ABSTRACT
Introduction: Significant need exists for effective, well-tolerated pharmacologic treatments for Tourette syndrome (TS). Medications that inhibit vesicular monoamine transporters (i.e. VMAT2 inhibitors) downregulate presynaptic packaging and release of dopamine into the neuronal synapse and are effective in treating hyperkinetic movement disorders such as Huntington’s chorea and tardive dyskinesia (TD); thus, they may be useful in treating TS.
Areas covered: This review describes the clinical program evaluating the safety and efficacy of valbenazine in the treatment of involuntary tics associated with TS in adult and pediatric subjects. While there was a trend in the 6 completed trials toward greater improvement in valbenazine-treated versus placebo subjects on the primary efficacy endpoint (Yale Global Tic Severity Scale Total Tic Score), this difference did not reach statistical significance. Valbenazine was generally well-tolerated in the studies, and treatment-emergent adverse events were consistent with valbenazine studies in TD.
Expert opinion: Due to the failure to meet the primary endpoint in these trials, further investigation of valbenazine for TS is unlikely. Given the need for safe and effective TS therapies and the key role of VMAT2 in modulating dopaminergic activity, it is reasonable for future studies to investigate other VMAT2 inhibitors as potential treatments for TS.
1. Introduction
Tourette syndrome (TS) is a childhood-onset neurodevelopment disorder characterized by the presence of motor and vocal tics that are sudden, involuntary, and nonrhythmic, and can wax and wane in frequency and severity [Citation1–3]. According to The Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-V) criteria, TS diagnosis requires the presence of multiple motor and 1 or more vocal tics that have persisted for more than a year, with an onset before age 18 years [Citation4]. Tics usually present at age 4 to 8 years and tend to increase in frequency and severity until age 10 to 12 years [Citation2,Citation3]. Most patients who present with tics before age 8 will experience a significant decrease in tic frequency during adolescence, often with a complete resolution of symptoms by adulthood [Citation2,Citation3]. TS can have a significant impact on the affected individual’s functioning and quality of life and is often associated with comorbid psychiatric conditions such as attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), anxiety, and depression [Citation2,Citation5–9]. Many patients with TS experience high levels of distress, frustration, and hopelessness, and an increased risk of suicidal thoughts and behaviors [Citation10,Citation11].
While the neuropathology of TS is not well understood, converging lines of empirical evidence implicate dopaminergic dysfunction and dysregulation within prefrontal cortex-basal ganglia circuitry [Citation12,Citation13]. Functional neuroimaging studies in patients with TS have identified a pattern of prefrontal cortex hypermetabolism and reduced striatal activity [Citation14–16]. The dopamine receptor blocking agents (DRBAs), haloperidol, pimozide, and aripiprazole, have demonstrated efficacy in TS, but their use is limited by the risk of adverse events such as sedation, weight gain, metabolic syndrome, and the potential to cause tardive dyskinesia (TD) [Citation17–21]. A range of off-label, non-dopaminergic therapies (e.g. alpha-2 adrenergic agonists, botulinum toxin, etc.) have been used for TS, but they have only limited efficacy [Citation2,Citation5,Citation17–19]. Thus, there is an unmet need for safer, more effective pharmacological treatments for TS.
Vesicular monoamine transporters (VMATs) are integral membrane proteins that play a critical role in packaging monoamines such as dopamine into presynaptic vesicles [Citation22,Citation23]. Two VMAT subtypes have been characterized: VMAT1, which is primarily expressed in neuroendocrine cells; and VMAT2, which is primarily expressed in the central nervous system [Citation24]. Medications that inhibit VMAT2 (i.e. VMAT2 inhibitors) reduce the availability of dopamine in the synapse by modulating the presynaptic release. VMAT2 inhibitors have been used to treat hyperkinetic movement disorders such as chorea associated with Huntington’s disease and TD, and were thought to be a potential therapeutic option for the treatment of TS [Citation25,Citation26].
Valbenazine, a VMAT2 inhibitor that is FDA-approved to treat TD in adults, is the valine ester of [+]-α-dihydrotetrabenazine (HTBZ) [Citation27,Citation28]. After oral administration, valbenazine is cleaved by hydrolysis into a valine ester, which improves passive absorption from the intestinal tract, and [+]-α-HTBZ, which is a potent and selective VMAT2 inhibitor and one of the active metabolites of tetrabenazine [Citation28,Citation29]. Unlike valbenazine, tetrabenazine is administered as a racemic mixture of 2 enantiomers and reduced to 4 active stereoisomers: [+]-α-HTBZ, [-]-α-HTBZ, [+]-β-HTBZ, and [-]-β-HTBZ [Citation28,Citation30,Citation31]. Of these, only [+]-α-HTBZ and [+]-β-HTBZ have any appreciable inhibitory effects on VMAT2 (the former having highest selectivity for VMAT2 of the 4 isomers but the lowest plasma concentration after oral administration of tetrabenazine), while [-]-α-HTBZ, [-]-β-HTBZ, and [+]-β-HTBZ have some off-target interactions at dopaminergic, serotonergic, and adrenergic receptor sites [Citation28,Citation31].
A clinical program was undertaken to investigate the safety and efficacy of valbenazine in the treatment of involuntary tics associated with TS. Six clinical trials were completed: one dose finding study in pediatric subjects; 3 randomized, double-blind, placebo-controlled trials (DBPC) (1 fixed-dose trial in adults, 1 fixed-dose trial in pediatric subjects, and 1 pediatric dose-optimization study); and 2 open-label, 24-week, rollover studies. An overview of these studies will be presented here.
