ABSTRACT
Objectives
The Asian PEONY trial showed that add-on pertuzumab to trastuzumab and chemotherapy significantly improved pathological complete response in the neoadjuvant treatment of patients with human epidermal growth factor receptor 2-positive (HER2+) early breast cancer (EBC). This study evaluated the cost-effectiveness of pertuzumab as an add-on therapy to trastuzumab and chemotherapy for neoadjuvant treatment of patients with HER2+ EBC in Singapore.
Methods
A six-state Markov model was developed from the Singapore healthcare system perspective, with a lifetime time horizon. Model outputs were: costs; life-years (LYs); quality-adjusted LYs (QALYs); incremental cost-effectiveness ratios (ICERs). Sensitivity/scenario analyses explored model uncertainties.
Results
The base case projected the addition of pertuzumab to be associated with improved outcomes by 0.277 LYs and 0.271 QALYs, increased costs by S$1,387, and an ICER of S$5,121/QALY. The ICER was most sensitive to the pCR rate, and the probabilistic sensitivity analysis showed that add-on pertuzumab had an 81.3% probability of being cost-effective at a willingness-to-pay threshold of S$45,000/QALY gained.
Conclusions
This model demonstrated that the long-term clinical impact of early pertuzumab use, particularly the avoidance of metastatic disease and thus avoidance of higher costs and mortality rates, make neoadjuvant pertuzumab a cost-effective option in the management of patients with HER2+ breast cancer in Singapore.
1. Introduction
Breast cancer has surpassed lung cancer to become the malignancy with the highest incidence globally, accounting for 11.7% of all cases in 2020 [Citation1]. Among women, breast cancer accounts for 1 in 4 cancer cases and 1 in 6 cancer deaths [Citation1]. In Singapore, breast cancer is also the cancer with the highest incidence among women of all ethnicities, accounting for approximately 30% of all female cancers [Citation2]. This represents one of the highest rates in Asia [Citation2,Citation3]. Furthermore, the prevalence of breast cancer is rising in Singapore, potentially driven by increasing life expectancy and the wider adoption of Western lifestyles [Citation2]: the age-standardized incidence rate of female breast cancer in Singapore rose from 20.1/100,000 people from 1968–1972 to 72.6/100,000 people in 2015–2019 [Citation4].
The clinical and psychological burden associated with breast cancer is substantial [Citation5], particularly in the late stages of the disease [Citation6]. The economic burden also increases with worsening disease severity: the average costs per patient in the year after diagnosis of breast cancer in the United States (US) have been reported to be US$60,637, US$82,121, US$129,387, and US$134,682 for disease stage 0, I/II, III, and IV, respectively [Citation7].
Human epidermal growth receptor-2 (HER2) overexpression is estimated to account for ~ 15% of early breast cancers (EBCs) and is associated with poorer prognosis in terms of overall survival (OS) and time to relapse compared to HER2-negative breast cancers [Citation8,Citation9]. Over the last few decades, meaningful strides have been made in the development of effective treatments that can improve OS in patients with HER2-positive (HER2+) EBC [Citation10]. Neoadjuvant therapy, administered prior to surgery, is recommended by the European Society for Medical Oncology (ESMO), American Society of Clinical Oncology and the National Comprehensive Cancer Network for breast cancer subtypes that are sensitive to chemotherapy, such as the HER2+ subtype [Citation11–13]. This approach helps to shrink the tumor to confer surgical resectability or reduce the extent of surgery required after neoadjuvant therapy. It also tests the biology of the tumor and identifies high-risk patients who have residual disease post-neoadjuvant chemotherapy and who may be further salvaged with escalated adjuvant therapy [Citation11].
Trastuzumab is a humanized monoclonal antibody that binds to an epitope on the extracellular domain of HER2 to interrupt HER2-mediated signaling, and was the first anti-HER2 directed therapy to be approved by the US Food and Drug Administration (FDA) for the treatment of HER2+ metastatic breast cancer [Citation14]. Pertuzumab is also a humanized monoclonal antibody but binds to a different HER2 epitope; in addition, pertuzumab is a first-in-class dimerization inhibitor, blocking the dimerization of HER2 with other HER family members, resulting in synergism and more effective and complete HER2 blockade when administered together with trastuzumab [Citation14]. While single-agent trastuzumab combined with neoadjuvant chemotherapy is a standard of care treatment for HER2+ EBC [Citation15,Citation16], landmark trials such as NeoSphere, TRYPHAENA, BERENICE and PEONY have shown that adding on pertuzumab can further improve clinical outcomes including pathological complete response (pCR) rate, event-free survival (EFS) and disease-free survival (DFS) compared to trastuzumab monotherapy and chemotherapy [Citation17–21]. PEONY was the first phase III randomized controlled trial (RCT) conducted in Asia to show that the addition of pertuzumab to neoadjuvant trastuzumab and docetaxel treatment significantly improved the pCR rate [Citation20]. In the intention-to-treat population, pCR rates were 39.3% (86/219) in the pertuzumab group and 21.8% (24/110) in the comparator group (difference: 17.5% [95% CI: 6.9–28.0%]; p = 0.001) [Citation20], concordant with other RCTs that recruited mainly non-Asian patients [Citation17–19].
These positive clinical outcomes supported pertuzumab receiving regulatory approval from Singapore’s Health Sciences Authority for both EBC and metastatic breast cancer (MBC) in 2014 [Citation22]. Singapore government subsidies cover up to 75% of the price of drugs on the Standard Drug List and Medicine Assistance Fund List and help to provide patients with affordable access to effective medicines [Citation23]. With the high prevalence of EBC in Singapore [Citation2], there is a need to evaluate the cost-effectiveness of available drugs to help inform the efficient allocation of healthcare resources [Citation24]. Although cost-effectiveness analyses (CEA) of pertuzumab for neoadjuvant treatment in HER2+ EBC have been reported from the US and Canadian health system perspective [Citation25,Citation26], there are currently no studies that investigate the cost-effectiveness of neoadjuvant pertuzumab for the Singapore health system.
