ABSTRACT
Introduction
We aimed to summarize evidence on the effect of poor medication adherence on clinical outcomes and health resource utilization (HRU) among patients with hypertension and/or dyslipidemia.
Areas covered
A systematic review of studies reporting clinical outcomes and HRU for patients by status of adherence to antihypertensives and/or lipid-lowering medications was searched using Embase, MEDLINE, and MEDLINE In-Process and supplemented by manual searches of conference abstracts. In total, 45 studies were included, with most being retrospective observational studies (n = 36). Patients with poor adherence to antihypertensives and lipid-lowering medications compared with those with good adherence showed less reduction of blood pressure (BP) and low-density lipoprotein cholesterol (LDL-c) after 6–12 months follow-up (∆ systolic BP: 1.2 vs. −4.5 mmHg; ∆LDL-c: −14.0 to −18.9 vs. −34.1 to −42.0 mg/dL). Poor adherence was also significantly associated with a higher risk of cardiovascular events (HR: 1.1–1.9) and mortality (HR: 1.4–1.8) in patients with hypertension and dyslipidemia and increased HRU (i.e. outpatient visits, risk of cardiovascular-related and all-cause hospitalization, annual inpatient days, total health-care costs).
Expert opinion
Poor adherence is associated with poor clinical outcomes and increased HRU, highlighting the need to enhance medication adherence in patients with hypertension and/or dyslipidemia.
Plain Language Summary
High blood pressure is a leading cause of death and disease burden followed by high lipid levels in blood. Due to the silent nature of the diseases, patients can fall short of optimal medicinal treatment adherence and persistence, leading to poor outcomes and disease complications. The effectiveness of medicinal interventions depends on the appropriate medication-taking behavior of patients as lower adherence can lead to poor treatment benefits. Research was conducted to look for published studies that assessed the effect of lower medication adherence on clinical outcomes and health resource use among patients with high blood pressure, high lipid levels in blood, or both. Researchers were able to find 45 already published studies, from which 32 evaluated the use of blood pressure lowering medications and 7 evaluated the use of lipid-lowering medications, while 6 included patients treated with both types of medications. Refill of pharmacy prescription records was the most common method of assessing treatment adherence. Researchers found that patients with lower adherence to these medications compared with those with good adherence showed less decrease in blood pressure levels and less improvement in blood lipid levels after 6–12 months of follow-up. Patients who had lower adherence also had higher rates of cardiovascular events and deaths and increased usage of health services including visits to outpatient clinics, getting admitted to hospitals, and a longer stay of hospitalizations, leading to a higher overall healthcare cost. These findings suggest lower adherence is associated with poor clinical outcomes and increased health-care resource usage, highlighting the need to improve medication adherence in patients with high blood pressure and high lipid levels in blood.
1. Introduction
Cardiovascular diseases (CVDs) including coronary heart disease, stroke, peripheral arterial disease, and aortic disease, are the leading cause of morbidity and mortality globally [Citation1–3]. CVD-related disease burden is driven by high clinical, patient, and economic burden and is expected to continue increasing [Citation2,Citation3]. Hypertension and dyslipidemia are major risk factors for CVDs [Citation4], contributing to increased morbidity and mortality. Raised blood pressure and cholesterol are estimated to cause 7.5 and 2.6 million annual deaths worldwide, respectively [Citation5,Citation6]. Multiple pharmacological interventions are available for the control of blood pressure or serum lipid levels, with most patients requiring a complex combination therapy to achieve blood pressure/serum lipid level goals [Citation7,Citation8]. The effectiveness of these pharmacological interventions depends on the appropriate medication-taking behavior of patients as poor adherence and non-adherence represents the most common cause of non-response to pharmacological medications [Citation9–12].
Several approaches are available to investigate the medication adherence which include subjective methods, such as self-reported questionnaires including the 8-item Morisky Medication Adherence Scale (MMAS-8), Brief Medication Questionnaire, the Hill-Bone Compliance scale or other questionnaires [Citation13], and objective methods, which can be indirect (e.g. formulations like proportion of days covered [PDC] or medication possession ratio [MPR]) or direct (i.e. measurement of drug concentrations in body fluids such as blood or urine) [Citation14,Citation15]. However, there is no gold standard method to measure medication adherence, and a discordance in the adherence levels has been reported with different methods, with subjective methods being more prone to underestimate the prevalence of poor adherence [Citation16,Citation17]. Furthermore, there is no consensus on the cutoff value that defines optimal adherence to antihypertensives and lipid-lowering medications, although the 80% threshold appears to be the most widely used criterion in clinical research and by policymakers [Citation18].
Despite the complexity associated with adherence measurement, it has been reported that up to 60% of patients with hypertension and up to 90% of those with dyslipidemia have poor medication adherence [Citation19,Citation20]. To our knowledge, this is the first SLR analyzing the impact of poor adherence to both clinical outcomes (e.g. control of blood pressure/serum lipid levels) and health resource utilization in patients treated with antihypertensives and lipid-lowering medications. Previous systematic reviews and meta-analyses investigating this relationship focused only on the association between adherence and the risk of CVD events or mortality [Citation21–23]. Therefore, we aimed to perform a systematic review to better understand the effect of medication adherence on outcomes of patients with hypertension and/or dyslipidemia. This could help tailor the effect of interventions by increasing medication adherence.
