ABSTRACT
Introduction: Deep brain stimulation (DBS) has become a standard therapy for the treatment of select cases of medication refractory essential tremor and Parkinson’s disease however the effectiveness and long-term outcomes of DBS in other uncommon and complex tremor syndromes has not been well established. Traditionally, the ventralis intermedius nucleus (VIM) of the thalamus has been considered the main target for medically intractable tremors; however alternative brain regions and improvements in stereotactic techniques and hardware may soon change the horizon for treatment of complex tremors.
Areas covered: In this article, we conducted a PubMed search using different combinations between the terms ‘Uncommon tremors’, ‘Dystonic tremor’, ‘Holmes tremor’ ‘Midbrain tremor’, ‘Rubral tremor’, ‘Cerebellar tremor’, ‘outflow tremor’, ‘Multiple Sclerosis tremor’, ‘Post-traumatic tremor’, ‘Neuropathic tremor’, and ‘Deep Brain Stimulation/DBS’. Additionally, we examined and summarized the current state of evolving interventions for treatment of complex tremor syndromes.
Expert commentary: Recently reported interventions for rare tremors include stimulation of the posterior subthalamic area, globus pallidus internus, ventralis oralis anterior/posterior thalamic subnuclei, and the use of dual lead stimulation in one or more of these targets. Treatment should be individualized and dictated by tremor phenomenology and associated clinical features.
1. Introduction
Tremor is defined as a rhythmic, sinusoidal oscillation of a body part and has been reported as one of the most common movement disorders encountered in clinical practice. The classification and differentiation of atypical, intricate, and uncommon tremors can be challenging, and the differential diagnosis hinges on the acquisition of a detailed examination that focuses attention on tremor topography, frequency, amplitude, and associated features. Furthermore, as most complex tremors can be underpinned by central nervous system pathology, a variable combination of neural elements is usually implicated, and the result can be a wide variety of tremor phenomenologies.
Traditionally, the ventralis intermedius nucleus (VIM) of the thalamus has been considered the target for deep brain stimulation (DBS) for most tremor syndromes. This clinical practice of using VIM has been largely based on the excellent tremor outcomes in patients with refractory essential tremor (ET) and Parkinson’s disease (PD) [Citation1]. Nevertheless, despite the exceptional results observed in some patients, thalamic stimulation might not successfully treat all patients. Additionally, uncommon forms of tremors remain a treatment challenge. Thalamic lesioning surgery and DBS for complex tremor syndromes has yielded mixed and in many cases disappointing results [Citation2,Citation3]. Larger lesions (e.g. thalamotomy) or areas of stimulation have been commonly required to treat both distal and the more difficult problem of proximal tremor [Citation4,Citation5]. Larger lesions or wider zones of electrical stimulation can potentially lead to an increased risk of adverse events [Citation6]. Complex tremors are commonly refractory and can recur over the course of months to years [Citation7–Citation10]. There has also been the concern of a delayed loss of efficacy or tolerance over weeks to months following DBS surgery, as tremor commonly reappeared despite repeated DBS programming sessions along with limited improvement in proximal tremors [Citation11,Citation12]. Recently, there was a study that showed that this phenomenon in ET was related more to disease progression than to tolerance [Citation13].
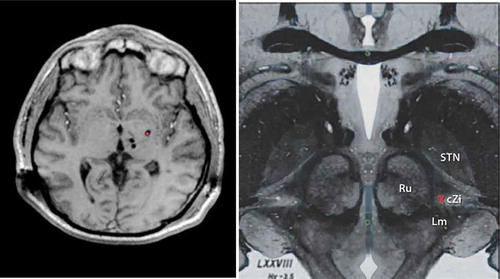
As a result of these many challenges, other nuclei and/or combinations of stereotactic targets have been explored with promising results. Recently explored targets include the prelemniscal radiations and the nearby zona incerta [Citation14] as multiple reports since the 1960s described tremor arrest following small destructive lesions aimed at interrupting the thalamic, red nucleus, zona incerta, or pallidal connections [Citation15]. Limitations in lesioning the posterior subthalamic target have included the induction of a transient or even permanent neglect of the contralateral extremities in patients with advanced PD [Citation16], but advancement of DBS techniques has renewed the interest in this target for treatment of other complex tremor syndromes. Additionally, the globus pallidus internus (GPi) and [Citation17,Citation18] the use of dual DBS leads have been proposed for refractory cases (). The addition of a second electrode to stimulate different regions of thalamus (with connections to different circuitry), such as cerebello-thalamic afferents in the Raprl or pallidal receiving neurons in the ventralis oralis anterior (Voa) nucleus [Citation2,Citation19], has emerged as a potential strategy. This approach might improve tremor control by expanding the volume of tissue affected by stimulation, or by differentially affecting two circuits which both play a role in tremor genesis [Citation20].
Figure 1. LEFT. T1 Weighted Axial Magnetic Resonance image showing multi-target DBS with bilateral thalamic (VIM and VoP) leads along with ipsilateral Gpi DBS (red dot). RIGHT. Upper midbrain anatomical axial image showing the relevant Posterior Subthalamic area DBS anatomy and neurosurgical target (Red X). Red Nucleus (Ru), Subthalamic nucleus (STN), Caudal zona Incerta (cZi), medial lemniscus (Lm). Full color available online
We want to emphasize that Holmes tremor (HT), dystonic tremor (DT), cerebellar outflow tremors, rubral tremor, midbrain tremors, and tremors due to multiple sclerosis (MS) are a complex group of disorders. The literature can be misleading, especially when there is a lack of videotape evidence to confirm the phenomenology. In many cases, there are overlapping features with dystonia, chorea, or even ballismus. Physiology often reveals multiple peaks on the power spectral analyses (e.g. MS tremor) likely due to the presence of multiple tremor generators. Disorders with multiple tremor generators may be more amenable to dual and multiple DBS lead approaches. In this article, we aim to review the current literature to assess the various stereotactic brain targets for several complex tremor disorders. We performed a PubMed search using different combinations between the terms ‘uncommon tremors,’ ‘dystonic tremor,’ ‘Holmes tremor,’ ‘midbrain tremor,’ “rubral tremor,’ ‘cerebellar tremor (CT),’ ‘outflow tremor,’ ‘multiple sclerosis tremor,’ ‘posttraumatic tremor,’ ‘neuropathic tremor (NT),’ and ‘deep brain stimulation/DBS.’ The respective medical subheading in the search strategy was included when available. Relevant case reports, case series, and studies that were published in peer-reviewed journals and available in full text and written in English were included in our review. Additionally, we will also present practical considerations for the treatment of complex tremor syndromes and discuss outcomes and future directions.
1.1. Holmes tremor
HT is characterized by large and irregular amplitude, low-frequency (<3–4 Hz) rest as well as prominent action and postural tremors, with tremor often affecting predominantly the proximal upper extremity(s). HT has been the preferred nomenclature by some experts, as other terms such as rubral or midbrain tremor have been considered to be anatomically misleading, since injury to multiple cortical and subcortical areas can underpin HT [Citation21]. Alternatively, many authors use the other terms because of the wide and variable presentations of these types of tremors.
HT has been described in several diverse conditions, including neurodegenerative etiologies (e.g. MS), trauma, tumors, and infectious or vascular conditions such as cerebral hemorrhage or cerebral vascular malformations. The medical treatment of HT is complex and often unsatisfactory. A variety of medications have been utilized in an attempt to modulate dopaminergic or cerebello-thalamic pathways including the use of antiepileptic drugs, levodopa, anticholinergics, or dopamine agonists. The time course has also been variable; however, if a causative lesion can be identified, the tremor appears from weeks to a few years afterward. Response to treatment has been inconsistent and has been commonly defined by transient benefit, limited follow-up, or conflicting results. Alternatively, in many cases, there is an immediate but unsustained benefit. Although there is limited data regarding the precise prevalence of HT, it accounted for approximately 1.6% of cases in a large series [Citation22]. The exact pathophysiology of HT remains unknown, but lesions affecting the cerebello-thalamo-cortical or dentato-rubro-olivary pathways with superimposed dysfunction in the nigrostriatal pathway may account for the resting component [Citation23–Citation25].
