26,Citation27,Citation29,Citation30,Citation33,Citation34]. Using the EQ-5D questionnaire, utility values of 0.70 ± 0.23 in patients with knee OA [Citation30] and of 0.71 in patients with hip and knee OA [Citation26] were reported. A third study on hip and knee OA [Citation33] also used the EQ-5D tool and reported values for each domain of the EQ-5D and compared them between patients with knee and hip OA. Generally, patients with hip OA reported better values in each domain of the EQ-5D compared to patients with knee OA. Using the SF-36, a study on knee & Hip OA [Citation34] reported PCS values of 45.6 ± 7.9 and MCS values of 53 ± 8.6. In generalized OA (i.e. shoulder, elbow, hand, neck, spine, hip, knee or foot OA) Cupertus et al. [Citation27] reported PCS values of 37.4 ± 6.9 and MCS values of 47.8 ± 10.5. Authors also indicated that scores were worst for subscales including physical function, physical role limitations, bodily pain and vitality; and that highest scores were obtained for subscales of mental health and emotional role limitations. The same observation was reported by Picavet et al. [Citation33] who also reported scores for all domains of the SF-36 for patients with knee OA and with hip OA. Generally, all SF-36 values of patients with knee OA were worse than those for patients with hip OA.
Table
4. Discussion
As essential for policy-making on resource allocation, this scoping review mapped the evidence on the prevalence or incidence of osteoarthritis in the Netherlands, the economic burden of osteoarthritis in the Netherlands, and the impact of osteoarthritis on the health-related quality of life of Dutch people affected by this disease. This scoping review allowed us to identify seven population-based cohort studies developed in the Netherlands. The development of such cohorts of individuals seems essential to understand a disease, to assess its association with risk factors or consequences, and thus to define the individual or societal burden of a disease.
Although not all cohorts included a representative sample of the population, they have provided data on the prevalence of OA and confirmed that OA is a chronic, prevalent disease that continues to impose a significant burden on patients and health care systems.
4.1. Prevalence of OA in the Netherlands
Based on the data from the population-based cohort studies, a prevalence of knee OA ranging from 6% to 18%, a prevalence of hip OA ranging from 4% to 7% and a prevalence of hand OA ranging between 12% and 56% was reported. The high prevalence of hand OA is probably biased by the sample of studies reporting hand OA, as all of these studies included older individuals and none of them was based on a representative sample of the population. This prevalence should therefore be interpreted with caution. In addition to the 12 studies included in this scoping review, the 2019 Global Burden of the Disease (GBD) study [Citation1] provides comprehensive and systematic assessments of age- and sex-specific incidence, prevalence, mortality, life-years lost, life-years lived with disability, and disability-adjusted life-years (DALYs) for 369 diseases, including osteoarthritis, in 204 countries and territories from 1990 to 2019. Because the 2019 GBD study is not a population cohort performed in the Netherlands, it was not identified by our search strategy. Nevertheless, this study provides prevalence data as it integrated all available data, including published data, gray literature data, survey data, and hospital and clinical data. Using data from the 2019 GBD study [Citation1], the age-standardized prevalence rate of osteoarthritis in the Netherlands was 4.16% in 1990, compared with 4.38% in 2019, representing a change in absolute numbers of 66.67% and an estimated annual percentage change of 5% [Citation36]. Higher changes in absolute number between 1990 and 2019 were highlighted for knee OA (+72.01%) and hip OA (+74.15%) compared to hand OA (+54.1%) [Citation36]. Regarding annual incidence of OA, the GBP indicated an incidence of 9.15% in 2019 (ranging from 0% in population younger than 30 years to 11.9% for population aged 70 to 74 years) [Citation37].
In addition to the scientific literature, the Netherlands Institute for Health Services Research (NIHSR) also provides prevalence and incidence data based on registered data from hundreds of primary care providers throughout the country. Assuming that people with ailments and conditions would eventually visit their general practitioner and/or other primary care providers, this institute hypothesized that the figures recorded by these general practitioners would provide an adequate picture of health in the Netherlands. According to this Institute, the prevalence of knee OA in 2021 in the Netherlands is of 4.35% (https://www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/cijfers-over-aandoeningen/jaarcijfers-aandoeningen-huisartsenregistraties). This prevalence is higher in women compared to men (i.e. 5.44% vs.3.25%). The annual incidence for 1000 persons is of 2.5 (men 1.9; women 3.1). Numbers reported by this Institute for hip OA are lower with a total prevalence of 2.77% (men 2.05%, women 3.48%) and a total annual incidence for 1000 persons of 1.6 (men 1.3, women 2.0). It is relevant to highlight, once again, that Arslan et al. [Citation9,Citation17] have demonstrated that prevalence data obtained from routine primary care data may be underestimated. As reliable estimates are needed for health policy maker to respond to the increasing demand for health care relating to OA, Arslan et al. [Citation9,Citation17] shown that the addition of narrative data could provide a more realistic picture of the current burden of OA. Nevertheless, the use of such data may be challenging due to data protection and to the fact that coding systems may differ between countries.