2. Methods
We searched electronic databases including Embase, MEDLINE, and MEDLINE In-Process from inception to 1 February 2022, using structured search terms. The complete search strategy for each database is presented in Table S1, which included a combination of keywords such as ‘hypertension,’ ‘dyslipidemia,’ and ‘medication compliance/adherence/persistence.’ Electronic searches were supplemented with manual searches of Google Scholar and conference proceedings, including the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), the European Society of Cardiology (ESC), and the American Heart Association (AHA), to identify studies published from January 2020 onwards. Other sources used included bibliography of included studies and of relevant SLRs published in the last 3 years [Citation23–35].
Two independent reviewers (L.Z. and J.L.) assessed the eligibility of all identified publications, first based on title and abstract screening and then the full-text review (with conflicts resolved by discussion or consultation with a third reviewer [M.K.B.]), following pre-defined inclusion and exclusion criteria based on the PICOS framework (Table S2). In brief, eligible studies were observational studies or randomized controlled trials (RCTs) in adult patients with hypertension and/or dyslipidemia receiving any pharmacological intervention for either conditions administered as a standalone therapy or in combination with any other pharmacological or non-pharmacological interventions.
In addition, studies were included if medication adherence was reported as a categorical (not continuous) variable using any cutoff value, with good and poor adherence as defined by the authors, and was measured using one of the following methods: (1) subjective/self-reported adherence using MMAS-8, (2) indirect methods including PDC/MPR or similar formulations, or (3) any direct method such as blood, plasma, or urine drug monitoring. Eligibility was further restricted to studies that reported clinical outcomes (i.e. changes in the proportion of patients with controlled blood pressure/serum lipid levels, cardiovascular events, and survival/mortality) and/or health resource utilization/costs. Articles published in peer-reviewed journals with no restriction on the publication date were included. For conference abstracts, only publications from January 2020 onwards were included.
After a full-text review, a list of the excluded studies was created with the reason for exclusion. Two independent researchers (L.Z. and J.L.) extracted data from the included studies into a pre-defined tabular summary, which included publication details, study and patient characteristics, and outcomes of interest. A third researcher (M.K.B.) checked the extracted data from 20% of studies for accuracy. For this SLR, we narratively described the findings of the included studies using descriptive statistics, presenting findings for hypertension and dyslipidemia separately. Based on the identified evidence, we assessed the feasibility of conducting a network meta-analysis, but it was deemed not feasible due to the high heterogeneity between studies. Therefore, no quantitative analysis was performed.
3. Results
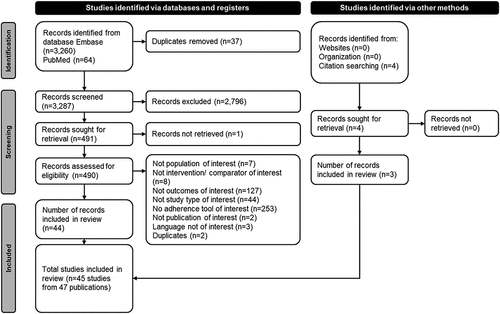
After the removal of duplicates, a total of 3,287 records from electronic databases were identified. In addition, four publications were identified via hand searches. Following eligibility assessment, 45 studies from 47 publications were included in this SLR. The study selection process and reasons for exclusion are summarized in . All studies were published between 2005 and 2021. Most were retrospective observational studies (n = 36) and with a sample size larger than 1,000 (n = 36). From the 45 studies included, 32 evaluated the use of antihypertensives and 7 evaluated the use of lipid-lowering drugs, while 6 included patients treated with both types of medications. The included studies employed a range of methods to assess medication adherence, with prescription refill records being the most common, followed by self-reporting and direct methods. Adherence for prescription refill records was based on two indirect methods: PDC or MPR formulations (n = 37). Different thresholds were applied to measure adherence in the included studies, and >80% was the most commonly applied cutoff value for good adherence (n = 24). Detailed characteristics of each study are provided in Table S3, including year of publication, study type, sample size, study country, pharmacologic intervention, adherence measurement method, and the threshold applied to measure adherence.
3.1. Hypertension
A total of 38 studies reported the impact of poor adherence to antihypertensive medications (AHMs) on clinical outcomes, including change in blood pressure or blood pressure control (n = 14 studies), cardiovascular events (n = 6), and survival and mortality (n = 6), as well as on health resource utilization and costs (n = 18).
3.1.1. Impact of poor adherence on blood pressure
Several studies have consistently shown that patients with poor adherence to AHMs had higher blood pressure values and less reduction in blood pressure at follow-up (6–12 months) compared with baseline (Table S4) [Citation36–40]. As reported by Bhagavathula et al. (2021), patients with newly diagnosed hypertension and with good adherence (defined as PDC ≥ 80%) had a significant reduction in systolic and diastolic blood pressure (DBP) of −4.5 mmHg (95% confidence interval [CI]: −5.40, −3.77) and −6 mmHg (95% CI: −6.5, −5.4), respectively, compared with the poor adherence group after 6 months of treatment [Citation37].
