ABSTRACT
Introduction: Neurocysticercosis (NC) is a neglected disease that contributes substantially to neurological morbidity/mortality in lower-income countries and increasingly among high-income countries due to migration. Many advances have been made in understanding NC, but unanswered questions remain
Areas covered: This review discusses the epidemiology, pathophysiology, immunology, diagnosis, treatment, and eradication of NC.
Expert commentary: The global NC prevalence remains unknown and needs proper ascertainment. Further understanding of the pathophysiology of extraparenchymally located cysts is needed to improve management. The role of inflammation, which is required for parasite death and reabsorption, but may lead to severe complications, must be better understood. Valid screening tools including immunological and molecular tests need to be developed to reduce the reliance on neuroimaging which is not usually accessible in endemic areas. Prognosis for people with parenchymal NC is generally good after treatment, but there are no sufficiently powered randomized trials evaluating antiparasitic treatment for extraparenchymal NC. Most people with seizures do not develop epilepsy. Overemphasizing NC as the main cause of epilepsy could increase stigmatization with potential medico/social implications. Several tools for prevention and control of taeniasis/cysticercosis are available, but strategies to eradicate NC must be created with the involvement of all stakeholders.
1. Introduction
Neurocysticercosis (NC), the larval phase of Taenia solium in the human central nervous system (CNS), is a neglected and usually poverty-related disease of high public health importance [Citation1]. NC is likely the most common parasitic disease involving the CNS worldwide and remains a substantial burden in terms of morbidity and mortality not only in endemic lower-income countries in Latin America, Africa, and Asia but also increasingly in high-income countries due to migration [Citation2–Citation4]. Despite increasing awareness about NC, there has been a surprising lack of investment in cost-effective eradication measures at a community level [Citation5]. So far, there are no sustainable, reliable long-term programs to eradicate this parasitic disease, apart from palliative short-term measures [Citation6–Citation9].
Although NC has often been described as a unique disease, it is crucial to ascertain the parasite location in the human host’s CNS to differentiate between parenchymal and extraparenchymal NC, which are two distinct entities from clinical, immunological, and pathophysiological perspectives [Citation10–Citation12]. Due to the heterogeneity of NC, it may not be well understood nor adequately managed by medical providers, with the subsequent risk that individuals undergo unnecessary or even dangerous procedures, such as surgery to remove parasites in degenerative or calcified forms [Citation13,Citation14]. This clinical uncertainty, together with the high percentage of individuals with NC who are asymptomatic, makes it difficult for policy makers to recommend and implement measures to eradicate NC [Citation15–Citation18].
We have carried out a critical review of current NC knowledge, mainly based on original research studies and some updated reviews to obtain a more objective view and to provide recommendations to the medical and public health communities.
2. Epidemiological facts about taeniasis/cysticercosis: a challenging issue
Cysticercosis was estimated to affect 50 million people and cause 50,000 deaths in 1990 [Citation19]. More recent data estimate that 370,710 (95% confidence interval [CI]: 282,937–478,123) individuals were affected by NC in 2010 and that it was the cause of death of 28,114 (95% CI: 21,059–36,915) individuals [Citation20]. These are rough estimations (as shown by the CIs), as it is difficult to make such determinations in endemic countries that do not have the diagnostic tools required for accurate diagnosis. Additionally, there are relatively few population-based data on prevalence and incidence of NC [Citation21–Citation23]. A review of NC focused mainly on community-based studies reported prevalence estimates in Latin America, Asia, and sub-Saharan Africa ranging from 13% to 54% [Citation16]. A number of issues exist with estimating NC prevalence, including lack of an adequate case definition. Some studies have based the diagnosis of NC on the visualization of calcification on computed tomography (CT) scans; however, calcifications alone are not a definitive diagnostic criterion for NC. Other studies have relied on serum immunological tests (e.g. enzyme-linked immunoelectrotransfer blot [EITB]) as diagnostic when it is known that these tests alone do not necessarily indicate presence of active NC [Citation14].
Another issue in estimating the prevalence of NC is that a large proportion of affected people are apparently asymptomatic, empirically assumed to be the case in about half of individuals with NC [Citation16]. A study in an endemic area in Mexico found that 9.1% of apparently healthy participants had calcifications, probably due to NC [Citation24]. Another study from India showed that about 15% of all asymptomatic individuals in a pig farming community had NC with different stages of cysts in the brain, with the majority of them (64.4%) in the calcified stage [Citation25]. A recent study carried out in a highly endemic village in Peru reported that the prevalence of brain calcifications on CT scan among adults was 18.8% and that most (83.3%) were asymptomatic [Citation26].
Studies related to mortality are rare. There are a few hospital-based studies reporting deaths mainly among people with extraparenchymal NC [Citation27] and limited population-based studies [Citation28]. However, a recent study from Brazil reported a systematic assessment of the epidemiological patterns, time trends, and spatial distribution of NC-related mortality [Citation29]. In this study, the age-adjusted mortality rate for the period 2000–2011 was 0.97 deaths/1,000,000 inhabitants (CI: 0.83–1.12). Disability-adjusted life years (DALYs) have been calculated for NC within the Global Burden of Disease study and were estimated at 7 DALYs/100,000 people globally, which puts NC into the lower range compared with other neglected parasitic diseases [Citation16].
Much information has been generated in different parts of the world but, unfortunately, there is still uncertainty regarding NC prevalence and incidence. A global study with adequate sampling methods and a uniform and valid case definition is needed.
3. Pathophysiology of cysticercosis: in need of a new approach
According to the location of the parasite, NC can be divided into the parenchymal location (in the brain and medulla tissues) and the extraparenchymal location (principally in the intraventricular and subarachnoid spaces, in between the leptomeninges, arachnoids, and pia mater, where the cerebrospinal fluid [CSF] is circulating). The parenchymal and extraparenchymal forms of NC have entirely different clinical, therapeutic, and prognostic outcomes () [Citation10,Citation12,Citation30–Citation33].
Table 1. Differences between parenchymal and extraparenchymal neurocysticercosis.
The improved current knowledge about the pathophysiology of NC relates to parenchymal NC. The evolution of the parasite in this location has been well documented, described by anatomo-pathological and imaging studies through visualization of vesicular, colloidal, granular-nodular, and calcified phases of the parasite [Citation32,Citation33] (). These classical stages of the parasite evolution in the brain parenchyma, however, which correspond to the cysticercus cellulosae form, have not been observed in the extraparenchymal location, especially in the basal cisterns where the parasite adopts the racemose form [Citation32] and appears as a cluster of grapes without scolex. The lack of information regarding extraparenchymal NC pathophysiology is probably, at least in part, related to the difficulty in its diagnosis. Consequently, although there have been many small retrospective studies, case reports, and reviews regarding pathological, clinical, and neuroimaging aspects of the disease, there is a dearth of prospective studies.
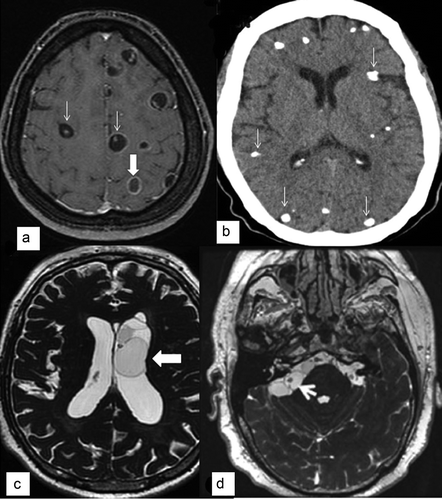
Figure 1. Imaging of parenchymal and extraparenchymal neurocysticercosis.