The objective of this economic evaluation was to assess the cost-effectiveness of pertuzumab as an add-on therapy to trastuzumab and chemotherapy for neoadjuvant treatment of patients with HER2+ EBC in Singapore.
2. Methods
2.1. Model design
A health economic analysis plan was developed to outline the planned adaptations of a previously published cost-effectiveness model to the Singapore setting [Citation26]. The model was based on a Markov state transition model, developed in Microsoft Excel, to model the transition of a cohort of patients through six health states: (1) EFS before surgery/invasive disease-free survival (iDFS) after surgery; (2) non-metastatic recurrence; (3) remission; (4) first-line treatment in MBC; (5) subsequent treatment lines of MBC; and (6) death (). Ethical approvals were not applicable to this study as this economic evaluation is based on published literature and does not contain any new studies conducted on human research subjects or animals.
Figure 1. Model structure Abbreviations: EFS: event-free survival; iDFS: invasive disease-free survival; MBC: metastatic breast cancer pCR: pathological complete response.
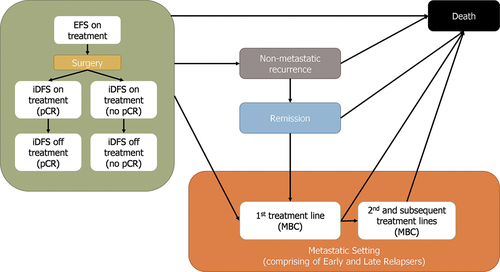
All patients entered the model in the EFS state, receiving neoadjuvant treatment. Following surgery, patients moved into the iDFS state and received adjuvant treatment. Adjuvant treatment received was dependent on the patients’ pCR status. In the iDFS state, patients were modeled as ‘on treatment’ initially and then ‘off treatment’ after the completion of adjuvant therapy. This distinction recognized that patients’ quality of life may be different depending on whether they are on or off treatment. Patients could remain in the EFS/iDFS state, progress to non-metastatic recurrence, progress to first-line MBC, or die. The non-metastatic recurrence state was a tunnel state, where patients resided for a fixed period of one year before automatically moving to the remission state, in which patients were modeled as being off treatment and assumed to have no further sign of the disease unless they experienced further recurrence. It was assumed that patients could not experience contralateral or locoregional recurrence again; this was a simplifying assumption taking into account data availability. If a patient developed another non-metastatic recurrence, it was treated as MBC. In the first line MBC state, patients were at risk of further disease progression to subsequent lines of treatment in MBC, or death. In the further lines of the MBC treatment state, patients could only remain in this state or die. Death was an all-absorbing state that patients could enter from any other model state. Further details of the model structure have been reported in a previous evaluation of neoadjuvant targeted treatments for HER2+ EBC from the US health system perspective [Citation26].
This model utilized the patient characteristics from the intention-to-treat (ITT) population of the PEONY RCT, which included female patients with HER2+ EBC [Citation20]. In the base case, a time horizon of 35 years was chosen, which considered the mean age that patients entered the model (50 years; based on the PEONY RCT [48.8 years] and previous Asian cohort studies [approximately 47–52 years]) and the average life expectancy of women in Singapore (approximately 85 years) [Citation20,Citation27–31]. This lifetime time horizon was expected to adequately capture clinical outcomes and costs associated with neoadjuvant pertuzumab in EBC. For the calculation of drug doses that informed drug costs, average inputs for body surface area and body weight in Singapore (1.60 m2; 60 kg) were used [Citation32]. Patients’ mean age, body surface and body weight were validated by local clinicians. Additionally, a lower body surface area and body weight (1.50 m2; 55 kg) were also tested in scenario analyses to explore the range of inputs provided by local clinicians.
This analysis adopted the Singapore health system perspective [Citation33]. Costs and benefits were discounted at a rate of 3% per year [Citation33]. A cycle length of 1 month was used for the Markov model and a half-cycle correction was applied. While a cycle length of 3 weeks could also have been appropriate to align to the treatment cycles, the current model cycle length of 1 month is aligned to the model cycle length used for the pertuzumab technology appraisal submission to United Kingdom’s (UK) National Institute for Health and Care Excellence (NICE) [Citation34].
2.2. Treatment arms and clinical inputs
In line with the anti-HER2 targeted therapy regimens in the PEONY RCT and the Singapore Health Sciences Authority-approved doses [Citation20,Citation22,Citation35], patients in the intervention arm of the model received intravenous pertuzumab (840 mg loading dose and 420 mg maintenance doses), trastuzumab (8 mg/kg loading dose and 6 mg/kg maintenance doses) and chemotherapy (PHT) as their neoadjuvant therapy; patients in the comparator arm received trastuzumab and chemotherapy (HT) as their neoadjuvant therapy. As the model captured the full treatment pathway from neoadjuvant treatment through to metastatic breast cancer, the model also included other treatments such as trastuzumab emtansine, chemotherapy only and lapatinib and chemotherapy, that are used later in the treatment pathway in the Singapore setting ().
Table 1. Summary of treatments considered at different health states.
2.2.1. Survival in the neoadjuvant setting (EFS survival)
EFS data were taken from the PEONY RCT to estimate the risk of disease recurrence or death in the EFS state before finishing neoadjuvant treatment [Citation20]. The Kaplan-Meier (KM) data from the first 3 months of the study were used to directly inform EFS survival. Pooled estimates from PEONY were used, resulting in an EFS survival of 98.3% at 3 months for both the PHT and HT arms in the model. This model input was expected to have a minor impact on the results, as most events were expected to occur after the neoadjuvant treatment period.
