ABSTRACT
Introduction
Levodopa remains the gold-standard Parkinson’s disease (PD) treatment, but the inevitable development of motor complications has led to intense activity in pursuit of its optimal delivery.
Areas covered
Peripheral inhibition of dopa-decarboxylase has long been considered an essential component of levodopa treatment at every stage of illness. In contrast, only relatively recently have catechol-O-methyltransferase (COMT) inhibitors been utilized to block the other major pathway of degradation and optimize levodopa delivery to the brain. First and second-generation COMT inhibitors were deficient because of toxicity, sub-optimal pharmacokinetics or a short duration of effect. As such, they have only been employed once ‘wearing-off’ has developed. However, the third-generation COMT inhibitor, opicapone has overcome these difficulties and exhibits long-lasting enzyme inhibition without the toxicity observed with previous generations of COMT inhibitors. In clinical trials and real-world PD studies opicapone improves the levodopa plasma profile and results in a significant improvement in ON time in ‘fluctuating’ disease, but it has not yet been included in the algorithm for early treatment.
Expert opinion
This review argues for a shift in the positioning of COMT inhibition with opicapone in the PD algorithm and lays out a pathway for proving its effectiveness in early disease.
1. Introduction
Levodopa is the most efficacious drug for the treatment of the motor symptoms of Parkinson’s disease (PD) and the ‘gold standard’ therapy required by almost every patient affected by this common neurodegenerative disorder [Citation1,Citation2]. However, its utility is often limited by the development of motor fluctuations (e.g. ‘wearing-off,’ ‘ON-OFF’) and motor complications (dyskinesia – chorea, dystonia, athetosis) [Citation3]. While the incidence of troublesome dyskinesia appears to be declining [Citation4], wearing-off, which can develop within a few years from treatment initiation, remains a common feature of PD. Wearing-off involves both motor and non-motor symptoms, that are underrecognized by patients and their caregivers [Citation5], and underdiagnosed by physicians [Citation6]. Modern cohort studies estimate the 5-year cumulative incidence of motor fluctuations ranges between 29% and 54% [Citation7–9], increasing to 100% at 10 years [Citation9] and, though the impact of motor fluctuations on daily life can be variable [Citation10], numerous studies consistently show that they have a detrimental impact on quality of life [Citation11–14], with their effective management remaining a significant unmet need [Citation3,Citation15]. This is illustrated by the fact that once motor fluctuations develop, cumulative daily OFF time can account for up to 50% of a patient’s waking day [Citation16]. Indeed, wearing-off is reported by patients as a more significant and inconvenient component of current treatment than non-troublesome dyskinesia [Citation17].
Even today, definitions of ON and OFF remain a matter of debate. Individual physicians use differing terminology, with the term ‘wearing-off’ being used to cover a variety of circumstances related to inadequate levodopa dosing, end of dose deterioration or ON-OFF phenomena. However, a good working definition of the wearing-off phenomenon would be a decrease in the duration of effect of each individual dose of levodopa with increasing disease progression and duration of drug treatment. While often held to be a complication of later disease, there is compelling evidence that it can emerge within months of starting levodopa therapy, but, despite intense research, the pathophysiological mechanisms responsible for wearing-off remain unclear and patient risk factors poorly defined [Citation18–20]. Pharmacodynamic factors involving both presynaptic and post-synaptic changes in dopaminergic and basal ganglia function appear responsible as opposed to any change in the peripheral pharmacokinetics of levodopa over time. Nevertheless, even with the uncertainties, it is well accepted that a key contributing factor to the development of wearing-off is the short plasma half-life of levodopa, leading to a non-physiological, ‘pulsatile’ stimulation of striatal dopamine receptors, which in turn is thought to result in a disorganized striatal output and disruption of the motor programs that control voluntary movement [Citation21,Citation22].