The use of stereotactic thalamic lesions for symptomatic HT treatment has been in general disappointing [Citation6,Citation26], but multiple reports have highlighted the efficacy of thalamic VIM DBS in HT ().
Table 1. Case reports and series of thalamic and subthlamic deep brain stimulation in patients with Holmes tremor.
However, there have been concerns about the recurrence of tremor over time, and the limited effect on proximal or intentional tremors. Other targets besides thalamic VIM have thus been proposed in HT. In most cases of HT, the tremor is distributed in both the distal and the proximal musculature, and a larger stimulation area, involving multiple pathways, may be required for adequate control of HT. Furthermore, it has been reported that patients with HT require higher DBS voltage levels than patients with other types of tremor [Citation34] with reports of improved tremor control following treatment with dual stimulation, mainly in another part of the thalamus perhaps due to multiple tremor generators. Foote et al. [Citation43] proposed to implant unilateral ventrolateral (VL)/ventralis anterior (VA) (VIM plus Voa/Vop) thalamic DBS leads to override a hypothesized abnormality in both the pallido-thalamic and cerebello-thalamic circuits. Dual thalamic stimulation resulted in a significant improvement in tremor scales without rebound at 6-month follow-up. Kobayashi et al. [Citation41] reported the additive effect in tremor management when combining VIM and PSA stimulation. In two patients, thalamic stimulation alone was superior to PSA; however, the PSA alone was better in another two patients and this highlights the complexities in approaching these disorders. The best effect on tremor was, however, achieved with dual stimulation. Romanelli et al. [Citation28] performed unilateral stimulation of both the VIM nucleus and the STN in a single patient with HT. The resting component of the tremor was not improved after VIM DBS though there was a 66% improvement in tremor with a dual approach.
In cases where the thalamus is severely damaged by the primary insult responsible for HT or in cases with associated dystonia or chorea, GPi DBS has been proposed as a potential target. Martinez et al. [Citation42] and Kilbane et al. [Citation18] reported the long-term outcome of 11 HT patients treated with GPi DBS. In both case series, an overall improvement of 64% and 78% in tremor scales was observed, and in a few exceptional cases, there was a complete tremor resolution. Kilbane et al. hypothesized that modulation of the basal ganglia outflow pathways (GPi) might be superior to thalamic DBS alone. This notion was initially supported by two case reports showing a beneficial effect of pallidal lesioning. Although outcome scales and follow-up varied among studies, the average overall improvement in tremor was 76% with an average age of 41 years (range 11 to 84 years), HT duration of 6 years (range 6 months to 32 years), and an average follow-up of 3 years (range 6 months to 12 years).
1.2. Dystonic tremor
DT, though a relatively new nosologic entity, has been recognized by expert movement disorder clinicians and has been broadly defined as a tremor occurring in a patient with dystonia [Citation21]. The diagnosis of DT can be challenging, in part because of subtle and overlapping features shared with other clinical tremor syndromes, particularly tremor-predominant early PD. The current consensus statement from the Movement Disorders Society lists dystonic tremor as commonly being characterized by three features: (1) an associated dystonic posture, (2) irregular amplitudes and frequency (usually <7 Hz), and (3) postural/intentional tremor rather than resting tremor [Citation21]. In our experience, there can be other features such as a null point or an inverse intentional component (e.g. the tremor gets better at target and worse when approaching the body or nose on a finger to nose maneuver). Dystonic tremor is under-recognized and commonly mistaken as ET or PD, depending on the location of the issue and the specific tremor characteristics [Citation44]. Some additional features have been proposed that may assist with the diagnosis, including the presence of a ‘null point’ (i.e. a specific posture that when held by the patient alleviates the tremor), sensory tricks, tremor directionality, unilateral arm tremor, hand ‘spooning,’ and atypical features for ET [Citation45,Citation46]. Neurophysiologic recordings have shown that tremor in dystonia (involving affected or unaffected body parts) usually manifests during action and while subjects maintain sustained postures.
Tremor associated with dystonia (TAD) is another type of similar tremor, which is present in a body region not affected by dystonia, but dystonia is present elsewhere (e.g., bilateral hand tremor in a patient with cervical dystonia). This is a relatively symmetric, postural, and kinetic tremor usually showing higher frequencies than typical DT [Citation47]. Prevalence rates for tremor in dystonia varied greatly among different studies ranging from 11% to 87% [Citation48]. Interestingly, tremor may be more frequent in patients with late-onset dystonia than in those with early-onset dystonia. Tremor oscillations usually have a low frequency (below 7 Hz). Unlike ET, in which tremor is regular in frequency and amplitude, DT is usually rhythmic but may be irregular in terms of symmetry about a joint and can vary in amplitude or even presence based on positioning [Citation49,Citation50]. Neurophysiologic investigations in patients with dystonia and tremor show reduced reciprocal inhibition between agonist and antagonist upper limb muscles, a lack of brainstem interneuronal inhibition, and abnormal sensory integration [Citation48].
The treatment outcome in DT is highly variable, depending on the specific type of intervention and tremor distribution [Citation42]. There are a paucity of studies specifically addressing the treatment of DT. In addition, it is impossible to know whether the reviewed studies reliably distinguished TAD and DT. Medical treatment has in general been disappointing with a moderate but variable effect observed with anticholinergics, tetrabenazine, clonazepam, β-blockers, and primidone; levodopa is only efficacious in tremor due to dopamine-responsive dystonia; however, botulinum toxin injections provide marked improvement in patients with head or vocal cord DT [Citation42]. DBS has been utilized in refractory cases with mixed but generally positive results. Most cases of DBS for DT reported in the literature are retrospective reviews in patients with generalized, multifocal, segmental, and even secondary dystonia. The specific effects on tremor control can be difficult to extract from the data unless presurgical standardized tremor scores were collected and reported. Most available studies lacked objective tremor assessments. None of the larger DBS trials for dystonia have specifically taken into account the effect on tremor. This likely reflects a lack of sensitivity of most dystonia rating scales (total score) for assessing limb tremors. This lack of sensitivity is seen in the Burke–Fahn–Marsden Dystonia Rating Scale motor (BFMDRS-M) scale and the Unified Dystonia Rating Scale.
However, based on the increasing evidence demonstrating the effectiveness of GPi targeting in treatment of generalized and segmental dystonia, there is an increasing interest in the potential benefits of pallidal DBS in cases of DT. Most reports specify treatment of DT only if VIM DBS was considered for DT or if tremors persisted after GPi DBS ().
Table 2. Case reports and series of thalamic and subthalamic deep brain stimulation (DBS) in patients with dystonic tremor..
Several DT cases required implantation of additional thalamic leads due to poor tremor response to pallidal stimulation despite dystonia reduction in other areas, indicating the potential need to modulate another circuit (e.g. cerebellar input). Hedera et al. [Citation42] reported 10 patients with primary DT and excluded individuals whose DT was considered secondary. VIM DBS was performed in four patients, GPi DBS was targeted in six patients bilaterally, and a combined bilateral GPi and unilateral VIM surgery was performed in two patients. The best tremor control was observed in the subgroup of patients who underwent VIM DBS, with an average improvement of 84.7% in the tremor rating scale (TRS). GPi DBS resulted in a significantly lower improvement in tremor score at 39.8% but effectively reduced dystonia by 63.5%. Morishita et al. [Citation65] reported moderate improvement in TRS in three patients with DT, one of them after failure to suppress tremor with bilateral GPi DBS. Similarly, Woehrle et al. [Citation63] reported marked benefit in two patients with segmental dystonia with predominant DT following treatment with VIM DBS. In one of the largest case series of DBS for dystonia, tremor was reported as suppressed after being treated with VIM or alternatively ventro-lateral posterior thalamic DBS in all six cases where the patient possessed a ‘dystonic tremor’ phenotype [Citation54]. Several reports have emerged to highlight the potential benefit of VL thalamic DBS (Voa/Vop) for treatment of DT, particularly in PWT [Citation58,Citation68]. DBS of PSA has been reported as being successful in a handful of patients with DT, but available data are insufficient to support its use beyond an experimental approach.