In addition, in studies monitoring adherence over time, patients who switched from non-adherent to adherent status experienced a decrease in systolic blood pressure (SBP) measured in the office-setting; in contrast, patients who became non-adherent after being adherent experienced an increase in SBP [Citation16,Citation41]. Patients who remained adherent over time showed stable blood pressure values [Citation16,Citation41]. To reinforce these findings, a lower percentage of patients with controlled blood pressure or a higher proportion of patients with uncontrolled blood pressure was found in the poor adherence group () [Citation16,Citation36,Citation37,Citation42–45].
Table 1. Number of patients with controlled or uncontrolled BP by adherence treated with antihypertensives.
Wu et al. (2018) showed that an increase in adherence levels in patients who had poor adherence at baseline led to a significantly larger reduction in SBP and DBP over 24 months of follow-up (Figure S1) [Citation46]. In this study, the use of non-pharmacological interventions (e.g. phone-based health coaching) led to improvement in adherence, with 64% of patients with poor adherence at baseline switching to good adherence over the follow-up period [Citation46].
Conversely, Ewen et al. (2015) reported that medication adherence measured using direct methods did not impact blood pressure in patients with resistant hypertension and undergoing catheter-based renal denervation [Citation47].
3.1.2. Impact of poor adherence on cardiovascular events
In studies that aimed to evaluate the impact of adherence to AHMs on cardiovascular events, patients with good adherence had lower incidence and risk of experiencing overall and specific CVD events compared to poor adherence () [Citation48–52]. A threshold effect of adherence measured using PDC or MPR at approximately 80% was reported for good adherence in all these studies; however, three studies categorized adherence into good, intermediate, and poor based on PDC ≥ 80%, 40–79%, and <40%, respectively [Citation48,Citation50,Citation52] one study used four categorization PDC ≥ 80%, 61–80%, 41–60%, and <40% [Citation51], and the fifth study used MPR ≥ 80% and <80% to categorize good and poor adherences [Citation49]. Four studies reported approximately 40–50% reduction in any CVD events in the good adherence group (PDC ≥ 80%) compared with low adherence group defined as PDC < 40% and the results were statistically significant in all these studies. Likewise, in a study with good and poor adherence defined as MPR ≥ 80% and <80%, a higher risk of chronic heart failure and cerebrovascular disease was reported with an HR of 1.42 (1.27–1.58) and 1.13 (1.03–1.25), respectively, in patients with MPR < 80% compared with MPR ≥ 80%. For the intermediate adherence level (PDC 40–79%), a relative reduction in any CVD events was reported compared with low adherence (PDC < 40%) in three studies; however, the statistical significance was reached in only one study. An additional study by Ho et al. (2018), which aimed to compare the impact of using a single-pill combination (SPC) vs. a free-equivalent combination (FEC) of the AHMs on the incidence of clinical outcomes, reported that patients using SPC had lower incidence of cardiovascular and renal-related events compared with FEC; however, good adherence had higher incidence rates compared with those with poor adherence in both the SPC and FEC cohorts, although statistical significance was not reported [Citation53].
Table 2. Incidence and risk of CVD events by adherence in patients on antihypertensives.
3.1.3. Impact of poor adherence on survival and mortality
Two studies reported the impact of adherence to AHMs on survival. In a study by Bailey et al. (2014), adherence was categorized as per 80% threshold, and the 5-year survival rate was higher in the good adherence group (medication refill adherence [MRA] ≥80%) compared with poor adherence (MRA < 80%) (0.938 vs. 0.933, p < 0.001) [Citation54]. Meanwhile, Tsoi et al. (2014) categorized adherence into three different groups: PDC < 40%, 40–69%, ≥70% and reported higher proportion of survivors in the moderate adherence (PDC 40–69%) group compared with patients with good (PDC ≥ 70%) or poor adherence (PDC < 40%) to AHMs (88.6% vs. 71.1% vs. 81.2%, statistical significance was not reported) [Citation55]. However, the baseline characteristics were not available to ascertain the differences in these two studies. Five studies evaluated the impact of adherence of AHMs on mortality and the findings were mostly consistent. Four out of five studies reported a lower rate or risk of all-cause or CVD-related mortality with improved adherence to AHMs, with statistical significance reached in three out of the four studies () [Citation51,Citation56–58].
Table 3. Mortality data by adherence in patients on antihypertensives.
3.1.4. Impact of poor adherence on health resource utilization and costs
Poor adherence to AHMs resulted in increased health resource utilization and costs across all 18 studies identified (). Health resource utilization reported in the studies included medical service use (including inpatient and outpatient services) [Citation59,Citation60] and incidence and risk of all-cause (n = 3 studies) and CVD-related (n = 10 studies) hospitalization [Citation11,Citation19,Citation43,Citation49,Citation53,Citation56,Citation58,Citation61–63]. Regarding costs in good adherence vs. poor adherence groups, although higher expenditure on medication was observed due to adequate doses taken by adherent patients, the overall health-care costs including all-cause and CVD-related medical costs were significantly lower in patients with good adherence [Citation11,Citation19,Citation49,Citation59,Citation61–66].
