ABSTRACT
Introduction: The burden of non-motor symptoms (NMS) is a major determinant of health-related quality of life in Parkinson’s disease (PD), particularly at its late stage.
Areas covered: The late stage is usually defined as the period from unstable advanced to the palliative stage, characterized by a combination of emerging treatment-resistant axial motor symptoms (freezing of gait, postural instability, falls and dysphagia), as well as both non-dopaminergic and dopaminergic NMS: cognitive decline, neuropsychiatric symptoms, aspects of dysautonomia, pain and sleep disturbances (insomnia and excessive day-time sleepiness). Here, the authors summarize the current knowledge on NMS dominating the late stage of PD and propose a pragmatic and clinically focused approach for their recognition and treatment.
Expert opinion: The NMS progression pattern is complex and remains under-researched. While dopamine-dependent NMS may improve with dopamine replacement therapy, non-dopamine dependent NMS worsen progressively and culminate at the late stages of PD. Furthermore, some PD specific features could interact negatively with other comorbidities, multiple medication use and frailty – the evaluation of these aspects is important in the creation of personalized management plans in the late stage of PD.
1. Introduction
1.1. Defining the late stage of Parkinson’s disease – Is it possible?
Parkinson’s disease (PD) is still classically defined as a movement disorder and diagnosed based purely on the presence of its hallmark motor features – tremor, rigidity and bradykinesia. Yet the burden of non-motor symptoms (NMS), recognized and described by Dr James Parkinson himself in 1817, has increasingly been acknowledged as a key unmet need and a major determinant of health-related quality of life in people with Parkinson’s (PwP) [Citation1–3]. Typically, the natural history of PD is now defined as a succession of a variable prodromal period to early stable phase, unstable, advanced and palliative phase and the late stage conceptually comprises unstable, advanced and palliative phase [Citation1,Citation2,Citation4]. From a clinical, pathophysiological and management angle, in addition to the profound loss of dopaminergic neurons in the ventrolateral substantia nigra pars compacta, involvement of multiple neurotransmitters becomes increasingly evident in the late stage of PD, giving rise to a complex medley of motor symptoms and NMS [Citation3,Citation5–12]. Varying degrees of neurodegeneration affecting different nuclei promote non-motor endophenotypes of PD, as described recently, and add to the heterogeneity of the late phase of PD [Citation6,Citation7].
1.2. Clinical assessments to aid defining late stage of PD
Although biomarkers (clinical, laboratory or imaging based) might be important surrogates aiding clinical characterization of the late stage of PD, a single biomarker cannot accurately reflect the late stage. Disease duration, although traditionally used, is not an optimal biomarker of severity of PD [Citation13]. Instead, a multimodal approach is required and Antonini et al. have, based on the Delphi-panel method, identified key clinically important indicators defining the late stage of PD [Citation8]. A tool called the Manage-PD (https://www.managepd.com/) has been developed based on this algorithm and may reflect advanced PD and the late stage with reasonable sensitivity and specificity, as confirmed in the Observe PD study [Citation9].
Martinez-Martin and Chaudhuri have proposed a simple and time efficient clinical PD grading system based on the Hoehn and Yahr (HY) staging for motor symptoms coupled with staging of the non-motor burden using the NMSQuestionnaire (NMSQuest) [Citation10–14]. This paradigm, established based on multi-center data analysis enrolling over 1000 People with Parkinson’s (PwP) internationally, aims to alert the healthcare professionals in the primary care to identify PwP requiring a referral to a tertiary center [Citation13]. .
Figure 1. Clinical indicators of the late stage of Parkinson’s disease using the Martinez-Martin-Chaudhuri grading system. NMSQuest – Non-motor Symptoms Questionnaire [Citation12,Citation14] A moderate to severe burden of non-motor symptoms (NMS) indicated by NMSQuest should trigger a specific personalized treatment pathway, based on identified NMS. Hoehn and Yahr stages 4–5 would indicate late stages of PD
![Figure 1. Clinical indicators of the late stage of Parkinson’s disease using the Martinez-Martin-Chaudhuri grading system. NMSQuest – Non-motor Symptoms Questionnaire [Citation12,Citation14] A moderate to severe burden of non-motor symptoms (NMS) indicated by NMSQuest should trigger a specific personalized treatment pathway, based on identified NMS. Hoehn and Yahr stages 4–5 would indicate late stages of PD](/cms/asset/fc612b0f-673c-4cde-b617-d14249db2a36/iern_a_1883428_f0001_oc.jpg)
Numerous assessment tools (scales and questionnaires) are available to flag up and quantify individual NMS and can be applied at the late stage of PD. A detailed discussion is beyond the scope of this review, but some specific assessment tools for NMS relevant at the late stage of PD, endorsed by International Parkinson and Movement Disorders Society (MDS) task force publications on rating scales are summarized in [Citation15–19].
Table 1. Assessment tools for non-motor symptoms (NMS) in Parkinson’s disease as recommended by International Parkinson and Movement Disorders Society (MDS) Taskforce on rating scales. In addition, specific items of Non-motor Symptoms Questionnaire (NMSQuest) and Non-motor Symptoms Scale (NMSS) as a part of the holistic approach can be applied. PD – Parkinson’s disease
In addition, wide range of laboratory- and imaging-based biomarkers for PD exists; however, none is specific for the late stage of the disease.
1.3. Late stage Parkinson’s related non-motor symptoms
Here, we focus on some of the key NMS dominating the late stage of PD, as summarized in , and outline the evidence on available treatment strategies for this particular population. Clearly, there is a substantial overlap between multiple NMS arising early in the course of PD, and persisting throughout late stage, with NMS typically appearing at late stages. A combined discussion would once again be beyond the remit of this review. A pragmatic theoretical charting of the motor and non-motor natural history of PD is proposed in ., comprising both dopaminergic and non-dopaminergic aspects of NMS burden, along with non-motor fluctuations, that usually accompany motor fluctuations, but might also occur isolated in some PwP.
Figure 2. A theoretical progression pattern of aspects of non-motor symptoms burden (NMSB) and motor symptoms across the life span in people with Parkinson’s (PwP). A progressive deterioration of motor symptoms if untreated is charted (loosely corresponding to Hoehn and Yahr stage, blue line), modified by in life dopamine replacement therapies (DRT; orange line), showing an altered trajectory but with increasing burden in late stage. NMSB is shown in two grades: dopamine-dependent NMSB (gray line) starts in the prodromal stage and improves in the early stages, as reported in several studies (e.g. DeNoPa [Citation20]), remaining at plateau, but further deteriorating in the late stages. Non-dopaminergic NMS (yellow line) deteriorate through the course of PD, becoming dominantly manifest in late stages, alongside non-motor fluctuations (NMF, light blue dotted line)
![Figure 2. A theoretical progression pattern of aspects of non-motor symptoms burden (NMSB) and motor symptoms across the life span in people with Parkinson’s (PwP). A progressive deterioration of motor symptoms if untreated is charted (loosely corresponding to Hoehn and Yahr stage, blue line), modified by in life dopamine replacement therapies (DRT; orange line), showing an altered trajectory but with increasing burden in late stage. NMSB is shown in two grades: dopamine-dependent NMSB (gray line) starts in the prodromal stage and improves in the early stages, as reported in several studies (e.g. DeNoPa [Citation20]), remaining at plateau, but further deteriorating in the late stages. Non-dopaminergic NMS (yellow line) deteriorate through the course of PD, becoming dominantly manifest in late stages, alongside non-motor fluctuations (NMF, light blue dotted line)](/cms/asset/db3d58f5-6944-4d44-ab2d-741392a7b985/iern_a_1883428_f0002_oc.jpg)
Table 2. Non-motor symptoms (NMS) dominating the late stage of Parkinson’s Disease, including those appearing as part of non-motor fluctuation
Some of the NMS dominating the late stage of PD are discussed below.
2. Specific NMS dominating the late stages of Parkinson’s disease
2.1. Cognitive impairment
A certain degree of decline in cognitive functions, which can ultimately evolve in overt dementia, is a common non-motor feature of PD. Cohort study-based data analysis has found that 46% of the patients may have dementia after 10 years of disease and more than 80% after 20 years [Citation21,Citation22]. The prevalence of dementia, however, seems to be increased in patients older than 50 years at the time of diagnosis [Citation23].
The spectrum of cognitive impairment in PD ranges among subjective cognitive decline, mild cognitive impairment and dementia, and it is now established that cognitive dysfunction can present as a single or multiple domain syndrome [Citation24].
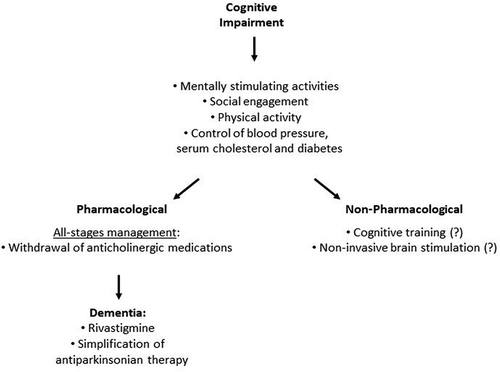
2.1.1. Can self-help management strategies help?
Although great research efforts are being made to effectively manage and prevent the onset of cognitive impairment in PD, the individual patient’s contribution to this cause could potentially work in synergy with the delivery of an effective treatment. . There is preliminary evidence, for instance, that physical activity, especially in the form of aerobic exercise, might benefit cognitive functions [Citation25–27]. However, research on this topic has focused on mild to moderate stages of PD, and, to our knowledge, no studies have specifically looked at the effects of exercise on cognition in late stage PD. This could be directly explained by the intrinsic motor limitations of late stage PD. A recent UK study funded by the Wellcome Trust addressing the effect of dance therapy (ballet) in PD will however aim to include patients with late stage PD in the program (clinicaltrials.gov, NCT04719468).