(A) MRI post-gadolinium T1 sequence showing viable cysts with scolex (thin arrows) and a transitional or degenerative-choloidal cyst (wide arrow).(B) CT scan showing multiple parenchymal calcifications (arrows).(C) 3D MRI sequence (FIESTA) showing cysts into the left ventricle (arrow).(D) 3D MRI sequence (FIESTA) showing cysticerci at the right cerebellopontine cistern (arrow).
It is also unknown whether the parasites in their racemose forms have the capacity of gemmation (asexual reproduction by budding). The appearance of clusters of grapes has long been noted; it is not known whether each ‘grape’ has the potential to individualize. In some people, new parasites are seen over time, sometimes after treatment, and generally in the proximity of the first recognized parasites. One of the reasons could be reinfection, but gemmation cannot be excluded, as it is known to occur in a closely related parasite Taenia crassiceps.
Cysts located in the subarachnoid space over the convexity of the hemispheres, although located extraparenchymally, have a clinical behavior similar to cysts located inside the parenchyma. For example, the effectiveness of cysticidal treatment is also similar to the treatment response of cysts located inside the parenchyma [Citation12]. Conversely, the effectiveness of cysticidal treatment of cysts located in basal cisterns and the intraventricular space is very poor [Citation31,Citation34,Citation35]. This is explained by the fact that in the subarachnoid space over the convexity of the hemispheres, although parasites are located extraparenchymally, they are in closer contact with the parenchyma due to lack of space, a situation that permits contact with a higher concentration of cysticidal drugs and close interaction with the immune cells of the host, necessary for their destruction.
4. Immunology and inflammation: further research is needed
NC is associated with an important immunoinflammatory reaction at both local and systemic levels. Wherever the parasite location, the intensity of the reaction will depend principally on the stage of the parasites. The colloidal stage, when parasites are degenerating, is associated with a higher inflammatory reaction. This is frequently seen at a clinical level with the debut of symptoms, and at a radiological level by the presence of edema and ring contrast enhancement (in parenchymal locations). Consequently, individuals are frequently diagnosed with parenchymal NC when parasites are in this stage () [Citation36–Citation39] as they remain asymptomatic when they have vesicular parasites. In extraparenchymal locations, the situation is different. Inflammation is generally not the cause of initial symptoms which are more frequently related to the mass effect of the parasites, arresting the normal flux of CSF, causing hydrocephalus [Citation12]. This difference is probably due to the distinct characteristics of the environments in which parenchymal and extraparenchymal parasites lie. The growth of extraparenchymal parasites is favored, in part at least, by the ampler available space in the extraparenchymal compartment. Also, the development of inflammatory reaction between the two compartments will be different. In the extraparenchymal space, the presence of parasites is probably recognized early by the host immune system, but the immunological response is less effective than in the parenchymal compartment in which parasites are surrounded by cerebral tissue and in contact with the local immunological cells (microglia, particularly). In CSF, inflammation will progressively increase and will be involved in more late complications (mainly arachnoiditis and vascular events), eventually favored by the administration of cysticidal treatment that may trigger the intensification of the inflammation.
Thus, it is clear that neuroinflammation in NC is a ‘double-edged sword,’ required to cause the death of parasites, but potentially causing an increase in symptomatology and severe complications [Citation40]. Restraining the immunoinflammatory reaction in these patients by using corticosteroids could contribute to treatment nonresponse, despite reducing the risk of inflammation-related symptoms and complications. More studies are needed to describe and understand the role of the different components of the inflammatory reaction, allowing control of the negative side effects of inflammation (involved in complications) while maintaining the benefits of inflammation (death of parasites), with the objective of creating more specific molecules that improve the response to treatment.
5. Clinical aspects: traditional concepts under revision
Prospective studies of NC specifically designed to estimate the incidence of clinical manifestations show that seizures, headache, motor deficits, and cognitive impairment [Citation33,Citation41] are the main symptomatology. It is now clear that clinical manifestations of parenchymal NC are different from those of extraparenchymal NC [Citation12]. When the parasites are localized in the parenchyma, the main clinical manifestations are seizures or focal neurological deficits, which are usually transient, occurring over a few days, weeks, or months, with periods of remission and relapse, most likely due to different evolutionary stages of the parasite [Citation35]. Parenchymal NC is only rarely associated with the development of intractable epilepsy [Citation42,Citation43]. A recent study showed that refractory epilepsy due to calcified parenchymal NC is similar to other structural brain lesions [Citation44]. Extraparenchymal NC (around 15–30% of NC cases reported in different hospital studies) has different clinical manifestations, including intracranial hypertension, cranial nerve abnormalities, and hydrocephalus [Citation33,Citation40] ().
An early report found that when hydrocephalus due to cysticercotic meningitis is present, mortality is high (50%), with most individuals dying within 2 years of CSF shunting [Citation27]. For this reason, ventricular and basal cisternal locations are considered to be malignant forms of NC [Citation31]. With the improvement of diagnosis and treatment, the situation is probably less dire today, although data on this topic are lacking. In NC meningitis, CSF analysis commonly shows mild elevation of protein, hypoglycorrhachia and lymphocytic pleocytosis, which is similar to other chronic basal meningitis, such as tuberculosis and mycosis. When cysticerci are located inside the ventricular system, life-threatening acute intracranial hypertension as a result of hydrocephalus may occur. Cysts in the subarachnoid space may lodge in the Sylvian fissure or basal cisterns and grow to a large size (racemose form), causing a mass effect and intracranial hypertension ().
It is also known that clinical manifestations are different according to age. Most children with NC have mild-to-moderate symptomatology and single lesions; seizures are more frequent in children, and intracranial hypertension and headaches are more frequent in adults [Citation38,Citation45]. Most children with NC have a single transitional or enhancing cyst, also named solitary cysticercus granuloma (SCG). SCG is a common finding in people with newly identified seizures in endemic countries. These individuals, mainly children and young adults, have some benign and transitory clinical manifestations, predominantly partial or partial secondary generalized seizures, and occasionally Todd’s paresis or focal neurological deficits. Clinical and radiologic features consistent with a diagnosis of SCG have been well described elsewhere [Citation38,Citation46]. Resolution of the SCG tends to occur spontaneously, since the parasite is already in the degenerative phase and will eventually disappear or become calcified.
The relationship between NC and a wide range of diverse symptoms and signs reported in people with symptomatic NC is less clear. These symptoms include involuntary movements, extrapyramidal signs, stroke-like symptoms, manifestations of brainstem dysfunction, Klüver–Bucy syndrome, and cortical blindness [Citation47]. Most reports, however, are based on retrospective studies, anecdotal reports or case series, without definitions nor reliable diagnostic criteria [Citation15], which makes it difficult to assume causality between these pathologies and NC. Cysticercotic encephalitis, a rare but severe form of parenchymal NC, usually affects children and young people in the first two decades of life. These patients have hundreds of viable or degenerating cysts with a diffuse inflammatory showed by imaging procedures, and treatment is symptomatic and prognosis uncertain.
Prospective cohort studies using validated diagnostic criteria are needed to establish the frequency of clinical manifestations, especially in the extraparenchymal forms of the disease.
6. Seizures, epilepsy, and epileptogenesis of NC: more questions than answers
More than two decades ago, it was suggested that NC could be considered a human model of epileptogenesis [Citation48], a hypothesis that was recently revisited [Citation49]. The multifactorial concept proposed [Citation50] based on the triad of epileptogenic abnormality, seizure threshold, and precipitating factors could be applied to the development of seizures/epilepsy in people with NC (). This model emphasizes the features of genetic predisposition to a low or high seizure threshold in people with NC contributing to the development of epilepsy, which would explain the large number of people who are asymptomatic [Citation51]. It should be a priority to explore potential biomarkers of NC at different cyst phases of evolution of the parasite, which might have a predictive value for development of seizures or epilepsy; these might include serial magnetization transfer imaging [Citation52], serum matrix metalloproteinase-9 levels [Citation53], toll-like receptor polymorphism [Citation54], gel filtration fraction [Citation55]. Development of relevant animal models also needs to be considered [Citation56].