Compared to worldwide data on overall prevalence of OA (i.e. estimated at 3.8% for knee OA and 0.85% for hip OA) [Citation1], prevalence in the Dutch population seems a bit higher. Prevalence data obtained from population-based cohorts included in this scoping review were also a bit superior than prevalence data obtained from the NIHRS. The reason is that most of the cohort studies identified by our scoping review included adults population, children being excluded from the figures.
4.2. Economic burden of OA in the Netherlands
OA, as the 15th highest cause of years lived with disability worldwide, is no doubt associated with substantial costs (consisting of direct medical costs and indirect productivity costs) due to the health care consumption and restrictions in work participation such as sick leave (absenteeism) and sickness/productivity loss while at work (presenteeism). The impact of OA on work force participation can be reflected in various aspects from requiring more assistance at paid work to withdrawal from the work force, the heterogeneity was also identified in our review, making it difficult to quantify the general work participation loss in the Netherlands.
OA is nevertheless a costly disease in economic terms because of its far higher prevalence given two main factors: aging and obesity. Approximately one-third of direct OA expenditures are allocated for medications, much of which goes toward pain-related agents [Citation38]; hospitalization and surgery costs account for the largest part of direct costs. Besides scientific literature identified in this scoping review, vzinfo.nl reported data estimated by the Dutch National Institute for Public Health and the Environment [Citation39]. They estimated the direct medical costs for OA in the Netherlands in 2019 at €1.1 billion (€495 million and €446 million for knee and hip OA, respectively). Most of the expenditures relates to hospital care. This corresponds to 19.3% of the costs for musculoskeletal diseases, and 1.1% of the total annual health care costs. Compared to other chronic diseases, OA is associated with greater indirect costs (productivity costs) which is largely attributable to the effects of disability and related restrictions in work participation. This is confirmed by the study of Hermans et al. [Citation24] as productivity costs accounted for 83% of the total knee-related costs of conservatively treated symptomatic knee OA patients with paid employment in the Netherlands. In addition, it was indicated that performing physically intensive work were significantly associated with more productivity loss and higher productivity costs. However, we found well-documented information on direct and indirect OA-related costs in the Netherlands are largely scarce, the lack of evidence on indirect costs possibly prevails because no reliable national registries for sick leave and absenteeism data, more information are expected to be provided by future studies.
4.3. Burden of OA in health-related quality of life
OA is associated with significant physical disabilities and psychological disorders like depression, anxiety or sleep disturbance [Citation1,Citation40,Citation41]. It is therefore not surprising to observe a negative impact of OA on HRQOL, as highlighted by a recent systematic literature review and meta-analysis [Citation42]. A previously published meta-analysis of 6 studies [Citation42] (7094 patients with any OA, 12100 healthy controls) using the SF-36 questionnaire to measure HRQoL revealed a significantly impaired state in each dimension of HRQoL for OA patients in comparison to the general population. Authors of this meta-analysis also showed that physical health is more likely to be affected by OA than mental health (mean difference (MD) between patients with OA and healthy controls of −31.24% (95%CI −43.49;-18.99) for physical function vs MD of −12.55% (95%CI −18.1;-7.00) for mental health) [Citation42]. Due to strict inclusion criteria used by authors (i.e. restriction to cross-sectional studies, to studies provided results of the 8 domains of the SF-36, to studies reporting results as mean ± SD, etc.), none of the 6 studies comparing a group of patients with OA to a healthy controls group identified through our scoping review, was included in the MA of Yan et al. [Citation42]. Nevertheless, results from those 6 studies seems concordant to those provided by Yan et al. [Citation42], with a significant reduction in HRQoL of patients suffering from OA, mainly for the physical component score of the SF-36. Another interesting point to discuss is the fact that none of the studies included in our scoping review directly reported quality-adjusted life years (QALYs) for OA patients, which is one of the HRQoL indicator expressed as an unique measure of utility assigned to different health states [Citation43]. Only two studies [Citation26,Citation30] from our scoping review provided a global utility score from the EQ-5D questionnaire for patients with OA (respectively 0.70 and 0.71) but the cross-sectional design of the study does not allow the computation of cumulative QALYs over time. In the US, Losina et al. [44] estimated a mean losses of 1.71 QALYs per person with OA. A gap of evidence in the estimation of QALYs for Dutch people suffering from OA from Dutch cohort studies is therefore identified. This observation is