ABSTRACT
Introduction
Sensor-based digital health technology (DHT) has emerged as a promising means to assess patient functioning within and outside clinical trials. Sensor-based functional outcomes (SBFOs) provide valuable insights that complement other measures of how a patient feels or functions to enhance understanding of the patient experience to inform medical product development.
Areas covered
This perspective paper provides recommendations for defining SBFOs, discusses the core evidence required to support SBFOs to inform decision-making, and considers future directions for the field.
Expert commentary
The clinical outcome assessment (COA) development process provides an important starting point for developing patient-centered SBFOs; however, given the infancy of the field, SBFO development may benefit from a hybrid approach to evidence generation by merging exploratory data analysis with patient engagement in measure development. Effective SBFO development requires combining unique expertise in patient engagement, measurement and regulatory science, and digital health and analytics. Challenges specific to SBFO development include identifying concepts of interest, ensuring measurement of meaningful aspects of health, and identifying thresholds for meaningful change. SBFOs are complementary to other COAs and, as part of an integrated evidence strategy, offer great promise in fostering a holistic understanding of patient experience and treatment benefits, particularly in real-world settings.
1. Introduction
Inclusion of the patient voice has become an increasingly vital component of medical product development [Citation1,Citation2]. Patients provide uniquely valuable insights into their lived experiences with a disease or treatment. Gathering their perspectives on symptoms, impacts, and treatment outcomes ensures that medical product development is more aligned with the needs of patients and that the resulting information about treatment risks and benefits is tailored to better inform treatment decision-making in clinical practice [Citation2,Citation3].
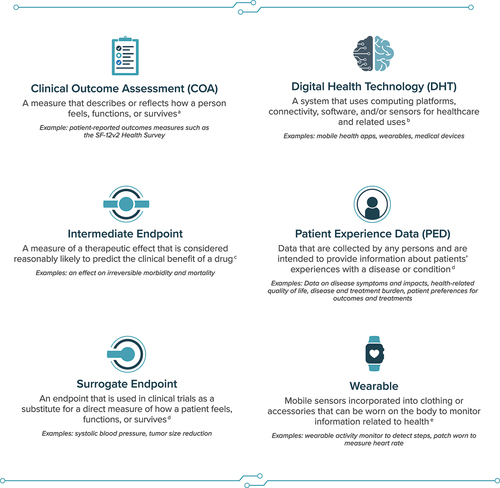
Clinical research and development efforts to measure outcomes aligned with what matters most to patients must prioritize patient engagement as well as the collection and integration of patient experience data (PED). Generation of these data can include the use of clinical outcome assessments (COAs), which are measures that ‘describe or reflect how a person feels, functions, or survives’ () [Citation4]. There are currently 4 types of COAs: patient-reported outcome (PRO) measures, observer-reported outcome measures, clinician-reported outcome measures, and performance outcome (PerfO) measures [Citation5]. These COAs may be collected through electronic COA systems or platforms (e.g. electronic PRO measures) using digital health technology (DHT), broadly defined as ‘a system that uses computing platforms, connectivity, software, and/or sensors for healthcare and related uses’ [Citation6]. In addition to the use of DHTs to collect COA data electronically, sensor-based DHTs have emerged as a promising means to gather high-quality clinical data efficiently from patients in their daily lives via sensors.
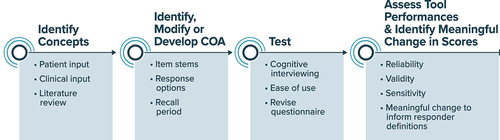
Figure 1. Definitions and Terminology Related to SBFOs.
Sensor-based DHTs may be external, ingestible, or implantable and can exist on, outside, or within a patient [Citation7]. Notably, sensor-based DHTs enable use in real-world settings and allow for frequent or continuous data collection [Citation8]. Therefore, these tools are of particular interest for gathering data on a variety of clinical measures outside of a clinical setting. Recently, sensor-based functional outcomes (SBFOs) have emerged as a means to obtain data directly from patients on key non–task-based functional outcomes both within and outside the clinic, providing a valuable opportunity to reflect a patient’s lived experience. This perspective paper outlines recommendations for defining SBFOs, describes how SBFOs enhance understanding of the patient experience, discusses the importance of a patient-centric focus and an integrated approach to SBFO development, and considers future directions for the field.