2. Pediatric open-label dose-finding study
2.1. Study design
A phase 1b, open-label trial (T-Force, NCT02256475) was conducted to explore the appropriate dosing in pediatric patients with TS (). Eligible subjects were 6–18 years of age and met the following criteria for TS: DSM-IV or -V diagnosis of TS, at least moderate tic severity (Clinical Global Impression of Tics-Severity [CGI-Tics-Severity] score ≥4 or Yale Global Tic Severity Scale [YGTSS] Total Tic Score [TTS] ≥20), and TS symptoms that impair school, occupational, and/or social function. Subjects could remain on stable doses of medications to treat TS symptoms or other TS spectrum comorbidities (except the use of dopamine antagonists and VMAT2 inhibitors, which were prohibited).
Table 1. Valbenazine studies in Tourette syndrome
Children (age 6–11 years) and adolescents (age 12–18 years) were assigned to receive fixed doses of valbenazine (5 mg, 10 mg, and 25 mg [children]; 10 mg, 25 mg, and 50 mg [adolescents]) for 14 days. The doses were chosen based on pharmacokinetic (PK) modeling using exposure data from completed studies of valbenazine in adult healthy volunteers and in patients with TD (data on file). After completion of the 14-day dosing period of the first dosing cohort, there was a 2-week interim period for an independent medical monitor to review all safety and PK results and to determine if the maximum tolerated dose (MTD) had been reached before starting the next dosing cohort dosing period. Subsequent decisions to dose escalate were also based on an independent medical monitor review of safety and PK data, and an assessment of MTD.
Pharmacodynamic (PD) assessments included the YGTSS TTS and the Clinical Global Impression of Change-Tourette Syndrome-Improvement (CGI-TS-Improvement) score. The YGTSS assesses tic severity during the prior week and includes 2 components: the TTS and the Impairment Score. The TTS has a total of 5 domains (number, frequency, intensity, complexity, and interference) that are assessed for both motor and phonic (vocal) tics separately [Citation32]. Each domain is rated on a scale of 0–5 (with higher scores representing greater severity); thus, the maximum TTS score is 50. CGI-TS-Improvement assesses overall improvement from baseline in TS symptoms on a 7-point scale (range 1 ‘very much improved’ to 7 ‘very much worse’).
Safety assessments included monitoring treatment emergent adverse events (TEAEs), vital signs, 12-lead electrocardiograms (ECGs), laboratory tests, and suicidality using the Columbia-Suicide Severity Rating Scale (C-SSRS).
This study and all other studies described in this paper adhered to International Conference on Harmonization Guidelines for Good Clinical Practice and US Food and Drug Administration guidelines. Study protocols were reviewed and approved by the institutional review board for each participating center. All study subjects provided voluntary written informed consent.
2.2. Baseline characteristics
A total of 28 subjects enrolled in the study, 11 children (valbenazine 5 mg, n = 6; valbenazine 10 mg, n = 5) and 17 adolescents (valbenazine 10 mg, n = 6; valbenazine 25 mg, n = 6; valbenazine 50 mg, n = 5) (). Mean age was 9.5 years for children and 14.2 years for adolescents. Most subjects were white (92.9%) and male (85.7%). Mean YGTSS TTS scores at baseline (Day −1) were 32.7 (valbenazine 5 mg) and 35.4 (valbenazine 10 mg) in children and 26.5 (valbenazine 10 mg), 30.2 (valbenazine 25 mg), and 34.0 (valbenazine 50 mg) in adolescents.
2.3. Pharmacodynamics
Mean YGTSS TTS decreased from baseline to Day 14 by 5.7 points (valbenazine 5 mg) and 12.8 points (valbenazine 10 mg) in children, and by 10.7 points (valbenazine 10 mg), 10.0 points (valbenazine 25 mg), and 7.7 points (valbenazine 50 mg) in adolescents. On Day 14, CGI-TS-Improvement scores were ≤2 (‘much improved’ or ‘very much improved’) in 3/11 (27.3%) children and 9/15 (60.0%) adolescents, and ≤3 (‘minimally improved,’ ‘much improved,’ or ‘very much improved’) in 11/11 (100.0%) children and 13/15 (86.7%) adolescents.
2.4. Safety and tolerability
A total of 5 (45.5%) children and 14 (82.4%) adolescents reported TEAEs during treatment. In children, the most frequently reported TEAEs (n [%]) were headache (3 [27.3%]) and tic (2 [18.2%]); in adolescents, the most frequent TEAEs were fatigue (8 [47.1%]), somnolence (3 [17.6%]) and headache (2 [11.8%]). Two adolescent subjects in the valbenazine 50 mg group discontinued due to TEAEs. There were no deaths or serious adverse events (SAEs) during the study, and no clinically relevant changes in mean values for clinical laboratory tests, vital signs, body weight, or ECGs parameters.
3. Adult and pediatric fixed-dose trials
Based on the safety and tolerability profile and promising pharmacodynamic (PD) results from the T-Force study, two phase 2, randomized, DBPC trials were conducted to evaluate the efficacy and safety of valbenazine in the treatment of TS: 1 fixed-dose study in adults (T-Forward [NCT02581865]) and 1 fixed-dose study in adolescents and children (T-Force GREEN [NCT02679079]).
3.1. Study designs
In the adult study (T-Forward), subjects 18 to 64 years of age were randomized to receive 8 weeks of placebo, 8 weeks of 40 mg valbenazine, or 1 week of 40 mg valbenazine and 7 weeks of 80 mg valbenazine followed by a 2-week no-treatment follow-up period (). In the pediatric study (T-Force GREEN), subjects 6 to 17 years of age were randomized to receive placebo, ‘lower’ dose valbenazine (10 mg in children [6 to 11 years of age] and 20 mg in adolescents [12 to 17 years of age]), or ‘higher’ dose valbenazine (20 mg in children and 40 mg in adolescents) for 6 weeks, and then a 2-week, no-treatment follow-up period.