Most current pharmacological strategies to improve motor function are based on the premise that there is inadequate and discontinuous stimulation of striatal post-synaptic dopamine receptors and that providing more continuous dopaminergic stimulation leads to an increase in ON time. In clinical practice, physicians employ a range of strategies to try to improve levodopa delivery to the brain and to maintain dopamine receptor stimulation. These can include levodopa modification strategies such as increasing the levodopa dosage, increasing oral levodopa dosing frequency, and the use of controlled or sustained release preparations of the drug. While these levodopa approaches are inexpensive and usually effective in the short-term, they do not address the problem of low levodopa trough levels and can instead worsen pulsatility and further affect basal ganglia output. Continuous intra-duodenal delivery of levodopa is often highly effective, but is invasive and cannot be employed in all patients [Citation23,Citation24]. An alternative is to use a longer acting oral dopamine agonist drug, such as ropinirole or pramipexole, to provide more continuous receptor stimulation [Citation25,Citation26] or to deliver a dopamine agonist by subcutaneous infusion or transdermal administration as in the case of apomorphine pumps and rotigotine [Citation27,Citation28]. However, dopamine agonists bring other potentially significant adverse events (such as hallucinations, confusion and impulse control disorders) into the risk-benefit equation and are therefore not usually employed in an older (>65–70 years old) PD population [Citation29]. Recently, non-dopaminergic approaches to altering basal ganglia function have been suggested as effective in improving wearing-off such as the adenosine A2A antagonist, istradefylline [Citation30] and the NMDA antagonist, amantadine [Citation31]. As with the dopamine agonists, while these non-dopaminergic approaches usually reduce the severity of fluctuations, they do not affect the pharmacokinetic profile of levodopa and therefore do not address the underlying problem of pulstaility.
One tried and tested strategy has proven consistently effective in enhancing the plasma profile of levodopa and its delivery to the brain and the duration of effect of each dose – and that is through the use of enzyme inhibitors controlling the activity of the key catabolic pathways that determine the efficacy of levodopa. The first of these were the peripheral decarboxylase inhibitors, carbidopa and benserazide, which are used as standard of care to increase levodopa availability to brain at all stages of the disease. Subsequently, the irreversible monoamine oxidase B (MAO-B) inhibitors, selegiline and rasagiline were developed and are now commonly used as early monotherapy and as adjuncts to levodopa to prolong the duration of effect of endogenous dopamine and dopamine formed from levodopa in brain [Citation32]. More recently, the reversible MAO-B inhibitor safinamide has also been introduced into therapy as an adjunct to levodopa [Citation32].
The catechol O-methyl transferase (COMT) inhibitors, entacapone, tolcapone and opicapone were specifically developed for the management of wearing-off as they act to protect levodopa from its major peripheral pathway of metabolism by the COMT enzyme. Although tolcapone has been shown to inhibit central COMT, its clinical efficacy seems to be mainly mediated through inhibition of peripheral COMT and depends on concomitant use of exogenous levodopa [Citation33]. Tolcapone and entacapone were introduced in the 1990’s, and have mainly been used for more advanced patients with chronic motor fluctuations. However, neither compound has turned out to be ideal – tolcapone being associated with hepatic toxicity and entacapone having a short plasma half-life requiring dosing with every administration of levodopa [Citation34]. Opicapone is a third-generation COMT inhibitor rationally designed to reduce the risk of toxicity and improve COMT inhibitory potency and peripheral tissue selectivity compared with other COMT inhibitors [Citation35]. It was first approved in Europe for the management of motor fluctuations in 2016, and since been approved for use in the USA, Japan, South Korea, Australia and other countries. Despite its obvious advantages over earlier COMT inhibitors, it has also been largely reserved for use in later stage patients with wearing-off where other treatment strategies have failed.
In this article, we review the rationale for the use of enzyme inhibitors in the treatment of wearing-off in PD, focusing in particular on the role of peripheral enzymatic degradation and the significance of the inhibition of COMT. We examine the clinical actions of the latest peripheral COMT inhibitor to be introduced, opicapone, and make the argument for the positioning of effective COMT inhibition earlier in the PD treatment paradigm based on the early occurrence of wearing-off and the key principle of maximizing the delivery of levodopa to the brain to avoid motor fluctuations appearing.
