ABSTRACT
Background
Antineutrophil cytoplasmic antibody-associated vasculitis (AAV) are rare autoimmune diseases characterized by inflammation of blood vessels. This study aimed to assess the cost-utility of avacopan in combination with rituximab (RTX) or cyclophosphamide (CYC) compared with glucocorticoids (GC) for the treatment of severe, active AAV in Spain.
Methods
A 9-state Markov model was designed to reflect the induction of remission and sustained remission of AAV over a lifetime horizon. Clinical data and utility values were mainly obtained from the ADVOCATE trial, and costs (€ 2022) were sourced from national databases. Quality-adjusted life years (QALYs), and incremental cost-utility ratio (ICUR) were evaluated. An annual discount rate of 3% was applied. Sensitivity analyses were performed to examine the robustness of the results.
Results
Avacopan yielded an increase in effectiveness (6.52 vs. 6.17 QALYs) and costs (€16,009) compared to GC, resulting in an ICUR of €45,638 per additional QALY gained. Avacopan was associated with a lower incidence of end-stage renal disease (ESRD), relapse and hospitalization-related adverse events. Sensitivity analyses suggested that the model outputs were robust and that the progression to ESRD was a driver of ICUR.
Conclusions
Avacopan is a cost-effective option for patients with severe, active AAV compared to GC in Spain.
1. Introduction
Antineutrophil cytoplasmic antibody-associated vasculitis (AAV) are autoimmune conditions characterized by inflammation and destruction of small and medium blood vessels, particularly those in the renal and respiratory systems [Citation1]. It is a group of rare, and often organ or life-threatening diseases, with microscopic polyangiitis (MPA) and granulomatosis with polyangiitis (GPA) being the most prevalent forms of AAV [Citation1,Citation2]. In Spain, the prevalence of GPA and MPA is estimated to be 15.8 and 23.8 cases per million populations, respectively, and the incidence ranges from 2.1–2.9 to 3.4–7.9 new cases per million populations per year, respectively [Citation3,Citation4].
Renal involvement is the most frequent severe clinical manifestation in patients with AAV (56% in GPA; 86% in MPA) [Citation5] and is an important risk factor of morbidity and mortality [Citation6]. In Spain, progression to end-stage renal disease (ESRD) was observed in 35% of patients with AAV with renal involvement at 5 years [Citation7]. Baseline estimated glomerular filtration rate (eGFR) and relapses are independent risk factors for progression to ESRD [Citation8,Citation9].
The management of AAV consists of a two-stage approach: a remission induction phase and a remission maintenance phase. Over the last decades, guidelines recommended glucocorticoids (GCs) in combination with cyclophosphamide (CYC) or rituximab (RTX) for induction of remission [Citation10]. Despite treatment, relapse rates are high, and patients suffer from morbidity associated with disease activity and treatment toxicity. High-dose GCs are associated with complex dose regimens, adverse events (AEs) and complications, including infections, osteoporosis, new-onset diabetes and hypertension, neuropsychiatric effects or increased cardiovascular risk [Citation11,Citation12].
The updated European League Against Rheumatism (EULAR) guideline published in 2023 stated that avacopan in combination with CYC or RTX may be considered for induction of remission in GPA/MPA, as part of a strategy to substantially reduce GC exposure [Citation13]. Avacopan is an orally administered small molecule that is a highly selective inhibitor of the human complement C5a receptor 1 (C5aR1). This inhibition reduces the pro-inflammatory effects of the anaphylatoxin C5a, which include neutrophil activation, migration, and adherence to sites of small blood vessel inflammation, vascular endothelial cell retraction and permeability [Citation14]. In the phase 3 ADVOCATE trial, avacopan was non-inferior to the prednisone taper regimen in inducing remission at week 26 (72.3% vs. 70.1%; p<0.001) and was superior to the prednisone taper regimen in sustaining remission at week 52 (65.7% vs. 54.9%; p<0.007) in patients with AAV concurrently treated with immunosuppressive drugs [Citation15]. Avacopan has been recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of severe, active GPA and MPA in adult patients [Citation16]. Its clinical benefit has also been acknowledged by the Gemeinsame Bundesausschuss (G-BA) in Germany [Citation17] and by the Haute Autorité de Santé (HAS) in France [Citation18]. Among others, avacopan is reimbursed in these countries as well as recently in Spain [Citation17–19].
The aim of this study is to evaluate the cost-utility of avacopan in the management of severe, active AAV (GPA/MPA) from the perspective of the Spanish National Health System (NHS).
2. Patients and methods
2.1. Patient population
The target patient population for this analysis was adults (≥18 years old) with newly diagnosed or relapsing AAV (GPA or MPA) receiving RTX or CYC. Baseline patient demographic and clinical characteristics were obtained from the intention-to-treat (ITT) population of the phase 3 ADVOCATE trial [Citation15]. Most patients (81%) had renal involvement () [Citation15].