2.2.2. Survival in the adjuvant setting (iDFS survival)
After completing neoadjuvant treatment and subsequent surgery, patients were modeled to remain in the iDFS health state as long as they remained disease free and were still alive. To determine the duration of disease-free survival, a key effectiveness input was the pCR status; the probability of a patient remaining in the iDFS health state was modeled as dependent on whether the patient had achieved pCR or not. pCR was defined as the absence of residual invasive cancer in the resected breast specimen and the axillary lymph nodes [Citation36].
Data to estimate and extrapolate a lifetime risk of survival was sourced from alternative publications due to the absence of survival data (e.g. OS) from the PEONY RCT. For patients who did not achieve pCR, iDFS data from the Phase III KATHERINE study (trastuzumab arm) was extrapolated over the model’s time horizon [Citation37]. The KATHERINE study compared trastuzumab against trastuzumab emtansine in patients with EBC [Citation37]. A lognormal parametric distribution was applied in the base case as it was determined to provide the best fit overall to the KATHERINE RCT after assessing the Akaike Information Criterion and Bayesian Information Criterion values. For patients who achieved pCR, data from a pooled analysis by Swain et al. 2020 was applied to the iDFS distribution from KATHERINE (trastuzumab arm) to create an indirect estimate of the iDFS survival (hazard rate [HR]: 0.33) that reflected the decreased risk of progression for patients who achieve pCR compared to those with residual disease (non-pCR patients), regardless of hormone receptor status or clinical stage [Citation36]. The survival estimates for non-pCR versus pCR patients were applied to both arms of the model. The difference in the proportion of patients who achieved pCR, as reported in the PEONY RCT, was the key driver of the difference between the two model arms ().
Table 2. Clinical inputs.
As well as taking into account pCR status, the iDFS projections also adjusted for the treatment received in the adjuvant setting (). The adjuvant treatment mixes in this model were validated with local clinicians and were reflective of the real-world usage of different adjuvant treatments in Singapore. In the PHT arm, where patients achieving pCR could receive adjuvant pertuzumab and trastuzumab or adjuvant trastuzumab only, iDFS for patients that continued treatment with pertuzumab and trastuzumab in the adjuvant setting (up to 18 cycles in total), was adjusted (relative to iDFS for pCR patients receiving adjuvant trastuzumab) based on a HR from the latest 6-year follow-up from Phase III APHINITY study (HR: 0.72), reflecting the benefit of improved iDFS for patients who continue treatment with pertuzumab and trastuzumab treatment versus trastuzumab treatment only [Citation38]. Patients who did not achieve pCR either received adjuvant trastuzumab or trastuzumab emtansine only. In these patients, survival extrapolations continued to be based on the iDFS data from the KATHERINE RCT [Citation37].
2.2.3. Cure proportion adjustment and treatment effect assumption
As the lognormal extrapolation for the trastuzumab arm resulted in a lower 10-year disease-free survival compared to the 10-year disease-free survival data observed in the BCIRG-006 study [Citation46], the iDFS survival curve was further adjusted to account for the decrease in recurrence risk over time (a cure proportion adjustment), to align to the longer-term BCIRG-006 and HERA RCT results [Citation46,Citation47]. The input for the cure adjustment that was preferred in the NICE appraisal of pertuzumab was used for both pCR and non-pCR patients in the model () [Citation34,Citation39]. Cured patients were subject to background mortality rates equivalent to the age-matched Singapore population [Citation31]. Background mortality rates were based on the 2021 life tables for the Singapore Resident Population provided by Singapore’s Department of Statistics [Citation31]. In one of the scenario analyses, the cure proportion was assumed to be 0% to explore the impact on model results of assuming no potential for cure.
Although the treatment duration of trastuzumab does not exceed 1 year, long-term data from the BCIRG-006 and HERA RCTs suggest that the incremental effect of trastuzumab was maintained over time [Citation46,Citation47]. It was expected that pertuzumab and trastuzumab emtansine would similarly be associated with the maintenance of treatment effect beyond treatment discontinuation as both drugs target the same pathways. The NICE-preferred inputs for treatment effects were used in this model, where the treatment effect was assumed to be maintained for 4 years and then linearly decreased to null at year 7 [Citation39]. Additionally, an alternative treatment effect assumption from a published pertuzumab CEA from another region (treatment effect to be maintained for 7 years and then linearly decreasing to be null at year 10) was tested in scenario analyses [Citation48].
2.2.4. Recurrence (iDFS events)
Rates of recurrence (proportion of non-metastatic and metastatic recurrence) for patients in the iDFS state were also based on the KATHERINE RCT (); however, no adjustments for risk of recurrence were made for treatment received as no meaningful difference was observed between treatment arms in terms of iDFS events [Citation37]. In the model, the same rates of recurrence were used regardless of pCR status.
A distinction was also made to differentiate patients who had incurred metastatic recurrence from the iDFS state within 18 months (Early Relapsers) versus after 18 months (Late Relapsers) as there is a difference in post-progression survival between these two groups, as shown by the HERA RCT [Citation47].
2.2.5. Progression and survival following non-metastatic recurrence
Patients in the non-metastatic recurrence and remission health states were at risk of death based on background mortality [Citation31]. The risk of a second malignancy was based on Hamilton et al. 2015, which estimated the risk of a second malignancy after adjuvant therapy in a cohort of 12,836 early breast cancer patients [Citation40].
2.2.6. Progression and survival following metastatic recurrence (early recurrence)
Transition probabilities derived from the pooled survival data of the EMILIA RCT (fast-progressing subpopulation) were used to model the progression and survival of Early Relapsers in the MBC setting [Citation41]. The transition probabilities for Early Relapsers were independent of treatment received in the metastatic setting.