Both studies included subjects who met standard inclusion and exclusion criteria for clinical trials, as well as the following criteria for TS: DSM-IV or -V diagnosis of TS, at least moderate tic severity (CGI-Tics-Severity score ≥4 or YGTSS TTS ≥20), and TS symptoms that impair school, occupational, and/or social function. Subjects could remain on stable doses of medications to treat TS symptoms or other TS spectrum comorbidities (except the use of dopamine antagonists and VMAT2 inhibitors, which were prohibited).
The primary efficacy outcome was change from baseline to end of treatment in the YGTSS TTS, as scored by the certified site rater (). Secondary or exploratory endpoints included the clinician-rated CGI-Tics-Severity, which rates overall tic severity on a 7-point scale (range 1 ‘normal, not at all ill’ to 7 ‘among the most extremely ill patients’), and CGI-TS-Improvement score. Statistical analysis was conducted based on the intent-to-treat (ITT) population using mixed-effect model repeated measures (MMRM) analysis for the YGTSS TTS (primary efficacy endpoint) and CGI-Tics-Severity. CGI-TS-Improvement was analyzed using MMRM analysis (T-Force GREEN) and the analysis of variance (ANOVA) model (T-Forward).
Safety assessments included monitoring TEAEs, vital signs, ECGs, laboratory tests, and suicidality using the C-SSRS. Plasma concentrations of valbenazine and its major metabolite ([+]-α-HTBZ) were assessed throughout the treatment period.
3.2. Disposition and baseline characteristics
Of 124 subjects enrolled in the adult study (T-Forward), 97 (78.2%) completed the study (Supplemental Table 1). The most common reasons for discontinuation were adverse events and withdrawal of consent. Baseline characteristics were similar across treatment groups (Supplemental Table 2). Mean age was 35.2 years and mean age at TS diagnosis was 19.9 years. The most common comorbid psychiatric conditions were ADHD (26.6%), anxiety (18.5%), and depression (18.5%), and the most common concomitant medications were antidepressants (34.7%), nonsteroidal anti-inflammatories (28.2%), and psychostimulants/alpha-2 adrenergic agonists (21.8%). Mean baseline YGTSS TTS score was 31.1.
Of 98 subjects enrolled in the pediatric study (T-Force GREEN), 90 (91.8%) completed the study (Supplemental Table 1). The most common reasons for discontinuation were adverse events and withdrawal of consent. Demographic and baseline disease characteristics were similar across treatment groups (Supplemental Table 2). Mean age was 11.7 years and mean age at TS diagnosis was 9.1 years. More than half (55.7%) of subjects reported a comorbid psychiatric diagnosis of ADHD, and 17.5% and 15.5% reported insomnia and anxiety, respectively. The most common concomitant medications in pediatric subjects were psychostimulants/alpha-2 adrenergic agonists (44.3%) and antidepressants (33.0%).
3.3. Efficacy
In the adult fixed-dose study (T-Forward), change from baseline to Week 8 in YGTSS TTS (primary efficacy endpoint) was not statistically different from placebo in either the 40 mg valbenazine (LS mean difference [SEM], −1.4 [1.8], P= 0.433) or 80 mg valbenazine (LS mean difference [SEM], −2.5 [1.9], P= 0.184) group ( and )). However, there was a trend toward greater improvement over placebo in both valbenazine treatment groups, and subjects in the 80 mg valbenazine group experienced statistically significant improvement versus placebo at Week 4 (P= 0.025) and Week 6 (P= 0.018) ()). CGI-TS-Improvement in the valbenazine 80 mg group achieved P< 0.05 at Week 6 (LS mean difference [SEM], −0.7 [0.3], P= 0.006) and Week 8 (end of treatment, LS mean difference [SEM], −0.7 [0.3], P= 0.015), but these results could not be considered statistically significant due to failure to meet the primary endpoint based on the prespecified multiplicity adjustment. Differences in CGI-Tics-Severity did not reach P< 0.05 between valbenazine (40 or 80 mg) and placebo.
Figure 1. Change from baseline in YGTSS TTS by visit and treatment group in double-blind, placebo-controlled studies (primary outcome)
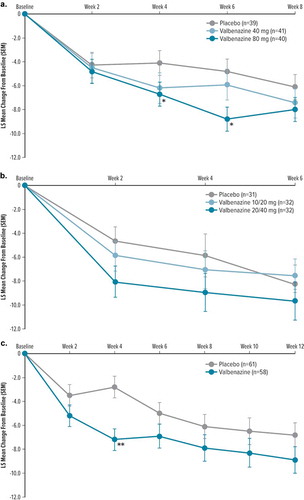
Table 2. Primary and select additional efficacy outcomes in double-blind, placebo-controlled studies
In the pediatric fixed-dose study (T-Force GREEN), the change from baseline to Week 6 in YGTSS TTS (primary efficacy endpoint) was not significantly improved relative to placebo for either the lower or higher dose valbenazine groups (10/20 mg valbenazine, LS mean difference [SEM], 1.5 [2.1], P= 0.467; 20/40 mg valbenazine, LS mean difference [SEM], −0.3 [2.1], P= 0.888) ( and )). Similarly, CGI-TS-Improvement scores at Week 6 for valbenazine did not differ substantially from placebo (10/20 mg valbenazine, LS mean difference [SEM], 0.1 [0.3], P= 0.669; 20/40 mg valbenazine, LS mean difference [SEM], −0.4 [0.3], P= 0.212). There was a nominally significant difference in Week 6 CGI-Tics-Severity scores in the 10/20 mg valbenazine group compared with placebo (LS mean difference [SEM], 0.6 [0.2], P= 0.009; placebo showed a greater decrease), but not significant in the 20/40 mg valbenazine group compared with placebo (LS mean difference [SEM], 0.2 [0.2], P= 0.413).