Mentally stimulating activities, such as volunteering, visiting friends, computer use, crafts, attending church or reading, have shown to be associated with better cognitive performance, independently of education, severity of motor disease, nigrostriatal dopaminergic, and cortical cholinergic degeneration [Citation28]. Moreover, social engagement, which has already been associated with decreased risk to develop dementia in the aging population, seems to contribute to individual`s cognitive reserve [Citation29]. This can, in turn, prevent and modulate the effects of PD pathology on cognition [Citation30]. Management of vascular risk factors such as hypertension, hypercholesterolemia and diabetes can be partially achieved through lifestyle changes.
2.1.2. Pharmacological management
Currently, no approved interventions for non-dementia cognitive impairment are available, but several steps can be taken [Citation31,Citation32]. Anticholinergic medications, such as, for instance, trihexyphenidyl, benztropine and orphenadrine, should not be prescribed, as they may further impair an already compromised cholinergic system. Use of the Anticholinergic Cognitive Burden Scale could guide the clinician in assessing and potentially reducing the patient’s individual anticholinergic load [Citation33,Citation34]. This is specifically relevant if a cholinergic subtype of PD is identified clinically [Citation35].
Management of other NMS and common comorbidities associated with cognitive impairment in PD, such as depression, orthostatic hypotension, and obstructive sleep apnea, might also have a beneficial effect in terms of cognitive gains for the patient [Citation36,Citation37].
For PwP with dementia, only rivastigmine, a cholinesterase inhibitor, has been proved efficacious in a randomized placebo-controlled trial (RCT), moderately improving cognitive performance, at the expenses of higher rates of nausea, vomiting, and tremor [Citation38]. Better results in terms of efficacy were obtained with the capsules compared to the patch formulation, potentially due to the different pharmacokinetic parameters between the two forms, although the difference could be overcome by increasing the patch dose [Citation39].
Other cholinesterase inhibitors have been investigated for the treatment of dementia in PD, namely donepezil and galantamine [Citation40,Citation41], as well as the N-methyl-D-aspartate antagonist memantine [Citation42–44], but there is still insufficient evidence to validate their efficacy in PD dementia [Citation32]. However, because of the established efficacy and license of donepezil and galantamine outside dementia in PD, the practice implications for these cholinesterase inhibitors have been defined as ‘possibly useful’ by the recent International Parkinson and Movement Disorder Society Evidence-Based Medicine (MDS-EBM) review on the treatment of NMS in PD [Citation32].
In relation to late stage PD patients with cognitive impairment who need advanced therapies, levodopa/carbidopa intestinal gel infusion may be the best option based on the EuroInf and EuroInf 2 studies [Citation45,Citation46]. Interestingly, some preliminary data indicates a potential anti-amyloid effect of apomorphine, although robust data and clinical translation are lacking [Citation47,Citation48].
2.1.3. Non-pharmacological interventions
Among the non-pharmacological interventions that could provide an improvement in cognitive functions for PD patients, cognitive training is increasingly being investigated. Although existing studies are limited by substantial methodological issues, such as small sample sizes, scarcity of evaluation of long-term effects, difficulty in designing reliable RCT as well as mixed study findings, there are preliminary data on the effectiveness of cognitive training in PD [Citation49–51]. As an example, data on the effectiveness of computerized, multi-domain cognitive training (with a frequency of 2–3 times/week for 3–12 weeks) showed statistically significant improvements in working memory, visual memory, attention, processing speed, executive function, and visuospatial skills, although there are limitations related to the study design, software used and nature of the control groups (active, treatment-as-usual) [Citation52]. Moreover, the existing evidence for cognitive training has mainly been gathered for PwP at the mild-moderate stage or for subjects with specific cognitive profiles (e.g. mild cognitive impairment and dementia). It is therefore unclear whether late-stage PD patients may benefit from such interventions and further research is needed. Some evidence suggests that transcranial direct current stimulation and repetitive transcranial magnetic stimulation may produce statistically significant improvement in several cognitive domains [Citation53]. However, no stronger recommendation can be made so far on these techniques. .
2.2. Psychiatric non-motor symptoms: anxiety and psychosis
2.2.1. Anxiety
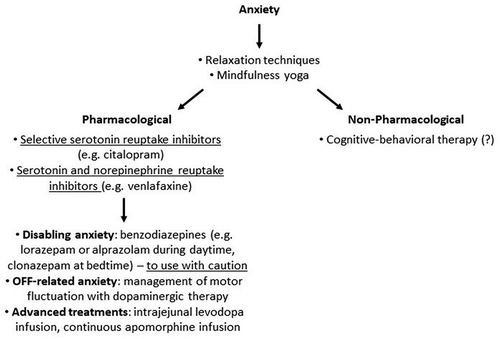
Anxiety is a common and troublesome neuropsychiatric feature of all stages of PD and, in the late stages, may comprise the cognitive aspects of non-motor fluctuations [Citation54]. In late stage PD, anxiety can present as generalized anxiety disorder, social phobia, and panic disorder. Of note, the COVID-19 pandemic has led to a substantial worsening of anxiety in many older PwP with late stage condition, both in those affected by the virus and in unaffected patients owing to the pandemic-related restrictions [Citation55,Citation56].
2.2.1.1. Self-help management
A recent comprehensive review on clinical trials assessing self-management approaches for the non-pharmacological treatment of anxiety identified thirteen interventions, and a focused use of breath appeared to have a positive outcome [Citation57].
2.2.1.2. Pharmacological management
There are currently no recommended treatments for anxiety in PD [Citation32]. For anxiety associated with OFF episodes in the context of non-motor fluctuations, an adjustment of the patient’s dopamine replacement therapy is recommended. Selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), such as citalopram, paroxetine and venlafaxine, are often used in PD patients to manage moderate to severe anxiety symptoms, but no clinical trials so far have demonstrated a statistically significant effect of these treatment on the symptom [Citation58]. Currently, ADepT-PD, a national RCT study in the UK, is addressing the evidence base (clinicaltrials.gov, NCT03652870) but is not specific to late stage of PD.
2.2.1.3. Non-pharmacological interventions
Given the lack of pharmacological options for the management of PD-related anxiety, there is an increasing need for non-pharmacological interventions, specifically in relation to COVID-19-related effects. There are no studies of the effectiveness of cognitive behavioral therapy (CBT) for anxiety in late-stage PD, although there are preliminary results for the general PD population, with mixed findings [Citation58–62]. .
2.2.2. Psychosis
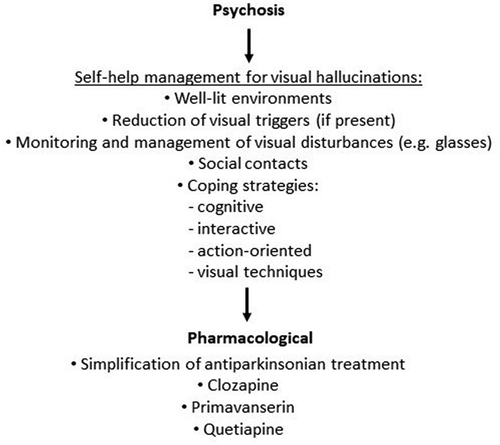
Late stage PD may be complicated by psychotic symptoms, in particular hallucinations and delusions, which are associated with reduced quality of life, increased rates of institutionalization and increased mortality [Citation63]. A prevalence of hallucinations and delusions of 44% and 25%, respectively, has been observed in a recent longitudinal multicenter study conducted on a cohort of patients with advanced PD with a mean disease duration of 15.4 years [Citation64]. In an acute setting, delirium may also occur in hospitalized patients, as evident in PwP affected by COVID-19 [Citation55].
2.2.2.1. Self-help management
A person with PD can put in place several measures in order to reduce the risk for visual hallucinations to occur, such as improving lighting at home, avoiding visual triggers (if present), monitoring visual function and wearing glasses if required, engaging in social contacts at the time of the day in which is more probable to experience hallucinations [Citation65]. Coping strategies can include visual techniques, such as better focusing on the hallucinatory object, focusing at another object, or looking away from the hallucination; cognitive techniques, such as self-initiated reactions like turning the lights on, being aware that the hallucinations are not real or self-reassuring that they will resolve shortly; interactive techniques, which require discussions with other people in order to feel reassured and to verify the non-reality of the hallucinations [Citation66,Citation67]. .
2.2.2.2. Pharmacological management
The first step is adjusting and simplifying therapeutic regimen such as decreasing and eventually stopping non-essential PD medications before the introduction of antipsychotic medication [Citation68]. In particular, a gradual and stepwise discontinuation of PD medication should start with stopping anticholinergics, followed by monoamine oxidase B inhibitors (MAO-Bi), amantadine, dopamine agonists (DAs), catechol-O-methyltransferase (COMT) inhibitors, and, as the last resource, by reduction in levodopa intake [Citation33]. In addition, monitoring and management of potential systemic comorbidities should be included in the first approach to PwP with psychosis [Citation68].
Only two compounds have been identified as ‘efficacious’ and ‘clinically useful’ for PD-related psychosis, namely clozapine and primavanserin [Citation32]. However, the risk of agranulocytosis, sedation, sialorrhea and orthostatic hypotension make the use of clozapine hazardous [Citation32]. Primavanserin, a selective 5-HT2A receptor inverse agonist with no dopaminergic receptor-blocking properties, has been found to be effective in improving psychotic symptoms in PD [Citation69]. However, no studies have assessed its safety and tolerability in late-stage PD as well as its impact on mortality, especially in the case of concomitant dementia [Citation33]. In addition, it is currently available only in the United States.