2. Terminology and definition of an SBFO
While the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) provide a broad definition of DHTs [Citation6,Citation9], a lack of consensus remains on the appropriate term to describe assessments of functional outcomes leveraging measures derived from sensor-based DHTs. Establishing a common terminology would promote innovation within this space by ensuring effective communication and collaboration among stakeholders. In recent years, several different terms have been used to describe such outcome assessments [Citation6,Citation8,Citation10–12]. Additionally, recent review articles have highlighted the need to standardize terminology to encourage more effective communication around these technologies [Citation13–15]. Notably, the European Federation of Pharmaceutical Industries and Associations raised a call to action among stakeholders, proposing the establishment of a common lexicon to support standardization and increase regulatory acceptance in this field [Citation14].
The term SBFO is proposed here as having the advantages of including the term sensor, which acknowledges the source or type of DHT data, and focusing on sensor-measurable aspects of clinical benefit (i.e. functioning) as opposed to feeling or survival, while excluding the term device, which has a specific meaning within regulatory context unrelated to sensor technologies for remote patient monitoring [Citation16]. SBFOs can be defined as non–task-based functional outcomes collected and derived using mobile sensor technology directly from patients in both clinical and real-world settings ().
Figure 2. Continuum of evidence measuring direct or indirect clinical benefit.

It is important to distinguish an SBFO (i.e. the outcome) from the type of sensor-based DHT being used to assess the SBFO (i.e. the technology). Sensor-based DHTs provide a means to collect data in a free-living setting. While some of these technologies may be used by and benefit both patients and consumers, SBFOs specifically use sensors to measure patient outcomes (i.e. non–task-based functional outcomes) that are linked to clinical benefits meaningful from the patient perspective. For instance, wearables are a type of sensor-based DHT that offer a means to collect data on gait parameters (e.g. walking bouts at a defined cadence); using these data, an SBFO evaluates a specific functional outcome that matters to patients (e.g. functional impact of disease on sustained walking ability). Because SBFOs are assessments that describe how a person functions, it is feasible that they may be considered another type of COA.
In addition, sensor-based DHTs can also be used to administer and measure PerfOs (e.g. performing a range of motion exercise directed by an app) [Citation4], measure surrogate or intermediate endpoints in clinical trials (e.g. sensor-based outcomes predictive of mortality or hospitalization, such as real-world walking speed [Citation12,Citation17,Citation18]), or measure an indicator of normal biological or pathogenic processes or biological responses to an exposure or intervention (e.g. sensor-based biomarkers [blood pressure and other hemodynamics; blood glucose data collected via a continuous glucose monitor]) [Citation12,Citation19]. Importantly, these various uses fall along distinct points on a continuum providing evidence of direct to indirect clinical benefit – for instance, biomarkers provide indirect evidence of clinical benefit, while certain SBFOs may relate directly or more indirectly to concepts of interest that are important to patients and therefore must always be linked to clinical benefit with appropriate evidence (). Aligning on these definitions and distinctions is essential for collaboration in SBFO development efforts.
3. SBFOs to enhance understanding of patient experience
SBFOs may be erroneously perceived as introducing greater objectivity by replacing subjective human observations or responses. However, thoughtfully developed, patient-centered SBFOs do not replace the patient’s voice but rather enhance it by offering a different approach to PED collection, including the potential to collect information on novel health concepts, or a new way to measure existing health concepts more accurately, conveniently, or frequently. Furthermore, data generated by SBFOs complement the evidence generated from COA measures reported directly from patients, clinicians, or observers. Key examples are SBFOs that passively measure a patient’s physical activity during routine daily living via wearables (mobile sensors incorporated into clothing or accessories worn on the body [Citation7]), providing additional context to patient-reported measures of physical activity and activities of daily living. Other examples include external movement monitors and under-mattress pressure mats, which may be used to complement COAs evaluating sleep parameters such as total sleep time, sleep disturbance, and sleep quality.