Table 1. Baseline patient demographic and clinical characteristics. Source: Jayne et al. 2021 [Citation15].
2.2. Intervention and comparator
The intervention evaluated was a treatment regimen of avacopan (30 mg twice per day for 52 weeks) in combination with RTX or CYC followed by azathioprine (AZA) (hereafter referred to as ‘Avacopan arm’). The comparator was the current standard of care: GC (i.e. oral prednisone; 60 mg per day tapered to discontinuation by week 21) in combination with RTX or CYC followed by AZA (hereafter referred to as the ‘GC arm’). In both arms, the dose for intravenous RTX was 375 mg/m2/week for 4 weeks; and for intravenous CYC was ranging between 15 mg/kg and 1.2 g on day 1 and at weeks 2, 4, 7, 10 and 13. From week 15 onwards, oral AZA at a target dose of 2 mg/kg/day was administered as maintenance therapy. The distribution of treatment use was 65% for RTX and 35% for CYC based on clinical expert opinion and ADVOCATE trial data [Citation15].
2.3. Model structure
A Markov model was designed to reflect clinical practice for induction of remission in patients with AAV, with up to three induction courses. Markov models are appropriate for estimating long-term outcomes and chronic diseases. The model consisted of 9 health states, including an active disease state where patients start, 3 remission and 3 relapse states, ESRD and death (). According to the updated European League Against Rheumatism (EULAR) guideline [Citation13], the treatment algorithm for GPA and MPA recommends reinduction of remission in patients who relapse, so different remission and relapse states were included to reflect this. A health state for ESRD was included to account for the potential reduction in the risk of ESRD in patients treated with avacopan. Death was an absorbing state. In addition, patients can experience GC-related AEs, with the probability of having an event depending on the treatment arm.
Figure 1. Markov model for AAV.
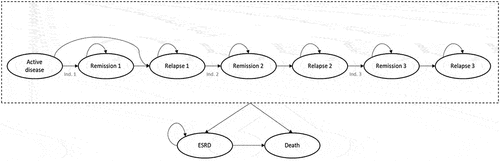
All patients who entered the model received a first course of induction therapy. Depending on treatment response, patients either move to ‘Remission’ (i.e. responders) or ‘Relapse’ (i.e. non-responders). Remission was defined as patients achieving a Birmingham vasculitis activity score of 0 and not taking GCs within 4 weeks of the end of the 6-month induction period. In a relapse state, patients can only be re-induced once and, if patients did not response, they were considered to have refractory disease and stayed in the relapse state for the remainder of the time horizon. Thus, patients in a relapse state cannot receive multiple consecutive induction courses.
AAV treatment is provided in the hospital setting. Therefore, the analysis adopted a Spanish NHS perspective, including only direct healthcare costs. The cycle length was 4 weeks and therefore a year (52 weeks) consisted of 13 cycles. A lifetime horizon was considered. A 3% annual discount rate was applied for both costs and health effects, in accordance with the Spanish recommendations for economic evaluation of drugs [Citation20]. The incremental cost-utility ratio (ICUR) was defined as the difference in costs and quality-adjusted life years (QALYs) between treatment arms.
2.4. Clinical efficacy
Clinical parameters were mainly derived from the ADVOCATE trial [Citation15]. Remission and relapse rates in both the avacopan arm and GC arm were based on proportions of patients in remission at week 26 and 52 observed from the ADVOCATE trial [Citation15]. The hazard ratio for the relapse rate between week 52 and 60 was derived from extension data. As there are no data on the efficacy of avacopan beyond the extension data, it cannot be assumed that the effect of avacopan will be sustained throughout the time horizon. Therefore, a 6-month extension of the effect of avacopan after treatment discontinuation was estimated based on clinical expert opinion. After that period, a constant tapering of the avacopan effect over time was assumed. Remission rates for RTX and CYC were considered equal based on non-inferiority of RTX in the RAVE trial [Citation21].