Figure 2. Multifactorial basis of epilepsy in patients with neurocysticercosis. (A) Three factors involved in epileptogenesis in NC. (Top) Sinuous line indicates seizure threshold or propensity for a seizure to occur. (Middle) Represents structural lesion due to the parasite evolutionary phase that changes over time: a, viable; b, colloidal; c, granular-nodular; and d, calcified cyst. The evolution of cysts phases lasts around 6 months on average, from the viable to calcified stage. (Bottom) Arrows illustrate potential precipitating factors or a cascade of mechanisms that may contribute to trigger seizures. Someone with a high threshold may have parasitic epileptogenic lesions and never have seizures (i.e. asymptomatic patients). (B) Someone with a low threshold could have acute seizures, as indicated by the red lines around the cysts (i.e. ictogenesis) due to parasitic epileptogenic lesions and precipitating factors, and seizures recur over time since there is a genetic predisposition to maintain low threshold (i.e. epileptogenesis). (C) Someone with a low threshold could have acute seizures due to parasitic epileptogenic lesions and precipitating factors, but seizures do not recur, since there is no genetic predisposition to maintain a low threshold and they do not evolve into epilepsy. Republished with permission from [Citation51].
![Figure 2. Multifactorial basis of epilepsy in patients with neurocysticercosis. (A) Three factors involved in epileptogenesis in NC. (Top) Sinuous line indicates seizure threshold or propensity for a seizure to occur. (Middle) Represents structural lesion due to the parasite evolutionary phase that changes over time: a, viable; b, colloidal; c, granular-nodular; and d, calcified cyst. The evolution of cysts phases lasts around 6 months on average, from the viable to calcified stage. (Bottom) Arrows illustrate potential precipitating factors or a cascade of mechanisms that may contribute to trigger seizures. Someone with a high threshold may have parasitic epileptogenic lesions and never have seizures (i.e. asymptomatic patients). (B) Someone with a low threshold could have acute seizures, as indicated by the red lines around the cysts (i.e. ictogenesis) due to parasitic epileptogenic lesions and precipitating factors, and seizures recur over time since there is a genetic predisposition to maintain low threshold (i.e. epileptogenesis). (C) Someone with a low threshold could have acute seizures due to parasitic epileptogenic lesions and precipitating factors, but seizures do not recur, since there is no genetic predisposition to maintain a low threshold and they do not evolve into epilepsy. Republished with permission from [Citation51].](/cms/asset/b7a95f40-c4f1-4f31-99a4-f45e1f5a25c5/iern_a_1451328_f0002_oc.jpg)
It is important when considering the relationship between NC and epilepsy to differentiate between seizures and epilepsy, since not all people with seizures have epilepsy [Citation57,Citation58]. Seizures related to NC may be categorized as either acute symptomatic seizures or as unprovoked seizures. Acute symptomatic seizures result from transitional or degenerating cysts and are a consequence of the acute inflammatory response of the brain. In contrast, an individual with seizures who has viable cysts and/or inactive, non-inflamed calcified cysts is categorized as having unprovoked seizures [Citation14]. Unfortunately, the distinction between seizures and epilepsy (i.e. recurrence of seizures) has not always been well recognized, in spite of the fact that this distinction is essential, as they are different entities with regard to etiology, treatment, and prognosis [Citation57,Citation58]. Most people with NC likely have acute symptomatic seizures, which do not necessarily evolve to epilepsy [Citation14]. A recent report from the International League Against Epilepsy includes NC as a comorbid condition associated with epilepsy [Citation57].
According to the International League Against Epilepsy [Citation59], epilepsy is defined as
a disease of the brain defined by any of the following conditions: 1. At least two unprovoked (or reflex) seizures occurring > 24 hours apart; 2. one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; or 3. diagnosis of an epilepsy syndrome.
A study carried out in children with seizure due to SCG showed that a relative risk of seizure recurrence in a child with abnormal CT and electroencephalography prior to antiepileptic drugs (AED) withdrawal was 26.2 [Citation60]; however, there are no prospective cohort studies that have measured the risk of seizure recurrence in patients with viable NC. Prospective cohort studies are needed to determine the risk of developing epilepsy among people with parenchymal viable cysts.
There is no doubt that seizures are the most frequent symptom of parenchymal NC, but the course and outcome of NC-associated seizures and epilepsy remain poorly understood. It is possible to overemphasize NC as a cause of epilepsy [Citation17,Citation18], which may lead to other potential causes or risk factors for acquiring epilepsy being ignored and could lead to inappropriate clinical management of people with epilepsy [Citation17]. Furthermore, people with NC might be erroneously labeled as ‘epileptic,’ and may undergo unwarranted long-term treatment subjecting them to potential side effects and have significant unnecessary adverse biopsychosocial and employment-related implications [Citation61].
Prospective and case-control studies designed to analyze the relationship and determinants of epilepsy related to NC are scarce [Citation17,Citation18]. Comparing results of studies based on NC as etiology of epilepsy is difficult because of differences in the definitions used and, until recently, lack of validated diagnostic criteria for NC. Most studies do not provide information on the latency between the first acute symptomatic seizure and the first unprovoked seizure, or on age at onset of seizures and at diagnosis of NC. Of course, it is also important to measure incident seizures, as temporality is critical to ascertain [Citation14,Citation18]. In countries with a high prevalence of NC, there is a substantial lack of diagnostic tools and consequently genetic causes of epilepsy and etiologies where magnetic resonance imaging (MRI) is mandatory for diagnosis, such as mesial temporal sclerosis and cortical malformations, are probably underreported [Citation17].
Recent critical reviews [Citation17,Citation18] investigated bias in reports about the relationship between epilepsy and NC by assessing results of meta-analyses [Citation23,Citation62]. These critical reviews suggested that these meta-analyses were controversial and that a significant weakness of many studies included in the meta-analyses was that they relied only on serologic tests as a proxy for NC diagnosis among people with epilepsy; furthermore, the people with epilepsy included in these studies may have had other putative etiologies aside from NC.
A meta-analysis exploring the association between NC exposure and epilepsy based on studies in Africa reported an odds ratio (OR) of 3.4, indicating more than threefold odds of having epilepsy in individuals infected with NC [Citation63]. This review, however, did not specify imaging as an inclusion criterion, instead relying almost exclusively on serological data, which may introduce misclassification as seropositivity does not infer a definitive diagnosis of NC. A recent meta-analysis also analyzed the relationship between NC and the development of epilepsy [Citation18] finding an OR of 2.8, indicating greater odds of epilepsy in a person with NC than an uninfected individual. An important conclusion from this study was that data were inadequate to enable inferences about a causal relationship and also concluded that studies had numerous limitations, including a general lack of consistency in diagnostics and lack of accurate epidemiological data. A recent paper aimed to assess the prevalence of NC and its relationship with epilepsy and concluded that people with epilepsy had three times the odds of having NC than those without epilepsy [Citation64], which replicated similar results of other studies and shared methodological limitations [Citation17,Citation18].
How calcified granulomas behave in the host CNS remains an enigma. Calcified lesions are often seen on CT scans of asymptomatic individuals [Citation24]. Conversely, single calcified lesions have been associated with mesial temporal lobe epilepsy due to hippocampal sclerosis [Citation65]. Parasite location may be remote from the apparent epileptogenic region and there is no clear correlation between the lesion burden and epilepsy severity [Citation14]. The mechanism by which calcified lesions cause epilepsy is not known, but it has been attributed to residual perilesional gliosis that results in chronic epileptogenic foci [Citation66].
