?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
This study assessed the feasibility of using the Milano-Torino staging (MiToS) system for conducting economic evaluation to measure health outcomes in amyotrophic lateral sclerosis (ALS).
Methods
A Markov model was developed using the MiToS system and evaluated with a hypothetical treatment versus standard of care. Health utilities and transition probabilities were derived from the literature. Four-time horizons (1, 5, 10, and 20 years) were examined. Treatment effects of 20–35% relative risk reduction (RRR) of progressing to the next MiToS stage were assessed. Three patient distribution scenarios were tested: (1) all patients began in stage 0; (2) patient distribution based on real-world TONiC study; (3) distribution based on the PRO-ACT database. Health outcomes (quality-adjusted life-years [QALYs], life-years [LYs]) were reported with a 3% discount rate.
Results
A time horizon of 10 years fully captured treatment benefits: incremental QALYs were 0.28–0.60, 0.21–0.45, and 0.26–0.55 for scenarios 1–3, respectively; incremental LYs were 0.56–1.17, 0.46–0.97, and 0.53–1.11, respectively.
Conclusion
MiToS-based staging can be used for conducting economic analyses in ALS. Estimated incremental QALY and LY gains were meaningful within the context of ALS, for hypothetical treatments with RRR of 20–35%.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder of motor neurons in the brain and spinal cord [Citation1]. On average, people with ALS survive 3–5 years from symptom onset, but there are considerable differences in progression rates, and approximately 5–10% of people live longer than 10 years [Citation2,Citation3]. The drugs currently approved by the United States Food and Drug Administration (FDA) for the treatment of ALS – riluzole, edaravone, and sodium phenylbutyrate-taurursodiol (AMX0035) – show only modest effects on slowing disease progression and do not cure or reverse the condition [Citation4,Citation5]. For the subset of adults with ALS who have a rare genetic form resulting from a mutation in the superoxide dismutase 1 gene, the FDA has also approved the antisense oligonucleotide, tofersen. Accelerated approval was granted based on a substantial reduction in the plasma neurofilament light chain, and the studies completed to date demonstrate only modest effects on disease progression in exploratory analyses [Citation6].
In addition to the impact on quality of life, the economic burden associated with ALS disease progression is substantial for patients and their families [Citation7]. The economic impact for health care systems is also large, even though ALS is a rare disease with global prevalence of 4.42 cases per 100,000 persons and incidence rate of 1.59 per 100,000 person-years [Citation8]. To facilitate reimbursement and access to therapies as they are approved, it is thus essential to conduct economic evaluation of new treatments in ALS. To some extent, this has been hampered by lack of an established scale to measure disease progression. The most widely used clinical measure for assessing functional status of people with ALS is the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R), which is also frequently used as the primary endpoint in randomized clinical trials [Citation9]. However, it has several limitations including nonlinearity, multidimensionality, a ‘floor-effect’ and not able to satisfy rigorous psychometric measurement standards [Citation10].
To overcome some of the limitations of the ALSFRS-R, a number of staging methods have been developed for the assessment of ALS disease progression [Citation10]. Of these, the Milano-Torino staging (MiToS) system and the King’s College system are the most commonly used [Citation11]. MiToS characterizes the functional burden of the disease as defined by loss of autonomy in four key domains: walking/self-care, swallowing, communicating, and breathing [Citation12]. The number of domains impaired determines the MiToS stage: stages 0–4 correspond to 0–4 domains impaired, respectively, with stage 5 being death. King’s staging is based on disease burden as measured by clinical milestones and considers how many of the three anatomical regions are involved (bulbar, upper limbs, and lower limbs; stage 1 for one region involved, stage 2 for two, and stage 3 for three) and the need for gastrostomy (stage 4A) and noninvasive ventilation (stage 4B) [Citation13]. More recently, researchers have proposed an additional staging approach, named Fine’til 9 (FT9), based on the proportion of ALSFRS-R subscores that are 9 or lower [Citation14]. The stages for all the systems can be determined retrospectively from the individual item scores on the ALSFRS-R [Citation12,Citation14,Citation15], and the value of MiToS and King’s staging in assessing treatment efficacy and their potential utility as outcome measures in ALS clinical trials have recently been demonstrated [Citation16,Citation17]. To date, economic evaluation using King’s staging and the FT9 staging systems in ALS have been reported [Citation18,Citation19].
The value of MiToS for economic evaluation of health care interventions in ALS has not been established. The objective of this study was to assess the feasibility of using MiToS for developing a Markov model to conduct an economic analysis in ALS, specifically looking at quality-adjusted life-years (QALYs) and life-years (LYs). Demonstrating the feasibility of the MiToS staging system for economic modeling will facilitate future economic evaluations to support an informed healthcare decision for comparison of two or more alternatives as to which is the best option.