1.3. Cerebellar tremor
CT represents a syndrome characterized by pure or dominant intention tremor (occurring during target-directed movement when the tremor amplitude increases during visually guided movements toward the target); unilateral or bilateral, with a tremor frequency usually below 5 Hz. Postural tremor may be present, but no rest tremor which differentiates this condition from HT. CT is a rare tremor syndrome accounting for 3.2% of patients with non-parkinsonian tremors [Citation22]. The tremor resulting from cerebellar abnormalities is often associated with dysmetria and dyssynergia, or alternatively hypotonia [Citation21].
The utility of DBS in patients with CT is considered to be limited by its inability to treat underlying ataxia, which often, but not always, overshadows tremor. The limited effectiveness for proximally located postural tremor, proximal or distal intention tremor, cerebellar outflow tremor, and potential worsening of speech and gait ataxia, along with the development of tolerance, are valid concerns when applying VIM DBS in CT [Citation69,Citation70]. PSA DBS has been considered a potential option in patients with CT based on the reciprocal connections among basal ganglia and multiple cerebellar nuclei with Zi [Citation35]. Additionally, PSA DBS provides the potential anatomical advantage of stimulating a large proportion of cerebello-thalamic afferents, which can be affected by a small volume of current spread prior to the physical location where the fibers spread out and innervate the entire VIM nucleus [Citation71]. PSA stimulation would therefore mainly interrupt afferent (axonal) fibers, thereby theoretically blocking the cerebello-thalamic pathway. In contrast, in the VIM, afferent fibers presumably diverge and connect to dendrites and somata of thalamo-cortical neurons and interneurons [Citation72]. Small numbers of cases of CT managed with DBS have been reported with positive results ().
Table 3. Case reports and series of thalamic and subthalamic deep brain stimulation (DBS) in patients with cerebellar tremor.
It is likely that a reporting bias accounts for the limited number of cases in the literature, as unsuccessful cases are likely not reported. The development of tolerance remains a valid concern when applying VIM DBS in CT [Citation69,Citation70]. PSA DBS has been considered a potential option in patients with CT based on the reciprocal connections among basal ganglia and multiple cerebellar nuclei with Zi [Citation35]. Additionally, PSA DBS provides the potential anatomical advantage of stimulating a large proportion of cerebello-thalamic afferents, which can be affected by a small volume of current spread prior to the physical location where the fibers spread out and innervate the entire VIM nucleus [Citation71]. PSA stimulation would therefore mainly interrupt afferent (axonal) fibers, thereby theoretically blocking the cerebello-thalamic pathway. In contrast, in the VIM, afferent fibers presumably diverge and connect to dendrites and somata of thalamo-cortical neurons and interneurons [Citation72].
Fragile X-associated tremor/ataxia syndrome (FXTAS) is an inherited, X-linked, adult-onset neurodegenerative disorder caused by a moderately expanded trinucleotide repeat (CGG block lengths 55–200) in a noncoding region of the fragile X mental retardation 1 (FMR1) gene. The clinical manifestations of FXTAS – which occur predominantly in men – include action tremor, gait and limb ataxia, cognitive and neuropsychiatric dysfunction, parkinsonism, dysautonomia, and peripheral neuropathy [Citation81].
Several patients with FXTAS have been reported in the literature, mostly treated with VIM DBS. Most reports include tremor improvement with either no benefit or worsening of the ataxia and balance. Tremor improvement ranged from 30% to 70% among FXTAS cases. Other reports indicated that unilateral VIM stimulation safely reduced tremor in a FXTAS patient without aggravating other neurological deficits, and even improving cerebellar ataxia [Citation69]. Larger trials are needed to validate these observations. In single patients, PSA DBS has shown potentially higher rates of CT control.
1.4. Multiple sclerosis tremor
MS tremor most commonly manifests as postural tremor and/or intention tremor. MS tremor typically involves the upper limbs, although tremor can also involve the head, neck, vocal cords, and trunk [Citation82]. Titubation is defined as a nodding head tremor with a frequency of 3–4 Hz; it may be observed in midline cerebellar disease and can occur in isolation or combined with a postural tremor elsewhere, especially in the arms [Citation83]. Proximal tremors can be large in amplitude resembling ballism. True rest tremor, task-specific tremors, or HT are uncommon in patients with MS and are observed in fewer than 1% in recent prevalence studies [Citation84,Citation85]. However, tremor is not unusual in MS patients and studies have provided estimated of the relatively high prevalence of tremor in the MS population that range widely between 25% and 58% [Citation86]. It is important to emphasize that essential, dystonic, and tardive tremors can occur in MS patients, and the presence of these specific syndromes is often coincidental. Treatment should be dictated by the specific tremor characteristics. Multiple lines of evidence support the role of cerebellar dysfunction in the pathogenesis of MS tremor. In MS patients, tremor severity has correlated with the degree of dysarthria, dysmetria, and dysdiadochokinesia [Citation85]. MS intention tremor may be modulated by sensory information and cooling of the arms markedly reduced intention tremor severity in patients with MS, further supporting the notion that the cerebellum is involved in the production of tremor [Citation87].
A number of retrospective case series have described the outcome of thalamic DBS in MS-associated tremor, with over 100 patients reported and recently summarized in detail by Koch et al. [Citation86]. Most studies were small observational retrospective studies with predominantly short-term follow-up (1 year or less) and with an absence of standardized outcome measures, adverse effects, or information on long-term functional status and tremor-associated disability. Most reports described clear improvement in tremor scales, but long-term outcomes were less certain. Torres et al. [Citation9] reported 10 MS patients treated with either unilateral or bilateral DBS. At 36 months, only three patients continued to benefit from stimulation, with two having >50% improvement. Of the six symptomatic sides that did not benefit at 1 year, three failed to have initial benefit and three had a transient improvement lasting <1 year. Patients in their cohort had advanced disease, with all but one patient being reported as wheelchair bound. Moreover, Hassam et al. [Citation10] retrospectively analyzed the 12-year follow-up of nine patients treated with unilateral thalamotomy (six patients) or VIM thalamic DBS (three cases) at the Mayo Clinic. The study showed that the benefit resulting from surgery was overall short lived (median 3 months), with a poor long-term prognosis. At 12-year follow-up, the Expanded Disability Status Scale had progressed in all patients, and five patients were deceased. Zakaria et al. [Citation88] reported the results in quality of life and tremor control in 16 patients (9 female, 7 male) with MS tremor treated with VIM thalamic DBS. The mean tremor reduction was 39% overall, with a change in TRS ranging from no benefit to 87% at 1-year follow-up. One-third of the patients noted at least 50% tremor reduction. The mean follow-up was 11.6 months (range 3–80). There was a trend for statistical improvement in the activities of daily living scale and in improved quality of life as well as in function in those who responded.
In other recent reports published after the Koch review, the effectiveness of PSA DBS has been highlighted. Detailed studies analyzing different tremor components and the most effective DBS contacts for stimulation have emerged. Herzog et al. [Citation89] performed kinematic analysis of 11 patients with refractory MS tremors. These authors reported a 50.4% average reduction of the preoperative TRS in MS patients. Thalamic DBS significantly reduced the pathological features of postural tremor and also improved the unsteadiness in the terminal phase of goal-directed movements, which was a typical feature of movements in cerebellar dysfunction. These effects were stronger for electrode contacts located below the AC–PC line (ZI or subthalamic area) as compared with stimulation at the standard VIM target within the VL thalamus. In a similar report, Hamel et al. [Citation90] observed the effects of thalamic and subthalamic stimulation in a series of 11 patients with refractory intention tremor, with two cases reported secondary to MS. The distal electrode contacts were more effective than the proximal contacts. The most effective intention tremor suppression (>75% postoperative improvement) was achieved at or below the intercommissural plane. Plaha et al. [Citation35] reported the effects of DBS of caudal Zi in four patients with MS tremor. The tremor was characterized by proximal upper limb kinetic and intention tremor with truncal ataxia. These authors reported 87% and 57.2% improvement in postural and intention components of tremor, respectively, with improvement in elements of ataxia. Nandi et al. [Citation91] reported their experience in 15 MS patients who received DBS that targeted both the Vop nucleus of the thalamus and the Zi. Data were available for 10 patients, with a mean follow-up of 15 months. The authors used a single DBS electrode straddling the two structures for combined stimulation. Foote et al. [Citation2] reported on a single MS tremor patient in whom improvement was seen following dual stimulation of the VIM and Voa/Vop nuclei of the thalamus. Mehanna et al. [Citation19] reported their experience using dual target stimulation in two patients with refractory MS tremors and three patients with ET. The patients in this report received an additional lead in Vop or PSA after tremor recurrence over time following the initial VIM DBS. MS patients with MS tremor had marginal (18%) improvement with dual VIM + Vop stimulation and no benefit with PSA lead. Foote et. al. have a recently completed NIH study on dual lead treatment of MS tremor (VIM plus Vo) and the study is listed as completed on clinicaltrials.gov (NCT00954421) and we expect the results to be published soon.