2.2.7. Progression and survival following metastatic recurrence (late recurrence)
The risk of disease progression and death for patients with metastatic recurrence (late recurrence) was extrapolated using an exponential distribution based on data from various RCTs (). There were three treatment regimens available for patients in the 1st line MBC setting: PHT; HT; and chemotherapy only (). Patients in the 2nd line MBC setting had four treatment regimens available: PHT; HT; chemotherapy only; and trastuzumab emtansine. For PHT and HT treatments, the transition probability from 1st line MBC to subsequent treatment lines of MBC and from subsequent treatment lines of MBC to death was derived from the CLEOPATRA RCT data [Citation42]. For chemotherapy only, the transition probabilities were derived from the M77001 RCT data [Citation43]. For trastuzumab emtansine, the transition probability from subsequent treatment lines of MBC to death was derived from the EMILIA RCT [Citation41]. The model considered the treatment mix in the 1st line and subsequent lines of MBC to derive weighted ‘cohort’ transition probabilities (). This simplifying approach was taken as this CEA aimed to model the EBC setting rather than model the MBC setting in detail.
2.3. Utilities
Utilities for EBC health states were obtained from the KATHERINE and APHINITY RCTs (), due to the absence of EQ-5D (EuroQol 5 Dimensions) or patient-reported outcomes data from the PEONY RCT and based on the assumption of no difference in health state utilities between pCR and no pCR states. For the MBC setting, utility values were derived from Lloyd et al. 2006 [Citation44]. For the base case, pooled estimates were used, where the same quality of life estimates were applied to intervention and comparator arms, irrespective of what treatment was received in the neo-adjuvant and adjuvant setting of the model. Disutilities for adverse events (AEs) were not modeled, because reported AEs in the RCTs were not considered to have a substantial impact on patient quality of life. UK preference weights are recommended in the Singapore ACE’s reference case and were used to derive the utility values used in this evaluation [Citation33].
2.4. Healthcare costs and resource use
All costs were reported in 2022 Singapore dollars (S$). Drug acquisition costs of targeted treatments (pertuzumab, trastuzumab and trastuzumab emtansine) were obtained from Roche’s data on file. The biosimilar cost was used for the cost of trastuzumab. Chemotherapy drug acquisition costs and costs of monitoring, adverse event management and treatment administration (e.g. intravenous infusion) were obtained from Roche’s data on file, the Singapore Agency for Care Effectiveness (ACE) resource sheet or published literature [Citation49] (). Costs and healthcare resource use were validated with local clinicians.
Table 3. Cost and healthcare resource use inputs.
For the purposes of the model, patients could receive either sequential anthracycline- or concurrent non-anthracycline-based chemotherapy (). The distinction between chemotherapy regimens was only made to estimate the drug cost.
2.5. Outcomes
Outcomes of interest were total life years (LYs), total quality-adjusted life years (QALYs) and the incremental cost-effectiveness ratio (ICER).
2.6. Scenario analyses
The present study also explored the impact of modeling different adjuvant therapies on the cost-effectiveness of neoadjuvant add-on pertuzumab. The adjuvant treatment mix amongst patients who achieved pCR after neoadjuvant add-on pertuzumab was varied, to reflect different proportions of patients using a continuation dual blockade regimen. Other scenarios, including varying the cure proportion and time horizon, were also assessed.
2.7. Sensitivity analyses
A probabilistic sensitivity analysis (PSA) was conducted to determine the impact of uncertainty surrounding the model parameters using the variability around point estimates. Distributions were assigned to model inputs () and a Monte Carlo simulation was conducted (1000 simulation runs). The 10th and 90th percentiles of the distributions were used as the lower and upper bounds for the PSA. Results were presented using a cost-effectiveness acceptability curve (CEAC).
A univariate deterministic sensitivity analysis (UDSA) was also used to account for uncertainty surrounding efficacy rates, costs, time horizon, utilities and discount rates. The 10th and 90th percentile values of parameters tested in the PSA were used to inform the ranges in the UDSA. For variables not included in the PSA (i.e. time horizon), the base case inputs were increased or decreased by 10% to understand how sensitive the model was to changes in the input parameters.
3. Results
The results of the base case analysis are presented in . Compared to neoadjuvant trastuzumab and chemotherapy alone, pertuzumab as an add-on neoadjuvant treatment resulted in greater LYs and greater QALYs (mean LYs: 17.880 versus 17.604; mean QALYs: 15.365 versus 15.094). Using pertuzumab as an add-on neoadjuvant treatment resulted in ICERs of S$5,012/LY gained and S$5,121/QALY gained.
Table 4. Model base case results.
The results of the scenario analyses are presented in . Scenario analyses consistently supported the conclusions from the base case analysis. In the unlikely scenario in which 100% of patients with pCR continue pertuzumab usage in the adjuvant setting, the ICER only increased to S$35,795/QALY, which remains below the ranges of ICERs considered acceptable in appraisals published by Singapore’s ACE [Citation51].
Table 5. Scenario analyses.
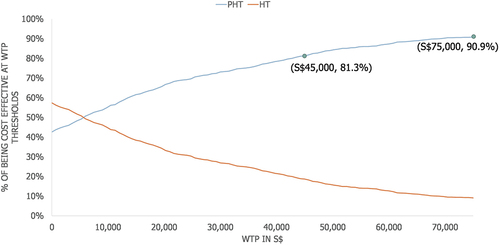
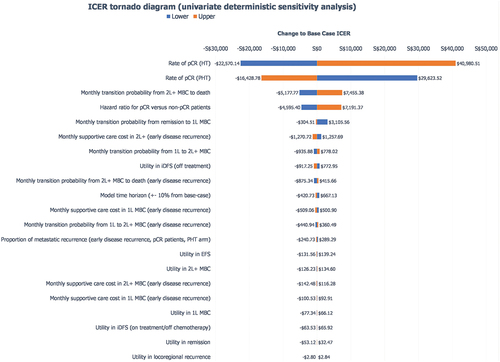
In the UDSA, the ICER was most sensitive to the following parameters: pCR rate, 2 L+ MBC to death transition probability and the hazard ratio for pCR patients versus non-pCR patients (). The CEAC generated by the PSA showed that pertuzumab in combination with trastuzumab and chemotherapy had an 81.3% probability of being cost-effective at a willingness-to-pay threshold of S$45,000/QALY and a 90.9% probability of being cost-effective at a willingness-to-pay threshold of S$75,000/QALY ().