Overall, SBFOs provide key insights into patients’ functioning in daily life and may foster a holistic understanding of patients’ experiences with their condition or treatment in both clinical and real-world settings. The use of these complementary data sources as part of an integrated evidence strategy has significant potential to enhance patient centricity in medical product development.
4. Importance of the patient perspective in SBFO development
As the field of SBFOs evolves and new technologies emerge, it is critical to ensure that the integration of the patient perspective remains at the forefront of SBFO development and that evidentiary requirements for robust COA development are considered alongside requirements for development of DHT. The good measurement principles for development of fit-for-purpose COAs detailed in the FDA’s Patient-Focused Drug Development (PFDD) guidance series [Citation20–23] are applicable to the development of any patient-centered measure, inclusive of SBFOs and regardless of the final reporter or means of data collection. Specifically, when developing an SBFO, evidence must be gathered to demonstrate that the SBFO captures or reflects aspects of health that are relevant and meaningful to patients [Citation20–23]. Additionally, the FDA’s guidance ‘Digital Health Technologies for Remote Data Acquisition in Clinical Investigations’ [Citation6], as well as the EMA’s qualification of digital technology-based methodologies guidance [Citation9], highlight the advancement of DHT for the remote capture of PED. In addition to FDA and EMA guidance, several initiatives are underway to generate guidelines and frameworks for the development of sensor-based DHTs [Citation24–26].
However, aligning SBFO development with PFDD guidance aimed at COA development () presents multiple challenges and considerations. Teams beginning to develop a new SBFO must consider how SBFOs fit into the COA development process, as well as how and when to engage patients to best inform SBFO development. Determining how to best incorporate key methodology (e.g. concept elicitation, cognitive debriefing) associated with COA development into SBFO development requires detailed considerations specific to sensor-based technologies. Like any COA, an SBFO should be built upon a well-defined and patient-centered concept of interest and context of use. As such, ensuring opportunities for patient engagement and early input from diverse and representative patients and caregivers is important for successfully developing an SBFO that measures what matters to patients.
Ideally, patient engagement research should be conducted to identify concepts that matter most to patients in the context of their lived experience; this knowledge may be used to inform all patient-centered measures and endpoints holistically across measure types. Following alignment on patient-centered outcomes relevant for a particular context of use, thoughtful determination of measures and endpoints is required to select and/or develop measurement tools that appropriately assess the concept of interest and yield interpretable scores in the intended context of use.
5. An integrated evidence approach for SBFO development
Developing an SBFO that contributes important evidence to enable a holistic understanding of the patient experience requires an integrated endpoint and evidence strategy that includes multiple complementary measures focused on patient-relevant outcomes. A multidisciplinary approach that brings together the skills of COA measurement, digital health, patient engagement, technology development, and regulatory science is critical for enabling the development of SBFOs that capture or reflect patient-centered outcomes, complement information provided by existing COAs, and meet regulatory requirements. Achieving alignment on the value of an SBFO in the context of a development program is essential before initiating development of the SBFO or incorporating it into an overall PED strategy and evidence generation plan.
SBFOs should be considered when they enable measurement of a new outcome, optimize the measurement of an existing outcome, improve the patient experience, or improve clinical trial agility or efficiency. When using SBFOs in clinical trial research to inform regulatory decision-making, the same evidentiary standards used in COA development to demonstrate relevance, reliability, validity, and interpretation must be followed to ensure the concept measured can be linked back to a patient-relevant outcome. There may be instances in which SBFOs are a necessary mode of assessing a concept of interest (e.g. an SBFO measuring continuous nocturnal scratching behavior in a real-world setting due to recall barriers during sleep [Citation27,Citation28]) or may singularly measure a concept of interest with robust evidence. However, in most cases, an SBFO will not replace the use of other COAs but instead will generate additional, complementary data to contextualize information provided by COAs and further support the evaluation of key concepts of interest. For example, an SBFO assessing sleep generates data to complement patient-reported measures of sleep quality and outcomes, such as daytime tiredness; an SBFO assessing scratching episodes generates data to complement patient-reported measures of itch severity. The use of SBFOs in combination with other COAs also enables clinical validation and the assessment of trends or correlations in similar outcomes across various measures.