Perilesional edema associated with calcified lesions after seizures was described in a retrospective study [Citation67], which hypothesized that persistent enhancement could represent a lesion in the process of completely resolving. This perilesional edema has also been proposed as being due to preservation of antigenic material, which provokes inflammatory response triggering seizures [Citation68]. Another study, not yet replicated, reported that 50% of people with brain calcifications also had perilesional edema [Citation69]. It is not clear whether this edema is the cause or the consequence of seizure [Citation66], so more research is needed.
7. We have many diagnostic tools, but do they really permit the diagnosis of the population at risk?
Diagnosis of NC has progressively improved in recent decades. In the 1950s, the complement fixation test and some aggressive radiological techniques (e.g. ventriculography and myelography) were available and of some use. Most diagnoses were made based on surgical findings. Introduction of CT scanning in the 1970s and of MRI in the 1980s completely changed this. In parallel, the deployment of other immunological techniques (particularly hemagglutination, radioimmunoassay, enzyme-linked immunosorbent assay [ELISA], dipstick ELISA, latex agglutination, and immunoblot) and the use of more specific antigens have improved diagnosis.
Currently, only neuroimaging can provide evidence for the presence of a cysticercus in the CNS and is indispensable before the administration of any specific treatment. For the diagnosis of parenchymal parasites, the benefit of neuroimaging has long been known. Different classifications and criteria for the use of neuroimaging have been published, and the advantages and disadvantages of CT scan and MRI are established for each of the parasite’s stages. The diagnosis of extraparenchymal forms is more challenging. In these locations cysts lie in CSF, and the intensity of vesicular fluid is similar to the intensity of CSF, thus CT scanning and classical MRI sequences do not have the necessary precision to distinguish the thin parasite membrane. Until recently, radiological diagnosis was almost always made taking into account indirect signs such the unilateral enlargement of a basal cistern, with the risk of errors that this entails. It was only recently shown that 3D MRI sequences (Fast Imaging Employing Steady-state Acquisition [FIESTA], Constructive Interference in Steady-State [CISS], Spoiled Gradient Recalled Echo [SPGR]), allowing an enhanced contrast within the subarachnoid space and the ventricular system, were ideal to discriminate membranes in these locations [Citation70,Citation71]. Today, these tools allow precise information regarding number, state, and location of extraparenchymal parasites.
Regarding immunological tools, currently, the two most commonly used techniques for antibodies detection are ELISA and immunoblot. More recently, ELISA antigen detection has also been developed. Antigen detection by ELISA in the CSF of individuals with NC is an important marker of disease activity, independent of the stage of the cysts [Citation72]. The main advantage of antigen detection is its specificity for diagnosis of viable parasites (i.e. those requiring specific treatment), while antibodies can persist after their elimination [Citation73,Citation74]. This is particularly relevant in extraparenchymal location as their radiological detection is difficult when there is no access to 3D MRI sequences. Immunological tools are helpful in diagnosis, but the main problem with their use is related to the location and cyst characteristics. Localization of the larvae of T. solium in the CNS causes the most severe presentation of cysticercosis [Citation31,Citation75], but parasites can also be lodged in other compartments, particularly muscles and subcutaneous tissue. In these situations, serological detection of antibodies and of antigens (if parasites are viable) will also be positive. To avoid the lack of specificity of serological diagnostic tools, CSF testing in case of extraparenchymal disease is recommended [Citation73,Citation76]. For diagnosis at a community level, this is not an option as lumbar puncture is invasive and only possible in specialized clinical settings. It is, however, relevant to note that in this compartment, ELISA and immunoblot have similar performance [Citation77]. Molecular techniques (DNA-based technology) for NC diagnosis have also been used. A recent prospective study demonstrated that a polymerase chain reaction (PCR) assay in CSF had high sensitivity and specificity for extraparenchymal forms of NC, which is of particular value since it could be used when neuroimaging techniques are inconclusive [Citation78].
Based on many of these advances, a new proposal for the diagnosis of NC has been recently validated for the first time [Citation36]. An attribute of the new criteria is that they permit the distinction between parenchymal and extraparenchymal NC (). Using the new criteria, there was high sensitivity (89.8%) and specificity (80.7%) for detecting parenchymal disease, but specificity increased to 94.9% for detecting extraparenchymal NC. Sensitivity for extraparenchymal NC was 65.9%, indicating there is still room for improvement. A recent study [Citation79] questioned the validity of the diagnostic criteria described above for ventricular NC; nevertheless, methodological problems of this biased study were clearly demonstrated [Citation80].
Table 2. New diagnostic criteria for symptomatic neurocysticercosis.
When making a diagnosis we have useful neuroimaging tools that can be complemented with helpful immunological and molecular tools. Unfortunately, the cost of these tools is high. Endemic areas, due to the characteristics of the life cycle of T. solium, are usually impoverished where poor sanitation, free pig rearing, and poor water quality persist. Most people living in these settings do not have the economic resources to allow them to access the appropriate diagnostic facilities. Neuroimaging and immunoblot studies are expensive and results of ELISA alone (as any immunological study) are not enough to start a specific treatment. Paradoxically, the EITB test is more expensive than a CT scan in some Latin American endemic countries.
The development of a more specific serological screening diagnostic tool would be useful; this could perhaps be the detection of a marker of neurological involvement in addition to the detection of specific antibodies. Such a test should be available at the community level and could permit better identification of people who should have a radiological study. In this sense, adaptation of this potential test to a lateral flow assay format will be of great help, as its feasibility was shown recently for antigen detection in CSF [Citation76].
8. How effective is current treatment for NC?
Treatment of NC should be individualized and symptomatic therapy, anthelminthic drugs (AHDs), and surgery should be considered depending on location and parasite viability and clinical manifestations (). Symptomatic therapy consists of AED for seizures, mannitol for high intracranial pressure, analgesics for headache, and steroids to reduce inflammation and edema around dying parenchymal cysts, as well as for arachnoiditis. The optimal dose, duration, and timing of administration of corticosteroids remain unknown [Citation34].
Table 3. Treatment of neurocysticercosis.
Treatment with AHDs has been available for at least 30 years, but their use is still controversial. Praziquantel (PZQ) was first used in Mexico [Citation81] and albendazole (ALB) first used in China [Citation82,Citation83]. At present, there is evidence that AHDs treatment (ALB, PZQ) is efficacious for viable cysts in the parenchyma, according to placebo-controlled clinical trials [Citation34,Citation84–Citation86]. ALB is effective in about 40% of people with parenchymal viable cysts, measured as disappearance of cysts in imaging studies (). Combining PZQ and ALB, rather than using them separately, has been suggested to increase the therapeutic efficacy of AHDs [Citation22,Citation87]. A study used this combined therapy versus ALB alone, in children with seizures and a single enhancing lesion [Citation88], but the differences were not statistically significant. Another study utilized the same combination and a similar design in individuals with viable parenchymal cysts [Citation22]; the combined treatment was more effective (64%) than ALB monotherapy (37%). shows there were no statistically significant differences in seizure remission between groups of all clinical trials.
Table 4. Controlled clinical trials regarding effects of albendazole on resolution of viable parenchymal cysts and seizures recurrence.
An evidence-based guideline for the treatment of parenchymal NC concluded that ALB therapy, used with or without corticosteroids, is probably effective in decreasing long-term seizure frequency and radiologically demonstrable cysts numbers in adults and children [Citation88]. There were, however, insufficient data to assess PZQ efficacy. One meta-analysis of response to AHDs concluded that studies provided evidence of a modest effect of NC treatment in viable parenchymal cysts, with relatively small effects of cysticidal treatment (OR 2.2 [95% CI: 1.10–5.01]) [Citation89]. A Cochrane review concluded that in individuals with viable lesions, evidence from trials of adults suggests that ALB may reduce the number of lesions [Citation90]. The outcome ‘reduction of the number of lesions’ to measure treatment effectiveness might be misleading. If a person with 10 viable cysts has been administered AHDs, and, 8 parasites die (80% reduction of lesions), 2 cysts still remain which may continue to cause seizures or headache; therefore, this outcome should not be considered successful. The most appropriate end point to evaluate the effectiveness of AHDs should be complete cyst disappearance. This review also concluded that clinical efficacy of AHDs is still in question as there are no randomized controlled trials (RCTs) to determine definitive doses and treatment duration.