2. Methods
2.1. Model development
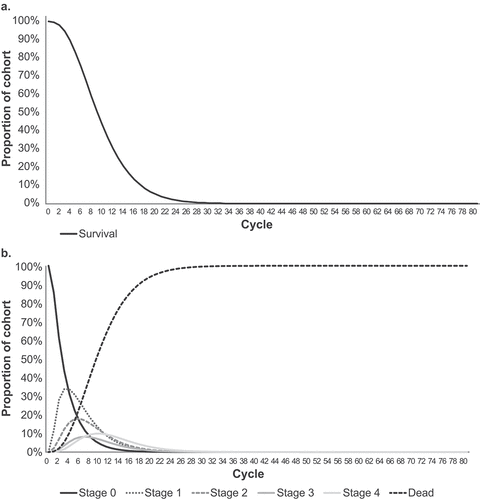
A Markov model was developed based on the MiToS clinical staging system to define progression of disease across six mutually exclusive health states: stage 0 indicates no functional domains lost, stages 1–4 indicate loss of 1 to 4 functional domains, respectively, and stage 5 is death (). A hypothetical ALS treatment was compared with standard of care. Since currently approved treatments for ALS do not cure or reverse the disease but may delay disease progression, the MiToS-based model structure allows patients either to stay at their current stage or move to later stages (forwards) as the disease progresses but not back to earlier stages (backwards).
Figure 1. MiToS-based model structure.
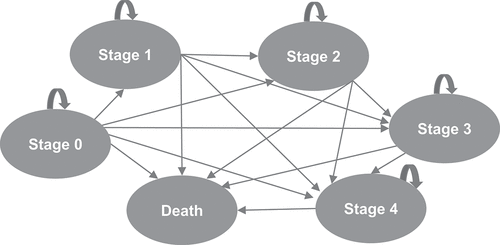
The model uses a 3-month cycle length, with half-cycle correction applied to account for midcycle transition [Citation20,Citation21]. Three-month cycle was previously adopted in a cost-effectiveness analysis of edaravone in ALS [Citation20]. Standard economic evaluation guidelines suggest to use a time horizon long enough to capture all consequences of an intervention [Citation22]. Time horizons of 1, 5, and 10 years and lifetime (20-year horizon) were compared, to explore the effect of different time horizons on the calculated health outcomes. A time horizon of 1 year was included as the shortest time, as it reflects the length of a typical phase 3 clinical trial in ALS. A 20-year horizon was chosen as the longest time as, although people with ALS survive an average of 3–5 years from symptom onset, a small proportion live longer than 10 years [Citation2,Citation3]; hence, a 20-year horizon ensured the economic model would continue until all patients reached the death state (i.e. a lifetime analysis).
2.2. Health state utilities and health state transition probabilities
The model used EQ-5D-5 L scores (mean along with 95% confidence intervals) for MiToS-based health states () previously reported from the Trajectories of Outcomes in Neurological Conditions (TONiC) study, a real-world cohort of 595 patients from the United Kingdom [Citation23]. To the best of our knowledge, this is the only study that reported and published health utilities based on MiToS in ALS [Citation23]. The 3-month transition probabilities (95% confidence intervals were not reported but recalculated using aggregated data) for standard of care () were obtained from a previously published analysis of the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT), a pooled dataset that includes data from 16 phase 2 and 3 clinical trials as well as 1 observational study in ALS [Citation14,Citation24].
Table 1. EQ-5D-5 L scores for MiToS stages (Moore et al. [Citation23]).
Table 2. Transition probability matrix for standard of care (Thakore et al. [Citation14]).
2.3. Treatment effect
The objective of this study was to assess the feasibility of using MiToS to conduct economic evaluation, and there was no intention to evaluate a particular treatment intervention in ALS. Hence, a range of hypothetical treatment effects was evaluated. The treatment effect for a hypothetical intervention was assumed to reduce the risk of progressing to the next MiToS stage. To test the feasibility of MiToS-based modeling, four options were evaluated, with a relative risk reduction (RRR) versus standard of care of 20%, 25%, 30%, or 35%. These values were chosen to reflect results of a survey of ALS clinicians and researchers, which found a new treatment that reduces disease progression by 20% or more would be clinically meaningful [Citation25]. Moreover, the assumed treatment effect is consistent with observed benefit in terms of reduction in disease progression for AMX0035 and edaravone in ALS [Citation26].
2.4. Patient distribution
For the distribution of patients, three starting scenarios were tested (), with patient distributions selected for evaluation based on observed distributions of MiToS staging in real-world and clinical trial settings [Citation14,Citation16,Citation23]. In scenario 1, all patients started in MiToS stage 0. This allowed for the possibility that all patients entered the model early in their disease course (i.e. with no functional loss), which is consistent with the patient distribution observed in a phase 3 trial in ALS [Citation16]. In scenario 2, the distribution was based on real-world evidence from TONiC study [Citation23]. In scenario 3, the distribution was based on a patient distribution reported for the pooled dataset from PRO-ACT [Citation14].
Table 3. Patient distributions in the three scenarios.
2.5. Model outcomes
Incremental QALYs and LYs gained were assessed with the hypothetical treatment compared with a standard-of-care treatment. Both outcomes were discounted at a rate of 3%.