Despite negative results in several clinical trials [Citation70–72], quetiapine, an atypical antipsychotic with better safety profile compared to clozapine, is probably the most used in clinical practice [Citation33]. Interestingly, statistically significant improvements in the perceptual problems/hallucination domain of the NMSS six months after initiation of APO infusion have been observed, possibly due to its piperidine moiety and 5-HT2A serotonin receptor antagonist effect [Citation46]. .
2.2.2.3. Non-pharmacological interventions
Despite evidence from the field of schizophrenia suggesting that structured psychosocial interventions may improve psychotic symptoms [Citation73], to our knowledge no clinical trials have investigated this therapeutic option in PD or late-stage PD.
2.3. Dysautonomia: orthostatic hypotension, urinary dysfunction, constipation and drooling
2.3.1. Orthostatic hypotension
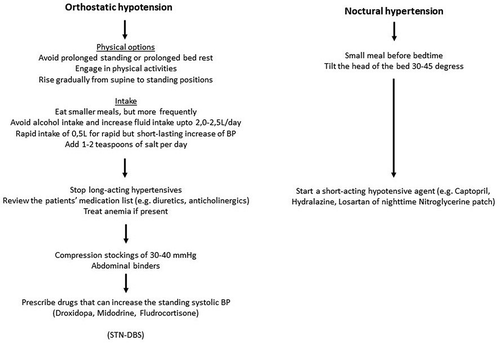
Orthostatic hypotension (OH) is defined as a decrease of ≥20 mmHg systolic or ≥10 mmHg diastolic blood pressure (BP) within 3 minutes when going from a supine to an upright position. The OH is called neurogenic (nOH) when there is a raise of heart rate < 20 bpm [Citation74]. As much as 30% to 50% of PwP are estimated to have nOH, but only a third have symptomatic OH. Symptoms, present on standing and less when sitting, can be light-headedness, dizziness, blurred vision, coat hanger pain and (pre)syncope. A recent paper highlights the significance of transient orthostatic hypotension (i.e. BP drop within the first minute after achieving a standing position) in PwP, as it may be as frequent as typical OH measured at 3 minutes upon standing [Citation75]. In contrast, some people may show delayed OH (later than 3 minutes upon standing). For these people, prolonged standing blood pressure measurements or prolonged tilt table testing are to be considered [Citation76]. nOH can be associated with supine hypertension [Citation74,Citation77]. Supine hypertension can present with a loss of normal nocturnal decrease of BP (non-dipping) or even a nighttime increase (reverse dipping) [Citation77].
Anemia, and particularly erythropoietin deficiency, should be excluded as this is an aggravating factor [Citation78]. Ambulatory BP monitoring can be performed to give a representative view of the diurnal pattern of the BP. The Orthostatic Hypotension Questionnaire is a validated questionnaire to assess OH [Citation79]. The goal of treatment should not be to normalize the standing BP, but rather to address the symptoms [Citation80]. There is, however, some evidence that also people with asymptomatic OH should be treated [Citation81].
2.3.1.1. Self-help management
Prolonged standing and prolonged bed rest should be avoided, if possible. Physical activity in recumbent position (e.g. stationary bicycle or in a swimming pool) is encouraged [Citation78]. The patient should be educated to rise gradually from supine to standing positions, especially in the morning, after meals and after urination or defecation to avoid effects of transient or delayed OH. Smaller and more frequent meals are recommended, and alcohol intake should be avoided [Citation74,Citation77]. Patients are encouraged to increase their fluid intake, preferably 2.0 to 2.5 L/day [Citation74,Citation77,Citation82]. Rapid intake of 0.5 L of water produces a rapid but short-lasting effect on the BP which can be beneficial in case of pronounced early morning OH. Addition of 1–2 teaspoons (2.3–4.6 g) of salt per day is also to be considered [Citation74].
In case of nocturnal hypertension, a snack before bedtime can induce postprandial hypotension which can attenuate the nocturnal hypertension [Citation77]. During sleep, head up tilting of the bed at 30–45 degrees can be helpful [Citation74]. .
2.3.1.2. Pharmacological management
If nOH is present, long-acting antihypertensives and medication known to cause hypotension should be avoided [Citation74]. In addition, agents that reduce intravascular volume (diuretics) or induce vasodilatation (phosphodiesterase E5 inhibitors, nitrates), alfa-blockers, central acting alfa-2-agonists and tricyclic antidepressants should be avoided, if possible [Citation82]. Anticholinergics may cause lowering of the BP as well [Citation76]. If the intake of antihypertensives is indicated for nocturnal hypertension, switching to short-acting overnight medication is preferred. DRT (levodopa and dopamine agonists) can also cause postural hypotension [Citation76].
The MDS Evidence-Based Medicine Committee classifies droxidopa, fludrocortisone and midodrine as ‘possibly useful’, but only droxidopa was categorized as efficacious in the short term [Citation32,Citation83]. A small double-blind cross-over RCT with fludrocortisone and domperidone showed a subjective improvement in orthostatic symptoms in both compounds [Citation84]. A meta-analysis on the effect of midodrine and droxidopa has shown an increase in standing systolic blood pressure in people with nOH, which was more pronounced in patients taking midodrine [Citation85]. Severely affected individuals may need a combination of pharmacological agents [Citation78].
In case of severe supine hypertension (systolic BP > 180 mmHg or diastolic BP > 110 mmHg), a short-acting antihypertensive can be considered at bedtime, e.g. Captopril, Hydralazine or Losartan [Citation74]. Nitro-glycerin patch at nighttime might also be considered [Citation74]. .
2.3.1.3. Non-pharmacological interventions
Compression stockings of 30–40 mmHg, preferably waist-high or thigh-high address peripheral venous pooling [Citation77]. However, no trial has been conducted in PD patients to quantify the effect of this intervention. A trial in 19 patients with autonomic failure (including 5 PwP) showed a similar effect of midodrine compared to an inflatable abdominal binder [Citation86]. Therefore, abdominal binders can be an alternative if compliance to compression stockings is low or can also be used in combination with compression stockings [Citation76,Citation87]. STN-DBS, in patients who qualify motorically, may also have a positive impact on orthostatic hypotension [Citation88]. .
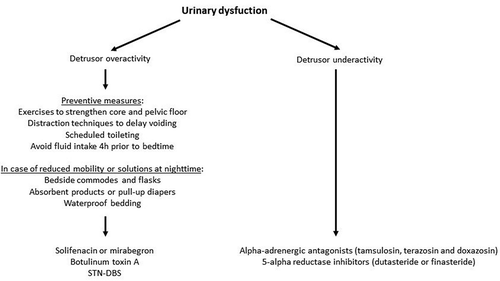
2.3.2. Urinary dysfunction
Urinary dysfunction in PD comprises urgency, frequency and nycturia. A recent observational study in late-stage Parkinson’s found these symptoms in, respectively, 68.5%, 60.7%, and 70.5% of the study population [Citation64]. Detrusor underactivity can coexist with detrusor overactivity and presents with decreased sensation during filling and incomplete emptying [Citation89]. Obstructed flow can also be present due to sphincter bradykinesia and delayed relaxation of the urethral sphincter and pelvic muscles [Citation89]. In case of new-onset urgency or frequency, pyuria and bacteriuria suggestive for a urinary tract infection should be excluded by a urinalysis [Citation89]. Obstructed flow should be excluded by uroflowmetry and in men complemented by ultrasonography as prostatic hypertrophy can cause obstructed flow as well [Citation89,Citation90].
2.3.2.1. Self-help management
Fluid intake in the 4 h prior to bedtime is discouraged, especially caffeine and alcohol should be avoided due to their diuretic effect [Citation89]. Urgency can be decreased by a strengthened core and pelvic floor for which exercise is recommended or by delaying the voiding by distraction techniques [Citation89]. Scheduled toileting can be beneficial as well [Citation91]. Bedside commodes and flasks can address frequency, especially for people with limited mobility [Citation90]. Incontinence can be addressed with absorbent products or pull-up diapers as well as waterproof bedding [Citation91]. .
2.3.2.2. Pharmacological management
Currently, only solifenacin is recommended by the MDS for treatment for urinary dysfunction [Citation32]. Solifenacin acts selectively on the M3 receptors of the bladder. .
Mirabegron is a recent drug working as a muscle relaxant via beta-3 adrenergic receptor and increasing the bladder capacity, but can cause orthostatic hypotension [Citation89]. A recent prospective study confirmed the safety and efficacy of mirabegron in PwP [Citation92].
Obstructive urinary symptoms due to prostatic hypertrophy can be treated by alpha-adrenergic antagonists (e.g. tamsulosin, terazosin and doxazosin) or by 5-alpha reductase inhibitors (such as dutasteride or finasteride) [Citation89]. These agents should be used with special care as they may cause orthostatic hypotension [Citation89]. .
Severe bladder overactivity can be potentially treated with Botulinum toxin A, but only in specialized centers with suitably trained injectors [Citation91]. Injections need to be repeated every 6–9 months and intermittent self-catheterization may be needed [Citation89,Citation91].
2.3.2.3. Non-pharmacological interventions
A recent RCT failed to show that behavioral therapy in 26 PwP (pelvic floor muscle exercises, bladder training, fluid and constipation management) decreases the weekly incontinency rate when compared to a control group (21 PwP). However, the quality of life was significantly improved in the behavioral group [Citation93]. An improvement of urinary symptoms has been observed following STN-DBS [Citation94]. Severe detrusor underactivity may need intermittent catheterization [Citation89]. .