Table 4. Health resource utilization and costs by adherence in patients on antihypertensives.
3.2. Dyslipidemia
A total of 13 studies reported the effects of poor adherence to lipid-lowering medication on clinical outcomes, serum lipid control (n = 5 studies), cardiovascular events (n = 3), and survival/mortality (n = 1), as well as health resource utilization and costs (n = 5).
3.2.1. Impact of poor adherence on serum lipid levels
Evidence consistently showed that good adherence led to improved control of serum lipid levels compared with poor adherence. Shani et al. (2019) reported that patients adherent to statins had significantly lower low-density lipoprotein cholesterol (LDL-c) levels compared with poorly adherent patients (PDC-like >75%: 84.0 mg/dL vs. PDC-like <75%: 103.0 mg/dL, p < 0.0001) [Citation38]. In the studies by Kazerooni et al. (2013) and Shalev et al. (2014), adherent patients experienced larger decreases in LDL-c levels compared with the poor adherence group (MPR/PDC <80%) at several time points (3–6 months: −34.1 to −42.0 mg/dL in good adherence vs. −14.8 to −18.7 mg/dL in poor adherence; 12 months: −37.2 mg/dL in good adherence vs. −18.9 mg/dL in poor adherence) [Citation67,Citation68]. Moreover, Shalev et al. (2014) also reported that a higher proportion of patients had controlled LDL-c levels (LDL-c <100–130 mg/dl depending on the patient cohort) in the good vs. poor adherence group (PDC 58–80% vs. 17–28%) [Citation68]. Similarly, in a study by Kim et al. (2017), where a total of four adherence groups were compared, the percentage of patients with controlled LDL-c levels (LDL-c <100 mg/dl) increased with increasing adherence levels (from 78.2% in MPR < 89% to 89.5% in MPR ≥ 97%) [Citation45].
3.2.2. Impact of poor adherence on cardiovascular events and survival/mortality
Three studies reported the impact of adherence on cardiovascular outcomes and lower incidence and risk of experiencing CVD events were reported in patients with good adherence to lipid-lowering medication across these studies (Table S5) [Citation69–71]. The risk of composite outcome of non-fatal and fatal CVD events was also significantly lower by 21% in patients with good adherence (MPR > 80%) compared with poor adherence in a study by Hero et al. (2020). Yeo et al. (2020) reported an increasing trend of stroke recurrence with a decrease in the adherence levels to statins with an adjusted HR of 2.41 (95% CI, 0.83–7.00), 2.68 (95% CI, 0.90–7.99), 3.44 (95% CI, 0.93–12.74) in the PDC 50–74%, 25–49%, and <25% adherence groups, respectively, compared with patients with good adherence (PDC ≥ 75%). Hero et al. (2020) also reported a higher risk of CVD-related mortality among patients with good adherence compared with those showing poor adherence (although statistical significance was not reached, p = 0.06;) and no difference in all-cause mortality between good and poor adherence (Table S5) [Citation70].
3.2.3. Impact of poor adherence on health resource utilization and costs
In studies reporting the impact of adherence to lipid-lowering medications on health resource utilization and costs, patients with poor adherence consistently showed higher levels of all-cause inpatient/outpatient services utilization, higher probabilities and higher risk of hospitalization, longer annual inpatient days, as well as higher medical and overall health-care costs, including inpatient/outpatient costs and CVD-related costs compared to patients with good adherence () [Citation11,Citation59,Citation63,Citation64,Citation72].
Table 5. Health resource utilization and costs by adherence in patients on lipid-lowering medications.
4. Discussion
In this SLR, we identified 45 studies comparing clinical outcomes and health resource utilization of patients with poor and good adherence to antihypertensives or lipid-lowering medications. Each of the outcomes were assessed in at least eight studies and most of the published evidence consistently reported suboptimal blood pressure and lipid levels, increased risk of cardiovascular events and stroke, and higher levels of health resource utilization in patients with poor medication adherence to antihypertensives and lipid-lowering therapies compared to those with good adherence. This highlights the importance of good adherence which will result in better outcomes.
In line with previous systematic reviews, this SLR reported the risk of any CVD event in the high adherence group compared to poor adherence to be reduced in the range of 40–80%. Unlike previous systematic reviews that focused only on the association between adherence and risk of CVD events or mortality, additional clinical outcomes including the impact of poor adherence on blood pressure and serum lipid levels were analyzed in this review. A significant reduction in the systolic BP by −4.5 mmHg in patients with good adherence to AHMs compared with an increase of 1.2 mmHg in the poor adherence group was reported. Similarly, higher reduction in the LDL-c levels in the range of −34.1 to −42.0 mg/dL in patients with good adherence to lipid-lowering medications compared to −14.0 to −18.9 mg/dL in those with poor adherence.