2.6. Sensitivity analyses
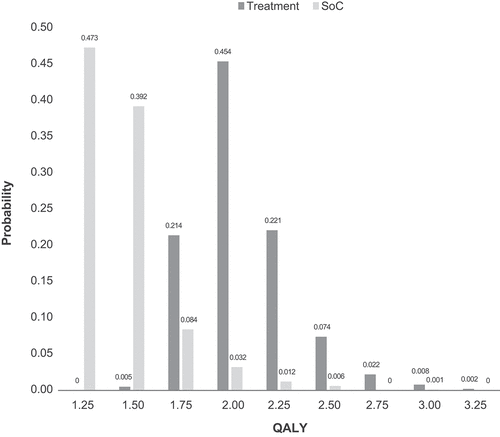
Uncertainty of input parameters was evaluated via one way (univariate) and probabilistic sensitivity analyses (PSA). In a one-way sensitivity analysis, the effect of uncertainty about parameter values on the base case model’s results for scenario 3 with 10-year horizon assuming treatment effect of 20% were individually assessed. Health utilities (95% CI), transition probabilities (recalculated, 95% CI), treatment effect (12%, 36%), and discount rates (1%, 5%) with reasonable assumptions were considered () [Citation29–31]. For transition probabilities and health utility parameters, 95% CI values were used to assess uncertainty as minimum and maximum values and their impact on model results. For treatment effect and discount rates reasonable values were assumed. In PSA, several parameters were allowed to change by specifying plausible distributions instead of selecting specific values [Citation32]. For probabilities and health utilities, a beta distribution was used, while for treatment effect we selected log normal distribution. Detailed information of each parameter related to standard error, minimum and maximum values, and distributions used for one-way sensitivity analysis and PSA is documented in . Results of the one-way sensitivity analyses were presented using a tornado diagram, which shows the key parameters that may have larger impact on the model base case results. Similarly, results of the 1000 simulations for QALYs were analyzed and reported using a histogram, which shows an effectiveness distribution of QALY of both the treatment and standard of care.
Table 4. Model input values for base case, one-way analysis sensitivity and probabilistic sensitivity analyses.
2.7. Model validation
Model validation is an important component of economic evaluation to create confidence of its use by decision makers or consumers of the ultimate model results [Citation33]. Model design, input data, and model outcomes were reviewed by a highly experienced neurologist and two health economists. While there was no change to the model structure from the first draft model, there were some changes to the appropriateness of input data, choice of comparators (hypothetical intervention versus actual treatment), and ultimately model outcomes. The experts recommended conducting effectiveness (QALY) comparisons of hypothetical treatment versus standard of care as an outcome instead of cost/QALY due to lack of generalizable cost data in the literature. Subsequently, the Markov model programmed in Microsoft Excel was subject to formal quality control, validation and testing by the two experienced health economists. Through this process, all data inputs were checked against sources for accuracy and minor programming errors were identified and corrected. Model assumptions were reasonable and justified.
As stated above, the model translation probabilities for patients with ALS were based on the PRO-ACT database. Survival predictions from our model were validated with the results from the FT9 staging-based cost-effectiveness model of Thakore et al. [Citation19], which used the same database to estimate transition probabilities. Furthermore, median survival was compared with a historical cohort or population-based studies [Citation19,Citation34–36]. No previous model based on MiToS staging was identified in the literature, and hence cross-validation of model outcomes was not possible. However, model results were compared to other published ALS staging (King’s staging and FT9) based economic evaluation [Citation18,Citation19].
3. Results
3.1. Time horizon
The incremental QALYs and LYs for all four of the time horizons for scenario 3 (patient distribution from the pooled dataset) are shown in . Results for scenarios 1 and 2 showed a similar pattern (Supplementary Tables S1 and S2). For each hypothetical treatment effect, maximum gains of QALYs and LYs were realized at the 10-year horizon in all three scenarios of patient distribution. The lifetime (20-year horizon) analysis did not improve either QALYs or LYs in any material way compared with the 10-year horizon.
Table 5. QALY and LY for a range of hypothetical treatment effects versus standard of care: patient distribution based on a pooled dataset (scenario 3).
3.2. Patient distribution scenarios
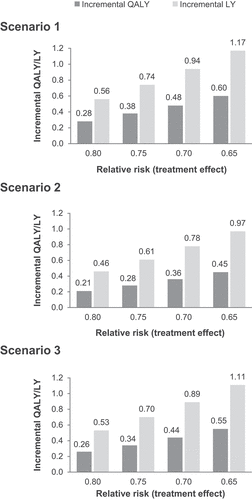
For a 10-year time horizon, incremental QALYs and LYs for the hypothetical treatment effects are shown in . For a patient distribution scenario with all patients starting in stage 0, the range of incremental QALYs with the range of treatment effects tested was 0.28–0.60, while the range of incremental LYs was 0.56–1.17. For scenarios 2 and 3, patient distribution was based on reported data as shown in . In scenario 2 (the real-world cohort study) more patients started in later stages compared with scenario 3 (the pooled dataset). For scenario 2, the range of incremental QALYs was 0.21–0.45 and the range of incremental LYs was 0.46–0.97 (). For scenario 3, the range of incremental QALYs was 0.26–0.55 and the range of incremental LYs was 0.53–1.11 (). For the hypothetical treatment effects of 20–35%, the incremental QALYs for scenario 3 increased by 21–24% compared with scenario 2 ().
Figure 2. Incremental QALYs and LYs for a range of hypothetical treatment effects versus standard of care over a 10-year horizon, for 3 scenarios of patient distribution.
3.3. Analysis by MiToS stage
Analysis of QALYs by MiToS stage showed the majority of gains occurred in earlier MiToS stages, especially stages 0, 1, and 2. For scenario 3 (the patient distribution scenario based on a pooled dataset), of the 0.55 QALY incremental gain over the 10-year horizon with a 35% RRR, 0.48 was accounted for during MiToS stages 0, 1, and 2 (). Details for patient distribution scenarios 1 and 2 are shown in Supplementary Table S3.
Table 6. QALY for a treatment with hypothetical RR of 0.65 versus standard of care for each MiToS stage: patient distribution based on a pooled dataset (scenario 3).