2.3.3. Constipation
The American College of Gastroenterology defines constipation as an “unsatisfactory defecation characterized by infrequent stools, difficult stool passage, or both„ [Citation95]. In PD, constipation, usually defined as less than three bowel movements per week, is reported in over 60% of the people with advanced Parkinson’s disease [Citation64,Citation96]. The causation is multifactorial, ranging from slow colonic transit to pelvic floor dyssynergia associated with impaired motor function and further compounded by the effects of concomitant use of antiparkinsonian, analgesic and antidepressant medications.
In case of new-onset constipation, the patient should be referred to a specialist for a colonoscopy to exclude secondary causes that can induce constipation (e.g. gastrointestinal tumor and inflammatory bowel disease) [Citation97].
2.3.3.1. Self-help management
Fruit, vegetables and olive oil should be included in the patient’s diet [Citation82]. In RCT enrolling 120 PwP, the use of probiotics and prebiotic fibers has shown a positive impact on the complete bowel movements per week [Citation98]. Physical activity and adequate hydration (1.5–2 liters of water a day) are recommended as well [Citation96]. .
Table 3. Management of drooling and constipation in People with Parkinson’s
2.3.3.2. Pharmacological management
If possible, opioids should be discontinued. The efficacy of Macrogol, an osmotic laxative, has been investigated in an RCT with 57 PwP. The significant effect on responder rate was found in the treatment arm but not in the placebo arm [Citation99]. An RCT with 54 PD patients, shows that lubiprostone, a synthetic ghrelin agonist has a significant effect on constipation when measured after a 4-week period [Citation100]. .
Prucalopride, a selective high-affinity 5-HT4 receptor agonist can help refractory chronic constipation but has not been specifically tested in PD [Citation101]. In general, bulk agents such as psyllium, non-absorbable sugars such as lactulose, and stimulant laxatives such as senna are all used in practice [Citation82].
2.3.4. Drooling
Daytime drooling (or sialorrhea) is reported in 10–84% of the people with Parkinson’s disease with a significant impact on patients’ quality of life [Citation32]. Sialorrhea can lead to silent aspiration of saliva and is thus a risk factor for respiratory infection [Citation102]. Drooling is not a result of hypersalivation, but rather of a reduction of swallowing [Citation102]. Hypomimia with a reduced lip seal and postural changes contributes to sialorrhea as well [Citation103]. .
2.3.4.1. Self-help management
PwP should be educated about the reduced swallowing as a cause of drooling and encouraged to swallow more often, e.g. with help of a swallow timer [Citation82]. The use of chewing gum, ice or lemon juice can also help to increase the swallowing frequency [Citation101]. .
2.3.4.2. Pharmacological management
Glycopyrrolate was classified as ‘possibly useful’ [Citation32]. A short-term RCT with a crossover design in 23 PwP (mean H&Y 2.5) demonstrated a decrease of sialorrhea on a 9-point drooling scoring scale [Citation104]. Sublingual atropine drops or ipratropium spray, through their topical anticholinergics effect might be helpful, although evidence is insufficient [Citation82,Citation101]. .
The MDS EBM classifies botulinum toxin A and B as ‘clinically useful’ [Citation32]. Botulinum toxin type B demonstrated clinical efficacy in 54 PD patients in a double-blind RCT, captured on the Drooling Frequency and Severity Scale, and a significant decrease in unstimulated salivary flow rates [Citation105]. Recently, the National Institute for Health and Care Excellence in the UK (NICE) recommended botulinum toxin type A for the treatment of chronic sialorrhea, administered by a trained physician [Citation32,Citation106]. As injection sites, the parotid gland and the submandibular glands are recommended [Citation107]. .
2.4. Chronic Pain
Pain is highly prevalent across all stages of PD, lingering and, in a substantial proportion of PwP, worsening in its late stages, although specific studies are lacking [Citation108]. PD-related pain is heterogeneous – PwP might suffer from nociceptive or neuropathic pain, or experience pain as a sensory aspect integral to non-motor fluctuations [Citation54,Citation109–111]. The origin of PD-related pain is multifactorial, as PD could affect pain processing at multiple levels. Experimental studies using functional magnetic resonance imaging (fMRI) highlighted that in PwP, functional interconnectivity between basal ganglia and the salience network is disturbed in both resting and in pain task state, unsettling the balance between intrinsic brain activity and external pain stimuli [Citation112]. Although evident already in early, de novo PD, diminished functional coupling between the basal ganglia and the salience network is likely related to the disease severity [Citation113].
The King’s PD Pain Scale (KPPS), currently the only PD-specific pain scale, classifies pain into seven subtypes (musculoskeletal, chronic, fluctuation-related, nocturnal, oro-facial, peripheral, radicular) [Citation114]. The overall pain burden, as measured by the KPPS total score, seems to correlate with advancing HY stages (correlation coefficient 0.24, p = 0.001) [Citation114]. Additionally, in elderly PwP with advanced disease, various musculoskeletal comorbidities (e.g. osteoarthritis, osteoporosis) may also promote worsening of pain.
2.4.1. Self-help advice
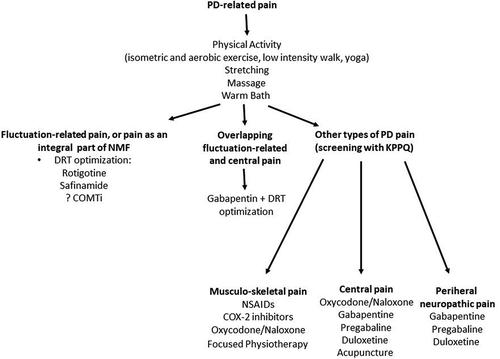
Some PwP might find stretching, massage, or warm bath beneficial for their pain. In PwP at H&Y stages II–III, yoga significantly improved low back pain and related disability [Citation115]. Both isometric and aerobic exercise, even a low intensity walks, reduce pain sensitivity in PwP. Hence, any mode and intensity of exercise chosen by PwP that best suits their needs, might be included as a component of the pain management [Citation116,Citation117]. Listening to pleasant music might possibly reduce pain intensity, although there is no evidence specifically in patients with a late stage PD [Citation118]. .
Figure 8. Possible treatment strategies for Parkinson’s Disease related pain. COMTi – catechol-o-methyltransferase inhibitor. DRT – dopamine replacement therapy, NMF – Non-motor fluctuations, KPPQ – King’s Parkinson’s Disease Pain Questionnaire, NSAID – non-steroidal anti-inflammatory drugs, COX-2 – cyclooxygenase-2
2.4.2. Pharmacological treatment
The high-quality, level I evidence on treatment options for PD-related pain is scarce [Citation110]. Based on a double-blind RCT of the analgesic efficacy of prolonged-release oxycodone/naloxone in PwP with chronic pain (PANDA study), the MDS EBM Committee classified oxycodone/naloxone as ‘possibly useful’, although this is not specific to late stages of PD [Citation32,Citation119]. In fact, in late stage, particularly in older PwP, caution is required as oxycodone/naloxone poses an ‘acceptable risk without specialized monitoring’, with dizziness, headache, fatigue, worsening cognitive dysfunction, and gastrointestinal tract symptoms (nausea, vomiting, and constipation) among potential side effects [Citation32,Citation120,Citation121].
In a smaller study which enrolled mainly (70%) participants with advanced PD, an improvement of fluctuation-related pain with rotigotine vs. placebo was observed [Citation122].
The multimodal selective MAO-Bi safinamide has shown a favorable effect on pain while opicapone, a third-generation COMT inhibitor is being investigated for dopaminergic pain relief in PD. Whether these will work in the late stages of PD is not known.
Several types of non-dopaminergic medication recommended in general elderly population might be useful also in PwP, although there is a lack of robust evidence on their analgesic efficacy specifically for PD-related pain. In two survey-based studies, nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase-2 inhibitors were the most commonly used drugs and self-rated by PwP as the most effective analgesics [Citation123,Citation124]. Gabapentin, pregabalin, and duloxetine (the later has showed a beneficial effect on PD pain in a small, open label study) are possibly useful in treatment of central and peripheral neuropathic pain in PwP [Citation125,Citation126]. .
2.4.3. Non-pharmacological interventions
Acupuncture may lead to pain relief in PwP via modulation of brain regions related to both sensory-discriminative and emotional aspects of pain processing, as highlighted in a fMRI study, and could be an option in late stages of PD [Citation127]. The implantation of percutaneous electrodes for spinal cord stimulation led to substantial improvement of chronic neuropathic pain in PwP (mean disease duration 17 years) [Citation128]. .
2.5. Sleep disturbances: insomnia and excessive daytime sleepiness
Two specific sleep problems are conspicuous at the late stages of PD: insomnia and excessive day-time sleepiness (EDS) [Citation129–131]. Prevalence of sleep disturbances raises progressively from 47.9% in PwP at H&Y stage I to 81.6% at H&Y stages IV–V; insomnia affects 44.5% and EDS 32.1% of PwP at late stages of the disease [Citation132,Citation133].
2.5.1. Insomnia
At late stages of PD, sleep maintenance insomnia with frequent awakenings and sleep fragmentation is common and, in some PwP, might be further influenced by nocturnal motor (e.g. night-time akinesia and rigidity) or NMS (urinary, e.g. nycturia, or neuropsychiatric issues) [Citation129,Citation134,Citation135]. Additionally, while low doses of levodopa act on pre-synaptic D2 receptors, facilitating sleep and reducing wakefulness, higher doses that are frequently used at the late stages of PD, act at post-synaptic D1 and D2 receptors, inhibiting sleep and increasing insomnia [Citation136].