Different probabilities of ESRD from active disease/relapse and remission were considered. In addition, the probability of relapse was adjusted to reflect renal outcomes in AAV based on eGFR data from the ADVOCATE trial and the risk of progression to ESRD. In the base case, the probability of ESRD was derived from Robson et al. (2015) [Citation11]. In this study, the risk of ESRD is substantially higher in the first 6 months after disease onset than in subsequent years. The transition probability in the active disease/relapse health state corresponds to the probability in the first six months after diagnosis observed in the study by Robson et al. [Citation11]. The 4-week transition probability, based on long-term data up to 7 years of follow-up, was a proxy for the probability of ESRD in remission. It was assumed that the probability of ESRD in refractory disease is equal to that of relapse, based on clinical expert opinion. In addition, the probability of ESRD from active disease and remission was adjusted based on the improvement in eGFR in the avacopan and GC arms observed between weeks 0 and 26, and weeks 0 and 52, respectively, in the ADVOCATE trial [Citation15]. The model assumes that relapse of AAV is associated with worsening renal outcomes. Thus, the probability of ESRD for each subsequent relapse was estimated considering an expected decline in eGFR of 20 mL/min/1.73 m2 at relapse based on clinical expert opinion and the corresponding hazard ratio for ESRD per eGFR unit estimated by Cui et al. [Citation22] (Table S1 in the Supplementary Material).
Background mortality was derived from the Spanish national life tables [Citation23]. To account for the increased mortality rate in the AAV population and in patients with ESRD, compared to the general population, relative risks from the published literature were applied [Citation24,Citation25] (Table S1 in the Supplementary Material). Mortality rates were assumed equal in the active disease, remission and relapse health states [Citation15].
In addition, as GCs are associated with numerous AEs and the toxicity increases with cumulative dose, the incidence of grade 3 or 4 AEs reported in the avacopan and GC arms of the ADVOCATE trial was included [Citation15].
2.5. Costs
A literature search was conducted to quantify the cost estimates required to adapt the model to the Spanish setting. All cost parameter values were obtained from national sources [Citation26–32] and were expressed in euros and updated to the year 2022 based on the Spanish consumer price index. In particular, the search was focused on the main Spanish cost databases, such as the Minimum Basic Database (CMBD) for hospital care costs [Citation26] and eSalud for unit costs [Citation27], Spanish medical journals and reports published in collaboration with Spanish scientific societies.
The acquisition cost of the different therapeutic options was estimated based on the recommended dosage for the management of AAV. The costs were derived from the unit price (ex-factory price), applying the deduction of the Royal Decree Law 8/2010 [Citation33,Citation34]. The costs of avacopan and prednisone were adjusted based on the adherence rates reported in the ADVOCATE trial (86.4% for avacopan; 98.4% for GCs) [Citation15]. For intravenous treatments (i.e. RTX and CYC), non-vial sharing was assumed, and an administration cost was considered equal to day hospital cost [Citation27]. The mean body weight was 72.88 kg and mean body surface area was 1.81 m2 [Citation35].
The length of the treatment was considered as 6 induction cycles and up to 26 cycles of maintenance. In the avacopan arm, patients receive avacopan for 6 cycles combined with CYC (4 cycles) or RTX (1 cycle) for the first induction phase, and avacopan for 7 cycles for the maintenance phase. In the GC arm, patients receive prednisone for 5 cycles combined with CYC (4 cycles) or RTX (1 cycle) for the induction phase. AZA was used as maintenance therapy from the fourth cycle. Patients who were in the relapse state following failure of induction therapy (i.e. refractory patients) received AZA for the remainder of the time horizon.
AAV disease management, ESRD treatment, AEs and hospitalization events were included in the analysis. Management of AAV requires regular monitoring to assess disease status, including blood test, liver function test, X-ray and computed tomography scan, as well as outpatient follow-up visits. Resource use per health state was obtained from a report of RTX in a NICE submission [Citation36]. No data were available for the ESRD state, so it was assumed to be the same as for the active disease state (). Annual ESRD management costs were calculated from the proportion of patients on peritoneal dialysis (5.4%), hemodialysis (43.8%) and renal transplant (50.8%) and their respective unit costs [Citation28,Citation31]. The costs of AEs were included in the hospitalization data derived from ADVOCATE trial. The mean number of hospital admissions (0.47 for avacopan arm; 0.68 for GC arm) and length of stay (13.8 days; 19.6 days) were obtained from each treatment arm in the ADVOCATE trial and combined with the unit costs [Citation26] to estimate the total cost of inpatient hospital treatment ().
Table 2. Costs and utilities included.
2.6. Utilities
The impact of AAV, ESRD and AEs on health-related quality of life (HRQoL) was assessed using utilities and utility decrements expressed as QALYs. Baseline utility was age-dependent and used the European Quality of Life-5 Dimensions (EQ-5D) questionnaire data from EuroQoL performed in Spain [Citation38]. The model included utility scores from EQ-5D-5L questionnaire from the ADVOCATE trial, stratified by health state (active disease, remission, and relapse) [Citation15]. Health state utilities were assumed to be treatment-specific and any difference in the utilities between treatment arms for the same health state was attributed to reduced use of GCs in the avacopan arm and related AEs. Utility for ESRD state by treatment (dialysis and transplant) was derived from the published literature [Citation37] ().