By using an integrated evidence approach, SBFOs provide valuable information that further enhances the patient voice in medical product development and informs both regulatory and real-world treatment decision-making. However, this is often tempered by the reality of the current drug development process within pharmaceutical companies, which is generally aligned by therapeutic areas and products. Without input from digital health experts, an SBFO may lack fundamental evidentiary support of verification, analytic validation, and usability; without input from experts in COA development and patient engagement research, SBFOs may lack clinical validity and meaningfulness to patients. Furthermore, siloed processes that lack an integrated approach result in insufficient sharing of key learnings and experiences across programs and therapeutic areas to appropriately inform SBFO development, potentially leading to unnecessary duplication of efforts or a lack of implementation of regulatory feedback across programs. This risk can be mitigated through multidisciplinary collaboration and engagement with stakeholders to share insights, ensuring patient-centered SBFOs provide value and complement information provided by other COAs.
Once an integrated strategy has been developed to meet evidentiary requirements for an SBFO, appropriate regulatory agencies should be engaged to further inform strategy and regulatory decision-making and promote long-term success. For many programs, regulatory input on a measurement strategy, inclusive of SBFOs, should be sought as early as possible and continued throughout drug development (e.g. Type C meetings [FDA], Innovation Task Force Meetings or Scientific Advice Working Party [EMA]). A lack of early engagement increases the risk of not obtaining regulatory alignment on the proposed SBFO for a specific context of use, delaying clinical program timelines, or not having enough evidence to support use as a primary or secondary endpoint.
Consortia efforts, such as the C-Path Digital Drug Development Tool (3DT) Consortium endeavoring to generate evidentiary support to develop an SBFO to assess early functional impact of Parkinson’s disease progression [Citation29], exemplify an integrated evidence strategy approach, supporting early regulatory engagement and data sharing in such a way that both industry and ultimately patients may benefit. The 3DT Consortium received key early regulatory feedback to conduct qualitative research among patients with Parkinson’s disease to support content validity of a potential measure. Qualitative research supported the relevance of the concepts of interest as measured to a patient’s daily lived experience with Parkinson’s disease (‘[The task relates to my] dexterity, fine motor skills … It’s very similar to typing’) [Citation30]. The Consortium’s early engagement with the FDA highlights the importance of patient-centered SBFO development as well as cross-industry consortia. In parallel with regulatory engagement, collaboration with pharmacoeconomic and health outcomes research experts may allow for better adoption of SBFOs for use in real-world decision-making. This may be accomplished through clinician and payer initiatives that focus on assessing a medical product’s value, risks, and benefits in real-world settings.
6. Conclusions
The unique ability of SBFOs to continuously capture key insights meaningful to patients and in settings outside the clinic may further enhance the patient’s voice in medical product development, our overall understanding of patients’ experiences with a disease or condition, and the effects of new interventions. When focused on outcomes that patients consider relevant and important, SBFOs can provide a critical tool to support the development of new therapies by providing high frequency, comprehensive data that complement and contextualize COAs. However, further refinement of existing frameworks for COA development is needed to reflect SBFO-specific considerations and attain the best approach to developing patient-centered SBFOs that are part of an integrated evidence plan and regulatory engagement strategy. Approaching the measurement of patient-centered outcomes with COAs and SBFOs without an integrated plan for evidence generation, implementation, and regulatory engagement risks the collection of data that are not clearly linked to outcomes that matter to patients and inhibits meaningful interpretation. With careful consideration of the COA development process and current evidentiary requirements, SBFOs may be incorporated in clinical development programs to support the evaluation of treatment benefit and regulatory decision-making and provide a more holistic understanding of the patient experience.