2.5.1.1. Self-help advice
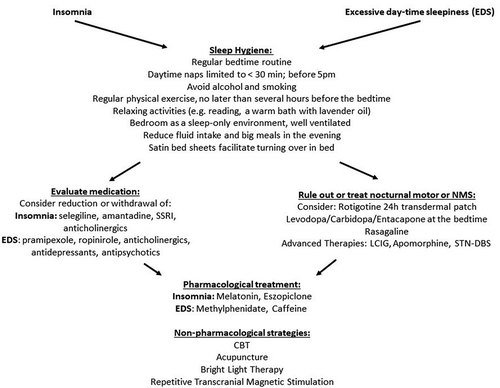
Firstly, PwP should adopt ‘sleep hygiene’, as shown in .
2.5.1.2. Pharmacological treatment
Certain medication, including amantadine, selegiline (presumably through its amphetamine metabolites), SSRIs and anticholinergics might potentially disrupt the nocturnal sleep, and should be reduced, withdrawn, or taken in the first part of the day in PwP suffering from insomnia [Citation135,Citation137,Citation138].
Furthermore, it is necessary to rule out or treat nocturnal motor and NMS potentially causing insomnia. This may be aided by objective monitoring of sleep using wearable sensors, polysomnography, or a sleep diary [Citation139]. When nocturnal motor intrusion is identified, a dopamine replacement therapy targeting night-time could be tried. Currently, rotigotine transdermal patch has Level 1 evidence on improvement of nocturnal and early morning motor impairment, as well as nocturnal sleep disturbances in PwP across H&Y stages I–IV and is classified as ‘likely efficacious’ by MDS EBM Task Force [Citation32,Citation140,Citation141].
The MDS EBM Task Force classified melatonin and eszopiclone as ‘possibly useful’ for treatment of insomnia in PwP, although with ‘insufficient evidence’ [Citation32,Citation142,Citation143].
2.5.1.3. Non-pharmacological strategies
Among complementary therapies, acupuncture, bright light therapy, and CBT might have a beneficial effect on quality of sleep and are currently being investigated [Citation134,Citation144,Citation145]. .
2.5.2. Excessive daytime sleepiness
EDS is a highly prevalent feature of late PD, strongly associated with higher age and higher HY stage, and, in some PwP, may culminate in unintended, sudden onset of sleep. Neurodegeneration of ascending arousal system of the brainstem and hypocretin system, reduced quality of nocturnal sleep, day-time immobility, and certain medication might all be causative [Citation129,Citation130,Citation135,Citation136,Citation138,Citation146]. In particular, dopamine agonists (DA) can contribute to EDS in a dose-dependent manner, most evidently during the dose-escalation phase and when coupled with levodopa therapy [Citation147–152].
2.5.2.1. Self-help advice
summarizes advice on sleep hygiene measures.
2.5.2.2. Pharmacological treatment
Following an education on sleep hygiene and ruling out any night-time causes of sleep disturbances, a careful review, and, if appropriate, reduction, change of timing or withdrawal of the current DA medication, along with antidepressants, hypnotics with antihistamine activity, benzodiazepines, and antipsychotics is necessary in PwP suffering from EDS [Citation137,Citation144,Citation153].
MDS EBM Task Force classified modafinil as ‘possibly useful’ for treatment of EDS in PwP, based on findings from two small-sample RCTs enrolling PwP up to H&Y stage IV [Citation154,Citation155]. Caffeine has been classified as ‘investigational’ due to ‘insufficient evidence’ based on a borderline improvement of EDS in two further RCTs [Citation32,Citation156,Citation157]. SNRI atomoxetine significantly improved EDS in PwP with disease duration 7.9 ± 6.6 years in a recent RCT [Citation158]. In addition, evidence from small, open-label studies implicates that istradefylline, methylphenidate and sodum oxybate might have a beneficial effect on EDS in PwP, eliciting an interest for further research [Citation138,Citation159–161].
In terms of complementary therapies, timed bright light therapy enhanced day-time alertness in PwP at H&Y stages II–IV [Citation144]. CBT, repetitive transcranial magnetic stimulation and STN-DBS might have similar effects [Citation138]. .
3. Drug-induced non-motor complications
A wide range of drug-induced non-motor complications can occur at late stage PD, including impulse control disorder, dopamine agonist withdrawal syndrome, psychosis and, rarely, drug interaction linked Parkinson-hyperpyrexia syndrome. Here, we would like to stress the importance of the awareness of these iatrogenic complications in PwP at the late stages of the disease, although a detailed discussion is beyond the scope of this review. Psychosis has already been alluded to in earlier sections while, of note, a recent study suggested that ICD may be common in PwP with dementia [Citation162].
4. Non-motor fluctuations
In PwP at the complex stages of the disease with long-term levodopa usage, non-motor fluctuations (NMF) might co-exist alongside more acknowledged levodopa-induced motor fluctuations, often being equally or even more disabling and negatively impacting the health-related quality of life [Citation54,Citation163]. .
In daily clinical practice, awareness of NMF and distinction between NMF and NMS not influenced by on/off fluctuations bears important therapeutic implications: while for NMF the approach should be similar to management of motor fluctuations – adjusting the DRT to achieve continuous, non-pulsatile dopaminergic stimulation, in the case of non-fluctuating NMS specific symptomatic treatment is the first-line option [Citation54,Citation164,Citation165].
A recently published MDS-Non-Motor Rating Scale (MDS-NMS) can aid the distinction, as it includes NMF-Subscale to assess changes in NMS linked to the timing of dopamine replacement therapy intake [Citation166–168].
5. Multimorbidity, frailty and polypharmacy
In the late stage PD, aspects of PD specific features interact negatively with comorbidities, medication use and frailty, unmasking NMS such as confusion, sleep dysfunction, and urinary urgency. Evaluation of these aspects is important in the creation of personalized management plans in the late stages of PD [Citation169].
6. Expert opinion
Knowledge of the natural history of PD has evolved greatly in the last decade. While previous concepts staged PD using motor-only approach based on Hoehn and Yahr stages, current concepts comprise both motor symptoms and NMS, the later underpinning the long prodromal stage in some, but not all PwP. The ‘in life manifest’ features of PD are unmasked at clinical diagnosis, often following a variable prodromal period stretching up to 20 years and the condition then appears to progress through the early untreated to stable treated, unstable, advanced and palliative stages. Late stage PD represents a period in PD natural history spanning from unstable to palliative stage. In the late stage, there is a considerable heterogeneity of clinical phenotypic presentations, as the complex pathophysiology (in terms of the range of neurotransmitters (not just dopamine) involved, as well as neuropathological changes, as there may be overlapping alpha synuclein, amyloid and tau deposition) gives rise to a range of clinical subtypes. While progression of the motor aspects of PD can be modified substantially with dopamine replacement therapy (as shown in .), the NMS progression pattern remains complex and under-researched. Broadly, dopamine-dependent NMS, similarly to motor symptoms, may improve with dopamine replacement therapy; however, non-dopamine dependent NMS worsen progressively through the course of the PD, culminating at the late stages. The clinical presentation at the late stage is therefore beset with worsening motor function complicated by levodopa-induced motor fluctuations, and further aggravated by a variety of NMS, ranging from cognitive impairment to dysautonomia features, as discussed in this review. In addition, aspects of PD specific features could interact negatively with other comorbidities, multiple medication use and frailty, unmasking NMS such as confusion, sleep dysfunction, and urinary urgency. Evaluation of these aspects is important in the creation of personalized management plans in the late stages of PD [169]. NMS dominating the late stage of PD tend to be mainly cognitive impairment, sleep disturbances (EDS and insomnia), dysautonomic features, and pain. Neuropsychiatric issues, ubiquitous across all stages of PD, are highly prevalent in the late stage, as well. Therefore, optimal management requires a clinically pragmatic recognition process for better definition of the advanced stages of PD. The recently proposed Antonini criteria, combining a simple motor paradigm with the NMS burden, alongside with functional impact may be the way forward. Furthermore, data from large cohorts suggest that addressing both motor and NMS in a busy clinic can be signposted using the Martinez-Martin & Chaudhuri clinical grading system, based on the combination of the Hoehn and Yahr staging for motor symptoms with scores obtained using the self-completed NMSQuest. Taken together, both scores may provide a healthcare professional with a clinically relevant alerting system, triggering a holistic pathway of care.
Specific management of the NMS dominating the late stages of PD, however, remains poorly evidence based when class 1 or level A evidence is sought, reflecting a general paucity of high-quality clinical trials addressing these major determinants of quality of life in PD. Only a few pharmacological options underpinned by a strong evidence base exist to address dementia, pain, insomnia, EDS and the features of dysautonomia. Recently, non-pharmacological management strategies such as exercise, physical therapy, or light therapy and noninvasive stimulation (brain stem modulation), some of which showed promising pilot data on improving certain aspects of NMS, came into the spotlight. Similarly, growing evidence is emerging from worldwide registry studies on the possible effects of conventional advanced therapies appropriate to the late stages of PD (levodopa/carbidopa intrajejunal infusion, apomorphine subcutaneous infusion, STN-DBS) on the NMS burden. Personalized management of the late stage of PD therefore needs to combine the best aspects of this multimodal therapeutic approach.