At the 2-week post-treatment follow-up visit in both T-Forward and T-Force GREEN, mean YGTSS TTS change from baseline increased (i.e. less improvement from baseline) compared to the end of treatment visit, indicating that TS symptoms returned toward baseline levels following discontinuation of valbenazine (Supplemental Table 3).
3.4. Safety and tolerability
In T-Forward, 84.5% of subjects experienced TEAEs, with a higher proportion of subjects in the 80 mg valbenazine group reporting TEAEs compared to the valbenazine 40 mg group and placebo (). The most frequently reported TEAEs were somnolence, fatigue, akathisia, and headache. TEAEs led to study discontinuation in 16 (19.0%) subjects. SAEs were reported in 4 subjects, 1 of which (hypersensitivity after 16 days on valbenazine 80 mg) was assessed as related to treatment and led to study discontinuation (the 3 other SAEs were judged by the investigator as unrelated to treatment).
Table 3. Treatment-emergent adverse events in double-blind, placebo-controlled studies
In T-Force GREEN, 61.9% of subjects reported TEAEs, with a higher incidence of TEAEs in subjects receiving valbenazine than placebo (). The most frequently reported TEAEs were headache, somnolence, and upper respiratory tract infection. TEAEs led to study discontinuation in 4 (4.1%) subjects. SAEs were reported in 1 subject who was in the placebo group and withdrew from the study.
No deaths occurred during either study, and there were no clinically important mean changes in clinical laboratory values, vital signs, or ECG findings in any of the DBPC studies. Serum prolactin concentrations tended to increase during treatment with valbenazine; however, these increases were generally small and not considered to be clinically relevant. No TEAE of hyperprolactinemia was reported in either study. Body weight increases from baseline were approximately 1–2 kg greater in the valbenazine groups than placebo.
Plasma concentrations of valbenazine and [+]-α-HTBZ were dose proportional in both T-Forward and T-Force GREEN, with steady-state concentrations generally achieved by Week 2 and remaining generally consistent throughout the treatment period.
4. Pediatric dose-optimization study
The balance of efficacy and safety data from the adult fixed-dose study (T-Forward), and the predominance of TS in pediatric patients compared to adults, did not support the continued evaluation of valbenazine in adult patients with TS. In the fixed-dose pediatric study (T-Force GREEN), preliminary exposure-response analysis indicated subtherapeutic plasma exposures in the lower dose valbenazine group (10/20 mg), and exposures at the lower end of desired therapeutic levels in the higher dose group (20/40 mg valbenazine) (data on file). Therefore, a phase 2b, DBPC dose-optimization study (T-Force GOLD [NCT03325010]) was conducted in pediatric subjects to enable an individualized, stepwise evaluation of the impact of higher valbenazine doses on TS symptoms.
4.1. Study design
Pediatric subjects 6 to 17 years of age were randomized to receive placebo or valbenazine. The starting valbenazine dose was 20 mg for subjects <50 kg in body weight at baseline and 40 mg for subjects ≥50 kg. For the first 6 weeks, doses could be escalated in 20 mg increments based on tolerability and persistence of TS symptoms to a maximum of 60 mg or 80 mg for subjects <50 kg and ≥50 kg, respectively. Subjects randomized to placebo had the same dose escalation requirements but received placebo only. After Week 6, subjects received their optimized dose of valbenazine or placebo for the 6-week maintenance period, followed by a 2-week, no-treatment follow-up period. Inclusion/exclusion criteria and efficacy and safety outcomes were the same as previously described for the fixed-dose studies.
4.2. Disposition and baseline characteristics
Of 127 subjects enrolled in T-Force GOLD, 98 (77.1%), completed the study (Supplemental Table 1). The most common reasons for discontinuation were adverse events and protocol deviations. Demographic and baseline disease characteristics were similar across the treatment groups (Supplemental Table 2). Mean age was 12.3 years and mean age at TS diagnosis was 8.5 years. More than half (56.2%) of subjects reported a comorbid psychiatric diagnosis of ADHD, and 17.4% and 19.8% reported anxiety and obsessive-compulsive disorder, respectively. The most common concomitant medications were psychostimulants/alpha-2 adrenergic agonists (49.6%), antihistamines (24.0%), and antidepressants (23.1%).
4.3. Efficacy
The difference in change from baseline to Week 12 in the YGTSS TTS favored valbenazine over placebo but was not statistically significant (LS mean difference [SEM], −2.1 [1.5], P= 0.177) ( and )). Results for secondary/exploratory endpoints showed similar results favoring valbenazine, but these results could not be considered for statistical analysis due to failure to meet the primary endpoint based on the prespecified multiplicity adjustment. Improvement from baseline to Week 12 in CGI-Tics-Severity was greater for valbenazine than placebo (LS mean difference [SEM], −0.3 [0.2], P= 0.110) (), and achieved P< 0.05 for valbenazine versus placebo at Weeks 2, 4, 6, and 10 ()). CGI-TS-Improvement scores showed significant improvement at Week 12 (LS mean difference [SEM], −0.5 [0.2], P= 0.025) and at every earlier assessment (P< 0.05) ( and )).