7. Expert opinion
By using SBFOs as part of an integrated evidence strategy to address outcomes that matter to patients (i.e. outcomes that patients find relevant and meaningful), we can gather more evidence from patients in real-world settings. While an SBFO can provide direct evidence of a meaningful aspect of health, it will more typically measure something that is reflective of, or a selected parameter of, a meaningful aspect of health. However, identifying concepts beneficial to measure with an SBFO may be challenging, as patients may not describe the concepts associated with their disease or condition in terms of a concept measured by an SBFO. For example, a patient may think about physical activity in terms of accomplishing tasks or relating to others (e.g. ‘Playing with grandchildren’ or ‘Doing more for myself around the house’) rather than as individual activity components, such as step counts or sit-stand transitions, that would be captured by activity monitor devices. In these situations, it is important for the research team to fully understand exactly what the sensor is measuring, what the outcome of interest is, and what evidence would be needed to show that the SBFO is indeed reflective of clinical benefit. Mixed methods research inclusive of both patient-derived and statistically derived measure identification can be useful in this context. After identifying the concept of interest, it is important to consider what types of DHT data will provide the most relevant information for assessing patient outcomes and to assess the feasibility and fit of the chosen DHT for recording the intended outcome measurements. However, navigating the complexities around ensuring that a measure appropriately assesses the concept of interest, yields interpretable scores, and measures meaningful aspects of health can be challenging.
SBFOs can complement other types of outcome measures in a holistic approach to data generation and not as a substitute for understanding and measuring what is directly meaningful to patients. For example, measuring step counts should not be a substitute for asking patients how difficult it is to walk; measuring scratching behavior should not be a substitute for asking patients about itch severity. Within an integrated evidence framework, SBFOs either can serve as their own standalone endpoint (e.g. an increase in physical activity volume) or be used to help interpret or contextualize another endpoint. For example, a pain study might include an endpoint that evaluates pain severity and incorporates SBFO-measured activity into the endpoint – if a patient’s low back pain level remains the same over time but the patient has become more active, this could indicate improvement. Conversely, a patient who chooses to significantly limit mobility to avoid low back pain may report reduced pain scores only because they are not moving, which could be more indicative of adaptation to low back pain than a true improvement in their condition.
As the field evolves, SBFOs may also be developed to interact with other types of COAs. For example, when a sensor detects movement (e.g. walking up the stairs) or activity level, it could trigger a PRO measure to assess shortness of breath or fatigue. This may be helpful in therapeutic areas marked by symptom exacerbations during activity (e.g. chronic obstructive pulmonary disease or heart failure) or muscle weakness/exercise intolerance (e.g. cancer, chronic fatigue syndrome). This type of interactive approach would complement and possibly improve upon PRO measures that are asked only at the end of each day or week.
Increasing interest in sensor-based DHTs has led to the development of multiple recommendation articles, guidelines, and frameworks describing their development, including those by the C-Path Institute’s Consortia, TransCelerate, Clinical Trials Transformation Initiative, Drug Information Association, and the Digital Medicine Society (DiME) [Citation7,Citation16,Citation24–26,Citation31]. For instance, the C-Path Institute PRO Consortium developed a conceptual model to support the foundation of a sensor-based assessment of physical activity for use in clinical trials assessing congestive heart failure [Citation32]. Additionally, DiME outlined a multi-stakeholder framework to address evidentiary requirements necessary for developing digital clinical measures in its playbook and defined a sequential framework of core principles for selecting and developing digital sensor–derived measurements that are meaningful for patients [Citation16,Citation33]. Furthermore, a multidisciplinary collaboration including the Clinical Trials Transformation Initiative, DiME, and industry authors defined evidentiary requirements to support the use of mobile sensor technology for COAs [Citation7] that parallel evidentiary requirements for COA development.
Several key elements of these existing frameworks apply to SBFO development and serve as a valuable starting point for developing an evidentiary framework for SBFOs. Importantly, all the existing frameworks begin with identifying key concepts considered meaningful from the patient perspective to inform endpoint definitions and selection of appropriate tools for assessment. However, to better support the alignment of SBFOs with COA development, we recommend further refinement of these existing frameworks.