3.4. Sensitivity analyses
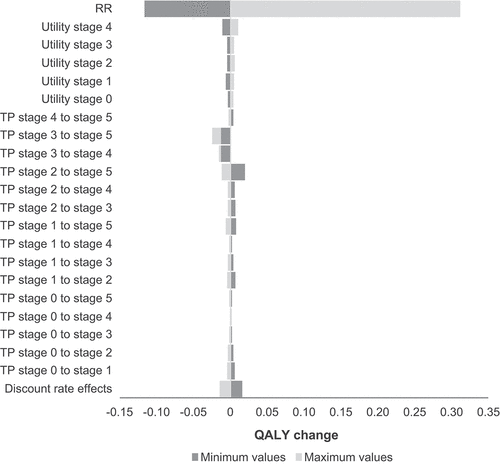
Results of the one-way sensitivity analyses showed that base case model results (QALYs) were most sensitive to treatment benefit variations with higher treatment effect associated with higher incremental QALYs and vice versa (). Health state utilities were positively correlated with QALY gains although there were variations in their effect to the model results. On the other hand, higher transition probabilities from earlier stage to later stages were negatively associated with incremental QALYs with some parameters having more effect than others (). These findings are consistent with disease progression of ALS as health-related quality of life is inversely related to disease stages [Citation12]. Finally, the discount rate was negatively correlated with the incremental QALYs.
Figure 3. Tornado diagram from one-way sensitivity: incremental QALYs of hypothetical treatment effect versus standard of care over a 10-year horizon, for scenario 3 of patient distribution.
Results from PSA showed that the simulated QALYs for the hypothetical treatment and standard of care overlapped only over a small portion of the effectiveness distribution (). The hypothetical treatment yielded higher QALYs compared with standard of care in 100% of the simulations indicating that the base case model results are relatively robust to plausible variations in the model parameters when allowed to change simultaneously.
3.5. Model validation
Median survival for standard of care was 30 months (), which falls within the range of median estimates from other studies [Citation4,Citation19,Citation34–36].
4. Discussion
The main objective of the current study was to assess the utility of MiToS staging for conducting economic modeling to quantify the economic value (health benefit) of a hypothetical treatment compared with standard of care in ALS. The use of MiToS has been shown to have several advantages over other staging systems, as it measures the patient’s function in each domain, is sensitive to smaller treatment effects, superior staging in later disease, is familiar to clinicians, and the stages have been shown to correlate with the generic measurement of quality of life (Short Form-36) [Citation37–39].
One study looked at an incremental QALYs of new drugs which received a favorable decision by the UK National Institute for Health and care Excellence (NICE) from 2010 to 2020 across disease areas [Citation40]. The authors reviewed 129 drugs and reported a median incremental value of 0.27 QALY (interquartile range: 0.07–0.73). Our results showed that over a 10-year time horizon, a hypothetical new treatment with 20–35% RRR using MiToS can lead to QALY gains of 0.21–0.60, equivalent to 77–219 days in perfect health when expressed in quality-adjusted life days (=QALY × 365 days [Citation41]). This incremental health benefit falls within the reported interquartile range and would likely be considered meaningful for a relentlessly progressive disease such as ALS. Unfortunately, there is very little published information available for comparison in ALS, but what is available suggests that incremental values for currently approved therapies would fall within these ranges [Citation18–20,Citation42]. Recently, the United States Institute for Clinical and Economic Review (ICER) developed a Markov model for ALS based on King’s staging [Citation18]. Populating this model with clinical trial data for oral edaravone gave a lifetime incremental QALY of 0.04 (equivalent to 15 days in perfect health); for AMX0035, the model gave a lifetime incremental QALY of 0.14 (equivalent to 51 days in perfect health). The Canadian Agency for Drugs and Technologies in Health (CADTH) conducted similar assessment of both drugs [Citation20,Citation42]. CADTH reported a lifetime incremental QALYs of 0.078–0.267 and 0.137 for edaravone and AMX0035, respectively. For riluzole, the cost-effectiveness compared with standard of care in ALS has been assessed using the FT9 staging system [Citation19]. At a 10-year horizon, riluzole contributed 0.281 QALY gains compared with standard of care, equivalent to 103 days in perfect health [Citation19]. Direct comparison between these results is limited, as there were a number of differences between studies in the designs of the models and inputs used, beyond the staging system used in each case. The purpose of the current study was to independently evaluate the potential use of MiToS staging systems for conducting economic evaluation in ALS using a hypothetical treatment and was not designed to evaluate the superiority of MiToS over other staging based economic evaluation in ALS. However, findings from these studies do suggest that the incremental QALYs with the MiToS-based model presented here are of a level considered meaningful in ALS. Despite the health benefits reported in incremental QALYs in the current study and previous studies of ALS falling within the range determined for drugs which received a favorable decision by NICE between 2010 and 2020 [Citation40], the cost-effectiveness of ALS drugs exceed the willingness to pay established for conventional diseases. This is commonly seen in diseases and is partially due to the high cost of drugs and disease management that made cost/QALY values not economically attractive [Citation43]. ICER reported base case cost/QALY gains of $11,981,000 and $2,136,000 for edaravone and AMX0035, respectively. The analogous numbers reported by CADTH were CA$1,440,786–3,152,352 and CA$2,086,658, respectively. Based on this, the incremental cost-effectiveness ratio of edaravone and AMX0035 far exceeds the commonly used threshold despite the health benefit measured by QALYs falling within the reported ranges in the literature. NICE and ICER have already started taking measurements including higher thresholds for ultra rare diseases of £300,000 and $500,000 per QALY, respectively, to this effect [Citation44]. Health technology assessment agencies should consider other factors as they are already doing for ultra rare diseases to lower the incremental cost-effectiveness ratio or increase the willingness to pay threshold when conducting pharmacoeconomic review for ALS treatments.