2. Peripheral enzyme inhibition is an essential factor that determines the effect of levodopa in the brain
The universal use of levodopa to treat the motor symptoms of PD and the effectiveness of its clinical actions is so widely accepted that the difficulties initially encountered in utilizing levodopa are often forgotten. Levodopa would, in all probability, not be developed for treating PD if it were discovered today. Being a prodrug of dopamine, it would not have suited the combinatorial chemistry and high throughput screening approach used in modern drug discovery. It was developed before the requirement for robust toxicology and it has never been subjected to current standards of testing. The molecule itself is chemically and enzymatically unstable; absorption only occurs from the upper gastro-intestinal tract and by an active transport process for large neutral amino acids. Consequently, absorption is poor, extensive enzymatic metabolism takes place and the plasma half-life is very short, with little drug reaching the brain. Levodopa itself is not a potent molecule and, when first introduced, doses of up to 8 g per day [Citation36] were needed to produce a clinically relevant antiparkinsonian effect and even this was not reliable or predictable. Nor were its effects subjected to randomized, blinded clinical trials – something that has happened only relatively recently [Citation37,Citation38]. Its conversion to dopamine in the periphery induces widespread and unpleasant side effects, notably nausea, vomiting and orthostatic hypotension that severely limit its practical utility [Citation39].
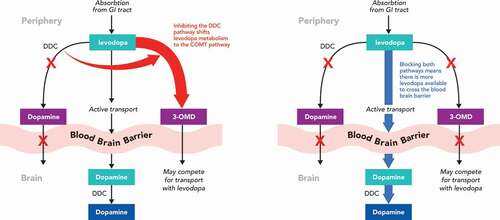
While it is hard to comprehend just how revolutionary the advent of the levodopa was in the management of PD, history shows that the pioneers who worked with the drug almost gave up in the pursuit of its use in PD and this is reflected in their publications. The major discovery that saved levodopa was the realization that preventing its decarboxylation to dopamine in the periphery would make levodopa a viable treatment option. Metabolism by the ubiquitous enzyme aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), in the gut, liver, kidneys and other organs removed much of each levodopa dose before it reached systemic circulation and could enter the brain. The discovery of peripheral DDC inhibitors, carbidopa and benserazide, changed everything, as the reduction of peripheral metabolism of levodopa allowed for a marked reduction in the administered dose, improving the plasma half life of the drug and increasing penetration into brain [Citation40–44] (). The reality is that it was this introduction of enzyme inhibition into the treatment of PD that was the real revolution that led to the modern approach to therapy.
Figure 1. Effect of enzyme inhibition on levodopa pharmacokinetics. (A) Effect of dopa decarboxylase inhibition (B) Effect of dopa decarboxylase + COMT inhibition
The combination of levodopa with a DDC inhibitor is now so embedded in clinical practice in PD that ‘levodopa monotherapy’ inevitably means levodopa plus a DDC inhibitor, and nobody would contemplate using levodopa without a DDC inhibitor from the very start of treatment. This is despite the fact that, again, these drugs were developed in an era that did not require the rigorous testing employed today and that many facets of their actions remain unclear. For example, benserazide is not the active DDC inhibitor – rather it is its major metabolite. Additionally, the precise site of their action is not clear and the ratios of DDC inhibitor to levodopa were deduced empirically [Citation44].
However, using levodopa with a DDC inhibitor does not overcome many of the inherent problems in its use. The oft quoted ‘short’ 90 minute half-life of levodopa, actually refers to the plasma pharmacokinetics of oral levodopa combined with carbidopa [Citation45] and the extent of brain penetration of levodopa remains low, reaching only 10% when combined with a DDC inhibitor. A major reason for these continued deficits in levodopa’s profile is linked to its other pathway of metabolism – namely through COMT. COMT is another ubiquitous enzyme found in the periphery and brain that is responsible for the O-methylation of a wide range of catechol-containing substrates. In peripheral tissues, COMT is mainly available in its soluble cytosolic form (S-COMT) with the highest activities being described in the liver, kidney and gastrointestinal tract, whereas its membrane bound form (MB-COMT) predominates in the CNS [Citation46]. As a consequence, peripheral COMT inactivates much of each levodopa dose before it can cross into the brain (). Indeed, COMT converts about 90% of levodopa to 3-O-methyldopa (3-OMD) which in contrast to levodopa itself, has a long plasma half-life and accumulates on repeated levodopa administration as it is not a substrate for DDC. While no adverse effects of 3-OMD have been conclusively reported, it may compete with levodopa for transport in to brain at the level of the blood–brain barrier [Citation47] and has been implicated in the pathogenesis of motor complications [Citation48,Citation49] as well as levodopa-induced peripheral neuropathy [Citation50]. It is an underappreciated fact that, when a peripheral DDC inhibitor is used, levodopa metabolism is shunted to the COMT pathway (and increases the formation of 3-OMD) such that only 5% to 10% of the administered drug reaches the brain [Citation51].