Figure 2. Univariate deterministic sensitivity analysis Notes: the 10th and 90th percentiles values of selected parameters in the PSA were used in the univariate deterministic sensitivity analysis. For variables not included in the PSA (i.e. time horizon), the base case inputs were increased or decreased by 10% to understand how sensitive the model was to changes in the input parameters. Abbreviations: 1 L: 1st treatment line; 2 L+: 2nd and subsequent treatment lines; EFS: event-free survival; HT: trastuzumab and chemotherapy; ICER: incremental cost-effectiveness ratio; iDFS: invasive disease-free survival; MBC: metastatic breast cancer; pCR: pathological complete response; PHT: pertuzumab, trastuzumab and chemotherapy.
4. Discussion
The high prevalence of EBC in Singapore places a particular demand on the availability of effective treatments that target the early stages of the disease and mitigate the substantial clinical and economic burden on patients and the healthcare system associated with the advanced stages of the disease [Citation6]. Neoadjuvant therapy has a role to play in reducing the tumor burden, improving the chances of breast-conserving surgery, and minimizing the need for axillary clearance [Citation52]. Pertuzumab has been granted regulatory approval by the FDA as a neoadjuvant treatment of HER2+ EBC in view of its mechanism of action providing dual blockade of HER2 signaling when used with trastuzumab [Citation14,Citation53], and its demonstrated efficacy based on the surrogate endpoint pCR and tolerable safety profile across a large number of patients [Citation17–19,Citation21].
Extensive evidence shows that the achievement of pCR improves long-term outcomes in EBC, including DFS and OS, particularly in high-risk subtypes of breast cancer such as HER2+ positive breast cancer [Citation54,Citation55]. The CTNeoBC pooled analysis of neoadjuvant breast cancer RCTs showed that pCR has prognostic value in predicting improved EFS [Citation54]. A recent meta-analysis of more than 27,000 patients further confirmed that pCR is associated with improved EFS (hazard ratio: 0.32; 95% prediction interval: 0.21–0.47) and OS (hazard ratio: 0.22; 95% prediction internal 0.15–0.30) [Citation55]. Reflecting this, pCR has been accepted as a valid surrogate endpoint for the granting of regulatory approval for pertuzumab in EBC [Citation34,Citation56].
The PEONY trial was the first phase III RCT that confirmed significant improvements in pCR rates when pertuzumab is used in combination with trastuzumab and chemotherapy in Asian patients with EBC or locally advanced breast cancer in the neoadjuvant setting, compared to trastuzumab and chemotherapy alone [Citation20]. The study was conducted in an Asian population, which strengthens the generalizability of results to the Singapore setting. While there are existing economic assessments of neoadjuvant add-on pertuzumab conducted in different geographical settings [Citation25,Citation26], the present cost-effectiveness study, based on the PEONY RCT, is the first analysis evaluating the cost-effectiveness of neoadjuvant pertuzumab as an add-on to trastuzumab in a dual HER2 blockade regimen for EBC in the Singapore setting. In the base case analysis, neoadjuvant pertuzumab increased LYs by 0.277, QALYs by 0.271, and costs by S$1,387, resulting in an ICER of S$5,121/QALY gained. Although there are currently no fixed willingness-to-pay thresholds for Singapore, these ICERs are below the typical ranges of acceptable ICERs in appraisals published by ACE (S$45,000–75,000/QALY) [Citation51], suggesting that pertuzumab as an add-on neoadjuvant treatment for EBC is likely to be cost-effective in the Singapore setting.
For patients with breast cancer, the prognosis worsens as the disease progresses [Citation6]. In our model, the cost-effectiveness of pertuzumab was driven by the additional survival for patients who avoided metastatic disease. The difference in the proportion of patients in the iDFS health state between arms was 1.4% at 5 years (pertuzumab arm: 84.0%; trastuzumab arm: 82.6%) and 2.1% at 10 years (pertuzumab arm: 77.6%; trastuzumab arm: 75.5%). The higher proportion of patients who remained in the iDFS health state without progressing to later stages of disease contributed to cost-offsets, driven by avoidance of supportive care costs (comprising treatment, administration and AE costs) in the metastatic health states and avoidance of end-of-life costs, as well as some QALY gains.
ESMO guidelines state that the choice of adjuvant treatment should be based on tumor characteristics (e.g. tumor burden and biology) that influence the risk of relapse, the risk-benefit profile of different treatment options (e.g. predicted sensitivity to different treatments considered, adverse event profile), and patient factors (e.g. age, health status, comorbidities, preferences) [Citation11]. Additionally, trastuzumab should be completed for a total of one year (across neoadjuvant and adjuvant settings) in the majority of HER2+ breast cancer patients, and dual blockade for a total of one year specifically in patients with node-positive disease [Citation52]. Our model explored the cost-effectiveness of different neoadjuvant-adjuvant treatment strategies, for patients who did or did not achieve pCR following neoadjuvant therapy. In the scenario assuming that 100% of patients who achieved pCR after neoadjuvant pertuzumab adopted the adjuvant dual blockade regimen (100% of patients who did not achieve pCR after neoadjuvant pertuzumab were modeled to receive adjuvant trastuzumab emtansine), the ICER increased to S$35,795/QALY. Compared to the base case analysis, this scenario reflected greater LY gains of 0.335 and greater QALY gains of 0.333, at greater incremental costs of S$11,927. The increased ICER remains lower than the willingness-to-pay threshold of countries such as Australia and the United Kingdom (the equivalent of S$45,000–75,000/QALY), which are commonly referenced by the national health technology assessment (HTA) body in Singapore, ACE [Citation51,Citation57]. While there is currently no consensus on whether continued neoadjuvant-adjuvant dual blockade should be more widely considered in patients who achieve pCR or if dual blockade should be stopped at surgery [Citation52], this scenario highlights that continued neoadjuvant-adjuvant dual blockade may be a cost-effective treatment strategy, generating clinical benefits that warrant consideration by prescribers, especially where the additional treatment cost can be accommodated. These results are also in line with a study that investigated the cost-effectiveness of different neoadjuvant-adjuvant regimens for HER2+ EBC from the US healthcare payer perspective: neoadjuvant-adjuvant regimens in which pertuzumab was used only in the neoadjuvant setting (but not the adjuvant setting) were associated with lower costs but also fewer total QALYs than when continued dual blockade was used (US$272,873–279,466 versus US$280,448–299,813; 14.494 QALYs and 14.585 QALYs, respectively) [Citation26].