The appropriate time horizon and its influence on the value assessment of pharmacological interventions are still debated [Citation22]. To capture all consequences (cost and health benefits) it is important to use time horizons that extend sufficiently into the future to capture the life span of people with ALS [Citation22]. The cost-effectiveness analysis of riluzole discussed above reported incremental QALYs of 0.182 and 0.281 for 5- and 10-year horizons, respectively [Citation19], and a 10-year horizon yielded higher health benefits than a 5-year horizon, which is consistent with our findings. In the present study, a 20-year horizon was also assessed as a lifetime analysis. However, the 20-year horizon did not improve either QALYs or LYs in any material way compared with the 10-year horizon. Based on our results, it appears that a 10-year time horizon is appropriate for MiToS-based economic analyses in ALS regardless of the treatment effect. This is consistent with the natural history of the disease, as few patients survive for more than 10 years from onset.
Most pivotal phase 3 trials report changes in ALSFRS-R rate of decline as the primary outcome to assess treatment effect in ALS [Citation45]. In the past, MiToS staging was rarely included in clinical trials as it is a relatively new instrument developed in 2015 [Citation12]. Hence, treatment effects reported as ALSFRS-R rate of decline should be translated to MiToS staging for use in economic decision models when conducting economic evaluation. Chio et al. developed a mapping algorithm that translates existing or prospectively collected ALSFRS-R data into MiToS staging [Citation12]. Recently, there is an increasing application of MiToS in phase 2 and 3 trials in ALS. MiToS has been used as a primary endpoint in a 6-month phase 2 clinical trial of guanabenz in patients with ALS [Citation46]. Gebrehiwet et al. and Al-Chalabi et al. retrospectively evaluated treatment effects based on MiToS staging using phase 2 and phase 3 trial data, respectively [Citation16,Citation17]. Recently, two largest trials in ALS (COURAGE-ALS and PHOENIX) included MiToS staging prospectively as secondary and exploratory end point, which indicates the increasing use of MiToS as clinical outcome measure in ALS clinical trials that can complement the ALSFRS-R [Citation47,Citation48].
The median survival of 30 months found in this study is in line with data from other studies. The FT9 staging-based decision model (which also used the PRO-ACT database to calculate transition probabilities) by Thakore et al. reported a median survival for standard of care treatment of 32 months [Citation19]. Two other studies reported a median survival from diagnosis between 1.4 and 2.2 years [Citation35,Citation36]. Another study reported a median survival from onset of 40.2 and 25.9 months for young (<65.2 years) and older patients (>65.2 years), respectively [Citation34]. In a review by Chio et al., the reported median survival from onset was 20–36 months based on data from population-based studies [Citation2]. Our model-based median estimate falls within this range.
Selecting an appropriate patient distribution is crucial as it may lead to a meaningful difference in health outcomes, as seen in this study. In our patient distribution scenario based on a real-world cohort, patients were at later stages when starting treatment compared with the scenario based on a pooled dataset. For the hypothetical treatment effects of 20–35%, the incremental QALYs for scenario 3 increased by 21–24% compared with scenario 2, clearly demonstrating the effects of start distribution on health outcome results as measured by QALYs. Our results also showed that most of the incremental QALYs were gained in MiToS stages 0, 1, and 2. This is consistent with the disease, as an increasing disease stage (0 to 4) is associated with a decline in quality of life in people with ALS [Citation12].
Despite the robustness of our study base case results as demonstrated by the one-way and probabilistic sensitivity analyses reported, several limitations should be considered when interpreting the results and conclusions from our findings. The limitations of this study largely resulted from a lack of robust published studies on the economic and humanistic burden in ALS, which are required for the model inputs. Economic evaluation in ALS should provide full consideration of the economic and humanistic burden associated with the condition. The consequence of ALS is beyond motor disability. It has significant impact on patients’ productivity due to absenteeism and decline in performance as well as mental and psychological consequences [Citation7]. It has also a societal impact such as on caregivers psychological and mental wellbeing and their level of productivity. Thus, cost of illness and health-related quality of life studies should include those factors and failing to account for those will underestimate the socio-economic impact of ALS. To the best of our knowledge, the only study that estimated cost of ALS using MiToS was Moore et al. [Citation23]. The authors of the study failed to consider the above factors; instead, the study reported only direct medical costs which we believe underestimate the true socio-economic burden of ALS and for this and other reasons, such as generalizability of the cost data reported, we focused on reporting QALY instead of cost/QALY in the current study. Schönfelder et al. [Citation49] conducted a cost of illness study in which the authors included both direct and indirect (societal) costs which we believe considered many of the societal costs including productivity loss. It was reported that the direct nonmedical cost and indirect cost in ALS account for 49% and 15% of the total cost, respectively. Thus, ignoring this considerable economic cost in cost-effectiveness analyses may lead to misleading results and conclusions as it underestimates the economic burden of ALS. In addition, results from the study were stratified and reported using King’s staging, not MiToS. Hence, data from Schönfelder et al. were not included in our economic evaluation. We believe that the results of our study are useful to show the feasibility of MiToS for conducting economic evaluation in ALS while highlighting the lack of generalizable and reliable cost data to conduct a full economic evaluation based on MiToS staging.