2.7. Sensitivity analysis and scenario analysis
We performed sensitivity and scenario analyses to assess the robustness of our results.
In the deterministic sensitivity analysis, parameters were varied within sensible and appropriate ranges to assess the impact of individual model parameters on the model outcomes. In the probabilistic sensitivity analysis (PSA), we fitted probabilistic distributions to each parameter and ran 1,000 iterations in a Monte Carlo simulation. We simultaneously varied the input parameters within their 95% confidence intervals, or by ± 10% when data were unavailable. The results were represented in a cost-effectiveness plane scatter diagram. A cost-effectiveness threshold of €60,000 per QALY gained was assumed [Citation39].
In the scenario analyses, different probabilities of ESRD were considered because of the high variability observed in the literature. Progression to ESRD was changed from 1.2% to 7.7% at 6 months and from 17.5% to 49.1% at 7.1 years based on three studies reporting data on progression to ESRD in the Spanish setting [Citation5,Citation7,Citation40].
This manuscript was validated against the ISPOR Consolidated Health Economic Evaluation Reporting Standards checklist in accordance with their reporting guidelines [Citation41] (see details in Table S2 in the Supplementary Material).
3. Results
3.1. Base case
Treatment with avacopan compared to GC arm was associated with an increase in QALYs gained (6.52 vs. 6.17, respectively) and an increase in life year gained (LYG) (9.72 vs. 9.43) over a lifetime horizon (). Total costs for the avacopan arm were estimated at €267,671 and for the GC arm at €251,662, indicating an increase of €16,009. The ICUR was estimated to be €45,638 per QALY gained, suggesting that avacopan was cost-effective compared to GC at a willingness-to-pay (WTP) threshold of €60,000 per QALY gained (). The incremental costs for the avacopan arm were mainly driven by a higher acquisition costs for the combination drugs (€ 33,415), but the avacopan arm was associated with significantly lower ESRD (-€16,794) and AAV management costs and hospitalization (-€613) as avacopan delayed the progression to ESRD and achieved better disease control by improving the rate of sustained disease remission, reducing relapses and promoting recovery of renal function in patients with AAV. The incidence of ESRD per 1,000 life years was 169 and 213 in the avacopan and GC arms, respectively.
Table 3. Base case results: avacopan vs. GC arm (€ 2022).
3.2. Sensitivity analyses
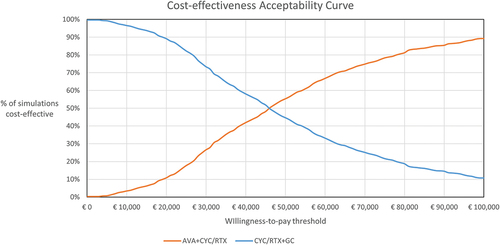
The results of the one-way sensitivity analysis confirmed the robustness of the base case to changes in individual parameters, with results most sensitive to assumptions related to progression to ESRD and discount rate (outcomes and costs) (). From the PSA, the resulting cost-effectiveness plane is presented in and the cost-effectiveness acceptability curve is presented in . The average incremental QALYs and costs were 0.35 and €16,050, respectively, yielding an ICUR of €45,906. At a WTP threshold of €60,000/QALY gained [Citation41], treatment with avacopan was estimated to be a cost-effective intervention in 66.9% of the simulations compared to GC.
Figure 2. Tornado diagram: avacopan vs. GC arm.
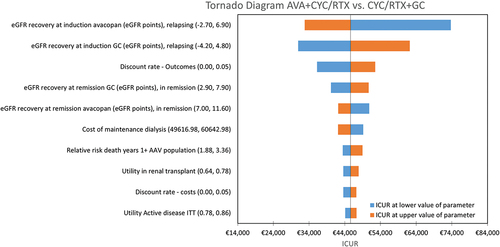
Figure 3. Cost-effectiveness plane: avacopan vs. GC arm.
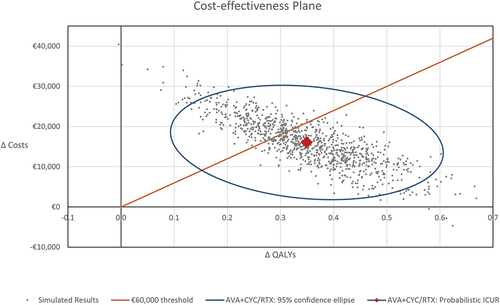
Figure 4. Cost-effectiveness acceptability curve: avacopan vs. GC arm.
Scenario analysis showed that the ICUR ranged from €45,638 to €62,538/QALY gained based on the alternative cumulative incidence of ESRD according to the literature [Citation5,Citation7,Citation40] (). These results showed the high impact of this parameter in the model.
Table 4. Scenario analysis: progression to ESRD (€ 2022).