There have been several concerns regarding the use of DBS to control MS tremor. First, the other symptoms of MS may cause worsened disability compared with tremor, leading to unrealistic expectations about the outcome of DBS [Citation92]. In addition, patients with MS may have altered neuroanatomy or neurophysiology that may render the DBS targeting more difficult. Other important considerations in MS tremor patients include a potentially higher risk of perioperative seizures and evidence of possible demyelination around the electrode lead [Citation93,Citation94]. MS exacerbations and new MS plaque formation have been reported following thalamotomy and DBS implantation, but the available data do not clearly indicate whether this represented a change in exacerbation frequency relative to the presurgical baseline.
1.5. Posttraumatic tremors
The term posttraumatic tremor refers to a series of atypical tremors that occur secondary to traumatic brain injury. However, posttraumatic tremors do not represent a uniform syndrome or disease, and various forms of tremor semiology have been reported. Typically, posttraumatic tremor is characterized by a combination of irregular resting, and a postural and intention tremor of large amplitude with slow frequency, though the presentation can be variable [Citation95]. Tremor is usually unilateral, it predominantly affects the proximal upper extremities, and it is markedly worsened by goal-directed movements. Posttraumatic tremor is one of the most common movement disorders resulting from severe head trauma with prevalence studies ranging from 19% to 45% [Citation38,Citation95].
Treatment is difficult, and additional concerns complicate the surgical treatment of posttraumatic tremor, namely, associated neurological deficits including cognitive deficits, ataxia, dysarthria, hemiparesis, and oculomotor problems since these symptoms can lead to severe disability [Citation6,Citation95]. Because traumatic brain injury is, by its nature, a heterogeneous insult, observed posttraumatic tremors are themselves distinct. Several reports have documented the effectiveness of stereotactic surgery to alleviate posttraumatic tremor and to improve functional disability (). Most case series have targeted the thalamic VIM with an average of 50–70% improvement in tremor in the short term. Other targets utilized by different authors include dual-lead VIM and Voa/Vop, Zi and Vop, Zi and Voa/Vop, and VIM + STN. To date, there have been encouraging results and a reported reduction in total TRS ranging from 38% to 80%.
Table 4. Case reports and series of thalamic and subthlamic deep brain stimulation (DBS) in patients with posttraumatic tremor.
1.6. Orthostatic tremor (OT)
OT is a rare syndrome characterized by unsteadiness on standing due to a high-frequency tremor involving the legs though notably tremor can occur in the upper extremities and mimic ET. Complaints of leg shaking activated by standing, orthostatic leg weakness, and unexplained imbalance should draw attention to a possible OT diagnosis. Additional features suggesting this condition include improvement with walking (which would not be expected in cases of simple imbalance), improvement with leaning, and transmission of the tremor to the arms on leaning. The prevalence of OT is unknown.
Electromyography provides a definitive diagnosis and demonstrates a unique tremor frequency (13–18 Hz) and a high coherence between antagonistic and contralateral muscle groups [Citation102]. The pathophysiology of OT is not completely understood, but cortical oscillatory activity centered in the motor cortex has been shown to be time-locked to tremor discharges in the lower limbs [Citation103], suggesting a role for thalamic DBS in the treatment of the disease. A myriad of medications have been used for the treatment of OT, all with limited success. Notably, benzodiazepines (primarily clonazepam) have been reported as being the most effective therapy, with about one-third of those treated reporting moderate or marked improvement [Citation104]. Eleven cases of refractory OT have been reported (). All patients underwent thalamic VIM stimulation, resulting in a marked clinical improvement in patients undergoing bilateral procedures. Some cases reported a long-term follow-up of up to 4 or 5 years.
Table 5. Case reports and series of thalamic deep brain stimulation (DBS) in patients with orthostatic tremor (OT).
Interestingly, tremor recurrence was noted at 3 months in a single patient treated with unilateral stimulation, suggesting the possibility that bilateral surgery in this condition may be more appropriate. Despite the observed clinical benefits, the pathological 15–18 Hz oscillatory activity and posturographic measures post-DBS seem to be unaltered in some studies. These findings suggest that chronic stimulation did not disrupt the tremor generator, but may have modulated its intensity. There has been speculation that spinal stimulation could be beneficial in OT, and to date most experts concede that long-term outcomes from VIM DBS have been mixed and inconsistent.
1.7. Neuropathic tremor
NT is defined as a kinetic tremor in association with peripheral neuropathy when no other neurological condition related to tremor is encountered [Citation55]. Tremor phenomenology reveals a primarily postural and kinetic tremor with a frequency between 3 and 6 Hz in arm and hand muscles [Citation112]. Weakness, areflexia, sensory changes, and propioceptive ataxia are commonly encountered. NT is observed mainly in patients with chronic inflammatory demyelinating polyneuropathies, hereditary motor and sensory neuropathies (HMSN), and IgM demyelinating paraprotein-related neuropathy, and is more common with kappa rather than lambda light chain disease [Citation113]. Several drugs have been evaluated for possible treatment of NT with disappointing results. Propranolol, primidone, or clonazepam can be considered. The mechanism responsible for neuropathic tremor remains uncertain and may not be the same for different types of neuropathy. A distorted peripheral sensory input misleading an otherwise intact central network might produce NT. Feedback of abnormal afferent information results in a faulty attempt by the cerebellum to correct the limb position and velocity which perpetuates the mistake and results in a variable tremor [Citation113]. More recently, reports showing significant corticomuscular coherence at tremor frequency (4 Hz) between limb muscles and contralateral motor cortex during postural tremor in a patient with NT has been described [Citation114]. Furthermore, tremor was suppressed with high frequency DBS. Their findings support the concept of maladaptive central motor processing in NT and provide a strong pathophysiological rationale for the use of VIM DBS. Reports suggesting a role of thalamic DBS in refractory NT cases have emerged. Ruzicka et al. reported the effect of unilateral VIM DBS in a patient with IgM paraproteinemic neuropathy. The patient noted 50% improvement in disability and tremor scores with sustained benefit at 12-month follow-up [Citation115]. Other reports have echoed the potential benefit of VIM DBS in NT with either unilateral or bilateral procedures. Breit et al. reported 30% reduction in tremor in one patient with HMSN type 1 using the Columbia University Assessment of Disability in Essential Tremor scale. Tremor control was limited by the occurrence of worsening gait ataxia with higher amplitudes or electrical charge despite multiple programming changes [Citation116]. Weiss et al. reported the outcome of a single patient treated with bilateral VIM DBS for NT associated with IgM paraproteinemia. There was a marked improvement in the Tremor Rating Scale (67% at 3 months, 59% at 12 months) and activities of daily living (31% at 12 months), but stimulation amplitude had to be consecutively raised within the first year to achieve tremor suppression. In their study, corticomuscular coherence at tremor frequency was completely suppressed by DBS. Additionally, one patients treated with unilateral PSA DBS have been reported by Blomstedt et al. [Citation60]. Tremor suppression of approximately 70% was achieved and sustained at 12 months. At the present time, there is limited evidence to determine the long-term efficacy of DBS in NT and the procedure is considered investigational.