As poor adherence to anti-hypertensives and lipid-lowering medications contribute to increased CVD-related disease burden, including clinical, patient, and economic burden, more attention should be paid to interventions that improve adherence [Citation2,Citation3]. Numerous studies have evaluated the impact of interventions that improve adherence to clinical outcomes [Citation25,Citation26,Citation33,Citation73]. Interventions include non-pharmacological interventions such as education of patients and health-care professionals, phone applications, and text messaging, but also pharmacological-based interventions such as the use of SPCs [Citation25,Citation26,Citation33,Citation73], resulting in better control of blood pressure in patients with hypertension or among patients with concomitant hypertension/hypercholesterolemia [Citation25,Citation73,Citation74]. Therefore, implementing measures to enhance medication adherence will improve the clinical care of patients with hypertension and dyslipidemia, both from the patient perspective (i.e. better control of blood pressure and serum lipid levels, as well as decreased risk of CVD events and mortality) and from the health system perspective (i.e. decreased health resource utilization and costs). In the study by Wu et al. (2018), the use of non-pharmacological interventions (e.g. phone-based health coaching) led to improved adherence (64% of patients with poor adherence at baseline switched to good adherence) over time, which was significantly associated with reductions of BP [Citation46].
A few studies have also reported contrasting findings (n = 3, i.e. poor adherence was associated with better clinical outcomes although with unknown statistical significance) [Citation53,Citation55,Citation70] and one study showed no differences between good and poor adherence groups [Citation47]. Several reasons may explain these findings, including: bias due to residual confounding, unmeasured, or inherited by the study design [Citation70]; particularities of the patient cohort studied (e.g. patients with resistant hypertension and undergoing catheter-based renal denervation or patients with type I diabetes) [Citation47,Citation70]; and lack of baseline characteristics by adherence groups that allow correct interpretation of the results [Citation53,Citation55].
This review has a few limitations. The method used to measure adherence, the definition of good and poor adherence group, and the number of adherence groups present in the study cohort lacked consistency across the included studies. Although 82% (37/45) of studies used indirect methods (PDC/MPR) to measure adherence, approximately half of the studies (17/37) had ≥3 groups of adherence (good, moderate, and poor, or further groups) rather than the dichotomous classification of good vs. poor adherence. Among the five studies that used direct measurement methods (urine/plasma drug monitoring), three defined poor adherence qualitatively – non-detection of at least one prescribed medication – while the other two used quantitative thresholds (one used <80% and one used <100%). Even within the three studies using self-reported methods of adherence (MMAS-8), the definition of good and poor adherence varied.
The lack of data at the same follow-up timepoint also constrained the feasibility of conducting quantitative analyses (e.g. meta-analysis) in our study. In most included studies, the follow-up time varied among studies assessing the same outcome, and most studies did not provide data for more than one follow-up visit. After conducting a formal feasibility assessment, performing a meta-analysis was not recommended based on the high heterogeneity observed in study design, population characteristics, adherence tool, and baseline characteristics reported across studies.
Nonetheless, our findings support the importance of good medication adherence in achieving better clinical outcomes as this SLR shows that poor medication adherence negatively impacts clinical outcomes and health resource utilization in patients with hypertension and dyslipidemia. The potential high prevalence of poor adherence worldwide emphasizes the effective use of interventions and an opportunity for cost saving in the healthcare system. This review also highlights that an agreement is needed on consistent methods to measure adherence. The most commonly used method was MPR; however, different definitions and thresholds were used to define good and poor adherence. Therefore, more research is needed to standardize the poor or good adherence thresholds. Additionally, due to different follow-up time periods in the included studies, a meta-analysis was not feasible, and more research is needed in this domain. Together with published SLRs on the same topic, these findings support the use of measures to improve adherence such as the use of SPCs, which could help to maximize the effectiveness of available antihypertensives and lipid-lowering medications in clinical practice, leading to improved patient outcomes, and optimize health resource utilization derived from related chronic conditions.
Declaration of interest
JB Briere is an employee of Servier International; Z M Khan was a paid consultant for Servier International at the time of the study; P Atanasov, L Zhu, J Li, and M K Bhatia were employees of Amaris Consulting at the time of the study, which received funding from Servier International to conduct the study; A P Kengne received honorarium for critical inputs into the study design and data interpretation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
Z Khan, A P Kengne, JB Briere, L Zhu, J Li, M K Bhatia, and P Atanasov were involved in the conception and design of this project; Z Khan, A P Kengne, JB Briere, M K Bhatia, J Li, and L Zhu were involved in interpretation of data and drafting of the paper or revising it critically for intellectual content; all authors provided final approval of the version to be published and also agree to be accountable for all aspects of the work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (5.7 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2266135.