Incorporating COA development methodology into the SBFO development pathway presents certain challenges and requires SBFO-specific considerations. These include accounting for potential technology barriers; providing evidence to support that an SBFO is measuring a concept that is meaningful in patients’ daily lives or is providing useful complementary information; including opportunities for statistically derived measure identification together with patient-driven approaches to development; and determining the method by which meaningful change is measured. Meaningful within-person change thresholds are essential to enable robust inferences from clinical trial data. However, challenges in identifying meaningful change thresholds may be more pronounced when using SBFO measures, as sensors may provide more indirect measures of clinical benefit compared with other COAs. There is also a need to bring COA development expertise together with DHT expertise, using both qualitative and quantitative research approaches to determine how to best incorporate SBFOs and other COAs into an integrated evidence strategy. Thus, we propose merging the unique aspects of the existing evidentiary frameworks with SBFO-specific considerations to develop one framework focused on (1) understanding the patient experience; (2) identifying the right way to measure the patient experience (e.g. COAs, SBFOs, or other approaches); and (3) developing measures, if needed, and unique considerations for SBFOs. Such a framework would facilitate the development of patient-centered SBFOs that reflect clinical outcomes that matter to patients.
We believe SBFOs hold tremendous potential that will be unlocked as we learn more about the selection and appropriate implementation of these measures in clinical trials and the analysis and reporting of the data generated in a way that maximizes their value to patients. Potentially hundreds of variables – and thousands of datapoints – can be generated by sensors. Statistically derived measure approaches, including ‘big-data’ analysis techniques such as machine-learning approaches, can yield important information about the variables and configurations of multiple variables that may predict important future outcomes. Outcome measure development derived from big data sets in this way may diverge from COA development, which starts with concept elicitation, but may still yield outcome measures that are informative and related to important aspects of health or survival. Relating statistically derived measures back to a conceptual framework (a holistic representation of the specific meaningful health aspects to support clinical benefit and how they will be measured) is an important step. This may be supported by existing published evidence, such as cognitive interviews with patients/caregivers, expert opinion, or data anchoring the predictive value of the measure in relation to other important clinical outcomes.
The data streams generated by sensors can be used in conjunction with other data sources to holistically assess patient experiences with treatments as part of clinical research and for regulatory decision-making. This real-world evidence allows for evaluating the effectiveness of drugs, providing complementary evidence toward the totality of the evidence for drug approval. As the field evolves, we envision SBFOs being regularly implemented in clinical trials and used to support endpoints that enhance the patient voice in medical product development.
Article highlights
Sensor-based functional outcomes (SBFOs) offer the ability to passively capture physical movement, activity levels, sleep measures, or other parameters in a manner associated with reduced patient burden.
SBFOs may offer a unique approach to collecting data on functional outcomes that cannot be accurately or conveniently collected using other methods.
SBFOs can substantially add to our understanding of treatment effects across a variety of therapeutic areas by providing further context to study endpoints.
The combination of SBFOs and other clinical outcome assessments (COAs), including patient-reported outcome measures, can provide a comprehensive, reliable, and valid evaluation of the patient’s experience.
Further refinement of existing frameworks for COA development is needed to reflect SBFO-specific considerations and attain the best approach to developing patient-centered SBFOs that are part of an integrated evidence plan and regulatory engagement strategy.
Declaration of interest
K.R. Keyloun, J. Abel, J. Garcia, E. J. Papadopoulos, and R. T. Carson are employees of AbbVie and may hold shares and/or stock options in the company. C. Gwaltney has received consulting fees from AbbVie. A. F. Slagle has received consulting fees from AbbVie. B. Byrom is an employee of Signant Health, which receives consulting fees from AbbVie, and holds Signant Health stock options. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Cassondra Saande, and Brian Samsell, of RTI Health Solutions for medical writing assistance. Ashley Slagle, Bill Byrom, Chad Gwaltney served as key expert authors in Regulatory Science, eCOA, and Digital Health Technology for the Expert Opinion Section.