2. Expert commentary
Uncommon and complex tremor syndromes represent a formidable obstacle in medication refractory cases. There have been only small case reports and series published and there has been a lack of evidence-based treatment guidelines. The majority of studies addressing treatment response include short case series, case reports, retrospective reviews, and all of the studies lack a randomized or even a controlled design. Additionally, the reporting of the actual stereotactic target and postoperative lead location has been inconsistent. There has been a paucity of clinical details in most reports of DBS for the treatment of rare tremors, and misdiagnosis is common because of the overlap with other syndromes [Citation117,Citation118]. It will be important in the future to report associated complications and suboptimal responses to DBS to better characterize appropriate candidates, refine targets and approaches, and to identify the risks of therapy.
The optimal target for treating HT remains debated, although most case series have shown positive short-term results with thalamic VIM or combined VIM and Vop or PSA stimulation. Furthermore, posterior, lateral, and ventral GPi DBS can be considered if the VIM nucleus anatomy is grossly disrupted by intracranial pathology (affecting stereotactic planning and theoretically affecting cerebello-thalamic-cortical loop effects of DBS), when intraoperative tremor control is unsatisfactory despite VIM high-intensity stimulation, and in patients with associated movements disorders such as chorea, parkinsonism, and dystonia. Reports indicate that unlike thalamic surgery, which is thought to interrupt the thalamocortical output that controls distal appendicular musculature, pallidal surgery might influence the control of otherwise inaccessible axial and proximal muscles [Citation12].
The specific mechanisms underlying the development of tremor in dystonia remain elusive, and it is unclear why GPi DBS fails to improve or may exacerbate tremor in some DT patients [Citation48]. DT may emerge at dystonia onset or thereafter, affects women more frequently than men, can manifest during posture or voluntary movements, and although less frequently, can also be observed at rest [Citation48]. In DT, based on typical DBS target trajectories and uncharacteristic programming settings, the most useful contacts for tremor control are located within the VL thalamus (VIM and Vop/Voa), and stimulation of both nuclei (e.g. a dual lead technique) might be necessary for tremor control. The majority of DT cases treated with DBS in the literature have been generalized or segmental dystonia with concomitant limb tremors. The difficulties frequently encountered when diagnosing DT have not facilitated the separation of DT from TAD or helped to draw concrete conclusions regarding a universal best target for DT. It is plausible that some of the DT patients reported in the literature were actually affected by TAD, or even ET [Citation12]. GPi should be viewed as the preferred target for stimulation in DT patients with generalized and segmental dystonia; however, thalamic stimulation may be added in dystonia cases with incomplete tremor control and when tremor is the main disabling feature. Thalamic DBS can occasionally lead to worsening of dystonia itself [Citation62,Citation65,Citation119]. If DT is highly specific or dystonia is mild and affecting the distal limbs, thalamic stimulation appears to be greatly effective, as illustrated in several cases of PWT [Citation51,Citation53,Citation67].
The management of intention tremor associated with prominent ataxia or cerebellar features can be challenging, and efforts to discern both components of the tremor have been critical for predicting the outcome. The effects of thalamic DBS on gait and balance are under investigation, but there are concerns about worsening cerebellar features [Citation120–Citation122]. Results have been mixed with thalamic stimulation, and PSA DBS could be considered in patients with refractory tremors with associated cerebellar features and proximal tremors as they both provide a theoretical advantage to thalamic DBS. In addition, bilateral stimulation has been associated with dysarthria and disequilibrium. Nevertheless, the latter was observed in a few cases of bilateral PSA DBS.
In MS tremor, the results from several case series support the clinical impression of a symptomatic benefit from thalamic DBS in at least a subpopulation of MS tremor patients. If severe disability or prominent cerebellar features are associated with MS tremor, the response can possibly be dampened. Hosseini et al. [Citation123] recently confirmed the higher efficacy of VIM DBS treatment of kinetic tremor in the subgroup of MS patients with minor or absent cerebellar dysfunction. Several preoperative issues are worthy of consideration in patients with MS tremors, including risk of seizures, balance, MS exacerbations, disease severity, and the presence of severe atrophy or demyelinating lesions in the thalamus. PSA DBS has emerged as a potential target in this population. Larger, long-term follow-up trials are needed to assess the efficacy of DBS of this population.
Patients with posttraumatic tremors must be evaluated carefully to determine the appropriate treatment and potential candidacy issues for surgery, along with the most appropriate surgical target. Target selections should be based on tremor characteristics and the associated neurological features, including the presence of cerebellar dysfunction, cognitive, sensory, and motor deficits, spasticity, and dystonia. Despite the predominant use of the VIM target in the literature, GPi or PSA DBS can be considered in select cases. Bilateral VIM DBS can be considered as a therapeutic option in patients with refractory OT though this may result in more cognitive, speech, and gait issues. Unilateral or thalamic VIM DBS can be entertained in selected cases of disabling NT but long-term efficacy and safety data are missing. Large, well-controlled trials are needed to determine the specific factors important for deciding on different targets for different patients. Individualized outcomes and setting expectations with patients is an important part of the therapy.
3. Five-year view
Despite improvement reported in most patients with thalamic VIM DBS for treatment of uncommon tremors, the potential benefits and applications of newer targets continue to emerge. Although the initial results are encouraging, comparative studies among different DBS targets are needed to better determine the specific advantages and effectiveness of various stereotactic techniques. The present review emphasizes the need for further research with well designed, prospective, controlled studies that will assist physicians to better manage refractory tremors and to appropriately select suitable candidates. These conditions are rare by definition, and the need for collaborative efforts among institutions and researchers is needed. A detailed assessment of the associated neurological signs and tremor phenomenology including cerebellar findings, dystonic postures, chorea, or abnormal muscle tone can all be crucial for an appropriate diagnosis and treatment selection. Advances in DBS technology will likely aid in the continued innovation and progress of DBS for difficult tremor cases. The tentative benefits of directional current steering, patterned stimulation, constant current stimulation, and interleaved stimulation need to be investigated in uncommon tremors but advantages in tremor control while minimizing side effects have been shown in PD and ET [Citation124–Citation126]. A recent study showed that the therapeutic window of STN neurostimulation increased up to twofold when using an ultra-short PW of 30 μs compared with the standard PW setting of 60 μs with concomitant improvement in rigidity [Citation127]. Even with appropriately placed DBS electrodes, the proximity to structures and white matter tracts might produce unwanted side effects. Side effects are determined by the structures stimulated and are specific to the target. With thalamic stimulation, paresthesias related to stimulation of sensory thalamus or medial lemniscus along with dysarthria due to internal capsule stimulation are common. Dysarthria, imbalance, and paresthesias are also common with PSA stimulation. Side effects encountered with pallidal stimulation are typically speech difficulties or tonic muscle contractions due to stimulation of the internal capsule. More recently, electrode design capable of directional stimulation steering current perpendicular to the lead axis toward the area to be stimulated and away from structures that might produce side effects has been developed [Citation128]. Directional stimulation may produce a wider therapeutic programming window at a lower current when compared with standard ring electrodes.
An important innovation applicable to tremor treatment will be the development of newer DBS leads. A newer DBS device (the Boston Scientific Vercise™) currently in clinical trials has eight independently powered electrodes per lead and allows two separate frequencies of stimulation at two different electrode locations. This system also facilitates fractionated constant current stimulation over multiple electrodes [Citation124]. Additionally, the availability to 8 contacts distributed over a 15.5 mm span might dictate different stereotactic targeting for tremor as more posteromedial or ventral coordinates can allow stimulation of the subthalamic area and thalamic nuclei using a single lead. Furthermore, adjustments in DBS trajectory and approach angles can spread out electrodes to be located more broadly within the thalamus. Stimulation of multiple sites along the same trajectory will allow the use of complex patterns of stimulation potentially minimizing side effects [Citation124]. Finally, it is possible that closed loop neuromodulation will be applied for some complex tremors and that this approach may minimize side effects particularly when two leads are required for tremor control.
Key issues
Medical treatment of complex tremors is usually disappointing and DBS should be considered in select refractory cases.
GPi DBS can be considered in HT if thalamic VIM nucleus anatomy is grossly disrupted by intracranial pathology and in patients with associated movements disorders like chorea, parkinsonism, and dystonia.
GPi should be viewed as the preferred target for stimulation in DT patients with generalized and segmental dystonia, but thalamic stimulation may be added in cases with incomplete tremor control.