In the case of SCG, the parasite is already dead and will resolve or calcify; therefore, regardless of its size or location, it should not be biopsied or removed (pathologies such as pyogenic abscess, tuberculoma, and metastatic brain tumors should be excluded). The treatment of SCG remains controversial. Several RCTs of AHDs and corticosteroids have been conducted in SCG, but most are small, without enough statistical power [Citation34]. A recent meta-analysis [Citation47] to determine the effect of different therapies on RCTs for SCG concluded that dual therapy of ALB and corticosteroids was the most efficacious regimen that could prevent seizure recurrence and promote lesion resolution in a follow-up period of around 1 year. However, the authors recommended this management of SCG until more high-quality evidence is available. A large, multicenter trial with sufficiently long follow-up, comparing outcomes with the use of AHDs with or without corticosteroids and corticosteroids alone in order to ascertain the benefits of these drugs, is missing.
Regarding treatment of extraparenchymal NC, there is much less information. This is related to the difficulty of its precise radiological localization and also to the lower frequency of these forms. Some case series have reported AHDs to be effective in extraparenchymal forms, although nonresponse was frequent even when using a high dose of ALB [Citation31]. In an RCT, disappearance of extraparenchymal cysts with ALB (15 mg/kg/day for 8 days) did not reach significance [Citation34]. One randomized study comparing 15 vs. 30 mg/kg of ALB for 8 days showed a significantly higher efficacy of the latter dose for reducing extraparenchymal cyst volumes [Citation35]. Neurosurgical intervention should be considered for hydrocephalus requiring ventricular-peritoneal shunt or intraventricular excision of a cyst (). This form of the disease has a worse prognosis and is associated with a higher fatality, and many questions remain about which anthelminthic agent (or potentially combination of agents), dose, and treatment duration are necessary. The use of corticosteroids in these forms should also be fully assessed. Their use is recommended to avoid the severe inflammatory complications that may occur (e.g. stroke and arachnoiditis), but their immunosuppressive function could favor nonresponse in some individuals [Citation31,Citation40]. RCTs are needed to evaluate this aspect and to assess more efficient anthelminthic therapeutic schemes.
The effect of AHDs on recurrence of seizures is still under debate. Most trials that specifically examined the risk of seizure recurrence during AHDs administration found that the risk was minimal and not significantly different from the control groups (). A Cochrane review found no significant difference when ALB was compared to no treatment for recurrence of seizures in people with viable parenchymal cysts [Citation90]; other studies found a modest effect of ALB in patients with degenerating cysts [Citation46]. Despite the lack of a statistically significant effect of AHDs on seizure recurrence, acute symptomatic seizures generally have a good prognosis in terms of remission [Citation30,Citation86,Citation91]. The risk of seizure recurrence after a first seizure due to NC is between 17% and 56%, depending on parasite viability, with greater risk with transitional cysts and decreased risk with viable or calcified cysts [Citation14,Citation53]. Overall, seizure recurrence affects up to a third of individuals. The current information is still incomplete and does not allow formulation of guidelines. It is therefore recommended that a large, multicenter trial be conducted with sufficiently long follow-up.
Another missing point is related to the treatment of seizures associated with NC. AEDs are routinely employed in the treatment of seizures associated with NC; however, there is no clear consensus regarding the choice and optimal duration of AED treatment [Citation92].
9. Eradication of NC: a socioeconomic problem
The World Health Organization (WHO) adopted a plan for the control and elimination of neglected diseases, including many parasites affecting the CNS, such as NC [Citation93]. Some principles for action addressed populations and interventions rather than specific diseases and the introduction of innovative tools for parasite detection and control. Many World Health Assembly resolutions have also provided a mandate for countries and other stakeholders to become more active in this area and included reference to NC and its prevention [Citation8]. T/C was eliminated in high- and middle-income countries, about a century ago; it has remained as an endemic disease in many low-income countries due to poverty and poor sanitation. Autochthonous transmission in high-income countries – although rare – has been reported in the Iberian Peninsula and areas of Eastern Europe and North America [Citation3–Citation5].
Mass drug administration (MDA) has long been proposed as a strategy to control human taeniasis and porcine cysticercosis. MDA was widely used in Latin America during the 1980s and 1990s [Citation9] as well as more recently [Citation7]. T/C is, however, still prevalent in all those countries and continues to be associated with high morbidity and mortality [Citation8]. MDA is a costly strategy that does not provide a long-term solution for eradicating T/C. It is a temporary solution since unhealthful conditions and poverty prevail in countries where these diseases are endemic. A systematic review of T/C control and elimination through community-based interventions concluded that there is little evidence to support MDA as an effective control of T. solium [Citation6]. This could be partly due to poor study design. This review also concluded that there is insufficient evidence to suggest any community-based intervention to control human and porcine cysticercosis. A recent study in northern Peru attempted to assess the feasibility of interrupting the parasite’s life cycle [Citation7]. The intervention involved repeated cestocide treatments of humans (niclosamide) and pigs (oxfendazole) in a single year, which would be unlikely to be a practical approach in other endemic settings [Citation94]. It is also likely that the reestablishment of T. solium could occur by transmission from surrounding areas.
Control of porcine cysticercosis, by the combined use of vaccines and chemotherapy, is a potentially effective method to control T. solium [Citation95,Citation96]. The main vaccines that have been assessed in field studies are a vaccine using a recombinant oncosphere antigen-based TSOL16 [Citation95], and a peptide-based S3PVac [Citation96]. Swine vaccination could be a good strategy to prevent transmission but the cost of an effective vaccine may be prohibitive for resource-poor communities where farmers often cannot even afford to feed their pigs [Citation97]. A recent development is an approach using transgenic papaya callus lines expressing the components of the S3Pvac vaccine representing a new hope to achieve an efficient and low-cost oral vaccine [Citation98].
These core approaches should be supplemented where possible by longer-term sustainable measures such as health education, focusing on the need for improvements in sanitation, pig husbandry, and meat inspection. Further research remains important to improve tools and permit easy application and standardization of intervention measures to effect control of T/C particularly in resource-poor regions [Citation1]. Ultimately, it should be kept in mind that eradication of T/C needs both a socioeconomic and political approach.
10. Expert commentary
Over the last three decades, there have been many positive achievements with regard to understanding NC; conversely, there have also been some negative points particularly due to limited study designs and their interpretation. There are still many aspects of NC for which information is missing and need to be resolved to improve the knowledge about this parasitic disease, as well inform measures for eradication.
There has been good progress in many areas allowing for some optimism regarding the worldwide situation of NC. A major achievement is that the diagnosis of NC has improved with the deployment of better neuroimaging techniques (CT scan and MRI) allowing the identification of the parasite in different evolutionary stages inside the host. There have been improvements in the immunological (Ab and Ag-ELISA, Ab-EITB) and molecular (PCR) screening which may help the diagnosis. These achievements, in turn, have allowed the delineation of two distinct forms of NC, parenchymal and extraparenchymal, which differ in their clinical presentation, treatment, and prognosis. Prospective cohort studies have shown that seizures, headache, motor deficits, and cognitive impairment are the most common symptoms mainly dependent on age and parasite location. The clinical prognosis in the parenchymal forms is usually good but dependent on the extent of inflammation resulting from parasite degeneration. For example, most people with seizures, the main presentation of parenchymal NC, do not develop epilepsy. During recent years, many studies have explored epileptogenesis by means of potential biomarkers of NC, as well as the potential genetic predisposition that might also be linked to increased risk of epilepsy. Treatment of NC using AHDs (either ALB or PZQ) has been showed to be effective in 30–40% of people with parenchymal NC. Combination of these two drugs is also probably effective in up to 60%. A new proposal to diagnose NC has been recently validated for the first time. Similarly, the WHO vision of controlling, eliminating and eradicating neglected diseases, such as NC, has gathered significant momentum over recent years.