Additional information
Funding
References
- Zvonareva O, Craveț C, Richards DP. Practices of patient engagement in drug development: a systematic scoping review. Res Involv Engagem. 2022 Jun 29;8(1):29. doi: 10.1186/s40900-022-00364-8
- Tegenge MA, Moncur MM, Sokolic R, et al. Advancing the science of patient input throughout the regulatory decision-making process. Learn Health Syst. 2017 Jul;1(3):e10032. doi: 10.1002/lrh2.10032
- Forsythe LP, Carman KL, Szydlowski V, et al. Patient engagement in research: early findings from the patient-centered outcomes research institute. Health Aff. 2019 Mar;38(3):359–367. doi: 10.1377/hlthaff.2018.05067
- US Food and Drug Administration. Clinical outcome assessments (COAs) in medical device decision making [Internet]. 2023 Oct 3 [cited 2023 Nov 17]. Available from: https://www.fda.gov/about-fda/cdrh-patient-science-and-engagement-program/clinical-outcome-assessments-coas-medical-device-decision-making
- US Food and Drug Administration. Patient-focused drug development glossary [Internet]. 2018 Jun 8 [cited 2024 Feb 29]. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/patient-focused-drug-development-glossary
- US Food and Drug Administration. Digital health technologies for remote data acquisition in clinical investigations [Internet]. 2023 Dec [cited 2024 Feb 4]. Available from: https://www.fda.gov/media/155022/download
- Walton MK, Cappelleri JC, Byrom B, et al. Considerations for development of an evidence dossier to support the use of mobile sensor technology for clinical outcome assessments in clinical trials. Contemp Clin Trials. 2020 Apr;91:105962. doi: 10.1016/j.cct.2020.105962
- Izmailova ES, AbuAsal B, Hassan HE, et al. Digital technologies: innovations that transform the face of drug development. Clin Transl Sci. 2023 Aug;16(8):1323–1330. doi: 10.1111/cts.13533
- European Medicines Agency. Qualification of digital technology-based methodologies to support approval of medicinal products. 2020 Jun 1 [cited 2023 Nov 17]. Available from: https://www.ema.europa.eu/en/documents/other/questions-answers-qualification-digital-technology-based-methodologies-support-approval-medicinal_en.pdf
- US Food and Drug Administration. Clinical outcome assessment (COA) qualification program [Internet]. 2023 Oct 27 [cited 2024 Jan 5]. Available from: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/clinical-outcome-assessment-coa-qualification-program
- Critical Path Institute. 14th annual patient-reported outcome consortium workshop [Internet]. 2023 [cited 2024 Jan 5]. Available from: https://c-path.org/14th-annual-patient-reported-outcome-consortium-workshop
- US Food and Drug Administration; National Institutes of Health Biomarker Working Group. BEST (Biomarkers, Endpoints, and other Tools) resource [Internet]. 2021 Nov 29 [cited 2024 Jan 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK326791
- Burrell A, Zrubka Z, Champion A, et al. How useful are digital health terms for outcomes research? An ISPOR special interest group report. Value Health. 2022 Sep;25(9):1469–1479. doi: 10.1016/j.jval.2022.04.1730
- Leyens L, Northcott CA, Maloney L, et al. Why language matters in digital endpoint development: harmonized terminology as a key prerequisite for evidence generation. Digit Biomark. 2024;8(1):1–12. doi: 10.1159/000534954
- Campbell CM, Webster C, Parisi M, et al. An aligned framework of actively collected and passively monitored clinical outcome assessments (COAs) for measure selection. NPJ Digit Med. 2024 Mar;7(1):71. doi: 10.1038/s41746-024-01068-x
- Digital Medicine Society. The playbook [Internet]. 2023 [cited 2023 Nov 22]. Available from: https://playbook.dimesociety.org/playbooks/the-playbook
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011 Jan;305(1):50–58. doi: 10.1001/jama.2010.1923