Additional information
Funding
References
- World Health Organization. Cardiovascular diseases (CVDs) [Internet]. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Roth Gregory A, Mensah George A, Johnson Catherine O, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
- Luengo-Fernandez R, Leal J, Burns R. P4659Economic burden of CVD across the European Union: trends over the last decade. Eur Heart J. 2017;38(suppl_1):ehx504.P4659. doi: 10.1093/eurheartj/ehx504.P4659
- World Health Organization. Regional Office for Europe. Global atlas on cardiovascular disease prevention and control: published by the World Health Organization in collaboration with the World Heart Federation and the World stroke Organization [Internet]. Copenhagen: World Health Organization. Regional Office for Europe; 2011 [cited 2022 Nov 30]. Available from: https://apps.who.int/iris/handle/10665/329516
- World Health Organization. Blood pressure/hypertension [Internet]. 2022. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3155
- World Health Organization. Raised cholesterol [Internet]. 2022. Available from: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3236
- Zodda D, Giammona R, Schifilliti S. Treatment strategy for dyslipidemia in cardiovascular disease prevention: focus on old and New drugs. Pharmacy. 2018;6(1):6. doi: 10.3390/pharmacy6010010
- Williams B, Mancia G, Spiering W, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339
- Liberopoulos EN, Florentin M, Mikhailidis DP, et al. Compliance with lipid-lowering therapy and its impact on cardiovascular morbidity and mortality. Expert Opin Drug Saf. 2008;7(6):717–725. doi: 10.1517/14740330802396984
- Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55
- Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af
- Souza AC, Borges JW, Moreira TM. Quality of life and treatment adherence in hypertensive patients: systematic review with meta-analysis. Rev Saúde Pública. 2016;50:71. doi: 10.1590/s1518-8787.2016050006415
- Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens Greenwich Conn. 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–1090. discussion 1073. doi: 10.1016/S0149-2918(99)80026-5
- Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi: 10.1155/2015/217047
- Hamdidouche I, Jullien V, Boutouyrie P, et al. Routine urinary detection of antihypertensive drugs for systematic evaluation of adherence to treatment in hypertensive patients. J Hypertens. 2017;35(9):1891–1898. doi: 10.1097/HJH.0000000000001402
- Osula D, Wu B, Schesing K, et al. Comparison of pharmacy refill data with chemical adherence testing in assessing medication nonadherence in a safety net hospital setting. J Am Heart Assoc. 2022;11(19):e027099. doi: 10.1161/JAHA.122.027099
- Gellad WF, Thorpe CT, Steiner JF, et al. The myths of medication adherence. Pharmacoepidemiol Drug Saf. 2017 [2017 Oct 11];26(12):1437–1441. doi: 10.1002/pds.4334
- Will JC, Zhang Z, Ritchey MD, et al. Medication adherence and incident preventable hospitalizations for hypertension. Am J Prev Med. 2016;50(4):489–499. doi: 10.1016/j.amepre.2015.08.021
- Lucas JE, Bazemore TC, Alo C, et al. An electronic health record based model predicts statin adherence, LDL cholesterol, and cardiovascular disease in the United States military health system. PLoS One. 2017;12(11):e0187809. doi: 10.1371/journal.pone.0187809
- Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295
- De Vera MA, Bhole V, Burns LC, et al. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014 [2014 Nov 5];78(4):684–698. doi: 10.1111/bcp.12339
- Feng Y, Zhao Y, Yang X, et al. Adherence to antihypertensive medication and cardiovascular disease events in hypertensive patients: a dose–response meta-analysis of 2 769 700 participants in cohort study. QJM: An International Journal Of Medicine. 2021;115(5):279–286. doi: 10.1093/qjmed/hcaa349
- Sorato MM, Davari M, Kebriaeezadeh A, et al. Reasons for poor blood pressure control in Eastern Sub-Saharan Africa: looking into 4P’s (primary care, professional, patient, and public health policy) for improving blood pressure control: a scoping review. BMC Cardiovasc Disord. 2021;21(1):123. doi: 10.1186/s12872-021-01934-6
- Tam HL, Wong EML, Cheung K, et al. Effectiveness of text messaging interventions on blood pressure control among patients with hypertension: systematic review of randomized controlled trials. JMIR mHealth uHealth. 2021;9(9):e24527. doi: 10.2196/24527
- Yao M, Zhou XY, Xu ZJ, et al. The impact of training healthcare professionals’ communication skills on the clinical care of diabetes and hypertension: a systematic review and meta-analysis. BMC Fam Pract. 2021;22(1):152. doi: 10.1186/s12875-021-01504-x
- Aguiar J, Ribeiro M, Pedro AR, et al. Awareness about barriers to medication adherence in cardiovascular patients and strategies used in clinical practice by Portuguese clinicians: a nationwide study. Int J Clin Pharm. 2021;43(3):629–636. doi: 10.1007/s11096-020-01174-2
- Van Truong P, Wulan Apriliyasari R, Lin M, et al. Effects of self‐management programs on blood pressure, self‐efficacy, medication adherence and body mass index in older adults with hypertension: Meta‐analysis of randomized controlled trials. Int J Nurs Pract. 2021;27(2):e12920. doi: 10.1111/ijn.12920
- Tan FCJH, Oka P, Dambha-Miller H, et al. The association between self-efficacy and self-care in essential hypertension: a systematic review. BMC Fam Pract. 2021;22(1):44. doi: 10.1186/s12875-021-01391-2
- Xu H, Long H. The effect of smartphone app–based interventions for patients with hypertension: systematic review and meta-analysis. JMIR mHealth uHealth. 2020;8(10):e21759. doi: 10.2196/21759
- Li R, Liang N, Bu F, et al. The effectiveness of self-management of hypertension in adults using mobile health: systematic review and meta-analysis. JMIR mHealth uHealth. 2020;8(3):e17776. doi: 10.2196/17776