PSA DBS can be considered in patients with refractory tremors with associated cerebellar features and in proximal tremors as it provides theoretical advantages to thalamic DBS.
MS patients with largely kinetic tremor with minor or absent cerebellar dysfunction might benefit from thalamic DBS. PSA DBS can be considered in selected cases.
In post-traumatic tremors, target selections should be based on tremor characteristic and associated neurological features, including cerebellar dysfunction, cognitive, sensory, and motor deficits as well as spasticity, and dystonia.
Closed loop neuromodulation may in the future be an option for complex tremors
Acknowledgment
The authors would like to thank Julia Prusik BS and Marcia Lamb for their assistance with the organization of the manuscript.
Declaration of interest
A Ramirez-Zamora has received consultant honoraria from Teva pharmaceuticals. The Phyllis E. Dake Endowed Chair in Movement Disorders supported this study. The institution and not Dr. Ramirez-Zamora receives grant support from Medtronic and Boston Scientific. Dr. Ramirez-Zamora has participated as a site PI and/or co-I for several NIH and industry sponsored trials over the years but has not received honoraria. MS Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun’s DBS research is supported by R01 NR014852 and R01NS096008. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, MedNet, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, Allergan, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Alesch F, Pinter MM, Helscher RJ, et al. Stimulation of the ventral intermediate thalamic nucleus in tremor dominated Parkinson’s disease and essential tremor. Acta Neurochir (Wien). 1995;136(1–2):75–81
- Foote KD, Seignourel P, Fernandez HH, et al. Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery. 2006;58(4 Suppl 2):ONS-280–285; discussion ONS-285–286.
- Berk C, Carr J, Sinden M, et al. Thalamic deep brain stimulation for the treatment of tremor due to multiple sclerosis: a prospective study of tremor and quality of life. J Neurosurg. 2002;97(4):815–820.
- Hirai T, Miyazaki M, Nakajima H, et al. The correlation between tremor characteristics and the predicted volume of effective lesions in stereotaxic nucleus ventralis intermedius thalamotomy. Brain. 1983;106(Pt 4):1001–1018.
- Nguyen JP, Degos JD. Thalamic stimulation and proximal tremor. A specific target in the nucleus ventrointermedius thalami. Arch Neurol. 1993;50(5):498–500.
- Krauss JK, Mohadjer M, Nobbe F, et al. The treatment of posttraumatic tremor by stereotactic surgery. Symptomatic and functional outcome in a series of 35 patients. J Neurosurg. 1994;80(5):810–819.
- Wishart HA, Roberts DW, Roth RM, et al. Chronic deep brain stimulation for the treatment of tremor in multiple sclerosis: review and case reports. J Neurol Neurosurg Psychiatry. 2003;74(10):1392–1397.
- Hooper J, Taylor R, Pentland B, et al. A prospective study of thalamic deep brain stimulation for the treatment of movement disorders in multiple sclerosis. Br J Neurosurg. 2002;16(2):102–109.
- Torres CV, Moro E, Lopez-Rios AL, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus for tremor in patients with multiple sclerosis. Neurosurgery. 2010;67(3):646–651. discussion 651
- Hassan A, Ahlskog JE, Rodriguez M, et al. Surgical therapy for multiple sclerosis tremor: a 12-year follow-up study. Eur J Neurol. 2012;19(5):764–768.
- Foote KD, Okun MS. Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: two leads may be better than one: technical note. Neurosurgery. 2005;56(2 Suppl):E445. discussion E445
- Goto S, Yamada K. Combination of thalamic Vim stimulation and GPi pallidotomy synergistically abolishes Holmes’ tremor. J Neurol Neurosurg Psychiatry. 2004;75(8):1203–1204.
- Favilla CG, Ullman D, Wagle Shukla A, et al. Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain. 2012;135(Pt 5):1455–1462.
- Velasco FC, Molina-Negro P, Bertrand C, et al. Further definition of the subthalamic target for arrest of tremor. J Neurosurg. 1972;36(2):184–191.
- Mundinger F. Stereotaxic interventions on the zona incerta area for treatment of extrapyramidal motor disturbances and their results. Confin Neurol. 1965;26(3):222–230.
- Velasco F, Velasco M, Ogarrio C, et al. Neglect induced by thalamotomy in humans: a quantitative appraisal of the sensory and motor deficits. Neurosurgery. 1986;19(5):744–751.
- Lim DA, Khandhar SM, Heath S, et al. Multiple target deep brain stimulation for multiple sclerosis related and poststroke Holmes’ tremor. Stereotact Funct Neurosurg. 2007;85(4):144–149.
- Kilbane C, Ramirez-Zamora A, Ryapolova-Webb E, et al. Pallidal stimulation for Holmes tremor: clinical outcomes and single-unit recordings in 4 cases. J Neurosurg. 2015;122(6):1306–1314.
- Mehanna R, Machado AG, Oravivattanakul S, et al. Comparing two deep brain stimulation leads to one in refractory tremor. Cerebellum. 2014;13(4):425–432.
- Yu H, Hedera P, Fang J, et al. Confined stimulation using dual thalamic deep brain stimulation leads rescues refractory essential tremor: report of three cases. Stereotact Funct Neurosurg. 2009;87(5):309–313.
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Dis: Off J Mov Disord Soc. 1998;13(Suppl 3):2–23.
- Regragui W, Lachhab L, Razine R, et al. A clinical study of non-parkinsonian tremor in Moroccan patients. Rev Neurol (Paris). 2014;170(1):26–31.
- Seidel S, Kasprian G, Leutmezer F, et al. Disruption of nigrostriatal and cerebellothalamic pathways in dopamine responsive Holmes’ tremor. J Neurol Neurosurg Psychiatry. 2009;80(8):921–923.
- Deuschl G, Bergman H. Pathophysiology of nonparkinsonian tremors. Move Dis. 2002;17(S3):S41–S48.
- Remy P, de Recondo A, Defer G, et al. Peduncular ‘rubral’ tremor and dopaminergic denervation: a PET study. Neurology. 1995;45(3 Pt 1):472–477.
- Kim MC, Son BC, Miyagi Y, et al. Vim thalamotomy for Holmes’ tremor secondary to midbrain tumour. J Neurol Neurosurg Psychiatry. 2002;73(4):453–455.
- Pahwa R, Lyons KE, Kempf L, et al. Thalamic stimulation for midbrain tremor after partial hemangioma resection. Mov Dis: Off J Mov Disord Soc. 2002;17(2):404–407.
- Romanelli P, Brontë-Stewart H, Courtney T, et al. Possible necessity for deep brain stimulation of both the ventralis intermedius and subthalamic nuclei to resolve Holmes tremor. Case report. J Neurosurg. 2003;99(3):566–571.
- Samadani U, Umemura A, Jaggi JL, et al. Thalamic deep brain stimulation for disabling tremor after excision of a midbrain cavernous angioma. Case report. J Neurosurg. 2003;98(4):888–890.
- Nikkhah G, Prokop T, Hellwig B, et al. Deep brain stimulation of the nucleus ventralis intermedius for Holmes (rubral) tremor and associated dystonia caused by upper brainstem lesions. Report of two cases. J Neurosurg. 2004;100(6):1079–1083.
- Piette T, Mescola P, Henriet M, et al. [A surgical approach to Holmes’ tremor associated with high-frequency synchronous bursts]. Rev Neurol (Paris). 2004;160(6–7):707–711.
- Bandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. J Neurosurg. 2008;109(4):635–639.
- Diederich NJ, Verhagen Metman L, Bakay RA, et al. Ventral intermediate thalamic stimulation in complex tremor syndromes. Stereotact Funct Neurosurg. 2008;86(3):167–172.
- Peker S, Isik U, Akgun Y, et al. Deep brain stimulation for Holmes’ tremor related to a thalamic abscess. Childs Nerv Syst. 2008;24(9):1057–1062.
- Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79(5):504–513.
- Acar G, Acar F, Bir LS, et al. Vim stimulation in Holmes’ tremor secondary to subarachnoid hemorrhage. Neurol Res. 2010;32(9):992–994.