4. Discussion
Our analysis shows that the addition of avacopan, as an add-on to RTX or CYC therapy, is a cost-effective alternative for the treatment of severe, active GPA or MPA. Patients treated with avacopan are expected to have sustained remission, lower relapse rates and a lower probability of ESRD, contributing to improved disease control and HRQoL. The inclusion criteria for the ADVOCATE trial did not require previous treatment failure in order to receive avacopan [Citation15]. The results of the current study showed that a regimen with avacopan resulted in an increase in LYG (9.72 vs. 9.43 LYG) and improvement in HRQoL (6.52 vs. 6.17 QALYs), compared to GC. The difference in total cost between avacopan and GC arms (+€ 16009) was driven by higher drug acquisition cost, partially offset by lower costs for the management of ESRD and AAV relapses and GC-related AEs.
Renal involvement is associated with an increased risk of death and healthcare expenditure in AAV patients and in the general population [Citation5,Citation31]. Patients with renal involvement may need renal replacement therapy as the disease progresses, which accounts for between 2.5% and 3.0% of the total Spanish NHS budget [Citation31]. The results from the ADVOCATE study showed greater improvement in patients with stage 4 chronic kidney disease treated with avacopan (5.6 ml/min/1.73 m2 [95% CI: 0.1.7–9.5]) [Citation15]. This result shows that a regimen with avacopan can improve renal function, which has a direct impact on reducing healthcare costs associated with GPA and MPA.
However, there is a high variability in the proportion of patients with AAV and renal involvement and, in particular, those who progress to ESRD. In Spain, we identified 3 publications reporting these data. Solans-Laqué et al. [Citation5] reported that 24.6% and 9.8% of patients with MPA and GPA required dialysis, respectively, at a median follow-up of 6.8 years. Marco et al. [Citation7] reported that 33.3% of patients with MPA and 35.5% with GPA developed ESRD at a median follow-up of 3.2 years. Finally, Villacorta et al. [Citation40] reported that 32.7% patients developed ESRD at a median follow-up of 3.4 years. This variability is also observed in other international publications, where the cumulative incidence of ESRD in patients with AAV ranged from 13.9% to 28.0% at a median follow-up of 3.1 to 7.1 years [Citation6,Citation8,Citation11,Citation42–45]. As observed in the scenario analyses, the probability of progression to ESRD is a highly sensitive parameter in the model, with an ICUR that ranged from €45,638 to €62,538/QALY gained [Citation5,Citation7,Citation40]. However, all the results were still very close to the WTP threshold of €60,000/QALY gained [Citation46]. The variability in the proportion of patients with AAV who progress to ESRD may also be related to differences in the profile of patients included in each study. It should be noted that in the case of rare diseases, the adopted WTP threshold may be even higher than the one assumed in this analysis. In 2017, the NICE adopted an upper threshold of between £100,000 and £300,000 per QALY gained for drugs indicated for very rare diseases [Citation47].
To our knowledge, this is the first study to assess the cost-effectiveness of avacopan from the Spanish NHS perspective. Thus, our study is the first to be published in this area and the results of this analysis may provide clinical and regulatory evidence for the use of avacopan in actual clinical practice. Other economic evaluations of avacopan have been conducted in other countries. In the United Kingdom (UK) and the Canadian settings, avacopan proved to be a cost-effective alternative [Citation48,Citation49]. In line with our analysis, the results from the UK perspective were sensitive to changes in the discount rate (outcomes and costs) and the cost of maintenance dialysis, which is the main component of the cost of ESRD in the UK model. In the Canadian setting, the assumptions about progression to ESRD were also the most sensitive [Citation49].
No other cost-utility analyses of current treatment options other than avacopan for patients with GPA or MPA were identified in Spain. However, other economic evaluations of AAV have been previously published at the European level. Montante et al. assessed the cost-effectiveness of RTX compared to AZA for maintenance treatment from a French perspective. RTX was cost-effective as a maintenance therapy to prevent AAV relapses, with an ICUR of €57,127/QALY at 28 months follow-up [Citation50]. Renal impairment was the main determinant of cost.
There are several limitations to our analysis. First, the clinical parameters included in the model were primarily derived from the ADVOCATE trial, where patients were followed for up to 52 weeks. The treatment effect was assumed to last for 6 months after treatment discontinuation. Beyond this period, the effect of avacopan was assumed to decline steadily over time. However, the results of the sensitivity analysis were robust to the base case, demonstrating that a regimen including avacopan is a cost-effective alternative. Further evidence is needed to confirm the long-term efficacy of avacopan.