Conversely, some points are still obscure. The global prevalence of NC is uncertain as probably more than 50% of people with NC are asymptomatic and diagnostic tools are frequently not available in endemic areas. Basic estimations of prevalence or incidence are only available in some countries. There is a myriad of NC-related clinical manifestations but many are derived from case reports/series or small studies which may be biased as temporality is usually unknown. Recent systematic reviews and meta-analyses have suggested the lack of reliable evidence to enable an accurate determination of how many cases of epilepsy are caused by NC. Some epidemiological studies have explored this association using immunologic-serological tests to diagnose NC in people with epilepsy, which may be problematic as it may overestimate the number of cases of NC. The efficacy of AEDs for either treating seizures or reducing the risk of subsequent seizures is also unclear. Neuroinflammation as a result of a dying parasite can potentially increase and complicate the symptomatology particularly in the extraparenchymal forms of NC. To date, there are no RCTs that establish the efficacy of medical or surgical treatment for extraparenchymal NC. Altogether, it is disappointing that many basic epidemiological, pathophysiological and clinical aspects of this condition remain unknown despite many years of research efforts.
A large amount of work remains to be done and those involved with NC should join forces to improve the knowledge of T/C to prevent and eradicate it. An international and multi-institutional study, using uniform methodology in different settings (allowing comparison of results across settings), is imperative to provide an unbiased view of the epidemiological profile of NC. Obtaining a serological tool available at the community level with a high specificity for NC diagnosis will be a great achievement. Improvement of therapeutic strategies, especially in the extraparenchymal forms of the disease, is also crucial. More studies to understand the role of different components of the inflammatory reaction are needed. We also need prospective cohort studies including those with new onset seizures and NC to establish how many develop epilepsy, using standardized definitions and a control group to elucidate the relationship between these diseases. T/C is a fecal-oral transmitted parasitic disease, related mainly to poor sanitation and hygiene; therefore, it cannot be solved by any drug alone. The objective of the WHO to scale up interventions for control and elimination of T. solium in selected countries by 2020 must be supported, particularly as it could advance the required knowledge necessary to eradicate this disease, which mainly affects the most vulnerable sector of the population.
11. Five-year view
Despite being considered a potentially eradicable disease, cysticercosis remains a challenge. It could be argued that its occurrence is unacceptable. The objective of the WHO to scale up interventions for control and elimination of T. solium in selected countries in Africa, Asia, and Latin America by 2020 may lead to a major change in the panorama. Particularly, preventive procedures including basic sanitation and strict food hygiene control must be enhanced. Promotion of the habit of using sanitary facilities and rearing pigs in complete separation from human waste are mandatory. National programs must be encouraged in which, particularly, the utilization of cheaper and effective pig vaccines should be promoted.
In the next years, the development of new and more specific serological diagnostic tools will be necessary, to make accessible the radiological tools to all the population. As NC has necessarily a systemic passage, immunological tests in the serum are not entirely reliable for the diagnosis of neurological disease. Research must be done to increase their specificity; this is perhaps possible coupling determination of specific Ab/Ag with a marker of neurological damage. Although use of CSF allows increased specificity of the tests, its collection is not free of risks; lumbar puncture must be performed in hospital settings and this test is not popular and often avoided in many places. Regarding imaging, new MRI sequences are improving diagnosis but the costs are unaffordable in regions where they are most needed. Making this more accessible will be another goal for the next years.
Two effective anthelmintic drugs, ALB and PZQ, are available. Their efficacy for parenchymal cysts is unquestionable; however, their performance is poor in extraparenchymal cysts. Therefore, research aimed at improving the efficiency of cysticidal drugs in this location must be part of the goals for the next 5 years. Conversely, we have been using steroids as the favorite anti-inflammatory drug with good efficacy. One issue in extraparenchymal disease is that steroids may be required over prolonged periods which may lead to undesirable effects. It will be a task for the next years to develop a safe and efficient steroid-like drug devoid of such side effects.
Key issues
Due to advances in neuroimaging technology, hospital diagnosis of NC has improved significantly over the last decades, but the need for diagnosis outside of specialized settings has created a need to identify and evaluate alternative diagnostic tools.
The parenchymal and extraparenchymal locations of NC are quite different from the immunological, pathophysiological, and clinical points of view, with entirely different therapeutic and prognostic scenarios.
More studies are needed to understand the role of the different components of the inflammatory reaction that causes death of parasites but can also cause increased risk of severe complications.
Overemphasis of NC as a cause of epilepsy may lead to other potential causes of epilepsy being ignored and could lead to inappropriate clinical management of epilepsy.
Prognosis of parenchymal NC is generally good, since in most people seizures do not evolve to epilepsy; conversely, the extraparenchymal forms of NC have a high morbidity and mortality with potentially permanent sequelae.
Double-blinded placebo-controlled clinical trials have shown efficacy of AHDs in around half of patients with parenchymal NC; however, AHDs has not been shown to significantly reduce risk of seizure recurrence. To date there are no standard treatment guidelines for extraparenchymal NC, as sufficiently powered trials are lacking.
Properly designed prospective studies are needed to better understand the natural history of NC and its epidemiology, as well as the distribution of clinical manifestations.
Adequate government health policies in endemic countries, including vaccination of pigs, and proper sanitation and hygiene to prevent T/C are needed.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Acknowledgments
We would like to thank Dr. Josemir W. Sander (NIHR University College London Hospitals, UK) for providing useful commentary on an early draft of this paper.
Additional information
Funding
References
- World Health Organization. Assembling a framework for intensified control of taeniasis and neurocysticercosis caused by Taenia solium. Report of an informal consultation. Geneva: WHO Headquarters; 2014 July 17-8.
- Fleury A, Moreno García J, Valdez Aguerrebere P, et al. Neurocysticercosis, a persisting health problem in Mexico. PLoS Negl Trop Dis. 2010;4:e805.
- Zammarchi L, Angheben A, Gobbi F, et al. Profile of adult and pediatric neurocysticercosis cases observed in five Southern European centers. Neurol Sci. 2016;37:1349–1355.
- Devleesschauwer B, Allepuz A, Dermauw V, et al. Taenia solium in Europe: still endemic? Acta Trop. 2017;165:96–99.
- Borzello M, Mateen FJ. Time for a new March of Dimes. Neurology. 2016;86:e85–e88.
- Carabin H, Traoré AA. Taenia solium taeniasis and cysticercosis control and elimination through community-based interventions. Curr Trop Med Rep. 2014;1:181–193.
- Garcia HH, O’Neal SE, Gilman RH, for the Cysticercosis Working Group in Peru. Elimination of Taenia solium transmission in Peru. N Engl J Med. 2016;374:2335–2344.
- Carpio A, Fleury A, Parkhouse M. Elimination of Taenia solium transmission in Peru. N Engl J Med. 2016;375:1196–1197.
- Okello AL, Thomas LF. Human taeniasis: current insights into prevention and management strategies in endemic countries. Risk Manag Healthc Policy. 2017;10:107–116.
- Carpio A, Romo ML, Parkhouse RM, et al. Parasitic diseases of the central nervous system: lessons for clinicians and policy makers. Expert Rev Neurother. 2016;16:401–414.
- Tuero I, Palma S, Cabeza F, et al.; for the Cysticercosis Working Group in Perú. A comparative study of peripheral immune responses to Taenia solium in individuals with parenchymal and subarachnoid neurocysticercosis. PLoS Negl Trop Dis. 2015;9:e0004143.