The logical consequence of needing to block both peripheral DDC and peripheral COMT to maximize the effect of levodopa in PD was recognized early on, but the concept proved difficult to translate into a viable medication. Early attempts to inhibit COMT using compounds such as pyrogallol showed these molecules to be nonspecific, inhibiting a range of enzyme systems and, more importantly, to be short acting and toxic [Citation52]. Only with the discovery of the nitrocatechols (the ‘capone’ series) did the clinical reality of selective COMT inhibition in PD start to appear. One of the first to be developed was nitecapone which was effective, but showed toxicity that prevented clinical development [Citation53]. Tolcapone is registered for use in treating levodopa wearing-off, but the subsequent discovery of its potential for liver damage limited its use with extensive monitoring required – despite subsequent extensive safety studies showing the usefulness of the compound [Citation54,Citation55]. Entacapone was also successfully registered for the treatment of PD, but since its half-life was as short as that of levodopa, the use of the two drugs had to be linked to achieve a successful inhibition of COMT. This practical limitation was overcome to some extent by the introduction of Stalevo as a combined levodopa/carbidopa/entacapone combination, but it can be difficult to use in patients who are on differing levodopa doses at different times of the day and with complicated dosing regimens. Moreover, entacapone is less effective than tolcapone and there are still marked peaks and troughs in levodopa plasma levels. Thus, while the second generation COMT inhibitors did start to address the pharmacokinetic limitations of levodopa, by inhibiting its peripheral metabolism and increasing levodopa delivery to the brain, they did not solve the issue of optimizing levodopa delivery.
3. Opicapone – experimental biochemistry and pharmacology
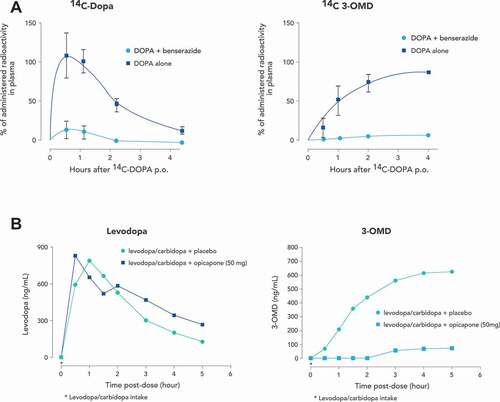
The search for a once daily, potent, selective and long acting peripheral COMT inhibitor with a low toxicity profile culminated in the development of opicapone as a third-generation molecule for the treatment of PD. Opicapone was designed as a 1,2,4-oxadiazole analogue with a pyridine N-oxide residue at position 3 and so is chemically distinct from the previous generation of nitrocatechols. Its unique pharmacophore resulted in high COMT inhibitory potency in the absence of cellular toxicity [Citation56]. In addition, opicapone has sub-picomolar binding affinity to S-COMT in peripheral tissues and does not appear to have any effect on COMT activity in brain [Citation56]. Opicapone does have a relatively short plasma half-life and would not immediately be expected to produce a long-lasting inhibition of COMT. However, its binding and interaction with S-COMT is prolonged and outlasts the clearance of the drug from the systemic circulation. Roughly translated, opicapone tightly binds to S-COMT, but it is a poor substrate and therefore inactivates enzyme activity for a prolonged period [Citation57]. The tight binding and slow complex dissociation characteristics of opicapone are fundamental to its COMT inhibitory potency and once-daily dosing frequency.