- Hong J, Tiu YC, Leung PYB, et al. Interventions that improve adherence to antihypertensive medications in coronary heart disease patients: a systematic review. Postgrad Med J. 2022;98(1157):219–227. doi: 10.1136/postgradmedj-2020-139116
- Tan JP, Cheng KKF, Siah RCJ. A systematic review and meta-analysis on the effectiveness of education on medication adherence for patients with hypertension, hyperlipidaemia and diabetes. J Adv Nurs. 2019;75(11):2478–2494. doi: 10.1111/jan.14025
- Shahaj O, Denneny D, Schwappach A, et al. Supporting self-management for people with hypertension: a meta-review of quantitative and qualitative systematic reviews. J Hypertens. 2019;37(2):264–279. doi: 10.1097/HJH.0000000000001867
- Cimmaruta D, Lombardi N, Borghi C, et al. Polypill, hypertension and medication adherence: the solution strategy? Int J Cardiol. 2018;252:181–186. doi: 10.1016/j.ijcard.2017.11.075
- Bramlage P, Ketelhut R, Fronk EM, et al. Clinical impact of patient adherence to a fixed-dose combination of olmesartan, amlodipine and hydrochlorothiazide. Clin Drug Investig. 2014;34(6):403–411. doi: 10.1007/s40261-014-0188-z
- Bhagavathula AS, Shah SM, Aburawi EH. Medication adherence and treatment-resistant hypertension in newly treated hypertensive patients in the United Arab Emirates. J Clin Med. 2021;10(21):5036. doi: 10.3390/jcm10215036
- Shani M, Lustman A, Vinker S. Adherence to oral antihypertensive medications, are all medications equal? J Clin Hypertens. 2019;21(2):243–248. doi: 10.1111/jch.13475
- De Jager RL, van Maarseveen EM, Bots ML, et al. Medication adherence in patients with apparent resistant hypertension: findings from the SYMPATHY trial. Br J Clin Pharmacol. 2018;84(1):18–24. doi: 10.1111/bcp.13402
- De Jager RL, De Beus E, Beeftink MMA, et al. Impact of medication adherence on the effect of renal denervation: the SYMPATHY trial. Hypertension. 2017;69(4):678–684. doi: 10.1161/HYPERTENSIONAHA.116.08818
- Gupta P, Patel P, Štrauch B, et al. Biochemical screening for nonadherence is associated with blood pressure reduction and improvement in adherence. Hypertension. 2017;70(5):1042–1048. doi: 10.1161/HYPERTENSIONAHA.117.09631
- Kebede B, Chelkeba L, Dessie B, Rate of blood pressure control and its determinants among adult hypertensive patients at Jimma University Medical Center, Ethiopia: prospective cohort study. SAGE Open Med. 2021; 9: doi: 10.1177/20503121211006000
- Wu PH, Yang CY, Yao ZL, et al. Relationship of blood pressure control and hospitalization risk to medication adherence among patients with hypertension in Taiwan. Am J Hypertens. 2009;23(2):155–160. doi: 10.1038/ajh.2009.210
- Overgaauw N, Alsma J, Brink A, et al. Drug nonadherence is a common but often overlooked cause of hypertensive urgency and emergency at the emergency department. J Hypertens. 2019;37(5):1048–1057. doi: 10.1097/HJH.0000000000002005
- Kim HJ, Kim E, Min KB, et al. Blood pressure and lipid target adherence in Korean patients receiving angiotensin II receptor blockers/statin regimens. Curr Med Res Opin. 2017;33(2):385–390. doi: 10.1080/03007995.2016.1257982
- Wu JR, Cummings DM, Li Q, et al. The effect of a practice-based multicomponent intervention that includes health coaching on medication adherence and blood pressure control in rural primary care. J Clin Hypertens. 2018;20(4):757–764. doi: 10.1111/jch.13265
- Ewen S, Meyer MR, Cremers B, et al. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol. 2015;104(12):1097–1105. doi: 10.1007/s00392-015-0905-5
- Yang Q, Chang A, Ritchey MD, et al. Antihypertensive medication adherence and risk of cardiovascular disease among older adults: a population-based cohort study. J Am Heart Assoc. 2017;6(6). doi: 10.1161/JAHA.117.006056
- Dragomir A, Côté R, Roy L, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care. 2010;48(5):418–425. doi: 10.1097/MLR.0b013e3181d567bd
- Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299
- Degli Esposti L, Saragoni S, Benemei S, et al. Adherence to antihypertensive medications and health outcomes among newly treated hypertensive patients. Clinicoecon Outcomes Res. 2011;3:47–54. doi: 10.2147/CEOR.S15619
- Kim J, Bushnell CD, Lee HS, et al. Effect of adherence to antihypertensive medication on the long-term outcome after hemorrhagic stroke in Korea. Hypertension. 2018;72(2):391–398. doi: 10.1161/HYPERTENSIONAHA.118.11139
- Ho CT, Tung YC, Chou SH, et al. Clinical outcomes in hypertensive patients treated with a single-pill fixed-dose combination of renin-angiotensin system inhibitor and thiazide diuretic. J Clin Hypertens. 2018;20(12):1731–1738. doi: 10.1111/jch.13413
- Bailey JE, Wan JY, Tang J, et al. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med. 2010;25(6):495–503. doi: 10.1007/s11606-009-1240-1
- Tsoi KKF, Wong MCS, Tam WWS, et al. Cardiovascular mortality in hypertensive patients newly prescribed perindopril vs. lisinopril: a 5-year cohort study of 15,622 Chinese subjects. Int J Cardiol. 2014;176(3):703–709. doi: 10.1016/j.ijcard.2014.07.114
- Lee H, Park JH, Floyd JS, et al. Combined effect of income and medication adherence on mortality in newly treated hypertension: nationwide study of 16 million person-years. J Am Heart Assoc. 2019;8(16). doi: 10.1161/JAHA.119.013148
- Wong MCS, Tam WWS, Wang HHX, et al. Predictors of the incidence of all-cause mortality and deaths due to diabetes and renal diseases among patients newly prescribed antihypertensive agents: a cohort study. Int J Cardiol. 2013;168(5):4705–4710. doi: 10.1016/j.ijcard.2013.07.174
- Kim S, Shin DW, Yun JM, et al. Medication adherence and the risk of cardiovascular mortality and hospitalization among patients with newly prescribed antihypertensive medications. Hypertension. 2016 [2016 Feb 13];67:506–512.