- Marcin Sierra M, Arroyo M, Cadena Torres M, et al. Extraparenchymal neurocysticercosis: demographic, clinicoradiological, and inflammatory features. PLoS Negl Trop Dis. 2017;11:e0005646.
- Fleury A, Escobar A, Fragoso G, et al. Clinical heterogeneity of human neurocysticercosis results from complex interactions among parasite, host and environmental factors. Trans R Soc Trop Med Hyg. 2010;104:243–250.
- Carpio A, Romo ML. The relationship between neurocysticercosis and epilepsy: an endless debate. Arq Neuropsiquiatr. 2014;72:383–390.
- Carabin H, Ndimubanzi PC, Budke CM, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop 14 Dis. 2011;5:e1152.
- Winkler AS, Richter H. Landscape analysis: management of neurocysticercosis with an emphasis on low- and middle-income countries. Geneva, Switzerland: Commissioned by the World Health Organization; 2015. Available from:: http://apps.who.int/iris/bitstream/10665/152896/1/WHO_HTM_NTD_NZD_2015.05_eng.pdf
- Tellez-Zenteno JF, Hernandez-Ronquillo L. Epidemiology of neurocysticercosis and epilepsy, is everything described? Epilepsy Behav. 2017;76:146–150.
- Gripper LB, Welburn SC. The causal relationship between neurocysticercosis infection and the development of epilepsy – a systematic review. Infect Dis Poverty. 2017;6:31.
- World Health Organization. Update: International Task Force for Disease Eradication. Weekly Epidemiological record 1992; 1992. 46. [cited 2017 September 1]. Available from: http://apps.who.int/iris/bitstream/10665/228533/1/WER6746_344-345.PDF
- Torgerson PR, Devleesschauwer B, Praet N, et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001920.
- Rodríguez-Morales AJ, Yepes-Echeverri MC, Acevedo-Mendoza WF, et al. Mapping the residual incidence of taeniasis and cysticercosis in Colombia, 2009-2013, using geographical information systems: implications for public health and travel medicine. Travel Med Infect Dis. 2017 Dec 27:pii: S1477-8939(17)30223–5. Epub ahead of print.
- Garcia HH, Gonzales I, Lescano AG, et al.; for the Cysticercosis Working Group in Peru. Efficacy of combined antiparasitic therapy with praziquantel and albendazole for neurocysticercosis: a double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14:687–695.
- Ndimubanzi PC, Carabin H, Budke CM, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870.
- Fleury A, Gomez T, Alvarez I, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–145.
- Prasad KN, Verma A, Srivastava S, et al. An epidemiological study of asymptomatic neurocysticercosis in a pig farming community in northern India. Trans R Soc Trop Med Hyg. 2011;105:531–536.
- Moyano LM, O’Neal SE, Ayvar V, et al.; for the Cysticercosis Working Group in Peru. High prevalence of asymptomatic neurocysticercosis in an endemic rural community in Peru. PLoS Negl Trop Dis. 2016;10:e0005130.
- Sotelo J, Marin C. Hydrocephalus secondary to cysticercotic arachnoiditis. A long-term follow-up review of 92 cases. J Neurosurg. 1987;66:686–689.
- Sorvillo FJ, DeGiorgio C, Waterman SH. Deaths from cysticercosis, United States. Emerg Infect Dis. 2007;13:230–235.
- Martins-Melo FR, Ramos AN Jr, Cavalcanti MG, et al. Neurocysticercosis-related mortality in Brazil, 2000-2011: epidemiology of a neglected neurologic cause of death. Acta Trop. 2016;153:128–136.
- Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59:1730–1734.
- Fleury A, Carrillo-Mezo R, Flisser A, et al. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011;9:123–133.
- Escobar A. The pathology of neurocysticercosis. In: Palacios E, Rodriquez-Carbajal J, Taveras JM, eds. Cysticercosis of the central nervous system. Springfield, Ill: Thom as; 1983. p. 27–54.
- Carpio A, Placencia M, Santillán F, et al. A proposal for a classification of neurocysticercosis. Can J Neuro Sci. 1994;21:43–47.
- Carpio A, Kelvin E, Bagiella E, et al.; for the Ecuadorian Neurocysticercosis Group. The effects of albendazole treatment on neurocysticercosis: a randomized controlled trial. J Neurol Neurosurg Psychiatry. 2008;79:1050–1055.
- Góngora-Rivera F, Soto-Hernández JL, González Esquivel D, et al. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology. 2006;66(3):436–438.
- Carpio A, Fleury A, Romo ML, et al. New diagnostic criteria for neurocysticercosis: reliability and validity. Ann Neurol. 2016;80:434–442.
- Kumar Garg R, Kumar Singh M, Misra S. Single-enhancing CT lesions in Indian patients with seizures: a review. Epilepsy Res. 2000;38:91–104.
- Sáenz B, Ruíz-Garcia M, Jiménez E, et al. Neurocysticercosis: clinical, radiologic, and inflammatory differences between children and adults. Pediatr Infect Dis J. 2006;25:801–803.
- Del Brutto OH. Neurocysticercosis in infants and toddlers: report of seven cases and review of published patients. Pediatr Neurol. 2013;48:432–435.
- Cárdenas G, Fragoso G, Rosetti M, et al. Neurocysticercosis: the effectiveness of the cysticidal treatment could be influenced by the host immunity. Med Microbiol Immunol. 2014;203:373–381.
- Ciampi de Andrade D, Rodrigues CL, Abraham R, et al. Cognitive impairment and dementia in neurocysticercosis: a cross-sectional controlled study. Neurology. 2010;74:1288–1295.
- Velasco TR, Zanello PA, Dalmagro CL, et al. Calcified cysticercotic lesions and intractable epilepsy: a cross sectional study of 512 patients. J Neurol Neurosurg Psychiatry. 2006;77:485–488.
- Goyal M, Chand P, Modi M, et al. Neurocysticercosis: an uncommon cause of drug-refractory epilepsy in North Indian population. Epilepsia. 2015;56:1747–1752.
- Leon A, Saito EK, Mehta B, et al. Calcified parenchymal central nervous system cysticercosis and clinical outcomes in epilepsy. Epilepsy Behav. 2015;43:77–80.
- Kelvin EA, Carpio A, Bagiella E, et al.; for the Ecuadorian Neurocysticercosis Group. The association of host age and gender with inflammation around neurocysticercosis cysts. Ann Trop Med Parasitol. 2009;103:487–499.
- Zhao BC, Jiang HY, Ma WY, et al. Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: a network meta-analysis. PLoS Negl Trop Dis. 2016 Feb 5;10:e0004418.
- Patel R, Jha S, Yadav RK. Pleomorphism of the clinical manifestations of neurocysticercosis. Trans R Soc Trop Med Hyg. 2006;100:134–141.
- Sotelo J, Rubio-Donnadieu F. Granuloma en parenquima cerebral. Un modelo humano para el estudio de la epilepsia. Gac Med Mex. 1989;125:31–35.
- Nash TE, Mahanty S, Loeb JA, et al. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia. 2015;56:177–183.
- Jr EJ, Pitkanen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia. 2013;54(Suppl. 4):61–69.
- Carpio A, Romo ML. Multifactorial basis of epilepsy in patients with neurocysticercosis. Epilepsia. 2015;56:973–974.
- de Souza A, Nalini A, Kovoor JM, et al. Perilesional gliosis around solitary cerebral parenchymal cysticerci and long-term seizure outcome: a prospective study using serial magnetization transfer imaging. Epilepsia. 2011;52:1918–1927.
- Lachuriya G, Garg RK, Jain A, et al. Toll-like receptor-4 polymorphisms and serum matrix metalloproteinase-9 in newly diagnosed patients with calcified neurocysticercosis and seizures. Medicine (Baltimore). 2016;95:e3288.