The persistent enzyme inhibition produced by opicapone translates into functional activity that can be seen both invitro and invivo experimental models. In liver and kidney homogenates from rats treated orally with opicapone, tolcapone or entacapone, opicapone produced a more marked and more sustained inhibition of COMT than the other drugs [Citation58,Citation59]. The effects on levodopa (in conjunction with a DDC inhibitor) metabolism also reflects the long-lasting inhibition of COMT produced by opicapone. Oral administration of opicapone with levodopa to rats resulted in a sustained increase in brain levodopa levels that was evident 24 hours after drug administration. Similar results were seen in the cynomolgus monkey, where administration of adjunct opicapone to levodopa/benserazide increased levodopa systemic exposure by 2-fold without changing Cmax values [Citation60,Citation61] and reduced both 3-OMD exposure and Cmax values by up to 7-fold [Citation60,Citation61]. These changes were accompanied by an up to ~85% reduction in erythrocyte COMT activity [Citation60,Citation61] and translated into an improvement in motor function in MPTP treated parkinsonian primates [Citation61]. ()[Citation62,Citation63]
4. Effect of opicapone on levodopa pharmacokinetic profile
Similar to in vitro and in vivo experimental models, the pharmacokinetics of opicapone in humans would not initially seem consistent with a drug for once daily administration. Single oral doses of opicapone ranging from 10 to 1200 mg administered to healthy male volunteers showed dose proportional exposure to the drug in plasma and a terminal elimination half-life of opicapone between 0.8 to 3.2 hours. However, the duration of COMT inhibition by opicapone was independent of the dose and the half-life of COMT inhibition in erythrocytes was 61.6 hours, reflecting the estimated dissociation of the COMT-opicapone molecular complex. Thus, despite a relatively short plasma half-life, opicapone markedly and sustainably inhibited peripheral S-COMT activity long after its plasma clearance [Citation63,Citation64]. This durable inhibition of COMT is reflected in changes in the plasma kinetics of levodopa. In patients with PD, administration of opicapone dose dependently increases levodopa bioavailability by up to 95% dependent on dose and duration of levodopa administration [Citation40,Citation43,Citation65]. As assessed by AUC, opicapone was more effective in increasing levodopa exposure than occurred after entacapone administration, reflecting its sustained COMT inhibition that endures over 24 hours () [Citation63]. Administration of opicapone also increased the minimum plasma concentration (Cmin) for individual levodopa doses by up to 3.1-fold [Citation65]. This is an important facet of opicapone’s action as the avoidance of low plasma levodopa trough levels is associated with a reduction in motor fluctuations [Citation66].
Figure 3. Mean S-COMT activity (% of baseline) versus time for once daily opicapone 50 mg, or placebo as adjunct to oral levodopa/carbidopa
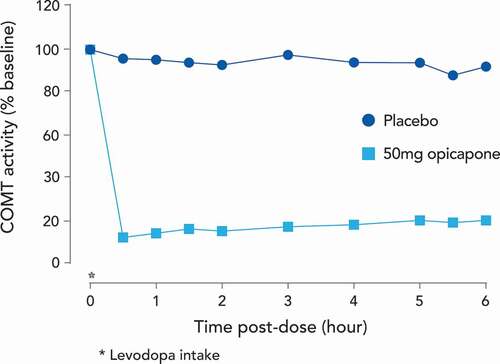
There may also be another advantage of the dissociation between opicapone’s pharmacokinetic profile and its functional activity. In some PD patients, entacapone absorption interferes with levodopa absorption, resulting in a delayed levodopa tmax and reduced Cmax on simultaneous administration [Citation67,Citation68]. This might explain an apparent lack of response to entacapone in some individuals [Citation67]. While there is also a potential interaction between levodopa and opicapone when given at the same time, its once daily bedtime administration (at least 1 hour before or after levodopa combinations) and rapid plasma clearance minimizes any interaction with levodopa based on drug absorption [Citation69]. Thus, the pharmacokinetic profile of opicapone, and its subsequent effect on levodopa availability, provides a treatment strategy based on once daily administration at a single effective dose where, unlike entacapone, COMT inhibition is not tied to the timing of levodopa dosing or to any specific levodopa product or dose. It is also easier and more convenient for patient compliance and drug cost. Recently, the UK National Institute for Health and Care Excellence (NICE) highlighted that using a once-daily administration of opicapone enables flexible dosing of levodopa without altering the opicapone dose [Citation70].