- Campbell PJ, Axon DR, Taylor AM, et al. Hypertension, cholesterol and diabetes medication adherence, health care utilization and expenditure in a medicare supplemental sample. Medicine (Baltimore). 2021;100(35):e27143. doi: 10.1097/MD.0000000000027143
- Lynch WD, Markosyan K, Melkonian AK, et al. Effect of antihypertensive medication adherence among employees with hypertension. Am J Manag Care. 2009;15(12):871–880.
- Pittman DG, Tao Z, Chen W, et al. Antihypertensive medication adherence and subsequent healthcare utilization and costs. Am J Manag Care. 2010;16:568–576.
- Yang Z, Howard DH, Will J, et al. Association of antihypertensive medication adherence with healthcare use and Medicaid expenditures for acute cardiovascular events. Med Care. 2016;54(5):504–511. doi: 10.1097/MLR.0000000000000515
- Roebuck MC, Liberman JN, Gemmill-Toyama M, et al. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff. 2011;30(1):91–99. doi: 10.1377/hlthaff.2009.1087
- Kymes SM, Pierce RL, Girdish C, et al. Association among change in medical costs, level of comorbidity, and change in adherence behavior. Am J Manag Care. 2016;22(8):e295–301.
- Qiao Y, Steve Tsang CC, Hohmeier KC, et al. Association between medication adherence and healthcare costs among patients receiving the low-income subsidy. Value Health. 2020;23(9):1210–1217. doi: 10.1016/j.jval.2020.06.005
- Campbell PJ, Axon DR, Taylor AM, et al. Associations of renin-angiotensin system antagonist medication adherence and economic outcomes among commercially insured us adults: a retrospective cohort study. J Am Heart Assoc. 2020;9(17). doi: 10.1161/JAHA.119.016094
- Kazerooni R, Watanabe JH, Bounthavong M. Association between statin adherence and cholesterol level reduction from baseline in a veteran population. Pharmacotherapy. 2013;33(10):1044–1052. doi: 10.1002/phar.1305
- Shalev V, Goldshtein I, Halpern Y, et al. Association between persistence with statin therapy and reduction in low-density lipoprotein cholesterol level: analysis of real-life data from community settings. Pharmacotherapy. 2014;34(1):1–8. doi: 10.1002/phar.1326
- Yeo SH, Toh MPHS, Lee SH, et al. Impact of medication nonadherence on stroke recurrence and mortality in patients after first-ever ischemic stroke: insights from registry data in Singapore. Pharmacoepidemiol Drug Saf. 2020;29:538–549. doi: 10.1002/pds.4981
- Hero C, Karlsson SA, Franzén S, et al. Adherence to lipid-lowering therapy and risk for cardiovascular disease and death in type 1 diabetes mellitus: a population-based study from the Swedish national diabetes register. BMJ Open Diabetes Res Care. 2020;8(1):e000719. doi: 10.1136/bmjdrc-2019-000719
- Perreault S, Ellia L, Dragomir A, et al. Effect of statin adherence on cerebrovascular disease in primary prevention. Am J Med. 2009;122(7):647–655. doi: 10.1016/j.amjmed.2009.01.032
- Pittman DG, Chen W, Bowlin SJ, et al. Adherence to statins, subsequent healthcare costs, and cardiovascular hospitalizations. Am J Cardiol. 2011;107(11):1662–1666. doi: 10.1016/j.amjcard.2011.01.052
- Parati G, Kjeldsen S, Coca A, et al. Adherence to single-pill versus free-equivalent combination therapy in Hypertens Dallas tex 1979: a systematic review and meta-analysis. Hypertension. 2021;77(2):692–705. doi: 10.1161/HYPERTENSIONAHA.120.15781
- Gallo G, Sarzani R, Cicero AFG, et al. An Expert opinion on the role of the Rosuvastatin/Amlodipine single pill fixed dose combination in cardiovascular prevention. High Blood Press Cardiovasc Prev. 2023;30(2):83–91. doi: 10.1007/s40292-023-00570-9