Second, another possible limitation is that, due to the lack of Spanish data, most of the inputs were collected from the international literature, except for costs and progression to ESRD. Thus, the results are subject to uncertainties related to how these sources are combined to inform the model parameters. In addition, some parameters were not identified specifically for patients with AAV, such as the distribution of patients by renal replacement therapy, which may overestimate patients with renal transplant. Nevertheless, our results are consistent with other economic evaluations of avacopan in other healthcare settings. Finally, indirect costs were not included because the analysis adopted the Spanish NHS perspective. However, the mean age of the patients included was over 60 years old and potential productivity losses may be small.
5. Conclusions
This study evaluates the potential benefits of avacopan for the treatment of patients with severe, active GPA or MPA, and rare diseases. The results indicate that, from the Spanish NHS perspective, avacopan was a cost-effective option for newly diagnosed or relapsed patients with severe, active GPA or MPA compared to GCs, at a WTP threshold of €60,000/QALY gained. Even though there were additional acquisition costs associated with a regimen including avacopan, ESRD management, AAV relapses and GC-related AEs costs were lower and disease control and quality of life were improved.
Article highlights
The aim of this analysis was to assess the cost-utility of avacopan in combination with rituximab or cyclophosphamide compared with glucocorticoids for the treatment of severe, active antineutrophil cytoplasmic antibody-associated vasculitis.
A Markov model with nine health states based on the ADVOCATE trial data was adapted from a Spanish payer perspective over a lifetime horizon.
Avacopan was associated with a lower incidence of end-stage renal disease, relapse and hospitalization-related adverse events.
Avacopan is more effective than glucocorticoids and results in an incremental cost-utility ratio of €45,638/QALY gained.
Avacopan is a cost-effective treatment option for patients with severe, active antineutrophil cytoplasmic antibody-associated vasculitis compared to glucocorticoids in Spain.
Declaration of interest
A Ramírez de Arellano is employed by CSL Vifor Switzerland. C Escribano is employed by CSL Vifor Spain. E Pomares Mallol and A García Castells are employed by PharmaLex Spain and received financial support from Vifor Pharma Spain for the development of this study. M Macía, M Díaz-Encarnación, and R Solans-Laqué received an honorarium from the sponsor and participated as independent consultants.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
All authors contributed to the conception and design of the study. E Pomares Mallol and A García Castells contributed to the analysis and interpretation of the data, and the drafting of the manuscript. M Macía, M Díaz-Encarnación and R Solans-Laqué contributed to the review and editing. All study authors meet the criteria for authorship as outlined by the journal policy and agree for the final version of the manuscript to be published.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are on the Advisory Board of Amgen. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (45.3 KB)Acknowledgments
We thank Susana Romero Yuste from the Rheumatology Service of the Complejo Hospitalario Universitario de Pontevedra (Spain), who provided insight and expertise that greatly assisted this study.
An abstract and poster of some of the material contained in the paper was previously presented at ISPOR Europe 2022, Vienna (Austria), 6-9 November 2022.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2023.2297790
Additional information
Funding
References
- Sociedad Española de Reumatología. Manual SER de diagnóstico y tratamiento de las enfermedades reumáticas autoinmunes sistémicas. 1a ed. 2014.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715
- Romero-Gómez C, Aguilar-García JA, García-de-Lucas MD, et al. Epidemiological study of primary systemic vasculitides among adults in Southern Spain and review of the main epidemiological studies. Clin Exp Rheumatol. 2015;33(2 Suppl 89):S–8.
- Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, et al. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the Chapel Hill Consensus Conference definitions. Arthritis Rheum. 2003;49(3):388–393. doi: 10.1002/art.11115
- Solans-Laqué R, Fraile G, Rodriguez-Carballeira M, et al. Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides. Medicine. 2017;96(8):e6083. doi: 10.1097/MD.0000000000006083
- Lionaki S, Hogan SL, Jennette CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis. Kidney Int. 2009;76(6):644–651. doi: 10.1038/ki.2009.218
- Marco H, Draibe J, Villacorta J, et al. Determinants of renal and patient outcomes in a Spanish cohort of patients with ANCA-associated vasculitis and renal involvement. Clin Rheumatol. 2018;37(4):1065–1074. doi: 10.1007/s10067-017-3973-2
- Wester Trejo MAC, Floßmann O, Westman KW, et al. Renal relapse in antineutrophil cytoplasmic autoantibody-associated vasculitis: unpredictable, but predictive of renal outcome. Rheumatology. 2019;58(1):103–109. doi: 10.1093/rheumatology/key260
- Slot MC, Tervaert JWC, Franssen CFM, et al. Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int. 2003;63(2):670–677. doi: 10.1046/j.1523-1755.2003.00769.x
- Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–1594. doi: 10.1136/annrheumdis-2016-209133
- Robson J, Doll H, Suppiah R, et al. Damage in the anca-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis. 2015;74(1):177–184. doi: 10.1136/annrheumdis-2013-203927
- Pujades-Rodriguez M, Morgan AW, Cubbon RM, et al. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLOS Med. 2020;17(12):e1003432. doi: 10.1371/journal.pmed.1003432
- Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2023:ard-2022–223764. doi:10.1136/ard-2022-223764
- European Medicines Agency. Avacopan SmPC; 2022 [cited 2022 Dec 9]. Available from: https://www.ema.europa.eu/en/documents/product-information/tavneos-epar-product-information_en.pdf
- Jayne DRW, Merkel PA, Schall TJ, et al. Avacopan for the treatment of ANCA-Associated vasculitis. N Engl J Med. 2021;384(7):599–609. doi: 10.1056/NEJMoa2023386
- National Institute for Health and Care Excellence. Avacopan for treating severe active granulomatosis with polyangiitis or microscopic polyangiitis [Internet]. 2022 [cited 2022 Dec 9]. Available from: https://www.nice.org.uk/guidance/ta825
- Gemeinsamer Bundesausschuss - the Federal Joint Committee. Resolution of the Federal Joint Committee on an amendment of the pharmaceuticals directive: annex XII – benefit assessment of medicinal products with new active ingredients according to section 35a SGB V avacopan (granulomatosis with polyangiitis or microscopic polyangiitis, combination with rituximab or cyclophosphamide). 2022.