- US Food and Drug Administration. Accelerated approval [Internet]. 2023 Feb 24 [cited 2024 Feb 28]. Available from: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/accelerated-approval
- Vasudevan S, Saha A, Tarver ME, et al. Digital biomarkers: convergence of digital health technologies and biomarkers. NPJ Digit Med. 2022 Mar;5(1):36. doi: 10.1038/s41746-022-00583-z
- US Food and Drug Administration. Patient-focused drug development: collecting comprehensive and representative input [Internet]. 2020 Jun [cited 2023 Nov 17]. Available from: https://www.fda.gov/media/139088/download
- US Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients [Internet]. 2022 Feb [cited 2023 Nov 17]. Available from: https://www.fda.gov/media/131230/download
- US Food and Drug Administration. Patient-focused drug development: selecting, developing, or modifying fit-for-purpose clinical outcomes assessments [Internet]. 2022 Jun. Available from: https://www.fda.gov/media/159500/download
- US Food and Drug Administration. Patient-focused drug development: incorporating clinical outcome assessments into endpoints for regulatory decision-making [Internet]. 2023 Apr [cited 2023 Nov 30]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-incorporating-clinical-outcome-assessments-endpoints-regulatory
- Byrom B, Watson C, Doll H, et al. Selection of and evidentiary considerations for wearable devices and their measurements for use in regulatory decision making: recommendations from the ePRO Consortium. Value Health. 2018;21(6):631–639. doi: 10.1016/j.jval.2017.09.012
- Clinical Trials Transformation Initiative. Developing novel endpoints generated by digital health technology for use in clinical trials. 2017 [cited 2023 Nov 22]. Available from: https://ctti-clinicaltrials.org/wp-content/uploads/2022/03/CTTI-Digital-Health-Trials-Novel-Endpoint-Acceptance-Recommendations.pdf
- Clinical Trials Transformation Initiative. Considerations for advancing the use of digital technologies for data capture & improved clinical trials. 2018 [cited 2023 Nov 22]. Available from: https://ctti-clinicaltrials.org/wp-content/uploads/2021/06/CTTI_Digital_Health_Technologies_Recs.pdf
- Digital Medicine Society. Driving adoption of nocturnal scratch as a digital endpoint and improving patients’ lives[Internet]. 2023 [cited 2024 Feb 26]. Available from: https://datacc.dimesociety.org/digital-measures-nocturnal-scratch
- Cesnakova L, Meadows K, Avey S, et al. A patient-centred conceptual model of nocturnal scratch and its impact in atopic dermatitis: a mixed-methods study supporting the development of novel digital measurements. Skin Health Dis. 2023;3(5):e262. doi: 10.1002/ski2.262
- Müller M, Cosman J, Adams J, et al. A progress update on the critical path for Parkinson’s consortium’s pre-competitive 3DT initiative [abstract]. 2022 [cited 2024 Feb 28]. Available from: https://www.mdsabstracts.org/abstract/a-progress-update-on-the-critical-path-for-parkinsons-consortiums-pre-competitive-3dt-initiative
- Mammen JR, Speck RM, Stebbins GM, et al. Mapping relevance of digital measures to meaningful symptoms and impacts in early Parkinson’s disease. J Parkinsons Dis. 2023;13(4):589–607. doi: 10.3233/jpd-225122
- TransCelerate Biopharma Inc. Patient technology implementation framework [Internet]. 2023 [cited 2023 Nov 22]. Available from: https://www.transceleratebiopharmainc.com/assets/patient-technology-implementation-framework
- Gwaltney CM, Trudeau J, Amchin W, et al. Endpoint construction from activity monitor data: chronic heart failure [Internet]. 2019 [cited 2023 Nov 22]. Available from: https://c-path.org/wp-content/uploads/2019_session4_endpointconstwearables-chf_final.pdf
- Manta C, Patrick-Lake B, Goldsack JC. Digital measures that matter to patients: a framework to guide the selection and development of digital measures of health. Digit Biomark. 2020 Sep;4:69–77. doi: 10.1159/000509725