The present cost-effectiveness analysis draws from an extensive literature evaluating the cost-effectiveness of anti-HER2 neoadjuvant and adjuvant treatments for EBC [Citation25,Citation26,Citation48], using a model structure that has been validated in multiple countries and accepted by well-established HTA agencies, such as Canadian Agency for Drugs and Technologies in Health (CADTH) in Canada and NICE in the UK [Citation34,Citation58,Citation59]. Assumptions used in the model were conservative and reflected clinical practice in Singapore as confirmed by clinical experts. The present analysis adopted the biosimilar cost across all health states and drug regimens involving trastuzumab, as biosimilar trastuzumab is commonly prescribed in Singapore even though the fixed-dose pertuzumab-trastuzumab subcutaneous injection and the intravenous branded trastuzumab are also available.
A previous Singapore cost-effectiveness analysis that evaluated the use of pertuzumab in the MBC setting reported ICERs that are above those that have typically been accepted in previous ACE appraisals [Citation32]. However, this model supports that pertuzumab can be a cost-effective treatment option when used early in the overall treatment strategy for HER2+ breast cancer. Therefore, consideration of improved coverage of neoadjuvant pertuzumab in the Singapore healthcare system could benefit patient access to cost-effective EBC treatments.
Some limitations of this study should be noted. Further evidence to confirm the relationship between pCR and clinical outcomes would be valuable to address any uncertainty around pCR as a surrogate marker for improved long-term outcomes. Furthermore, while preference weights based on the general population in the UK are recommended in the Singapore ACE’s reference case, the availability of local preference weights and/or utility values may improve the relevance of the estimated ICER within the local context [Citation33]. Lastly, the model required some assumptions that were challenging to validate in the local Singapore clinical practice, such as the cure proportion and treatment waning effects; however, sensitivity and scenario analyses were conducted and confirmed the robustness of the model results to changes in these variables.
Lastly, differences in EBC outcomes between races have been observed for the Singapore population, with evidence for poorer breast cancer outcomes in Singaporean Malay women [Citation60]. This includes a higher incidence of node-positive disease, higher grade tumors, a higher proportion of HER2+ subtypes and lowered 5-year and 10-year OS in Singaporean Malay women compared to Singaporean Chinese or Indian women [Citation60]. Effective breast cancer treatments may therefore also play a role in addressing racial health inequities in Singapore. However, as the present study was designed to assess cost-effectiveness in the whole population, it cannot provide information on cost-effectiveness by racial subgroup (e.g. Malay population versus Chinese or Indian population) or provide analysis of wider distributional impacts of the use of pertuzumab. Further studies looking at the distributional impacts of new breast cancer treatments, such as pertuzumab, across racial subgroups in Singapore would be of value.
5. Conclusions
Dual blockade with pertuzumab and trastuzumab in the neoadjuvant setting has been shown to improve pCR rates in patients with HER2+ breast cancer compared to the use of trastuzumab alone. Our model demonstrated that the long-term clinical impact from early pertuzumab use, particularly the avoidance of metastatic disease and thus avoidance of higher costs and mortality rates, means that add-on pertuzumab can be a cost-effective option in the neoadjuvant management of patients with HER2+ EBC in Singapore. The results from the scenario analyses further support that continued neoadjuvant-adjuvant dual blockade for HER2+ EBC patients who have achieved pCR may represent a cost-effective treatment strategy that can achieve higher LY and QALY gains compared to using dual blockade only in the neoadjuvant setting.
Article highlights
The present cost-effectiveness study, based on the Phase III PEONY RCT, is the first analysis evaluating the cost-effectiveness of neoadjuvant pertuzumab as an add-on to trastuzumab in a dual HER2 blockade regimen for EBC in the Singapore setting.
The cost-effectiveness study was developed in line with the recommendations in the Singapore ACE’s reference case.
Assumptions used in the model were conservative and reflected clinical practice in Singapore as confirmed by clinical experts.
The base case projected the addition of pertuzumab to be associated with improved outcomes by 0.277 LYs and 0.271 QALYs, increased costs by S$1,387, and an ICER of S$5,121/QALY.
The ICER was most sensitive to the pCR rate, and the probabilistic sensitivity analysis showed that add-on pertuzumab had an 81.3% probability of being cost-effective at a willingness-to-pay threshold of S$45,000/QALY gained.
This model demonstrated that the long-term clinical impact of early pertuzumab use, particularly the avoidance of metastatic disease and thus avoidance of higher costs and mortality rates, make neoadjuvant pertuzumab a cost-effective option in the management of patients with HER2+ breast cancer in Singapore.
Declaration of interest
E Hsuen Lim declares being a Consultant/Advisor for Roche Singapore Pte Ltd; Novartis; DKSH; Eisai; Eli Lilly.