As we discussed in the introduction, the ALFRS-R has several weaknesses. Thus, MiToS may be vulnerable to some of the same weaknesses as it is directly calculated from ALFRS-R. Despite these limitations, ALSFRS-R is currently used as the primary endpoint in 82% of the pivotal trials in ALS [Citation45]. The attractiveness of ALSFRS-R is that it can be easily mapped to MiToS [Citation12]. Further, the benefit of MiToS staging is that it is better suited to differentiate later stages of disease than the ALSFRS-R scores from which it is derived [Citation38]. However, MiToS may be less sensitive in early stages of the disease [Citation38], and this may impact the accuracy of QALY estimates in the current study. Furthermore, health utility inputs from Moore et al. [Citation23] for stage 3 and 4 (advanced stages) were low compared to earlier stages (stages 1,2 and 3) as a result we found small incremental QALYs in those advanced stages. Most of the QALY gains were seen in stages 0, 1, and 2. Consistent with ALS disease progression, later stages of MiToS are associated with lower health utilities compared with earlier stages [Citation12]. However, a study by Moore et al. [Citation23] used a relatively small sample for later (advanced) stages with <5% of the total sample contributing to calculate health utilities at stage 3 (3.03%) and 4 (0.84%). A small sample at stage 3 and 4 May make utility estimates in those stages less robust and may have impacted unfavorably on QALY calculations in this study. Unfortunately, Moore et al. [Citation23] is the only published study that reports health utilities for MiToS, hence we used these data in our study. Future real-world studies are needed to estimate the health utilities by MiToS with larger sample sizes to conduct robust QALY estimation.
The current base case results assumed constant treatment effect across all stages (from each alive stage to alive stages and to death) and one-way sensitivity results showed incremental QALYs were sensitive to changes to treatment effects. However, there is a possibility that there may be non-uniform treatment effects across baseline stages as observed in study by Fang et al. [Citation50], which evaluated whether there was variability of riluzole treatment benefit across stages based on King’s staging. The authors found that riluzole improved survival in patients with ALS in stage 4 but not in stages 2 and 3. Authors did not assess the benefit of riluzole for stage 1 because the lowest assigned clinical stage in the study was stage 2. Unfortunately, to the best of our knowledge, there are no published studies that report treatment effect of survival or slowing in disease progression for MiToS staging. Partially, this could be due to strict trials inclusion and exclusion criteria that leaves fewer patients assigned to either early or later stages which makes it difficult to accurately estimate the treatment benefit at each stage of ALS. Thus, in the absence of such data for MiToS, assuming non-uniform treatment effects (assigning specific treatment effects for each stage 0–4) in an economic evaluation will be challenging and unjustifiable. However, future studies based on real-world data e.g. from disease registries may be valuable to generate those data needed by recruiting larger number of patients as real-world studies usually have less stringent inclusion and exclusion criteria compared with clinical trials. Non-uniform treatment benefits are also possible depending on patients’ disease phenotype such as Bulbar or respiratory function with noninvasive ventilator (NIV) or level of disease severity as indicated by disease stages (early versus advanced stages).
Health utilities for the analysis were based on a sample from the United Kingdom and may not be generalizable to other settings. In addition, cost was not included in this analysis owing to lack of data; hence, cost per QALY could not be calculated. A hypothetical new treatment with an assumed 20–35% RRR was used to calculate both QALYs and LYs, but treatment effects for approved treatments may be different. Future studies should confirm our findings using actual treatment effects and costs to calculate cost per QALY.
5. Conclusions
This study demonstrates that MiToS can be applied as a measurement of disease progression when conducting economic modeling for existing or new therapies in ALS. Additionally, the study confirms the feasibility of using a MiToS-based staging for developing a Markov model. Based on the model developed, treatments associated with a 20–35% RRR of progression using MiToS can lead to incremental gains in QALYs and LYs that are meaningful in ALS.
Article highlights
Our aim was to assess the feasibility of using the Milano-Torino staging (MiToS) system for conducting economic evaluation measuring health outcomes in amyotrophic lateral sclerosis (ALS).
We developed a Markov model using the MiToS system and evaluated it with a hypothetical treatment versus standard of care using health utilities and transition probabilities from the literature.
We found that a 10-year time horizon entirely captured treatment benefits.
For hypothetical treatments with RRR of 20–35%, estimated incremental quality-adjusted life-years and life-years gains were meaningful within the context of ALS.
We therefore conclude that a MiToS-based staging can be used for conducting economic analyses in ALS.
Declaration of interest
P Gebrehiwet is an employee of and owns stock in Cytokinetics, Incorporated. S Aggarwal and O Topaloglu are employees at Novel Health Strategies and were financially compensated for their work by Cytokinetics, Incorporated. A Chiò serves on the advisory board for Amylyx, Biogen, Cytokinetics, Incorporated, Denali Pharma, and Mitsubishi Tanabe.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
P Gebrehiwet contributed to the study design and concept and originally implemented the model proposal in excel and drafted the manuscript. A Chiò gave expert opinion support. All authors contributed to the critical review of the manuscript and approval of the final version.
Ethics approval
This analysis was based on published data. As this was not an interventional study, approval from an ethics committee or institutional review board was not required.