- Castrop F, Jochim A, Berends LP, et al. Sustained suppression of holmes tremor after cessation of thalamic stimulation. Mov Dis: Off J Mov Disord Soc. 2013;28(10):1456–1457.
- Issar NM, Hedera P, Phibbs FT, et al. Treating post-traumatic tremor with deep brain stimulation: report of five cases. Parkinsonism Relat Disord. 2013;19(12):1100–1105.
- Follett MA, Torres-Russotto D, Follett KA. Bilateral deep brain stimulation of the ventral intermediate nucleus of the thalamus for posttraumatic midbrain tremor. Neuromodulation. 2014;17(3):289–291.
- Grabska N, Rudzińska M, Dec-Ćwiek M, et al. Deep brain stimulation in the treatment of Holmes tremor – a long-term case observation. Neurol Neurochir Pol. 2014;48(4):292–295.
- Kobayashi K, Katayama Y, Oshima H, et al. Multitarget, dual-electrode deep brain stimulation of the thalamus and subthalamic area for treatment of Holmes’ tremor. J Neurosurg. 2014;120(5):1025–1032.
- Espinoza Martinez JA, Arango GJ, Fonoff ET, et al. Deep brain stimulation of the globus pallidus internus or ventralis intermedius nucleus of thalamus for Holmes tremor. Neurosurg Rev. 2015;38(4):753–763.
- Foote KD, Seignourel P, Fernandez HH, et al. Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery. 2006;58(Supplement2):ONS-280–ONS-286.
- Elble R, Deuschl G. Milestones in tremor research. Mov Dis: Off J Mov Disord Soc. 2011;26(6):1096–1105.
- Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63(8):1100–1104.
- Jedynak CP, Bonnet AM, Agid Y. Tremor and idiopathic dystonia. Mov Dis: Off J Mov Disord Soc. 1991;6(3):230–236.
- Münchau A, Schrag A, Chuang C, et al. Arm tremor in cervical dystonia differs from essential tremor and can be classified by onset age and spread of symptoms. Brain. 2001;124(Pt 9):1765–1776.
- Defazio G, Conte A, Gigante AF, et al. Is tremor in dystonia a phenotypic feature of dystonia? Neurology. 2015;84(10):1053–1059.
- Elias WJ, Shah BB. Definitions of tremor–reply. JAMA. 2014;312(2):191–192.
- Deuschl G. Dystonic tremor. Rev Neurol (Paris). 2003;159(10 Pt 1):900–905.
- Minguez-Castellanos A, Carnero-Pardo C, Gómez-Camello A, et al. Primary writing tremor treated by chronic thalamic stimulation. Mov Dis: Off J Mov Disord Soc. 1999;14(6):1030–1033.
- Kitagawa M, Murata J, Kikuchi S, et al. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology. 2000;55(1):114–116.
- Racette BA, Dowling J, Randle J, et al. Thalamic stimulation for primary writing tremor. J Neurol. 2001;248(5):380–382.
- Vercueil L, Pollak P, Fraix V, et al. Deep brain stimulation in the treatment of severe dystonia. J Neurol. 2001;248(8):695–700.
- Deuschl G, Bergman H. Pathophysiology of nonparkinsonian tremors. Mov Dis: Off J Mov Disord Soc. 2002;17(Suppl 3):S41–48.
- Krause M, Fogel W, Kloss M, et al. Pallidal stimulation for dystonia. Neurosurgery. 2004;55(6):1361–1368. discussion 1368–1370
- Chou KL, Hurtig HI, Jaggi JL, et al. Bilateral subthalamic nucleus deep brain stimulation in a patient with cervical dystonia and essential tremor. Mov Dis: Off J Mov Disord Soc. 2005;20(3):377–380.
- Fukaya C, Katayama Y, Kano T, et al. Thalamic deep brain stimulation for writer’s cramp. J Neurosurg. 2007;107(5):977–982.
- Schadt CR, Charles PD, Davis TL, et al. Thalamotomy, DBS-Vim, and DBS-GPi for generalized dystonia: a case report. Tenn Med. 2007;100(2):38–39.
- Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochir (Wien). 2009;151(1):31–36.
- Jeong SG, Lee MK, Lee WH, et al. Deep brain stimulation of the subthalamic area for dystonic tremor. J Korean Neurosurg Soc. 2009;45(5):303–305.
- Kuncel AM, Turner DA, Ozelius LJ, et al. Myoclonus and tremor response to thalamic deep brain stimulation parameters in a patient with inherited myoclonus-dystonia syndrome. Clin Neurol Neurosurg. 2009;111(3):303–306.
- Woehrle JC, Blahak C, Kekelia K, et al. Chronic deep brain stimulation for segmental dystonia. Stereotact Funct Neurosurg. 2009;87(6):379–384.
- Torres CV, Moro E, Dostrovsky JO, et al. Unilateral pallidal deep brain stimulation in a patient with cervical dystonia and tremor. J Neurosurg. 2010;113(6):1230–1233.
- Morishita T, Foote KD, Haq IU, et al. Should we consider Vim thalamic deep brain stimulation for select cases of severe refractory dystonic tremor. Stereotact Funct Neurosurg. 2010;88(2):98–104.
- Lyons M, Shneyder N, Evidente V. Primary writing tremor responds to unilateral thalamic deep brain stimulation. Turk Neurosurg. 2013;23(1):122–124.
- Hedera P, Phibbs FT, Dolhun R, et al. Surgical targets for dystonic tremor: considerations between the globus pallidus and ventral intermediate thalamic nucleus. Parkinsonism Relat Disord. 2013;19(7):684–686.
- Goto S, Shimazu H, Matsuzaki K, et al. Thalamic Vo-complex vs. pallidal deep brain stimulation for focal hand dystonia. Neurology. 2008;70(16 Pt 2):1500–1501.
- Senova S, Jarraya B, Iwamuro H, et al. Unilateral thalamic stimulation safely improved fragile X-associated tremor ataxia: a case report. Mov Dis: Off J Mov Disord Soc. 2012;27(6):797–799.
- Ferrara JM, Adam OR, Ondo WG. Treatment of fragile-X-associated tremor/ataxia syndrome with deep brain stimulation. Mov Dis: Off J Mov Disord Soc. 2009;24(1):149–151.
- Sakai ST, Inase M, Tanji J. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol. 1996;368(2):215–228.
- Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation of the ventral intermediate nucleus in patients with essential tremor: stimulation below intercommissural line is more efficient but equally effective as stimulation above. Exp Neurol. 2011;230(1):131–137.
- Freund HJ, Barnikol UB, Nolte D, et al. Subthalamic-thalamic DBS in a case with spinocerebellar ataxia type 2 and severe tremor-A unusual clinical benefit. Mov Dis: Off J Mov Disord Soc. 2007;22(5):732–735.
- Joseph M, Ora F, Ondo WG. Treatment of fragile X associated tremor/ataxia syndrome with deep brain stimulation. Movement Disorders. 2009;25(1):149–150.
- Xie T, Goodman R, Browner N, et al. Treatment of fragile X-associated tremor/ataxia syndrome with unilateral deep brain stimulation. Mov Dis: Off J Mov Disord Soc. 2012;27(6):799–800.
- Mehanna R, Itin I. Which approach is better: bilateral versus unilateral thalamic deep brain stimulation in patients with fragile X-associated tremor ataxia syndrome. Cerebellum. 2014;13(2):222–225.
- Oyama G, Thompson A, Foote KD, et al. Deep brain stimulation for tremor associated with underlying ataxia syndromes: a case series and discussion of issues. Tremor Other Hyperkinet Mov (N Y). 2014;4:228.
- Oyama G, Umemura A, Shimo Y, et al. Posterior subthalamic area deep brain stimulation for fragile X-associated tremor/ataxia syndrome. Neuromodulation. 2014;17(8):721–723.
- Weiss D, Mielke C, Wächter T, et al. Long-term outcome of deep brain stimulation in fragile X-associated tremor/ataxia syndrome. Parkinsonism Relat Disord. 2015;21(3):310–313.
- dos Santos Ghilardi MG, Cury RG, Dosângelos JS, et al. Long-term improvement of tremor and ataxia after bilateral DBS of VoP/zona incerta in FXTAS. Neurology. 2015;84(18):1904–1906.