Declaration of interest
K Rukavina is supported by NIHR BRC and has received a consultancy fee from Valid Insight and Britannia. KR Chauduri has acted on advisory board for AbbVie, UCB, GKC, Bial, Cynapsus, Novartis, Lobsor, Stada, Medtronic, Zambon, Profile, Sunovion, Roche, Therevance, Scion and Britannia, and has received honoraria for lectures from AbbVie, Britannia, UCB, Mundipharma, Zambon, Novartis, Boeringer Ingelheim, and grants (Investigator Initiated) from Britania Pharmaceuticals, AbbVie, UCB, GKC, Bial, Aacdemic grants: EU, IMI EU, Horizon 2020, Parkinson’s UK, NIHR, PDNMG, EU (Horizon 2020), Kirby Laing Foundation, NPF, MRC, Welcome Trust. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers in this manuscript have no relevant financial or other relationships to disclose.
Article highlights
Burden of non-motor symptoms (NMS) is major determinant of health-related quality of life in Parkinson’s disease (PD)
The heterogeneity of the clinical phenomenology in the late stage of PD arises from overlapping neurotransmitter involvement (not just dopamine) as well as amyloid and tau deposition in addition to alpha synuclein
Late stage is dominated by treatment-resistant axial motor symptoms (freezing of gait, postural instability, falls and dysphagia) along with aggravating burden of dopaminergic and non-dopaminergic NMS, including cognitive decline, neuropsychiatric symptoms, aspects of dysautonomia, pain and sleep disturbances
A simple, time efficient and pragmatic assessment comprising motor and NMS in a busy clinic could be performed using the Martinez-Martin & Chaudhuri clinical grading based on the Hoehn and Yahr (HY) staging for motor symptoms coupled with staging of the non-motor burden using the NMSQuestionnaire (NMSQuest)
Optimal management of late stage PD requires a holistic, patient-centered approach with a personalized pharmacological and non-pharmacological therapeutic strategy tackling specific NMS and motor symptoms
Acknowledgments
The authors would like to thank the editorial support of Ms Aleksandra Podlewska for her help with design of figures as well as the Parkinson’s disease Non-motor Study Group (NMSG) of the International Parkinson’s and Movement Disorders Society. Authors, KK, LB, AAC and VB are part of the subtheme research groups of the NMSG.
References
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
- Titova N, Chaudhuri KR. Non-motor Parkinson disease: new concepts and personalised management. Med J Aust. 2018;208(9):404–409.
- Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004;61(7):1044–1053.
- Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. 2012;8(8):435–442.
- Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(8):509.
- Sauerbier A, Jenner P, Todorova A, et al. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord. 2016;22(Suppl 1):S41–6.
- Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord. 2016;31(8):1095–1102.
- Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–2073.
- Fasano A, Fung VSC, Lopiano L, et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019;19(1):50.
- Martinez-Martin P, Ray Chaudhuri K. Comprehensive grading of Parkinson’s disease using motor and non-motor assessments: addressing a key unmet need. Expert Rev Neurother. 2018;18(1):41–50.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17(5):427–442.
- Chaudhuri KR, Martinez-Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord. 2006;6(1):916–923. .
- Titova N, Martinez-Martin P, Katunina E, et al. Advanced Parkinson’s or “complex phase” Parkinson’s disease? Re-evaluation is needed. J Neural Transm. 2017;6(1):1529–1537.
- Chaudhuri KR, Sauerbier A, Rojo JM, et al. The burden of non-motor symptoms in Parkinson’s disease using a self-completed non-motor questionnaire: a simple grading system. Parkinsonism Relat Disord. 2015;21(3):287–291.
- Pavy-Le Traon A, Amarenco G, Duerr S, et al. The Movement Disorders task force review of dysautonomia rating scales in Parkinson’s disease with regard to symptoms of orthostatic hypotension. Mov Disord. 2011;26(11):1985–1992.
- Perez-Lloret S, Ciampi de Andrade D, KE L, et al. Rating Scales for Pain in Parkinson’s Disease: critique and Recommendations. Mov Disord Clin Pract. 2016;3(6):527–537.
- Friedman JH, Alves G, Hagell P, et al. Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson’s disease. Mov Disord. 2010;25(7):805–822.
- Schrag A, Barone P, Brown RG, et al. Depression rating scales in Parkinson‘s disease: critique and recommendations. Mov Disord. 2007;22(8):1077–1092.
- Hogl B, Arnulf I, Comella C, et al. Scales to assess sleep impairment in Parkinson’s disease: critique and recommendations. Mov Disord. 2010;25(16):2704–2716.
- Mollenhauer B, Zimmermann J, Sixel-Doring F, et al. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov Disord. 2019;34(1):67–77.
- Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844.
- Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258–1264.
- Cholerton BA, Zabetian CP, Quinn JF, et al. Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis. 2013;3(2):205–214.
- Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356.
- Lauze M, Daneault JF, Duval C. The Effects of Physical Activity in Parkinson’s Disease: a Review. J Parkinsons Dis. 2016;6(4):685–698.
- da Silva FC, Iop RDR, de Oliveira LC, et al. Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: a systematic review of randomized controlled trials of the last 10 years. PloS One. 2018;13(2):e0193113.
- Stuckenschneider T, Askew CD, Meneses AL, et al. The Effect of Different Exercise Modes on Domain-Specific Cognitive Function in Patients Suffering from Parkinson’s Disease: a Systematic Review of Randomized Controlled Trials. J Parkinsons Dis. 2019;9(1):73–95.
- Bohnen JLB, Muller M, Haugen J, et al. Mentally stimulating activities associate with better cognitive performance in Parkinson disease. J Neural Transm. 2017;124(10):1205–1212.
- Wang HX, Karp A, Winblad B, et al. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155(12):1081–1087.
- Hindle JV, Hurt CS, Burn DJ, et al. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson’s disease—a longitudinal cohort study. Int J Geriatr Psychiatry. 2016;31(1):13–23.
- Goldman JG, Weintraub D. Advances in the treatment of cognitive impairment in Parkinson’s disease. Mov Disord. 2015;30(11):1471–1489.
- Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord. 2019;34(2):180–198.
- Weintraub D, Caspell-Garcia C, Simuni T, et al. Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease. Ann Clin Transl Neurol. 2020;7(4):449–461.
- Cai X, Campbell N, Khan B, et al. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 2013;9(4):377–385.
- Marras C, Chaudhuri KR, Titova N, et al. Therapy of Parkinson’s Disease Subtypes. Neurotherapeutics. 2020;17(4):1366–1377.
- Centi J, Freeman R, Gibbons CH, et al. Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology. 2017;88(1):17–24.
- Kaminska M, Mery VP, Lafontaine AL, et al. Change in Cognition and Other Non-Motor Symptoms With Obstructive Sleep Apnea Treatment in Parkinson Disease. J Clin Sleep Med. 2018;14(5):819–828.
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351(24):2509–2518.
- Emre M, Poewe W, De Deyn PP, et al. Long-term safety of rivastigmine in Parkinson disease dementia: an open-label, randomized study. Clin Neuropharmacol. 2014;37(1):9–16.
- Dubois B, Tolosa E, Katzenschlager R, et al. Donepezil in Parkinson’s disease dementia: a randomized, double-blind efficacy and safety study. Mov Disord. 2012;27(10):1230–1238.
- Litvinenko IV, Odinak MM, Mogil’naya VI, et al. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial). Neurosci Behav Physiol. 2008;38(9):937–945.
- Leroi I, Overshott R, Byrne EJ, et al. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov Disord. 2009;24(8):1217–1221.
- Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8(7):613–618.
- Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969–977.
- Martinez-Martin P, Reddy P, Katzenschlager R, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510–516.
- Dafsari HS, Martinez-Martin P, Rizos A, et al. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353–365.
- Lim EW, Aarsland D, Ffytche D, et al. Amyloid-beta and Parkinson‘s disease. J Neurol. 2019;266(11):2605–2619.
- van Wamelen D, Politis M, Aarsland D, et al. APOmorphine infusion and aMYLoid deposition in Parkinson’s disease (APOMYL): preliminary clinical and amyloid imaging data (P3.8-032). Neurology. 2019;92(15Supplement):P3.8–032.
- Leung IH, Walton CC, Hallock H, et al. Cognitive training in Parkinson disease: a systematic review and meta-analysis. Neurology. 2015;85(21):1843–1851.
- Lawrence BJ, Gasson N, Johnson AR, et al. Cognitive Training and Transcranial Direct Current Stimulation for Mild Cognitive Impairment in Parkinson’s Disease: a Randomized Controlled Trial. Parkinsons Dis. 2018;4318475.
- Orgeta V, McDonald KR, Poliakoff E, et al. Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev. 2020;2:CD011961.
- Nousia A, Martzoukou M, Tsouris Z, et al. The Beneficial Effects of Computer-Based Cognitive Training in Parkinson’s Disease: a Systematic Review. Arch Clin Neuropsychol. 2020;35(4):434–447.
- Dinkelbach L, Brambilla M, Manenti R, et al. Non-invasive brain stimulation in Parkinson’s disease: exploiting crossroads of cognition and mood. Neurosci Biobehav Rev. 2017;75:407–418.
- Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology. 2013;80(9):800–809.
- Antonini A, Leta V, Teo J, et al. Outcome of Parkinson’s Disease Patients Affected by COVID-19. Mov Disord. 2020;35(6):905–908.
- Sulzer D, Antonini A, Leta V, et al. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Parkinsons Dis. 2020;6(1):18.
- Chandler SK, Robins JL, Kinser PA. Nonpharmacologic Interventions for the Self-Management of Anxiety in Parkinson’s Disease: a Comprehensive Review. Behav Neurol. 2019;8459579.
- Pontone GM, Dissanayaka N, Apostolova L, et al. Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. NPJ Parkinson’s Dis. 2019;5(1):30.