5. Efficacy in fluctuating Parkinson’s disease
5.1. Rationale for COMT inhibition in the management of motor fluctuations
There is a clear and obvious rationale for using a peripherally acting COMT inhibitor in patients treated with levodopa where motor fluctuations have appeared. The troughs in plasma levodopa levels that occur between doses directly correspond with OFF symptoms [Citation66,Citation71] and the treatment objective with a COMT inhibitor is to maintain levels above the threshold for ON. Adding a COMT inhibitor to the levodopa regimen helps to extend the benefit of each levodopa dose and avoid the fluctuations associated with oral levodopa therapy without unnecessarily increasing the dose or frequency of levodopa administration [Citation72]. The shunting of levodopa metabolism to the COMT pathway is avoided and results in increased drug exposure. Thus, by inhibiting both the major pathways of levodopa metabolism through the use of a DDC inhibitor and a COMT inhibitor, the delivery of levodopa to the brain can be maximized and more continuous drug delivery achieved.
5.2. Clinical studies of opicapone in patients with wearing-off
The efficacy of adjunct opicapone in reducing OFF time in patients with motor fluctuations has been well established in phase 3 clinical trials, as well as in observational studies, and has been extensively reviewed elsewhere [Citation73–75]. Three randomized, double-blind, placebo-controlled studies have examined the symptomatic effects of opicapone in PD patients with motor fluctuations, namely the BIPARK studies (I and II) [Citation76,Citation77] and, more recently, a phase 2b (COMFORT-PD) study conducted in the Japanese population [Citation78]. Key findings from the separate studies are given in .
Table 1. Key findings from pivotal studies of opicapone (25 and 50 mg doses) in patients with Parkinson’s disease and motor fluctuations
In a pooled analysis of the BIPARK studies, double-blind treatment with opicapone (25 and 50 mg) significantly reduced absolute daily OFF-time. The mean [95%CI] treatment effect versus placebo was −35.1 [−62.1, −8.2] minutes (p = 0.0106) for the opicapone 25 mg dose and −58.1 [−84.5, −31.7] minutes (p < 0.0001) for the 50 mg dose [Citation79] (). A statistically significant increase in ON time without dyskinesias was also observed, while troublesome dyskinesias did not increase [Citation79]. Such results were replicated in the Japanese study, which showed a placebo adjusted reduction in OFF time of −0.74 hours with the opicapone 25 mg dose group and −0.62 hours with the 50 mg dose (p < 0.05 for both opicapone groups). OFF-time was consistently and steadily reduced in both opicapone tablet groups from Week 1 up to the end of the double-blind period (14–15 weeks) [Citation78].
Figure 4. Reductions in OFF time with adjunct opicapone
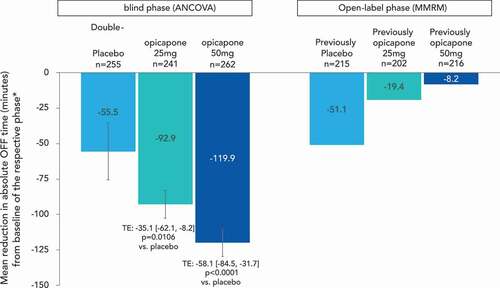
While the BIPARK I study was not designed to test for superiority of opicapone to entacapone (rather it tested for non-inferiority), statistically significant improvements were also seen in CGI-C and PGI-C scores for opicapone 50 mg versus the active comparator entacapone [Citation76]. Placebo-adjusted OFF time reductions in the study for entacapone (−40.3 minutes) were entirely consistent with prior studies (a meta-analysis of entacapone studies reports a reduction of −41 minutes [Citation80]) and the treatment difference for opicapone 50 mg versus entacapone of 26.2 minutes bordered statistical significance (p = 0.05) [Citation76]. Based on these data, the opicapone levodopa-equivalent dose (LED) was recently estimated to be 1.5, which is the same as for tolcapone and higher than the entacapone LED conversion factor of 1.3. Thus, opicapone’s LED is 140–150 mg for a 100 mg levodopa dose [Citation81]. Clinical differences between the products are further highlighted by the switch from entacapone in BIPARK I to opicapone in the open-label extension. Patients previously treated with entacapone in the double-blind phase had an average reduction of 40 minutes OFF-time, and subsequently experienced an additional improvement of 68 minutes OFF-time reduction in patients that ended open-label with opicapone 50 mg treatment [Citation82].