Figure 2. Secondary/exploratory efficacy outcomes by visit and treatment group in T-Force GOLD
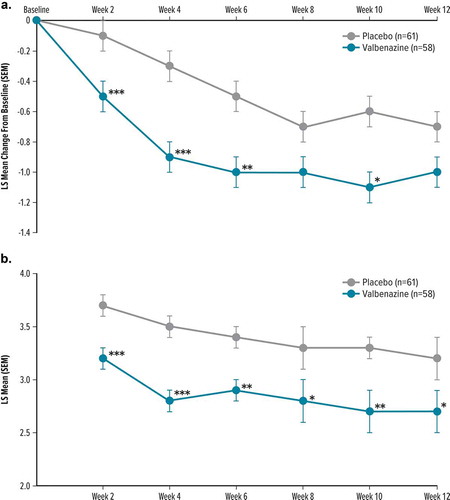
At the 2-week post-treatment follow-up visit, mean YGTSS TTS change from baseline increased (i.e. less improvement from baseline) compared to the end of treatment, indicating that TS symptoms returned toward baseline levels following discontinuation of valbenazine (Supplemental Table 3).
4.4. Safety and tolerability
A total of 66.9% of subjects reported TEAEs, with a higher incidence of TEAEs in subjects receiving valbenazine than placebo (). The most frequently reported TEAEs were somnolence, headache, fatigue, vomiting, and suicidal ideation. TEAEs led to study discontinuation in 11 (9.1%) subjects. SAEs were reported in 1 subject who was in the placebo group and withdrew from the study. No deaths occurred during the study, and there were no clinically important mean changes in clinical laboratory values, vital signs, or ECG findings.
5. Open-label extension trials
Two open-label studies were conducted to provide a sufficient long-term safety database to support chronic dosing of valbenazine in pediatric patients.
5.1. Study designs
Two phase 2, open-label, rollover studies (T-Fusion [NCT02879578] and T-Force GOLD+ [NCT03444038]) were conducted to assess the safety, tolerability, and PD of valbenazine administered once-daily for 24 weeks (). Adult and pediatric subjects who had completed the fixed-dose DBPC studies T-Forward and T-Force GREEN were eligible to enroll in T-Fusion. Subjects were started on valbenazine 10 mg (children, age 6–11 years), 20 mg (adolescents, age 12–17 years), or 40 mg (adults, age 18 to 64 years) and remained on these doses for 4 weeks. After Week 4, doses could be continued at the starting dose or increased based on tolerability and persistence of TS symptoms to a maximum of 20 mg in children, 40 mg in adolescents and 80 mg in adults. At any time after dose escalation, doses could be decreased to the starting dose. Pediatric subjects who completed the dose-optimization study T-Force GOLD were eligible to enroll in T-Force GOLD+. The starting valbenazine dose was 20 mg for subjects <50 kg in body weight at baseline or 40 mg for subjects ≥50 kg. Doses could be escalated at Week 2 or Week 4 in 20 mg increments based on tolerability and persistence of TS symptoms to a maximum of 60 mg or 80 mg for subjects <50 kg and ≥50 kg, respectively. After Week 4, subjects received their optimized dose of valbenazine for the 20-week maintenance period, followed by a 4-week no-treatment follow-up period.
Safety assessments included TEAEs (primary outcome), vital signs, ECGs, laboratory tests, and the C-SSRS rating of suicidal ideation or behavior. PD was assessed in adolescents and children using the YGTSS TTS, CGI-Tics-Severity score, and CGI-TS-Improvement score.
5.2. Disposition and baseline characteristics
A total of 74 pediatric and 81 adult subjects who had previously completed T-Force GREEN and T-Forward, respectively, enrolled in T-Fusion. Of these, 55 (74.3%) pediatric and 49 (60.5%) adult subjects completed T-Fusion. A total of 85 pediatric subjects who had previously completed T-Force GOLD enrolled in T-Force GOLD+; of these, 56 (65.9%) completed T-Force GOLD+. The most common reasons for discontinuation in the rollover studies were adverse events and withdrawal of consent in T-Fusion, and adverse events, withdrawal of consent, and lack of efficacy in T-Force GOLD+. Baseline characteristics for both studies are shown in Supplemental Table 4.
5.3. Pharmacodynamics
In T-Fusion, mean YGTSS TTS decreased from baseline to Week 24 by 9.6 points in children and 11.7 points in adolescents ()). CGI-TS-Improvement scores at Week 24 were ≤2 (‘much improved,’ or ‘very much improved’) in 13/23 (56.5%) children and 27/32 (84.4%) adolescents. CGI-Tics-Severity scores at Week 24 were ≤3 (‘normal, not at all ill,’ ‘borderline ill,’ or ‘mildly ill’) in 19/23 (82.6%) children and 29/32 (90.6%) adolescents.
Figure 3. Change from baseline in YGTSS TTS by visit in 24-week, open-label, extension studies
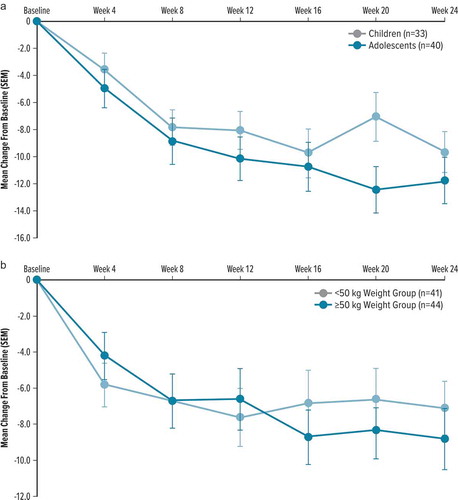
In T-Force GOLD+, mean YGTSS TTS decreased from baseline to Week 24 by 7.1 points in pediatric subjects <50 kg in body weight and by 8.8 points in pediatric subjects ≥50 kg ()). CGI-TS-Improvement scores at Week 24 were ≤2 in 18/30 (60.0%) subjects <50 kg and 15/27 (55.6%) subjects ≥50 kg. CGI-Tics-Severity scores at Week 24 were ≤3 in 23/30 (76.7%) subjects <50 kg and 16/27 (59.3%) subjects ≥50 kg.