- Dobkin RD, Menza M, Allen LA, et al. Cognitive-behavioral therapy for depression in Parkinson’s disease: a randomized, controlled trial. Am J Psychiatry. 2011;168(10):1066–1074.
- Yang S, Sajatovic M, Walter BL. Psychosocial interventions for depression and anxiety in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2012;25(2):113–121.
- Troeung L, Egan SJ, Gasson N. A waitlist-controlled trial of group cognitive behavioural therapy for depression and anxiety in Parkinson’s disease. BMC Psychiatry. 2014;14:19.
- Calleo JS, Amspoker AB, Sarwar AI, et al. A Pilot Study of a Cognitive-Behavioral Treatment for Anxiety and Depression in Patients With Parkinson Disease. J Geriatr Psychiatry Neurol. 2015;28(3):210–217.
- Weintraub D. Management of psychiatric disorders in Parkinson’s disease: neurotherapeutics — movement Disorders Therapeutics. Neurotherapeutics. 2020;17(4):1511–1524.
- Schrag A, Hommel A, Lorenzl S, et al. The late stage of Parkinson’s -results of a large multinational study on motor and non-motor complications. Parkinsonism Relat Disord. 2020;75:91–96.
- Collerton D, Taylor JP. Advances in the treatment of visual hallucinations in neurodegenerative diseases. Future Neurol. 2013;8(4):433–444.
- Diederich NJ, Pieri V, Goetz CG. Coping strategies for visual hallucinations in Parkinson’s disease. Mov Disord. 2003;18(7):831–832.
- Barnes J, Connelly V, Boubert L, et al. Behavioural coping patterns in Parkinson’s patients with visual hallucinations. J Neuropsychol. 2013;7(2):326–334.
- Thomsen TR, Panisset M, Suchowersky O, et al. Impact of standard of care for psychosis in Parkinson disease. J Neurol Neurosurg Psychiatry. 2008;79(12):1413–1415.
- Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540.
- Ondo WG, Tintner R, Voung KD, et al. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord. 2005;20(8):958–963.
- Rabey JM, Prokhorov T, Miniovitz A, et al. Effect of quetiapine in psychotic Parkinson’s disease patients: a double-blind labeled study of 3 months’ duration. Mov Disord. 2007;22(3):313–318.
- Shotbolt P, Samuel M, Fox C, et al. A randomized controlled trial of quetiapine for psychosis in Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:327–332.
- Zahodne LB, Fernandez HH. Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging. 2008;25(8):665–682.
- Kaufmann H, Palma JA. Neurogenic orthostatic hypotension: the very basics. Clin Auton Res. 2017;27(Suppl1):39–43.
- Fanciulli A, Campese N, Goebel G, et al. Association of transient orthostatic hypotension with falls and syncope in patients with Parkinson disease. Neurology. 2020;95(21):e2854–e65.
- Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–1582.
- Espay AJ, LeWitt PA, Hauser RA, et al. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol. 2016;15(9):954–966.
- Palma JA, Kaufmann H. Orthostatic Hypotension in Parkinson Disease. Clin Geriatr Med. 2020;36(1):53–67.
- Kaufmann H, Malamut R, Norcliffe-Kaufmann L, et al. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79–90.
- Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord. 2015;30(5):639–645.
- Merola A, Romagnolo A, Rosso M, et al. Orthostatic hypotension in Parkinson’s disease: does it matter if asymptomatic? Parkinsonism & related disorders. 2016;33:65–71.
- Palma JA, Kaufmann H. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord. 2018;33(3):372–390.
- Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328–335.
- Schoffer KL, Henderson RD, O’Maley K, et al. Nonpharmacological treatment, fludrocortisone, and domperidone for orthostatic hypotension in Parkinson’s disease. Mov Disord. 2007;22(11):1543–1549.
- Chen JJ, Han Y, Tang J, et al. Standing and Supine Blood Pressure Outcomes Associated With Droxidopa and Midodrine in Patients With Neurogenic Orthostatic Hypotension: a Bayesian Meta-analysis and Mixed Treatment Comparison of Randomized Trials. Ann Pharmacother. 2018;52(12):1182–1194.
- Okamoto LE, Diedrich A, Baudenbacher FJ, et al. Efficacy of Servo-Controlled Splanchnic Venous Compression in the Treatment of Orthostatic Hypotension: a Randomized Comparison With Midodrine. Hypertension. 2016;68(2):418–426.
- Fanciulli A, Goebel G, Metzler B, et al. Elastic Abdominal Binders Attenuate Orthostatic Hypotension in Parkinson‘s Disease. Mov Disord Clin Pract. 2016;3(2):156–160.
- Kurtis MM, Rajah T, Delgado LF, et al. The effect of deep brain stimulation on the non-motor symptoms of Parkinson’s disease: a critical review of the current evidence. NPJ Parkinsons Dis. 2017;3:16024.
- Norcliffe-Kaufmann L, Kaufmann H Autonomic dysfunction in Parkinson’s disease. Parkinson’s Disease: diagnosis, Motor Symptoms and Non-Motor Features: Future Medicine Ltd; 2013. P. 79–98.
- Batla A, Tayim N, Pakzad M, et al. Treatment Options for Urogenital Dysfunction in Parkinson’s Disease. Curr Treat Options Neurol. 2016;18(10):45.
- Madan A, Ray S, Burdick D, et al. Management of lower urinary tract symptoms in Parkinson’s disease in the neurology clinic. Int J Neurosci. 2017;127(12):1136–1149.
- Gubbiotti M, Conte A, Di Stasi SM, et al. Feasibility of mirabegron in the treatment of overactive bladder in patients affected by Parkinson’s disease: a pilot study. Ther Adv Neurol Disord. 2019;12:1756286419843458.
- Vaughan CP, Burgio KL, Goode PS, et al. Behavioral therapy for urinary symptoms in Parkinson’s disease: a randomized clinical trial. Neurourol Urodyn. 2019;38(6):1737–1744.
- Dafsari HS, Silverdale M, Strack M, et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson’s disease. Mov Disord. 2018;33(3):421–430.
- American College of Gastroenterology Chronic Constipation Task F. An evidence-based approach to the management of chronic constipation in. North America. Am J Gastroenterol. 2005;100(Suppl 1):S1–4.
- Mendoza-Velasquez JJ, Flores-Vazquez JF, Barron-Velazquez E, et al. Autonomic Dysfunction in alpha-Synucleinopathies. Front Neurol. 2019;10:363.
- Chen Z, Li G, Liu J. Autonomic dysfunction in Parkinson’s disease: implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis. 2020;134:104700.
- Barichella M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87(12):1274–1280.
- Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson‘s disease. A randomized placebo-controlled study. Mov Disord. 2007;22(9):1239–1244. .
- Ondo WG, Kenney C, Sullivan K, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology. 2012;78(21):1650–1654. .
- Fasano A, Visanji NP, Liu LW, et al. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–639.
- Reynolds H, Miller N, Walker R. Drooling in Parkinson’s Disease: evidence of a Role for Divided Attention. Dysphagia. 2018;33(6):809–817.
- van Wamelen DJ, Leta V, Johnson J, et al. Drooling in Parkinson’s Disease: prevalence and Progression from the Non-motor International Longitudinal Study. Dysphagia. 2020;35(6):955–961. .
- Arbouw ME, Movig KL, Koopmann M, et al. Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology. 2010;74(15):1203–1207. .
- Chinnapongse R, Gullo K, Nemeth P, et al. Safety and efficacy of botulinum toxin type B for treatment of sialorrhea in Parkinson’s disease: a prospective double-blind trial. Mov Disord. 2012;27(2):219–226.
- Sridharan K, Sivaramakrishnan G. Pharmacological interventions for treating sialorrhea associated with neurological disorders: a mixed treatment network meta-analysis of randomized controlled trials. J Clin Neurosci. 2018;51:12–17.
- Egevad G, Petkova VY, Vilholm OJ. Sialorrhea in patients with Parkinson’s disease: safety and administration of botulinum neurotoxin. J Parkinsons Dis. 2014;4(3):321–326.
- Valkovic P, Minar M, Singliarova H, et al. Pain in Parkinson´s Disease: a Cross-Sectional Study of Its Prevalence, Types, and Relationship to Depression and Quality of Life. PloS One. 2015;10(8):e0136541. .
- Buhidma Y, Rukavina K, Chaudhuri KR, et al. Potential of animal models for advancing the understanding and treatment of pain in Parkinson’s disease. NPJ Parkinsons Dis. 2020;6(1):1.
- Rukavina K, Leta V, Sportelli C, et al. Pain in Parkinsonʼs disease. Curr Opin Neurol. 2019;32(4):579–588. .
- Ghosh P, Imbriani P, Caputi N, et al. A Dual Centre Study of Pain in Parkinson’s Disease and Its Relationship with Other Non-Motor Symptoms. J Parkinsons Dis. 2020;10(4):1817–1825. .
- Tan Y, Tan J, Deng J, et al. Alteration of Basal Ganglia and Right Frontoparietal Network in Early Drug-Café Parkinson’s Disease during Heat Pain Stimuli and Resting State. Front Hum Neurosci. 2015;9:467.
- Putcha D, Ross RS, Cronin-Golomb A, et al. Altered intrinsic functional coupling between core neurocognitive networks in Parkinson’s disease. Neuroimage Clin. 2015;7:449–455.
- Chaudhuri KR, Rizos A, Trenkwalder C, et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: an international validation. Mov Disord. 2015;30(12):1623–1631. .