also valid for disability-adjusted life year (DALYs), a time-based measure that combines years of life lost due to premature mortality. The 2019 data reported by the GBD study highlighted a worldwide DALYs of hip osteoarthritis of 1.04 million and an age-standardized DALY rate of 12.57 per 100,000 person. The higher DALYs of hip OA were observed in the U.S.A., China and India. Data for the Netherlands were above the Mondial mean (i.e. 7,428 DALYs; age-standardized DALYs rate of 23,44 per 100,000 persons). Since we did not identify population cohorts in the Netherlands reporting DALYs, the comparison with the GBD study is therefore not possible. Finally, we observed that most of studies performed in the Netherlands used a generic HRQoL questionnaire (such as the SF-36, the EQ5D or the KOOS tool), partly due to the fact that the Dutch Healthcare Institute recommends the use of QALY in cost-effectiveness analyses. By definition, a specific HRQoL questionnaire is specific to a disease, more able to detect subtle effect of the disease on the HRQoL and therefore more sensitive to change. Further studies using specific instruments to measure HRQoL in Dutch patients suffering from OA may complement the available evidence. Policymakers can play a pivotal role in promoting the adoption of standardized tools, facilitating more robust and consistent assessments of quality of life in knee osteoarthritis. Addressing these research gaps and considering the implications for policymakers will not only enhance our understanding of the disease burden but also contribute to more informed decision-making, ultimately improving the quality of care and outcomes for patients with knee osteoarthritis.
4.4. Strength and limitations
This is the first time that an exhaustive synthesis of the burden of osteoarthritis at the Netherlands national level is conducted. Even though summaries of international data are worthwhile to be informed on the worldwide burden of OA, health-care decisions and resource allocation are usually made at a national level. Therefore, providing country-specific data concerning prevalence, costs and HRQoL of patients suffering from OA is important for national decision-making.
Although we carefully followed the PRISMA-ScR statement, there are some limitations to this work that should be taken into account. First, we only investigated two different bibliographic databases. Even if we supplemented our search with a manual search to identify the maximal available evidence, we may have missed some studies providing burden data of OA in the Dutch population. Second, data extraction was performed by only one researcher. Although a second reviewer carefully checked all data extracted, we could be prone to bias in collection of data. Third, we did not measure the quality of all individual studies involved in this scoping review. The large heterogeneity of included study designs prevent us to appraise the quality of included studies using a standardized tool. Nevertheless, quality appraisal of individual studies included in a scoping review is not mandatory, and therefore this methodological weakness of our work is limited. Four, we chosen not to include papers that reported costs data related to surgery. By excluding these studies, we might have overlooked valuable insights into the economic burden of knee osteoarthritis and the cost-effectiveness of surgical interventions. Surgical treatments, including joint replacement, are important aspects of disease management and can significantly impact healthcare resource allocation. Due to the specific focus of our scoping review on prevalence, incidence, and health-related quality of life, we made a strategic decision to exclude studies that primarily examined surgical costs. Although this decision limits the scope of our review, it allows us to concentrate on the broader burden of osteoarthritis and its impact on population health. Future research could consider conducting a separate review specifically focusing on the economic aspects of surgical interventions for osteoarthritis in the Netherlands.
5. Conclusion
The findings of this scoping review may have important implications for national health policy decision-making and resource allocation in the Netherlands. The prevalence and economic burden of osteoarthritis revealed in this study highlight the significant impact of this disease on individuals and the healthcare system. The high prevalence rates, particularly for knee and hip osteoarthritis, underscore the need for targeted interventions and allocation of resources to address the growing burden. These findings can inform policymakers in developing strategies that focus on prevention, early diagnosis, and effective management of osteoarthritis. Additionally, the negative impact of osteoarthritis on health-related quality of life emphasizes the importance of integrating patient-centered outcomes in policy discussions. By considering the physical disabilities, psychological disorders, and diminished quality of life associated with osteoarthritis, policymakers can prioritize interventions that aim to improve patients’ overall well-being. Ultimately, these insights can contribute to the development of comprehensive and evidence-based policies that address the burden of osteoarthritis and promote better health outcomes for individuals in the Netherlands.