5.4. Safety and tolerability
A total of 77% of subjects in T-Fusion and 83.5% of subjects in T-Force GOLD+ reported TEAEs during the 24 weeks of open-label valbenazine treatment (Supplemental Table 5). The most frequently reported TEAEs in the rollover studies were somnolence, fatigue, sedation, and headache. TEAEs led to study discontinuation in 17 (11.0%) subjects in T-Fusion and 12 (14.1%) subjects in T-Force GOLD+. In T-Fusion, there were no SAEs in children or adolescents; two adults experienced SAEs, one of which (extrapyramidal disorder) was considered possibly related to treatment and led to study discontinuation. The event resolved the same day, after treatment with intravenous diphenhydramine. The other SAE (chronic obstructive pulmonary disease) was considered not related to treatment. In T-Force GOLD+, 3 subjects reported SAEs, one of which (cognitive disorder) was judged possibly related to study drug and led to study discontinuation. The other 2 SAEs (Henoch-Schönlein purpura and infected spider bite) were considered unlikely or not related to treatment. There were no deaths reported during either study, and there were no clinically relevant mean changes in laboratory values, vital signs, body weight, or ECGs.
6. Conclusion
This paper describes the clinical program to evaluate the safety and efficacy of valbenazine in the treatment of TS in pediatric and adult subjects. First, a phase 2, open-label trial (T-Force) was conducted to explore the appropriate dosing in the pediatric population. Based on the favorable safety/tolerability and PD results from this study, two randomized, DBPC, fixed-dose trials (one in adults [T-Forward] and one in children/adolescents [T-Force GREEN]) were conducted. In these trials, there was a trend toward greater improvement in valbenazine-treated subjects versus placebo in the primary efficacy endpoint (YGTSS TTS), but this difference did not reach statistical significance. Results for the secondary and exploratory outcomes also generally favored valbenazine but did not reach statistical significance compared with placebo.
Exposure-response models of the data from T-Force GREEN indicated that therapeutic levels may not have been reached in pediatric patients; therefore, a dose-optimization study (T-Force GOLD) was conducted in pediatric patients. In this study, doses were escalated based on tolerability and persistence of TS symptoms to a maximum of 60 mg or 80 mg for subjects <50 kg and ≥50 kg body weight at baseline, respectively. Despite the optimization of dose/exposure, the primary efficacy endpoint (YGTSS TTS) was not met. Similar to the fixed-dose studies, there was a trend toward greater improvement in subjects receiving valbenazine on the primary outcome, but this difference did not reach statistical significance compared with placebo. On the secondary/exploratory outcomes, valbenazine groups achieved greater improvement compared to placebo in CGI-TS Improvement scores at Week 12 and every earlier assessment, and in CGI-Tics-Severity at Weeks 2, 4, 6, and 10.
Valbenazine was generally well-tolerated in both the placebo-controlled, 6- to 12-week studies and in the open-label, 24-week extension studies. There were no clinically important changes in mean values for clinical laboratory, ECG parameters, or vital signs, and TEAEs were generally consistent with those experienced in subjects treated with valbenazine in clinical studies of TD [Citation33–35]. TEAEs of suicidal ideation occurred sporadically during these studies, with 10 (2.9%) subjects reporting suicidal ideation in the DBPC trials, 6 of whom participated in the T-Force GOLD study. Of the 10 subjects with TEAEs of suicidal ideation in the DBPC studies, 6 had a prior history of suicidality (3 of whom participated in T-Force GOLD). In the four subjects with no prior history of suicidal ideation, the TEAEs reported during the study were isolated incidents (C-SSRS scores of 1 ‘wish to die’ or 2 ‘nonspecific active suicidal thoughts’), and the subjects completed the study with no further reports of suicidality and no TEAEs of depression. The interpretation of these findings is complicated by the relatively small clinical trial population, as well as the increased baseline risk of suicidality in individuals with TS [Citation10,Citation11]. Consistent with this increased baseline risk, 60 (17.5%) of the TS subjects in the DBPC studies of valbenazine had a history of suicidal thoughts or behaviors prior to their study participation.
Due to the failure to meet the primary endpoint in the phase 2 DBPC trials, further investigation of valbenazine for the treatment of TS is unlikely. However, given the continued need for tolerable and effective TS therapies and the key role of VMAT2 in modulating dopaminergic activity in the central nervous system, it is reasonable for future studies to investigate the suitability of other VMAT2 inhibitors as potential treatments for TS.
7. Expert opinion
The recent approval of the VMAT2 inhibitors, valbenazine and deutetrabenazine, for the treatment of TD has resulted in a renewed interest in the pathophysiology and treatment of other hyperkinetic movement disorders, including tics associated with TS. The only pharmacological treatments that are currently FDA approved for TS are the DRBAs, haloperidol, pimozide, and aripiprazole [Citation36–38]; however, these medications are not well-tolerated, especially in children and adolescents [Citation17–21]. In addition, DRBAs are associated with a risk of developing TD [Citation17–20], although evidence from a large, retrospective chart review suggested that the incidence of TD may be lower in patients with TS than other patient populations [Citation39]. Other off-label therapies for TS have shown only limited efficacy [Citation2,Citation5,Citation17–19]. Recent guidelines by the American Academy of Neurology did not find Level A evidence to recommend any available treatments for TS [Citation17,Citation18].