- Singh A, Garg RK, Jain A, et al. Toll like receptor-4 gene polymorphisms in patients with solitary cysticercus granuloma. J Neurol Sci. 2015;355:180–185.
- Nunes DS, Gonzaga HT, Ribeiro VS, et al. Usefulness of gel filtration fraction as potential biomarker for neurocysticercosis in serum: towards a new diagnostic tool. Parasitology. 2017;144:426–435.
- Fleury A, Trejo A, Cisneros H, et al. Taenia solium: development of an experimental model of porcine neurocysticercosis. PLoS Negl Trop Dis. 2015;9(8):e0003980.
- Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):S2–S26.
- Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–675.
- Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521.
- Singhi PD, Dinakaran J, Khandelwal N, et al. One vs. two years of anti-epileptic therapy in children with single small enhancing CT lesions. J Trop Pediatr. 2003;49:274–278.
- de Boer HM. Epilepsy stigma: moving from a global problem to global solutions. Seizure. 2010;19:630–636.
- Bruno E, Bartoloni A, Zammarchi L, et al.; for the COHEMI Project Study Group. Epilepsy and neurocysticercosis in Latin America: a systematic review and meta-analysis. PLoS Negl Trop Dis 2013;7(10):e2480.
- Quet F, Guerchet M, Pion SDS, et al. Meta-analysis of the association between cysticercosis and epilepsy in Africa. Epilepsia. 2010;51:830–837.
- Del Brutto OH, Arroyo G, Del Brutto VJ, et al. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: a large-scale, computed tomography-based population study in rural Ecuador. Epilepsia. 2017;58:1955–1961
- Bianchin MM, Velasco TR, Wichert-Ana L, et al. Neuroimaging observations linking neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res. 2015;116:34–39.
- Leite JP, Terra-Bustamante VC, Fernandes RM, et al. Calcified neurocysticercotic lesions and postsurgery seizure control in temporal lobe epilepsy. Neurology. 2000;55:1485–1491.
- Sheth TN, Pillon L, Keystone J, et al. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR Am J Neuroradiol. 1998;19:79–82.
- Gupta RK, Kumar R, Chawla S, et al. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia. 2002;43:1502–1508.
- Nash TE, Pretell EJ, Lescano AG, et al.; for the Cysticercosis Working Group in Peru. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–1105.
- Mont’Alverne Filho FE, Machado Ldos R, Lucato LT, et al. The role of 3D volumetric MR sequences in diagnosing intraventricular neurocysticercosis: preliminary results. Arq Neuropsiquiatr. 2011;69:74–78.
- Carrillo-Mezo R, Lara García J, Arroyo M, et al. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop. 2015;152:60–65.
- Abraham R, Pardini AX, Vaz AJ, et al. Taenia antigen detection in the CSF of patients with neurocysticercosis and its relationship with clinical activity of the disease. Arq Neuropsiq. 2004;62:756–760.
- Parkhouse RME, Carpio A, Campoverde A, et al. Reciprocal contribution of clinical studies and the HP10 antigen ELISA for the diagnosis of extraparenchymal neurocysticercosis. Acta Trop. 2017 Nov 18;178:119–123. Epub ahead of print.
- Zea-Vera A, Cordova EG, Rodriguez S, et al.; for the Cysticercosis Working Group in Peru. Parasite antigen in serum predicts the presence of viable brain parasites in patients with apparently calcified cysticercosis only. Clin Infect Dis. 2013;57:e154–e159.
- Cardenas G, Carrillo-Mezo R, Jung H, et al. Subarachnoidal Neurocysticercosis non-responsive to cysticidal drugs: a case series. BMC Neurol. 2010;10:16.
- Fleury A, Sastre P, Sciutto E, et al. A lateral flow assay (LFA) for the rapid detection of extraparenchymal neurocysticercosis using cerebrospinal fluid. Exp Parasitol. 2016;pii: S0014-4894(16)30277–6.
- Michelet L, Fleury A, Sciutto E, et al. Human neurocysticercosis: comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol. 2011;49:195–200.
- Carpio A, Campoverde A, Romo ML, et al. Validity of a PCR assay in CSF for the diagnosis of neurocysticercosis. Neurol Neuroimmunol Neuroinflamm. 2017;4:e324.
- Bustos JA, García HH, Del Brutto OH. Reliability of diagnostic criteria for neurocysticercosis for patients with ventricular cystic lesions or granulomas: a systematic review. Am J Trop Med Hyg. 2017 Sep;97:653–657.
- Fleury A, Carpio A, Romo ML, et al. Reproducibility of diagnostic criteria for ventricular neurocysticercosis. Am J Trop Med Hyg. 2017;97:1952.
- Robles C, Chavarría M. Presentación de un caso clínico de cisticercosis cerebral tratado medicamente con un nuevo fármaco: praziquantel. Salud Pub Mex. 1979;21:603–618.
- Xiao ZX, Zhao CY, Liu LP. Albendazole treatment in cerebral cysticercosis. Chin J Int Med. 1986;25:100–102.
- Wu W, Jia F, Wang W, et al. Antiparasitic treatment of cerebral cysticercosis: lessons and experiences from China. Parasitol Res. 2013;112:2879–2890.
- Romo ML, Wyka K, Carpio A, et al.; for the Ecuadorian Neurocysticercosis Group. The effect of albendazole treatment on seizure outcomes in patients with symptomatic neurocysticercosis. Trans R Soc Trop Med Hyg. 2015;109:738–746.
- Garcia H, Pretell E, Gilman R, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. N Engl J Med. 2004;350:249–258.
- Das K, Mondal GP, Banerjee M, et al. Role of antiparasitic therapy for seizures and resolution of lesions in neurocysticercosis patients: an 8 year randomised study. J Clin Neurosci. 2007;14:1172–1177.
- Kaur S, Singhi P, Singhi S, et al. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: a randomized, placebo-controlled double blind trial. Pediatr Infect Dis. 2009;28:403–406.
- Baird RA, Wiebe S, Zunt JR, et al. Evidence-based guideline: treatment of parenchymal neurocysticercosis: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:1424–1429.
- Del Brutto OH, Roos KL, Coffey CS, et al. Meta-analysis: cysticidal drugs for neurocysticercosis albendazole and praziquantel. Ann Intern Med. 2006;145:43–51.
- Abba K, Ramaratnam S, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. 2010;17:CD000215.
- Singhi P, Suthar R, Deo B, et al. Long-term clinical and radiologic outcome in 500 children with parenchymal neurocysticercosis. Pediat Infect Dis J. 2017;36:549–555.
- Singh G, Sharma R. Controversies in the treatment of seizures associated with neurocysticercosis. Epilepsy Behav. 2017;76:163–167.
- World Health Organization. Accelerating work to overcome the global impact of neglected tropical diseases – a roadmap for implementation (executive summary). 2012. [cited 2017 September 1]. Available from: http://whqlibdoc.who.int/hq/2012/WHO_HTM_NTD_2012.1_eng.pdf
- Lightowlers MW, Donadeu M. Designing a minimal intervention strategy to control Taenia solium. Trends Parasitol. 2017;33:426–434.
- Lightowlers MW. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol. 2010;40:1183–1192.
- Sciutto E, Fragoso G, Hernández M, et al. Development of the S3Pvac vaccine against porcine Taenia solium cysticercosis: a historical review. J Parasitol. 2013;99:686–692.
- Gabriël S, Dorny P, Mwape KE, et al. Control of Taenia solium taeniasis/cysticercosis: the best way forward for sub-Saharan Africa? Acta Trop. 2017;165:252–260.
- Fragoso G, Hernández M, Cervantes-Torres J, et al. Transgenic papaya: a useful platform for oral vaccines. Planta. 2017;245(5):1037–1048.