- Haute Autorité de Santé (HAS). Avacopan, Tavneos 10mg capsuse first assessment adopted by the Transparency Committee on 21 September 2022. 2022.
- Ministerio de Sanidad. Puntos destacados de la reunión de la Comisión Interministerial de Precios de los Medicamentos - 15 de junio de 2023 [Internet]. 2023 [cited 2023 Jul 18]. Available from: https://www.sanidad.gob.es/areas/farmacia/precios/comisionInteministerial/acuerdosNotasInformativas/docs/NOTAINFORMATIVACIPM_JUNIO23.pdf
- Ortega Eslava A, Marín Gil R, Fraga Fuentes MD, et al. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos [Internet]. 2016 [cited 2023 Aug 11]. Available from: https://gruposdetrabajo.sefh.es/genesis/genesis/Documents/GUIA_EE_IP_GENESIS-SEFH_19_01_2017.pdf
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905
- Cui Z, Zhao J, Jia X, et al. Clinical features and outcomes of anti–glomerular basement membrane disease in older patients. Am J Kidney Diseases. 2011;57(4):575–582. doi: 10.1053/j.ajkd.2010.09.022
- Instituto Nacional de Estadística. Tablas de mortalidad por año, sexo, edad y funciones. [Internet]. 2022 [cited 2022 Jul 25]. Available from: https://www.ine.es/jaxiT3/Tabla.htm?t=27153
- Wallace ZS, Lu N, Unizony S, et al. Improved survival in granulomatosis with polyangiitis: a general population-based study. Semin Arthritis Rheum. 2016;45(4):483–489. doi: 10.1016/j.semarthrit.2015.07.009
- UK Renal Registry. UK renal registry 23rd annual report – data to 31/12/2019 [Internet]. Bristol (UK); 2021 [cited 2022 Dec 9]. Available from: https://ukkidney.org/sites/renal.org/files/23rd_UKRR_ANNUAL_REPORT.pdf
- Ministerio de Sanidad. Portal Estadístico del SNS - Registro de Altas de los Hospitales del Sistema Nacional de Salud. CMBD [Internet]. 2022 [cited 2022 Jul 26]. Available from: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbdhome.htm
- Gisbert R, Brosa M. Base de datos de costes sanitarios y ratios coste-efectividad españoles: eSalud [Internet]. 2007 [cited 2022 Apr 12]. Available from: http://www.oblikue.com/bddcostes/
- Alianza frente a la Poliquistosis Renal Autosómica Dominante. Libro Blanco de la Poliquistosis Renal Autosómica Dominante (PQRAD) en España [Internet]. 2016 [cited 2023 Aug 11]. Available from: https://senefro.org/contents/webstructure/noticias/LIBRO_PQRAD.pdf
- Bartra A, Caeiro J-R, Mesa-Ramos M, et al. Cost of osteoporotic hip fracture in Spain per autonomous region. Rev Esp Cir Ortop Traumatol. 2019;63(1):56–68. doi: 10.1016/j.recote.2018.11.004
- Lopez-Bastida J, Oliva Moreno J, Worbes Cerezo M, et al. Social and economic costs and health-related quality of life in stroke survivors in the Canary Islands, Spain. BMC Health Serv Res. 2012;12(1):315. doi: 10.1186/1472-6963-12-315
- Consejerías de Sanidad de las Comunidades Autónomas. Documento Marco sobre Enfermedad Renal Crónica (ERC) dentro de la Estrategia de Abordaje a la Cronicidad en el SNS. 2015 [cited 2023 Aug 11]. Available from: https://www.sanidad.gob.es/organizacion/sns/planCalidadSNS/pdf/Enfermedad_Renal_Cronica_2015.pdf
- Fernández-de-Bobadilla J, López-de-Sá E. Carga económica y social de la enfermedad coronaria. Rev Esp Cardiol Supl. 2013;13:42–47. doi: 10.1016/S1131-3587(13)70079-7
- Consejo General de Colegios Oficiales de Farmacéuticos. Portal Farma. BotPLUS [Internet]. 2023 [cited 2023 Aug 10]. Available from: https://botplusweb.portalfarma.com/botplus.aspx