Reviewer disclosures
A reviewer on this manuscript has disclosed being an author of a manuscript on a similar topic that was submitted to this journal. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download PDF (182.8 KB)Acknowledgments
Editorial support for this manuscript was provided by Geraldine Thompson, PhD, and Andrea Schauenburg, PhD, on behalf of Engage Scientific Solutions, Horsham, UK, and was funded by Cytokinetics, Incorporated.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737167.2024.2306819
Additional information
Funding
References
- Brown RH, Al-Chalabi A, Longo DL. Amyotrophic lateral sclerosis. N Engl J Med. 2017 Jul 13;377(2):162–172. doi: 10.1056/NEJMra1603471
- Chio A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009 Oct;10(5–6):310–323.
- Lechtzin N, Cudkowicz ME, de Carvalho M, et al. Respiratory measures in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2018 Aug;19(5–6):321–330.
- Chio A, Mazzini L, Mora G. Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology. 2020 May 1;167:107986. doi: 10.1016/j.neuropharm.2020.107986
- Jiang J, Wang Y, Deng M. New developments and opportunities in drugs being trialed for amyotrophic lateral sclerosis from 2020 to 2022. Front Pharmacol. 2022;13:1054006. doi: 10.3389/fphar.2022.1054006
- Miller TM, Cudkowicz ME, Genge A, et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022 Sep 22;387(12):1099–1110. doi: 10.1056/NEJMoa2204705
- Achtert K, Kerkemeyer L. The economic burden of amyotrophic lateral sclerosis: a systematic review. Eur J Health Econ. 2021 Nov;22(8):1151–1166. doi: 10.1007/s10198-021-01328-7
- Xu L, Liu T, Liu L, et al. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol. 2020 Apr;267(4):944–953.
- Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J Neurol Sci. 1999 Oct 31;169(1–2):13–21. doi: 10.1016/S0022-510X(99)00210-5
- Tramacere I, Dalla Bella E, Chio A, et al. The MITOS system predicts long-term survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015 Nov;86(11):1180–1185.
- Goutman SA, Hardiman O, Al-Chalabi A, et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022 May;21(5):480–493.
- Chio A, Hammond ER, Mora G, et al. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015 Jan;86(1):38–44.
- Roche JC, Rojas-Garcia R, Scott KM, et al. A proposed staging system for amyotrophic lateral sclerosis. Brain. 2012 Mar;135(Pt 3):847–852.
- Thakore NJ, Lapin BR, Kinzy TG, et al. Deconstructing progression of amyotrophic lateral sclerosis in stages: a Markov modeling approach. Amyotroph Lateral Scler Frontotemporal Degener. 2018 Nov;19(7–8):483–494.
- Balendra R, Jones A, Jivraj N, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS functional rating scale. Amyotroph Lateral Scler Frontotemporal Degener. 2014 Jun;15(3–4):279–284.
- Al-Chalabi A, Chio A, Merrill C, et al. Clinical staging in amyotrophic lateral sclerosis: analysis of edaravone study 19. J Neurol Neurosurg Psychiatry. 2021 Feb;92(2):165–171.
- Gebrehiwet P, Meng L, Rudnicki SA, et al. MiToS and King’s staging as clinical outcome measures in ALS: a retrospective analysis of the FORTITUDE-ALS trial. Amyotroph Lateral Scler Frontotemporal Degener. 2023 May;24(3–4):304–310.
- Nikitin D, Makam AN, Suh K, et al. The effectiveness and value of AMX0035 and oral edaravone for amyotrophic lateral sclerosis: a summary from the institute for clinical and economic review’s midwest comparative effectiveness public advisory council. J Manag Care Spec Pharm. 2023 Feb;29(2):216–221.
- Thakore NJ, Pioro EP, Udeh BL, et al. A cost-effectiveness framework for amyotrophic lateral sclerosis, applied to riluzole. Value Health. 2020 Dec;23(12):1543–1551.
- Canadian Agency for Drugs and Technologies in Health Common Drug Review. Pharmacoeconomic review report edaravone (RADICAVA) (MItsubishi Tanabe Pharma Corporation). Indication: for the treatment of amyotrophic lateral sclerosis (ALS). Apr 2019 [cited 2023 Jun 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542526/pdf/Bookshelf_NBK542526.pdf
- Naimark DM, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making. 2008 Sep;28(5):706–712. doi: 10.1177/0272989X08315241
- Kim DD, Wilkinson CL, Pope EF, et al. The influence of time horizon on results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res. 2017 Dec;17(6):615–623.
- Moore A, Young CA, Hughes DA. Health utilities and costs for motor neurone disease. Value Health. 2019 Nov;22(11):1257–1265. doi: 10.1016/j.jval.2019.05.011
- Atassi N, Berry J, Shui A, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014 Nov 4;83(19):1719–25. doi: 10.1212/WNL.0000000000000951
- Castrillo-Viguera C, Grasso DL, Simpson E, et al. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1–2):178–180. doi: 10.3109/17482960903093710
- Tzeplaeff L, Wilfling S, Requardt MV, et al. Current state and future directions in the therapy of ALS. Cells. 2023 May 31;12(11):1523. doi: 10.3390/cells12111523
- Franco C, Little RJA, Louis TA, et al. Comparative study of confidence intervals for proportions in complex sample surveys. J Surv Stat Methodol. 2019 Sep;7(3):334–364.