- Myers PS, Harrison EC, Rawson KS, et al. Yoga Improves Balance and Low-Back Pain, but Not Anxiety, in People with Parkinson’s Disease. Int J Yoga Therap. 2020;30(1):41–48. .
- Nguy V, Barry BK, Moloney N, et al. Exercise-induced hypoalgesia is present in people with Parkinson’s disease: two observational cross-sectional studies. European Journal of Pain (London, England). 2019;23(7):1329–1339.
- NE A, Moloney N, van Vliet V, et al. The Rationale for Exercise in the Management of Pain in Parkinson’s Disease. J Parkinsons Dis. 2015;10(8):229–239.
- Skogar O, Lokk J. Pain management in patients with Parkinson’s disease: challenges and solutions. J Multidiscip Healthc. 2016;9:469–479.
- Trenkwalder C, Chaudhuri KR, Martinez-Martin P, et al. Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2015;14(12):1161–1170. .
- Thakur D, Dickerson S, Kumar Bhutani M, et al. Impact of Prolonged-release Oxycodone/Naloxone on Outcomes Affecting Patients‘ Daily Functioning in Comparison With Extended-release Tapentadol: a Systematic Review. Clin Ther. 2015;37(1):212–224.
- Guerriero F, Roberto A, Greco MT, et al. Long-term efficacy and safety of oxycodone-naloxone prolonged release in geriatric patients with moderate-to-severe chronic noncancer pain: a 52-week open-label extension phase study. Drug Des Devel Ther. 2016;10:1515–1523.
- Rascol O, Zesiewicz T, Chaudhuri KR, et al. A Randomized Controlled Exploratory Pilot Study to Evaluate the Effect of Rotigotine Transdermal Patch on Parkinson’s Disease-Associated Chronic Pain. J Clin Pharmacol. 2016;56(7):852–861. .
- Zella MAS, May C, Muller T, et al. Landscape of pain in Parkinson’s disease: impact of gender differences. Neurol Res. 2019;41(1):87–97. .
- Buhmann C, Wrobel N, Grashorn W, et al. Pain in Parkinson disease: a cross-sectional survey of its prevalence, specifics, and therapy. J Neurol. 2017;264(4):758–769. .
- Buhmann C, Kassubek J, Jost WH. Management of Pain in Parkinson’s Disease. J Parkinsons Dis. 2020;10(s1):S37–S48.
- Djaldetti R, Yust-Katz S, Kolianov V, et al. The effect of duloxetine on primary pain symptoms in Parkinson disease. Clin Neuropharmacol. 2007;22(9):1239–1244.
- Yu SW, Lin SH, Tsai CC, et al. Acupuncture Effect and Mechanism for Treating Pain in Patients With Parkinson’s Disease. Front Neurol. 2019;10:1114.
- Chakravarthy KV, Chaturvedi R, Agari T, et al. Single arm prospective multicenter case series on the use of burst stimulation to improve pain and motor symptoms in Parkinson’s disease. Bioelectron Med. 2020;6(18). DOI: 10.1186/s42234-020-00055-3.
- Loddo G, Calandra-Buonaura G, Sambati L, et al. The Treatment of Sleep Disorders in Parkinson’s Disease: from Research to Clinical Practice. Front Neurol. 2017;8:42.
- Peeraully T, Yong M-H, Chokroverty S, et al. Sleep and Parkinson’s disease: a review of case-control polysomnography studies. Mov Disord. 2012;27(14):1729–1737.
- Zhang Y, Zhao JH, Huang DY, et al. Multiple comorbid sleep disorders adversely affect quality of life in Parkinson’s disease patients. NPJ Parkinsons Dis. 2020;6(1):25. .
- Xu Z, Anderson KN, Saffari SE, et al. Progression of sleep disturbances in Parkinson’s disease: a 5-year longitudinal study. J Neurol. 2021;268(1):312–320.
- Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649.
- JRP Z, During EH. Sleep Issues in Parkinson’s Disease and Their Management. Neurotherapeutics. 2020 Oct;17(4):1480–1494.
- Dhawan V, Healy DG, Pal S, et al. Sleep-related problems of Parkinson’s disease. Age Ageing. 2006;35(3):220–228.
- Gros P, Videnovic A. Sleep and Circadian Rhythm Disorders in Parkinson’s Disease. 2017;3(3):222–234. Curr Sleep Med Rep.
- Garcia-Borreguero D, Larrosa O, Bravo M. Parkinson’s disease and sleep. Sleep Med Rev. 2003;7(2):115–129.
- Liu CF, Wang T, Zhan SQ, et al. Management Recommendations on Sleep Disturbance of Patients with Parkinson’s Disease. Chin Med J (Engl). 2018;131(24):2976–2985.
- Klingelhoefer L, Rizos A, Sauerbier A, et al. Night-time sleep in Parkinson’s disease — the potential use of Parkinson’s KinetiGraph: a prospective comparative study. Eur J Neurol. 2016;23(8):1275–1288.
- Pierantozzi M, Placidi F, Liguori C, et al. Rotigotine may improve sleep architecture in Parkinson’s disease: a double-blind, randomized, placebo-controlled polysomnographic study. Sleep Med. 2016;21:140–144.
- Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord. 2011;26(1):90–99.
- Menza M, Dobkin RD, Marin H, et al. Treatment of insomnia in Parkinson’s disease: a controlled trial of eszopiclone and placebo. Mov Disord. 2010;25(11):1708–1714.
- Dowling GA, Mastick J, Colling E, et al. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005;6(5):459–466.
- Videnovic A, Klerman EB, Wang W, et al. Timed Light Therapy for Sleep and Daytime Sleepiness Associated With Parkinson Disease: a Randomized Clinical Trial. JAMA Neurol. 2017;74(4):411–418.
- Zeng BY, Zhao K. Effect of Acupuncture on the Motor and Nonmotor Symptoms in Parkinson’s Disease—A Review of Clinical Studies. CNS Neurosci Ther. 2016;22(5):333–341.
- Gjerstad MD, Alves G, Wentzel-Larsen T, et al. Excessive daytime sleepiness in Parkinson disease: is it the drugs or the disease? Neurology. 2006;67(5):853–858.
- Amara AW, Chahine LM, Caspell-Garcia C, et al. Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88(8):653–662.
- Breen DP, Williams-Gray CH, Mason SL, et al. Excessive daytime sleepiness and its risk factors in incident Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84(2):233–234.
- Zesiewicz TA, Hauser RA. Sleep attacks and dopamine agonists for Parkinson’s disease: what is currently known? CNS Drugs. 2003;17(8):593–600.
- Hauser RA, Gauger L, Anderson WM, et al. Pramipexole-induced somnolence and episodes of daytime sleep. Mov Disord. 2000;15(4):658–663.
- Ferreira JJ, Galitzky M, Thalamas C, et al. Effect of ropinirole on sleep onset: a randomized, placebo-controlled study in healthy volunteers. Neurology. 2002;58(3):460–462.
- Paus S, Brecht HM, Koster J, et al. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson’s disease. Mov Disord. 2003;18(6):659–667.
- Stefani A, Hogl B. Sleep in Parkinson’s disease. Neuropsychopharmacology. 2020;45(1):121–128.
- Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson’s disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep. 2002;25(8):905–909.
- Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord. 2003;18(3):287–293.
- Postuma RB, Anang J, Pelletier A, et al. Caffeine as symptomatic treatment for Parkinson disease (Café-PD): a randomized trial. Neurology. 2017;89(17):1795–1803.
- Postuma RB, Lang AE, Munhoz RP, et al. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79(7):651–658.
- Weintraub D, Mavandadi S, Mamikonyan E, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology. 2010;75(5):448–455.
- Devos D, Krystkowiak P, Clement F, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(5):470–475.
- Suzuki K, Miyamoto M, Miyamoto T, et al. Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: an open-label, 3-month study. J Neurol Sci. 2017;380:230–233.
- Ondo WG, Perkins T, Swick T, et al. Sodium oxybate for excessive daytime sleepiness in Parkinson disease: an open-label polysomnographic study. Arch Neurol. 2008;65(10):1337–1340.
- Martinez-Martin P, Wan YM, Ray Chaudhuri K, et al. Impulse control and related behaviors in Parkinson’s disease with dementia. Eur J Neurol. 2020;27(6):944–950.
- Fauser M, Lohle M, Ebersbach G, et al. Intraindividual Variability of Nonmotor Fluctuations in Advanced Parkinson’s Disease. J Parkinsons Dis. 2015;5(4):737–741.
- Martinez-Fernandez R, Schmitt E, Martinez-Martin P, et al. The hidden sister of motor fluctuations in Parkinson’s disease: a review on nonmotor fluctuations. Mov Disord. 2016;31(8):1080–1094.
- Chaudhuri KR, Rizos A, Sethi KD. Motor and nonmotor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm. 2013;120(9):1305–1320.
- Martinez-Martin P, Schrag A, Weintraub D, et al. Pilot Study of the International Parkinson and Movement Disorder Society-sponsored Non-motor Rating Scale (MDS-NMS). Mov Disord Clin Pract. 2019;6(3):227–234.
- Martinez-Martin P, Rojo-Abuin JM, Weintraub D, et al. Factor Analysis and Clustering of the Movement Disorder Society-Non-Motor Rating Scale. Mov Disord. 2020;35(6):969–975.
- Chaudhuri KR, Schrag A, Weintraub D, et al. The movement disorder society nonmotor rating scale: initial validation study. Mov Disord. 2020;35(1):116–133.
- Tenison E, Henderson EJ. Multimorbidity and Frailty: tackling Complexity in Parkinson’s Disease. J Parkinsons Dis. 2020;10(s1):S85–S91.