- Ministerio de Sanidad. Listado de medicamentos afectados por las deducciones del Real Decreto-Ley 8/2010. Septiembre 2023 [Internet]. 2023 [cited 2023 Sep 10]. Available from: https://www.sanidad.gob.es/en/areas/farmacia/infoIndustria/infoDeducciones/ley8_2010/docs/Deducciones_Septiembre_23.pdf
- Instituto Nacional de Estadística. Encuesta Nacional de Salud (ENSE); 2017 [cited 2023 Aug 11]. Available from: https://www.ine.es/
- National Institute for Health and Care Excellence. Rituximab in combination with glucocorticoids for treating anti-neutrophil cytoplasmic antibody associated vasculitis [Internet]. 2014 [cited 2023 Aug 11]. Available from: https://www.nice.org.uk/Guidance/TA308
- National Institute for Health and Care Excellence. Patiromer for treating hyperkalaemia [internet]. 2020 [cited 2023 Aug 11]. Available from: https://www.nice.org.uk/guidance/TA623
- Instituto Nacional de Estadística. Encuesta Nacional de Salud (ENSE) 2011 [Internet]. 2011 [cited 2023 Aug 11]. Available from: https://www.ine.es/
- Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? Gac Sanit. 2020;34(2):189–193. doi: 10.1016/j.gaceta.2019.06.007
- Villacorta J, Diaz-Crespo F, Guerrero C, et al. Long-term validation of the renal risk score for vasculitis in a Southern European population. Clin Kidney J. 2021;14(1):220–225. doi: 10.1093/ckj/sfaa073
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31. doi: 10.1016/j.jval.2021.10.008
- Scott J, Canepa C, Buettner A, et al. A cohort study to investigate sex-specific differences in ANCA-associated glomerulonephritis outcomes. Sci Rep. 2021;11(1):13080. doi: 10.1038/s41598-021-92629-7
- Mohammad AJ, Segelmark M. A population-based study showing better renal prognosis for Proteinase 3 antineutrophil cytoplasmic antibody (ANCA)–associated nephritis versus myeloperoxidase ANCA–associated nephritis. J Rheumatol. 2014;41(7):1366–1373. doi: 10.3899/jrheum.131038
- Huang X, Chen L, Lan L, et al. Antineutrophil cytoplasmic antibody-associated vasculitis with acute kidney injury: short-term recovery predicts long-term outcome. Front Immunol. 2021;12:12. doi: 10.3389/fimmu.2021.641655
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Diseases. 2003;41(4):776–784. doi: 10.1016/S0272-6386(03)00025-8
- Powell T, O’Donell M. NICE appraisals of rare diseases [internet]. House Of Commons Library. 2019 [cited 2023 Aug 11]. Available from: https://researchbriefings.files.parliament.uk/documents/CDP-2019-0022/CDP-2019-0022.pdf
- Paulden M. Recent amendments to NICE’s value-based assessment of health technologies: implicitly inequitable? Expert Rev Pharmacoecon Outcomes Res. 2017;17(3):239–242. doi: 10.1080/14737167.2017.1330152
- Ramirez de Arellano Serna A, Berdunov V, Baxter G. EE382 cost-utility analysis of avacopan for the treatment of anca-associated vasculitis (AAV) patients in the UK. Value Health. 2022;25(12):S130. doi: 10.1016/j.jval.2022.09.628
- Lakhdari K, Vicente C, Melnyk P, et al. EE519 cost effectiveness analysis of Avacopan in antineutrophil cytoplasm antibody-associated vasculitis - a Canadian perspective. Value Health. 2022;25(12):S157–S158. doi: 10.1016/j.jval.2022.09.760
- Montante A, Le Bras A, Terrier B, et al. Economic evaluation of rituximab versus azathioprine for maintenance treatment of ANCA-associated vasculitis. A prospective, multicenter study. 2017 ACR/ARHP Annual Meeting; Nov 3–8; San Diego, CA; 2017. Abstract number: 1759.