- Hazra A. Using the confidence interval confidently. J Thorac Dis. 2017 Oct;9(10):4125–4130. doi: 10.21037/jtd.2017.09.14
- Attema AE, Brouwer WBF, Claxton K. Discounting in Economic Evaluations. PharmacoEconomics. 2018 Jul;36(7):745–758. doi: 10.1007/s40273-018-0672-z
- Gebrehiwet P, Eguale T, Segal A, et al. PDB27 the diabetes prevention program (dpp) quality of well being (qwb) utility measures and missing data considerations: available case analysis (aca) vs. manski lower and upper bounds (lmb and umb) results for utilities and quality adjusted life years (qalys). Value Health. 2020;23:S112–113. doi: 10.1016/j.jval.2020.04.215
- Gidwani R, Russell LB. Estimating transition probabilities from published evidence: a tutorial for decision modelers. PharmacoEconomics. 2020 Nov;38(11):1153–1164. doi: 10.1007/s40273-020-00937-z
- Drummond MFS, Sculpher M, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. Oxford, UK: Oxford University Press; 2015.
- Vemer P, Corro Ramos I, van Voorn GA, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. PharmacoEconomics. 2016 Apr;34(4):349–61.
- Kjaeldgaard AL, Pilely K, Olsen KS, et al. Prediction of survival in amyotrophic lateral sclerosis: a nationwide, Danish cohort study. BMC neurol. 2021 Apr 17;21(1):164. doi: 10.1186/s12883-021-02187-8
- Louwerse ES, Visser CE, Bossuyt PM, et al. Amyotrophic lateral sclerosis: mortality risk during the course of the disease and prognostic factors. J Neurol Sci. 1997 Oct;152(Suppl 1):S10–7.
- Punjani R, Larson TC, Wagner L, et al. Survival and epidemiology of amyotrophic lateral sclerosis (ALS) cases in the Chicago and Detroit metropolitan cohort: incident cases 2009-2011 and survival through 2018. Amyotroph Lateral Scler Frontotemporal Degener. 2023 May;24(3–4):203–211.
- Hartmaier SL, Rhodes T, Cook SF, et al. Qualitative measures that assess functional disability and quality of life in ALS. Health Qual Life Outcomes. 2022 Jan 21;20(1):12. doi: 10.1186/s12955-022-01919-9
- Fang T, Al Khleifat A, Stahl DR, et al. Comparison of the King’s and MiToS staging systems for ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2017 May;18(3–4):227–232.
- Genge A, Chio A. The future of ALS diagnosis and staging: where do we go from here? Amyotroph Lateral Scler Frontotemporal Degener. 2023 May;24(3–4):165–174. doi: 10.1080/21678421.2022.2150555
- Polak TB, Cucchi DGJ, Darrow JJ, et al. Incremental benefits of novel pharmaceuticals in the UK: a cross-sectional analysis of NICE technology appraisals from 2010 to 2020. BMJ Open. 2022 Apr 8;12(4):e058279. doi: 10.1136/bmjopen-2021-058279
- Gebrehiwet P, Meng L, Rudnicki SA, et al. Health utilities and quality-adjusted life years for patients with amyotrophic lateral sclerosis receiving reldesemtiv or placebo in FORTITUDE-ALS. J Med Econ. 2023 Jan;26(1):488–493.
- Canadian Agency for Drugs and Technologies in Health Common Drug Review. CADTH reimbursement review report sodium phenylbutyrate-ursodoxicoltaurine (Albrioza) (Amylyx Canada). Indication: for the treatment of amyotrophic lateral sclerosis (ALS). Oct 2022. [cited 2023 Aug 30]. Available from https://www.cadth.ca/sites/default/files/DRR/2022/SR0711-Albrioza.pdf
- Ginsberg G, Lowe S. Cost effectiveness of treatments for amyotrophic lateral sclerosis: a review of the literature. PharmacoEconomics. 2002;20(6):367–387. doi: 10.2165/00019053-200220060-00002
- Garrison LP, Jackson T, Paul D, et al. Value-based pricing for emerging gene therapies: the economic case for a higher cost-effectiveness threshold. J Manag Care Spec Pharm. 2019 Jul;25(7):793–799.
- van Eijk RPA, de Jongh AD, Nikolakopoulos S, et al. An old friend who has overstayed their welcome: the ALSFRS-R total score as primary endpoint for ALS clinical trials. Amyotroph Lateral Scler Frontotemporal Degener. 2021 May;22(3–4):300–307.
- Dalla Bella E, Bersano E, Antonini G, et al. The unfolded protein response in amyotrophic later sclerosis: results of a phase 2 trial. Brain. 2021 Oct 22;144(9):2635–2647. doi: 10.1093/brain/awab167
- ClinicalTrials.gov. Phase III trial of AMX0035 for amyotrophic lateral sclerosis treatment (Phoenix), 2023 Dec 19. Available from: https://clinicaltrials.gov/study/NCT05021536
- Shefner JM, Al-Chalabi A, Andrews JA, et al. COURAGE-ALS: a randomized, double-blind phase 3 study designed to improve participant experience and increase the probability of success. Amyotroph Lateral Scler Frontotemporal Degener. 2023 Aug;24(5–6):523–534.
- Schonfelder E, Osmanovic A, Muschen LH, et al. Costs of illness in amyotrophic lateral sclerosis (ALS): a cross-sectional survey in Germany. Orphanet J Rare Dis. 2020 Jun 12;15(1):149. doi: 10.1186/s13023-020-01413-9
- Fang T, Al Khleifat A, Meurgey JH, et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 2018 May;17(5):416–422.