Article highlights
Twenty-eight original studies were identified throught a scoping review process to inform about societal burden of osteoarthritis (OA) in the Netherlands
OA is a highly prevalent disease in the Dutch population
OA is associated with restrictions in work participation and substantial health care consumption, translating into costs of the lost productivity and healthcare costs
OA is responsible for a major health burden
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Authors contribution
C Beaudart, M Hiligsmann and A Boonen conceived the study and developed the search question. Protocol was developed by C Beaudart and reviewed by M Hiligsmann and A Boonen. Once the protocol was approved, C Beaudart ran the search strategy. Study selection and data extraction were done by C Beaudart and N Li. First draft of the manuscript was written by C Beaudart. All authors read and approved the final draft.
Availability of data
All data are available upon request.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Registration
A protocol was developed and published in Open Science Framework (https://osf.io/vgx4n/).
Additional information
Funding
References
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763
- Litwic A, Edwards M, Dennison E, et al. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:105–185. InternetAvailable from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3690438/
- Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. 2019;78:1463–1471. doi: 10.1136/annrheumdis-2019-215920
- Xie F, Kovic B, Jin X, et al. Economic and Humanistic Burden of Osteoarthritis: A Systematic Review of Large Sample Studies. PharmacoEconomics. 2016;34:1087–1100. doi: 10.1007/s40273-016-0424-x
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
- Malley H, O’Malley L. Theory & practice this is an electronic version of an article published in Arksey. Int J Soc Res Methodol. 2005;8:19–32. Available from: http://journalsonline.tandf.co.uk/OpenURLlinktothearticle:http://www.journalsonline.tandf.co.uk/openurl.asp?genre=article&eissn=1464-5300&volume=8&issue=1&spage=19
- Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–144. doi: 10.1017/S0266462312000086
- Saase JLCM V, Van Romunde LKJ, Cats A, et al. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271. doi: 10.1136/ard.48.4.271
- Arslan IG, Damen J, de Wilde M, et al. Incidence and prevalence of knee osteoarthritis using codified and Narrative data from electronic health records: a population-based study. Arthritis Care Res. 2022;74(6):937–944. doi: 10.1002/acr.24861
- Castell MV, Van Der Pas S, Otero A, et al. Osteoarthritis and frailty in elderly individuals across six European countries: results from the European project on OSteoArthritis (EPOSA). BMC Musculoskelet Disord. 2015;16(1): doi: 10.1186/s12891-015-0807-8
- Der Pas S V, Castell MV, Cooper C, et al. European project on osteoarthritis: design of a six-cohort study on the personal and societal burden of osteoarthritis in an older European population. BMC Musculoskelet Disord. 2013;14:138. doi: 10.1186/1471-2474-14-138
- Loef M, Kroon FPB, Böhringer S, et al. Percentile curves for the knee injury and osteoarthritis outcome score in the middle-aged Dutch population. Osteoarthritis Cartilage. 2020;28(8):1046–1054. doi: 10.1016/j.joca.2020.03.014
- Visser AW, De Mutsert R, Le Cessie S, et al. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis. 2015;74(10):1842–1847. doi: 10.1136/annrheumdis-2013-205012
- Picavet HSJ, Hazes JMW. Prevalence of self reported musculoskeletal diseases is high. Ann Rheum Dis. 2003;62:644–650. doi: 10.1136/ard.62.7.644
- Kwok WY, Kloppenburg M, Rosendaal FR, et al. Erosive hand osteoarthritis: its prevalence and clinical impact in the general population and symptomatic hand osteoarthritis. Ann Rheum Dis. 2011;70(7):1238–1242. doi: 10.1136/ard.2010.143016
- Dahaghin S, Bierma-Zeinstra SMA, Ginai AZ, et al. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64:682–687. doi: 10.1136/ard.2004.023564
- Arslan IG, Damen J, de Wilde M, et al. Estimating incidence and prevalence of hip osteoarthritis using electronic health records: a population-based cohort study. Osteoarthritis Cartilage. 2022;30(6):843–851. doi: 10.1016/j.joca.2022.03.001