A Lim declares being a Consultant/Advisor for Roche Singapore Pte Ltd.
J Singh Khara declares being a Stock Shareholder at Roche Singapore Pte Ltd and an employee at Roche Singapore Pte Ltd.
J Cheong declares being a Stock Shareholder at Roche Singapore Pte Ltd and an employee at Roche Singapore Pte Ltd.
J Fong declares being a Stock Shareholder at Roche Singapore Pte Ltd and an employee at Roche Singapore Pte Ltd.
S Sivanesan declares being a Stock Shareholder at Roche Singapore Pte Ltd and an employee at Roche Singapore Pte Ltd.
M Griffiths declares being a Consultant/Advisor for Roche Singapore Pte Ltd.
E New declares being a Consultant/Advisor for Roche Singapore Pte Ltd.
S Chin Lee declares being a Consultant/Advisor for Pfizer; Eisai; ACT Genomics; Novartis; AstraZeneca; Eli Lilly; MSD; Roche Singapore Pte Ltd; Daiichi Sankyo. S Chin Lee also declares receiving Grant/Research from Pfizer; Eisai; Taiho; ACT Genomics; Bayer; Karyopharm; Epizyme; Adagene; Novartis; MSD. They are also on the Speakers Bureau at Pfizer; Eisai; ACT Genomics; Novartis; AstraZeneca; Eli Lilly; MSD; Roche Singapore Pte Ltd.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
Substantial contributions to study conception and design: E Hsuen Lim, A Lim, J Singh Khara, J Cheong, J Fong, S Sivanesan, M Griffiths, E New, S Chin Lee.
Substantial contributions to analysis and interpretation of the data: E Hsuen Lim, A Lim, J Singh Khara, J Cheong, J Fong, S Sivanesan, M Griffiths, E New, S Chin Lee.
Drafting the article or revising it critically for important intellectual content: E Hsuen Lim, A Lim, J Singh Khara, J Cheong, J Fong, S Sivanesan, M Griffiths, E New, S Chin Lee.
Final approval of the version of the article to be published: E Hsuen Lim, A Lim, J Singh Khara, J Cheong, J Fong, S Sivanesan, M Griffiths, E New, S Chin Lee.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660
- Jara-Lazaro AR, Thilagaratnam S, Tan PH. Breast cancer in Singapore: some perspectives. Breast Cancer. 2010;17(1):23–28. doi: 10.1007/s12282-009-0155-3
- Shih V, Chan A, Xie F, et al. Economic Evaluation of Anastrozole Versus Tamoxifen for Early Stage Breast Cancer in Singapore. Value Health Reg Issues. 2012;1(1):46–53. doi: 10.1016/j.vhri.2012.03.013
- National Registry of Diseases Office. Singapore cancer Registry annual report 2019. Singapore: National Registry of Diseases Office.
- Chow WL, Tan S-M, Aung KCY, et al. Factors influencing quality of life of Asian breast cancer patients and their caregivers at diagnosis: perceived medical and psychosocial needs. Singapore Med J. 2020;61(10):532. doi: 10.11622/smedj.2019099
- Vondeling GT, Menezes GL, Dvortsin EP, et al. Burden of early, advanced and metastatic breast cancer in the Netherlands. BMC Cancer. 2018;18(1):262. doi: 10.1186/s12885-018-4158-3
- Blumen H, Fitch K, Polkus V. Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am Health Drug Benefits. 2016;9(1):23–32.
- Rakha EA, Pinder SE, Bartlett JM, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68(2):93–99. doi: 10.1136/jclinpath-2014-202571
- Slamon D, Clark G, Wong S, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106
- Martínez-Sáez O, Prat A. Current and future management of HER2-positive metastatic breast cancer. J Oncol Pract. 2021;17(10):594–604. doi: 10.1200/OP.21.00172
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173
- Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39(13):1485–1505. doi: 10.1200/JCO.20.03399
- National Comphrehensive Cancer Network (NCCN). Clinical practice guidelines in Oncology (breast cancer). United States: NCCN; 2022.
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69(24):9330–9336. doi: 10.1158/0008-5472.CAN-08-4597
- Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12(3):236–244. doi: 10.1016/S1470-2045(11)70033-X
- Takada M, Ishiguro H, Nagai S, et al. Survival of HER2-positive primary breast cancer patients treated by neoadjuvant chemotherapy plus trastuzumab: a multicenter retrospective observational study (JBCRG-C03 study). Breast Cancer Res Treat. 2014;145(1):143–53. doi: 10.1007/s10549-014-2907-9
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–2284. doi: 10.1093/annonc/mdt182
- Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646–653. doi: 10.1093/annonc/mdx773
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9
- Shao Z, Pang D, Yang H, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. 2020;6(3):e193692. doi: 10.1001/jamaoncol.2019.3692
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7
- Health Sciences Authority (HSA) Singapore. Pertuzumab registration (SIN14501P). Singapore: HSA; 2014.