- Hagerman RJ, Hall DA, Coffey S, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3(2):251–262.
- Alusi SH, Glickman S, Aziz TZ, et al. Tremor in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1999;66(2):131–134.
- Proudlock FA, Gottlob I, Constantinescu CS. Oscillopsia without nystagmus caused by head titubation in a patient with multiple sclerosis. J Neuroophthalmol. 2002;22(2):88–91.
- Pittock SJ, McClelland RL, Mayr WT, et al. Prevalence of tremor in multiple sclerosis and associated disability in the Olmsted County population. Mov Dis: Off J Mov Disord Soc. 2004;19(12):1482–1485.
- Alusi SH, Worthington J, Glickman S, et al. A study of tremor in multiple sclerosis. Brain. 2001;124(Pt 4):720–730.
- Koch M, Mostert J, Heersema D, et al. Tremor in multiple sclerosis. J Neurol. 2007;254(2):133–145.
- Labiano-Fontcuberta A, Benito-Leon J. Understanding tremor in multiple sclerosis: prevalence, pathological anatomy, and pharmacological and surgical approaches to treatment. Tremor Other Hyperkinet Mov (N Y). 2012;2:1–10.
- Zakaria R, Vajramani G, Westmoreland L, et al. Tremor reduction and quality of life after deep brain stimulation for multiple sclerosis-associated tremor. Acta Neurochir (Wien). 2013;155(12):2359–2364. discussion 2364
- Herzog J, Hamel W, Wenzelburger R, et al. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain. 2007;130(Pt 6):1608–1625.
- Hamel W, Herzog J, Kopper F, et al. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir (Wien). 2007;149(8):749–758. discussion 758
- Nandi D, Aziz TZ. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004;21(1):31–39.
- Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotherapeutics. 2008;5(2):331–338.
- Johnson RD, Qadri SR, Joint C, et al. Perioperative seizures following deep brain stimulation in patients with multiple sclerosis. Br J Neurosurg. 2010;24(3):289–290.
- Moore GR, Vitali AM, Leung E, et al. Thalamic stimulation in multiple sclerosis: evidence for a ‘demyelinative thalamotomy’. Mult Scler. 2009;15(11):1311–1321.
- Krauss JK, Jankovic J. Head injury and posttraumatic movement disorders. Neurosurgery. 2002;50(5):927–939. discussion 939–940
- Broggi G, Brock S, Franzini A, et al. A case of posttraumatic tremor treated by chronic stimulation of the thalamus. Mov Dis: Off J Mov Disord Soc. 1993;8(2):206–208.
- Hooper J, Simpson P, Whittle IR. Chronic posttraumatic movement disorder alleviated by insertion of meso-diencephalic deep brain stimulating electrode. Br J Neurosurg. 2001;15(5):435–438.
- Umemura A, Samadani U, Jaggi JL, et al. Thalamic deep brain stimulation for posttraumatic action tremor. Clin Neurol Neurosurg. 2004;106(4):280–283.
- Broggi G, Franzini A, Tringali G, et al. Deep brain stimulation as a functional scalpel. Acta Neurochir Suppl. 2006;99:13–19.
- Franzini A, Cordella R, Messina G, et al. Deep brain stimulation for movement disorders. Considerations on 276 consecutive patients. J Neural Transm (Vienna). 2011;118(10):1497–1510.
- Reese R, Herzog J, Falk D, et al. Successful deep brain stimulation in a case of posttraumatic tremor and hemiparkinsonism. Mov Dis: Off J Mov Disord Soc. 2011;26(10):1954–1955.
- McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve. 1993;16(11):1254–1260.
- Guridi J, Rodriguez-Oroz MC, Arbizu J, et al. Successful thalamic deep brain stimulation for orthostatic tremor. Mov Dis: Off J Mov Disord Soc. 2008;23(13):1808–1811.
- Hassan A, Ahlskog JE, Matsumoto JY, et al. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86:458–464.
- Espay AJ, Duker AP, Chen R, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Dis: Off J Mov Disord Soc. 2008;23(16):2357–2362.
- Magariños-Ascone C, Ruiz FM, Millán AS, et al. Electrophysiological evaluation of thalamic DBS for orthostatic tremor. Mov Dis: Off J Mov Disord Soc. 2010;25(14):2476–2477.
- Yaltho TC, Ondo WG. Thalamic deep brain stimulation for orthostatic tremor. Tremor Other Hyperkinet Mov (N Y). 2011;1:1–2.
- Lyons MK, Behbahani M, Boucher OK, et al. Orthostatic tremor responds to bilateral thalamic deep brain stimulation. Tremor Other Hyperkinet Mov (N Y). 2012;2:1–4.
- Contarino MF, Bour LJ, Schuurman PR, et al. Thalamic deep brain stimulation for orthostatic tremor: clinical and neurophysiological correlates. Parkinsonism Relat Disord. 2015;21(8):1005–1007.
- Hassan A, Ahlskog JE, Matsumoto JY, et al. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86(5):458–464.
- Coleman RR, Starr PA, Katz M, et al. Bilateral ventral intermediate nucleus thalamic deep brain stimulation in orthostatic tremor. Stereotact Funct Neurosurg. 2016;94(2):69–74.
- Bain PG. The management of tremor. J Neurol Neurosurg Psychiatry. 2002;72(Suppl 1):I3–I9.
- Bain PG, Britton TC, Jenkins IH, et al. Tremor associated with benign IgM paraproteinaemic neuropathy. Brain. 1996;119(Pt 3):789–799.
- Weiss D, Govindan RB, Rilk A, et al. Central oscillators in a patient with neuropathic tremor: evidence from intraoperative local field potential recordings. Mov Dis: Off J Mov Disord Soc. 2011;26(2):323–327.
- Růzicka E, Jech R, Zárubová K, et al. VIM thalamic stimulation for tremor in a patient with IgM paraproteinaemic demyelinating neuropathy. Mov Dis: Off J Mov Disord Soc. 2003;18(10):1192–1195.
- Breit S, Wächter T, Schöls L, et al. Effective thalamic deep brain stimulation for neuropathic tremor in a patient with severe demyelinating neuropathy. J Neurol Neurosurg Psychiatry. 2009;80(2):235–236.
- Deuschl G, Elble R. Essential tremor–neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Dis: Off J Mov Disord Soc. 2009;24(14):2033–2041.
- Fasano A, Bove F, Lang AE. The treatment of dystonic tremor: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85(7):759–769.
- Loher TJ, Krauss JK. Dystonia associated with pontomesencephalic lesions. Mov Dis: Off J Mov Disord Soc. 2009;24(2):157–167.
- Pahwa R, Lyons KE, Wilkinson SB, et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104(4):506–512.
- Fasano A, Herzog J, Raethjen J, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain. 2010;133(Pt 12):3635–3648.
- Earhart GM, Clark BR, Tabbal SD, et al. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Dis: Off J Mov Disord Soc. 2009;24(3):386–391.
- Hosseini H, Mandat T, Waubant E, et al. Unilateral thalamic deep brain stimulation for disabling kinetic tremor in multiple sclerosis. Neurosurgery. 2012;70(1):66–69.
- Barbe MT, Maarouf M, Alesch F, et al. Multiple source current steering–a novel deep brain stimulation concept for customized programming in a Parkinson’s disease patient. Parkinsonism Relat Disord. 2014;20(4):471–473.
- Barbe MT, Dembek TA, Becker J, et al. Individualized current-shaping reduces DBS-induced dysarthria in patients with essential tremor. Neurology. 2014;82(7):614–619.
- Ramirez-Zamora A, Kahn M, Campbell J, et al. Interleaved programming of subthalamic deep brain stimulation to avoid adverse effects and preserve motor benefit in Parkinson’s disease. J Neurol. 2015;262(3):578–584.
- Reich MM, Steigerwald F, Sawalhe AD, et al. Short pulse width widens the therapeutic window of subthalamic neurostimulation. Ann Clin Transl Neurol. 2015;2(4):427–432.
- Contarino MF, Bour LJ, Verhagen R, et al. Directional steering: a novel approach to deep brain stimulation. Neurology. 2014;83(13):1163–1169.