- Schellevis FG, van der Velden J, van Lisdonk ED, et al. Comorbidity of chronic disease in general practice. J Clin Epidemiol. 1993;46:469–473. doi: 10.1016/0895-4356(93)90024-U
- Teunissen JS, Wouters RM, Bierma-Zeintra SMA, et al. The prevalence, incidence, and progression of radiographic thumb base osteoarthritis in a population-based cohort: the Rotterdam study. Osteoarthritis Cartilage. 2022;30(4):578–585. doi: 10.1016/j.joca.2022.01.003
- Terpstra SES, Van De Stadt L, Boonen A, et al. Hand osteoarthritis is associated with limitations in paid and unpaid work participation and related societal costs: the HOSTAS cohort. RMD Open. 2022;8(2):e002367. doi: 10.1136/rmdopen-2022-002367
- Bieleman HJ, Oosterveld FGJ, Oostveen JCM, et al. Work participation and health status in early osteoarthritis of the hip and/or knee: a comparison between the cohort hip and cohort knee and the osteoarthritis initiative. Arthritis Care Res. 2010;62(5):683–689. doi: 10.1002/acr.20112
- Bieleman HJ, Reneman MF, Drossaers-Bakker KW, et al. Prognostic factors for Sustained work participation in early osteoarthritis: a follow-up study in the cohort hip and cohort knee (CHECK). J Occup Rehabil. 2013;23(1):74–81. doi: 10.1007/s10926-012-9384-y
- Hardenberg M, Speklé EM, Coenen P, et al. The economic burden of knee and hip osteoarthritis: absenteeism and costs in the Dutch workforce. BMC Musculoskelet Disord. 2022;23: doi: 10.1186/s12891-022-05306-9
- Hermans J, Koopmanschap MA, Bierma-Zeinstra SMA, et al. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care Res (Hoboken). 2012;64(6):853–861. doi: 10.1002/acr.21617
- Hoogeboom TJ, Snijders GF, Cats HA, et al. Prevalence and predictors of health care use in patients with early hip or knee osteoarthritis: two-year follow-up data from the CHECK cohort. Osteoarthritis Cartilage. 2012;20(6):525–531. doi: 10.1016/j.joca.2012.03.003
- Pelle T, Bevers K, van der Palen J, et al. Effect of the dr. Bart application on healthcare use and clinical outcomes in people with osteoarthritis of the knee and/or hip in the Netherlands; a randomized controlled trial. Osteoarthritis Cartilage. 2020;28(4):418–427. doi: 10.1016/j.joca.2020.02.831
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, et al. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35(5):871–877. doi: 10.1007/s00296-014-3149-1
- De Bock GH. Perifere artrose in de huisartspraktijk. Tijdschr Gerontol Geriatr. 1996;27:67–72 .
- de Bock C, Hermans J, van Marwijk H, et al. Health-related quality of life assessments in osteoarthritis during NSAID treatment. Pharm World Sci. 1996;8:130–136. doi: 10.1007/BF00717728
- Loef M, Damman W, de Mutsert R, et al. Health-related quality of life in patients with hand osteoarthritis from the general population and the outpatient clinic. J Rheumatol. 2020;47(9):1409–1415. doi: 10.3899/jrheum.190781
- Paget LDA, Tol JL, Kerkhoffs GMMJ, et al. Health-related quality of life in ankle osteoarthritis: a case-control study. Cartilage. 2021;13(1_suppl):1438S–1444S. doi: 10.1177/19476035211025814
- Picavet HSJ, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis. 2004;63:723–729. doi: 10.1136/ard.2003.010769
- Wesseling J, Welsing PMJ, Bierma-Zeinstra SMA, et al. Impact of self-reported comorbidity on physical and mental health status in early symptomatic osteoarthritis: the check (cohort hip and cohort knee) study. Rheumatology. 2013;52(1):180–188. doi: 10.1093/rheumatology/kes288
- Visser AW, De Mutsert R, Bloem JL, et al. Do knee osteoarthritis and fat-free mass interact in their impact on health-related quality of life in men? Results from a population-based cohort. Arthritis Care Res. 2015;67(7):981–988. doi: 10.1002/acr.22550
- Cronan TA, Shaw WS, Gallagher RA, et al. Predicting health care use among older osteoarthritis patients in an HMO. Arthritis Rheum. 1995;8(2):66–72. doi: 10.1002/art.1790080203
- Grindrod KA, Marra CA, Colley L, et al. After patients are diagnosed with knee osteoarthritis, what do they do? Arthritis Care Res. 2010;62(4):510–515. doi: 10.1002/acr.20170
- Ryan Bitton PM. The economic burden of osteoarthritis. Am J Manag Care. 2009;15:S230–5.