Studies in TS are complicated by the waxing and waning of symptoms and existence of comorbid conditions, as well as a lack of definitive target receptors for treatment. Recent evidence implicates dopaminergic dysfunction and dysregulation within prefrontal cortex-basal ganglia circuitry, and functional neuroimaging studies in patients with TS have identified a pattern of prefrontal cortex hypermetabolism and reduced striatal activity [Citation12–16]. VMAT2 inhibitors downregulate the presynaptic packaging and release of dopamine into the neuronal synapse and are effective in treating hyperkinetic movement disorders such as Huntington’s chorea and TD; thus they were considered as potentially useful agents in the treatment of TS [Citation25,Citation26]. In a recent retrospective chart review and patient questionnaire to assess real-world use of VMAT2 inhibitors in patients with hyperkinetic movement disorders, 49.6% of patients receiving a VMAT inhibitor had a diagnosis of TS, and >60% indicated that the VMAT2 inhibitors effectively controlled their hyperkinetic movement symptoms [Citation40]. In two retrospective, open-label studies, tetrabenazine treatment was associated with an improvement in TS symptoms or global improvement [Citation41,Citation42], but to date there have been no sufficiently powered, well-controlled trials to evaluate tetrabenazine for the treatment of TS. Deutetrabenazine, a deuterated molecular form of tetrabenazine, showed positive results in a small, phase 1, open-label, trial of patients with TS [Citation43]; however, it failed to meet the primary endpoint in large phase 2/phase 3 studies [Citation44].
The results from these studies presented here suggest that valbenazine may not be an effective treatment for the management of TS; thus, further investigation of valbenazine for the treatment of TS is unlikely. Future research is needed to better understand the neuropathology of TS, including the potential role of dopamine modulation. In addition, there is a continued need for well-powered safety and efficacy studies to evaluate potential new treatments for TS. Given the key role of VMAT2 in modulating dopaminergic activity in the central nervous system, future studies may investigate the suitability of other VMAT2 inhibitors as potential treatments for TS.
Article highlights
A clinical program was conducted to evaluate the safety and efficacy of valbenazine in the treatment of involuntary tics associated with TS in adults and children.
In the 6 completed valbenazine studies in TS, the primary efficacy endpoint was not met; however, some secondary and exploratory outcomes showed improvements in TS symptoms, and valbenazine treatment was generally well-tolerated.
Further investigation of valbenazine for TS is unlikely; however, given the continued unmet need for tolerable and effective TS therapies, future studies may be warranted to evaluate other VMAT2 inhibitors as potential treatments for TS.
Declaration of interest
The authors are all full-time employees of Neurocrine Biosciences, Inc., and own stock in Neurocrine Biosciences, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (59.3 KB)Acknowledgments
The authors wish to thank all investigators, patients, and families who participated in the T-Force, T-Forward, T-Force GREEN, T-Force GOLD, T-Fusion, and T-Force GOLD+ studies, and Roger Kurlan, Tanya Murphy, Barbara J. Coffey, Jorge L. Juncos, and Michael E. McManus for serving the program in an advisory capacity. The authors also thank Christopher O’Brien, former Chief Medical Officer at Neurocrine Biosciences, Inc., who contributed to the conception and design of the studies. Manuscript preparation and editorial support were provided by Jennifer Kaiser at Prescott Medical Communications Group (Chicago, IL) and funded by Neurocrine Biosciences, Inc.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to valbenazine being a product that has not been approved for the treatment of patients with Tourette syndrome but available from the corresponding author on reasonable request.
Supplementary materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Billnitzer A, Jankovic J. Current management of tics and tourette syndrome: behavioral, pharmacologic, and surgical treatments. Neurotherapeutics. 2020 Aug 27;17(4):1681–1693.
- Quezada J, Coffman KA. Current approaches and new developments in the pharmacological management of tourette syndrome. CNS Drugs. 2018 Jan;32(1):33–45.
- Mittal SO. Tics and Tourette’s syndrome. Drugs Context. 2020;9:1–7.
- Diagnostic and statistical manual of mental disorders. 5th ed. Washington D.C.: American Psychiatric Association. 2013.
- Seideman MF, Seideman TA. A review of the current treatment of tourette syndrome. J Pediatr Pharmacol Ther. 2020;25(5):401–412.
- Hirschtritt ME, Lee PC, Pauls DL, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015 Apr;72(4):325–333.
- Scahill L, Sukhodolsky DG, Williams SK, et al. Public health significance of tic disorders in children and adolescents. Adv Neurol. 2005;96:240–248.
- Claussen AH, Bitsko RH, Holbrook JR, et al. Impact of tourette syndrome on school measures in a nationally representative sample. J Dev Behav Pediatr. 2018 May;39(4):335–342.
- Storch EA, Merlo LJ, Lack C, et al. Quality of life in youth with tourette’s syndrome and chronic tic disorder. J Clin Child Adolesc Psychol. 2007 Apr–June;36(2):217–227.
- Johnco C, McGuire JF, McBride NM, et al. Suicidal ideation in youth with tic disorders. J Affect Disord. 2016 Aug;200:204–211.
- Storch EA, Hanks CE, Mink JW, et al. Suicidal thoughts and behaviors in children and adolescents with chronic tic disorders. Depress Anxiety. 2015 Oct;32(10):744–753.
- Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J Neurosci. 2011Aug31;31(35):12387–12395. .
- Pourfar M, Feigin A, Tang CC, et al. Abnormal metabolic brain networks in tourette syndrome. Neurology. 2011 Mar 15;76(11):944–952.
- Braun AR, Randolph C, Stoetter B, et al. The functional neuroanatomy of tourette’s syndrome: an FDG-PET study. II: relationships between regional cerebral metabolism and associated behavioral and cognitive features of the illness. Neuropsychopharmacology. 1995 Oct;13(2):151–168.
- Braun AR, Stoetter B, Randolph C, et al. The functional neuroanatomy of tourette’s syndrome: an FDG-PET study. I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology. 1993 Dec;9(4):277–291.
- Jeffries KJ, Schooler C, Schoenbach C, et al. The functional neuroanatomy of tourette’s syndrome: an FDG PET study III: functional coupling of regional cerebral metabolic rates. Neuropsychopharmacology. 2002 July;27(1):92–104.
- Pringsheim T, Okun MS, Muller-Vahl K, et al. Practice guideline recommendations summary: treatment of tics in people with tourette syndrome and chronic tic disorders. Neurology. 2019 May 7;92(19):896–906.
- Pringsheim T, Holler-Managan Y, Okun MS, et al. Comprehensive systematic review summary: treatment of tics in people with tourette syndrome and chronic tic disorders. Neurology. 2019 May 7;92(19):907–915.
- Roessner V, Plessen KJ, Rothenberger A, et al. European clinical guidelines for tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011 Apr;20(4):173–196.
- Factor SA, Burkhard PR, Caroff S, et al. Recent developments in drug-induced movement disorders: a mixed picture. Lancet Neurol. 2019 Sept;18(9):880–890.
- Tschoner A, Engl J, Laimer M, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract. 2007 Aug;61(8):1356–1370.
- Yaffe D, Forrest LR, Schuldiner S. The ins and outs of vesicular monoamine transporters. J Gen Physiol. 2018 May 7;150(5):671–682.
- Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011 July;31(4):483–519.
- Erickson JD, Schafer MK, Bonner TI, et al. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996 May 14;93(10):5166–5171.
- Citrome L. Tardive dyskinesia: placing vesicular monoamine transporter type 2 (VMAT2) inhibitors into clinical perspective. Expert Rev Neurother. 2018;18(4):323–332.
- Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016;17(18):2461–2470.
- Ingrezza [Prescribing Information]. San Diego (CA): Neurocrine Biosciences; 2020. [cited 2021 Mar 1]. Available from: https://www.neurocrine.com/assets/INGREZZA-full-Prescribing-Information.pdf.
- Grigoriadis DE, Smith E, Hoare SR, et al. Pharmacologic characterization of valbenazine (NBI-98854) and its metabolites. J Pharmacol Exp Ther. 2017 Apr 12;361(3):454–461.
- Harriott ND, Williams JP, Smith EB, et al. VMAT2 inhibitors and the path to Ingrezza (valbenazine). Prog Med Chem. 2018;57(1):87–111.
- Kilbourn MR, Lee LC, Heeg MJ, et al. Absolute configuration of (+)-alpha-dihydrotetrabenazine, an active metabolite of tetrabenazine. Chirality. 1997;9(1):59–62.
- Skor H, Smith EB, Loewen G, et al. Differences in dihydrotetrabenazine isomer concentrations following administration of tetrabenazine and valbenazine. Drugs R D. 2017 Sept;17(3):449–459.
- Leckman JF, Riddle MA, Hardin MT, et al. The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989 July;28(4):566–573.
- O’Brien CF, Jimenez R, Hauser RA, et al. NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Mov Disord. 2015 Oct;30(12):1681–1687.
- Hauser RA, Factor SA, Marder SR, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174:476–484.
- Factor SA, Remington G, Comella CL, et al. The effects of valbenazine in participants with tardive dyskinesia: results of the 1-year KINECT 3 extension study. J Clin Psychiatry. 2017 Nov 14;78:1344–1350.
- Orap [Prescribing Information]. Sellersville (PA): Teva Pharmaceuticals USA; 2008.
- Abilify [Prescribing Information]. Tokyo, Japan: Otsuka America Pharmaceutical Inc; 2016.
- Haloperidol. 2018 [cited 2018 Oct 31]. Available from: https://www.drugs.com/monograph/haloperidol.html
- Muller-Vahl KR, Krueger D. Does Tourette syndrome prevent tardive dyskinesia? Mov Disord. 2011 Nov;26(13):2442–2443.
- Niemann N, Jankovic J. Real-world experience with VMAT2 inhibitors. Clin Neuropharmacol. 2019 Mar/Apr;42(2):37–41.
- Kenney CJ, Hunter CB, Mejia NI, et al. Tetrabenazine in the treatment of tourette syndrome. J Pediatr Neurol. 2007;5(1):9–13.
- Porta M, Sassi M, Cavallazzi M, et al. Tourette’s syndrome and role of tetrabenazine: review and personal experience. Clin Drug Investig. 2008;28(7):443–459.
- Jankovic J, Jimenez-Shahed J, Budman C, et al. Deutetrabenazine in tics associated with tourette syndrome. Tremor Other Hyperkinet Mov (N Y). 2016;6:422.
- Teva announces registration trials of deutetrabenazine in pediatric patients with tourette syndrome did not meet the primary endpoint. Press Release, 2020 Feb 19. Available from: https://www.tevapharm.com/news-and-media/latest-news/teva-announces-registration-trials-of-deutetrabenazine-in-pediatric-patients-with-tourette-syndrome-did-n/.