- Singapore Ministry of Health Cancer Drug List [Internet]. Singapore: Singapore Ministry of Health; [cited 2023 Apr 28]. Available from: https://www.moh.gov.sg/home/our-healthcare-system/medishield-life/what-is-medishield-life/what-medishield-life-benefits/cancer-drug-list
- Pearce F, Lin L, Teo E, et al. Health technology assessment and its use in drug policies: Singapore. Value Health Reg Issues. 2019;18:176–183. doi: 10.1016/j.vhri.2018.03.007
- Attard C, Pepper A, Brown S, et al. Cost-effectiveness analysis of neoadjuvant pertuzumab and trastuzumab therapy for locally advanced, inflammatory, or early HER2-positive breast cancer in Canada. J Med Econ. 2015;18(3):173–188. doi: 10.3111/13696998.2014.979938
- Sussell JA, Roth JA, Meyer CS, et al. Assessment of the cost-effectiveness of HER2-targeted treatment pathways in the neoadjuvant treatment of high-risk HER2-positive early-stage breast cancer. Adv Ther. 2022;39(3):1375–1392. doi: 10.1007/s12325-022-02047-y
- Han H-S, Lee K-W, Kim JH, et al. Weight changes after adjuvant treatment in Korean women with early breast cancer. Breast Cancer Res Treat. 2009;114(1):147–153. doi: 10.1007/s10549-008-9984-6
- Sun LM, Chen HJ, Liang JA, et al. Association of tamoxifen use and increased diabetes among Asian women diagnosed with breast cancer. Br J Cancer. 2014;111(9):1836–1842. doi: 10.1038/bjc.2014.488
- Yeo W, Lei YY, Cheng AC, et al. Changes in body weight over 18-months follow-up among Chinese patients after breast cancer diagnosis. Ann Oncol. 2018;29:viii81–viii82. doi: 10.1093/annonc/mdy270.251
- Youlden DR, Cramb SM, Yip CH, et al. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11(2):101–115. doi: 10.7497/j.issn.2095-3941.2014.02.005
- Singapore Department of Statistics (Singstat). Complete life Tables for Singapore resident population. Singapore: Singstat; 2020-2021.
- Cheng LJ, Loke L, Lim EH, et al. Cost-effectiveness of pertuzumab and trastuzumab biosimilar combination therapy as initial treatment for HER2-positive metastatic breast cancer in Singapore. Expert Rev Pharmacoecon Outcomes Res. 2021;21(3):449–456. doi: 10.1080/14737167.2021.1880323
- Agency for Care Effectiveness (ACE). Procedures and guidelines for company submissions to the Agency for Care effectiveness for funding consideration. Singapore: ACE; 2022.
- National Institute for Health and Care Excellence (NICE). Pertuzumab for the neoadjuvant treatment of HER2-positive breast cancer (TA424). UK: NICE; 2016.
- Health Sciences Authority (HSA) Singapore. Trastuzumab registration (SIN11028P). Singapore: HSA; 2021.
- Swain SM, Macharia H, Cortes J, et al., editors. P1-18-01: risk of recurrence and death in patients with early HER2-positive breast cancer who achieve a pathological complete response (pCR) after different types of HER2-targeted therapy: a retrospective exploratory analysis. Poster presented at: San Antonio Breast Cancer Symposium; 2019 Dec 10-14; San Antonio, TX.
- von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017
- Piccart M, Procter M, Fumagalli D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448–1457. doi: 10.1200/JCO.20.01204
- National Institute for Health and Care Excellence (NICE). Trastuzumab emtansine for treating HER2-positive advanced breast cancer after trastuzumab and a taxane (TA458). UK: NICE; 2017.
- Hamilton SN, Tyldesley S, Li D, et al. Second malignancies after adjuvant radiation therapy for early stage breast cancer: is there increased risk with addition of regional radiation to local radiation? Int J Radiat Oncol. 2015;91(5):977–985. doi: 10.1016/j.ijrobp.2014.12.051
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1
- Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173
- Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–690. doi: 10.1038/sj.bjc.6603326
- Abdin E, Subramaniam M, Vaingankar JA, et al. Population norms for the EQ-5D index scores using Singapore preference weights. Qual Life Res. 2015;24(6):1545–1553. doi: 10.1007/s11136-014-0859-5
- Slamon D, Eiermann W, Robert N, et al., editors. S5-04: Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Abstract presented at: San Antonio Breast Cancer Symposium; 2015 Dec 8-12; San Antonio, TX.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2
- Garrison LP Jr, Babigumira J, Tournier C, et al. Cost-effectiveness analysis of pertuzumab with trastuzumab and chemotherapy compared to trastuzumab and chemotherapy in the adjuvant treatment of HER2-positive breast cancer in the United States. Value Health. 2019;22(4):408–415. doi: 10.1016/j.jval.2018.11.014
- Agency for Care Effectiveness (ACE). Process Methods and resource sheet. Singapore: ACE; 2022.
- Tan DS, Chan JJ, Hettle R, et al. Cost-effectiveness of olaparib versus routine surveillance in the maintenance setting for patients with BRCA-mutated advanced ovarian cancer after response to first-line platinum-based chemotherapy in Singapore. J Gynecol Oncol. 2021;32(2):e27. doi: 10.3802/jgo.2021.32.e27
- Viswambaram A, Wee YR, Lim S. PMU20 is there an implicit willingness-to-pay threshold in Singapore? Value Health Reg Issues. 2020;22:S72. doi: 10.1016/j.vhri.2020.07.378
- Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. Am J Clin Oncol. 2021;39(13):1485–1505. doi: 10.1200/JCO.20.03399
- Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878–5887. doi: 10.1158/0008-5472.CAN-08-0380
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8
- Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26(12):2838–2848. doi: 10.1158/1078-0432.CCR-19-3492
- National Institute for Health and Care Excellence (NICE). Pertuzumab for adjuvant treatment of HER2-positive early stage breast cancer (TA569). UK: NICE; 2019.
- Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–437. doi: 10.1002/hec.1481
- Pan-Canadian Oncology Drug Review (PCODR). Final economic guidance report: pertuzumab (Perjeta) neoadjuvant breast cancer. Canada: Canadian Agency for Drugs and Technologies in Health (CADTH); 2015.
- Pan-Canadian Oncology Drug Review (PCODR). Final Economic Guidance Report: Pertuzumab–Trastuzumab for Early Breast Cancer. Canada: Canadian Agency for Drugs and Technologies in Health (CADTH); 2018.
- Xin WR, Kwok LL, Yong WF. Screening uptake differences are not implicated in poorer breast cancer outcomes among Singaporean Malay women. J Breast Cancer. 2017;20(2):183–191. doi: 10.4048/jbc.2017.20.2.183