- Rijksinstituut voor Volksgezondheid en Milieu (RIVM). Artrose, Kosten, zorguitgaven [osteoarthritis, costs, healthcare expenses]; 2021. https://www.vzinfo.nl/artrose/zorguitgaven#:~:text=Grootste%20deel%20zorguitgaven%20voor%20artrose,59%20miljoen%20euro)%20naar%20ouderenzorg
- Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018
- Wallis JA, Taylor NF, Bunzli S, et al. Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open. 2019;9(9):e030060. doi: 10.1136/bmjopen-2019-030060
- Yan H, Guo J, Zhou W, et al. Health-related quality of life in osteoarthritis patients: a systematic review and meta-analysis related quality of life in osteoarthritis patients: a systematic review and meta-analysis. Psychol Health Med. 2022;27(8):1859–1874. doi: 10.1080/13548506.2021.1971725
- Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21. doi: 10.1093/bmb/ldq033
- Losina E, Walensky RP, Reichmann WM, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154(4):217. doi: 10.7326/0003-4819-154-4-201102150-00001
Appendix 1
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist
Appendix 2
Search Strategy:Ovid MEDLINE(R) – September 2022
exp Osteoarthritis/
osteoarth×.ti,ab,kf.
degenerative arthriti×.ti,ab,kf.
(arthroses or arthrosis).ti,ab,kf.
or/1-4
‘costs and cost analysis’/or ‘cost of illness’/or ‘global burden of disease’/or exp health care costs/or Cost-Benefit Analysis/or Health Expenditures/or Economics, Medical/or Economics, Pharmaceutical/
(cost or costs or costing or costly or expenditure or resource* or economic* or economy or pharmacoeconomic* or informal care or labor impact* or sick leave).ti,ab,kf.
Absenteeism/
‘Quality of Life’/
(quality adj2 life).ti,ab,kf.
(utilities or utility or ICER or eq-5d or HRQoL or SF-36 or ‘euroqol 5-dimension’ or qol).ti,ab,kf.
incidence/or prevalence/
burden.ti,ab,kf.
or/6-13
Netherlands/
netherlands.mp.
holland.mp.
dutch.mp.
or/15-18
20 5 and 14 and 19
Search Strategy:EMBASE – September 2022
#25. #22 AND #23 AND #24
#24. #20 OR #21
#23. #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#22. #1 OR #2 OR #3 OR #4 OR #5
#21. netherlands:ab,kw,ti OR holland:ab,kw,ti OR dutch:ab,kw,ti
#20. ‘netherlands’/de
#19. prevalence:ab,kw,ti OR incidence:ab,kw,ti OR burden:ab,kw,ti
#18. ‘incidence’/de
#17. ‘prevalence’/de
#16. (quality NEAR/2 life):ab,kw,ti
#15. utilities:ab,kw,ti OR utility:ab,kw,ti OR icer:ab,kw,ti OR ‘eq 5d’:ab,kw,ti OR hrqol:ab,kw,ti OR ‘sf 36’:ab,kw,ti OR ‘euroqol 5-dimension’:ab,kw,ti OR qaly:ab,kw,ti OR qalys:ab,kw,ti OR qol:ab,kw,ti
#14. ‘quality of life’/de
#13. (((cost:ab,kw,ti OR costs:ab,kw,ti OR costing:ab,kw,ti OR costly:ab,kw,ti OR expenditure:ab,kw,ti OR resource*:ab,kw,ti OR economic*:ab,kw,ti OR economy:ab,kw,ti OR pharmacoeconomic*:ab,kw,ti OR informal:ab,kw,ti) AND care:ab,kw,ti OR labour:ab,kw,ti) AND impact*:ab,kw,ti OR sick:ab,kw,ti) AND leave:ab,kw,ti OR burden:ab,kw,ti OR absenteeism:ab,kw,ti OR productivity:ab,kw,ti
#12. ‘cost effectiveness analysis’/de
#11. ‘pharmacoeconomics’/de
#10. ‘cost effectiveness analysis’/de
#9. ‘expenditures’/de
#8. ‘absenteeism’/de
#7. ‘disease burden’/de
#6. ‘cost’/de
#5. arthrosis:ab,kw,ti
#4. arthroses:ab,kw,ti
#3. (degenerative NEAR/1 arthritis):ab,kw,ti
#2. osteoarthr*:ab,kw,ti
#1. ‘osteoarthritis’/de
Appendix 3.
Prevalence of knee OA (A), hip OA (B) and hand OA (C) reported in scientific studies (classified by year of reported data on prevalence)