Using these and other data, Hansen etal. developed a Markov model to estimate cost-effectiveness of adjunctive opicapone treatment compared with adjunctive entacapone treatment in a synthetic cohort of 1,000 patients with PD taking levodopa/carbidopa in the United States [Citation83]. Over a 25-year horizon (for patients entering the model at age 64 with no difference in life expectancy between treatments), opicapone treatment was associated with an average of 1,187 fewer OFF-time hours per patient. While treatment with opicapone was associated with lower PD management costs, its acquisition cost was higher than the generic entacapone, resulting in slightly higher total lifetime costs (difference of $3,100) [Citation83]. While the increase in effectiveness of opicapone compared with entacapone was modest in terms of quality adjusted life year (0.07 QALYs), the difference in OFF hours was concluded to be meaningful since direct and indirect costs of care significantly increase with increasing OFF time [Citation84,Citation85].
5.3. Real-world, observational studies of opicapone in patients with wearing-off
Moving to the real-world observational setting, Reichmann and colleagues conducted the OPTIPARK study which included 506 patients treated at 68 centers in the UK and Germany [Citation86]. After 3 months of treatment with opicapone 50 mg, the majority of patients (71.3%) showed clinical improvement as judged by the investigators (CGI-C), with 43% reported as much or very much improved. For those UK patients (n = 95) who were also assessed at 6 months, 85.3% were judged as improved since commencing treatment (including 8.4% who were very much improved and 49.4% who were much improved) while 8.4% were judged as showing ‘no change’ and 6.4% as having worsened. Importantly, this high opinion of efficacy was corroborated by the patients themselves, of whom 76.9% reported they were improved at 3 months. Even though patients came into the study on optimized therapy (79% of patients were receiving levodopa plus another PD medication), the study found that adding opicapone therapy was associated with clinically relevant improvements in UPDRS motor and ADL scores (by 4.6 and 3.0 points, respectively) [Citation86]. These changes within the range of estimated clinically relevant differences of 2.0–5.2 points (for motor scores) and 0.5–2.3 points (for ADL scores) [Citation87], indicating that treatment with opicapone not only increases ON time, but also improves the quality of ON time.
Another interesting finding is that after 3 months of treatment with opicapone, most patients remained on the same total daily levodopa frequency (77.1% had no change, 10.4% had an increase and 12.5% had a decrease in dosing frequency), resulting in an overall mean reduction of approximately −10 mg/day. This is consistent with the pivotal studies where, for example, in the BIPARK II study almost two-thirds (63%) of patients were maintained on the same dose of levodopa, despite freedom to adjust dosing according to clinical need [Citation77]. The average number of daily levodopa intakes also remained stable during this phase, ranging from 4.7 to 4.8 over one year. In all studies, the most common reason for reducing the levodopa dose is to manage dopaminergic adverse events such as dyskinesia, while the maintenance of levodopa dosing with opicapone in the OPTIPARK [Citation86] and the year-long open-label extensions of the pivotal studies [Citation73,Citation79,Citation88] hint at a possible long-term delay of need for levodopa increases. In fact, this concept will be further explored in an ongoing study of early wearing-off (the ADOPTION study, see later).
Finally, an improved efficacy of opicapone versus entacapone has also been suggested in an audit of previous entacapone users at a single UK site [Citation89]. The audit included 20 patients who switched from entacapone to opicapone and 37 patients who had previously experienced a lack of efficacy or adverse events with entacapone. In those patients who continued with opicapone beyond 6 months, the mean reduction in OFF time of was reported to be ~2 hours per day as measured by interview. Patients who switched from entacapone to opicapone were more likely to remain on opicapone treatment than those who had experienced adverse events (AEs) with previous COMT inhibitors [Citation89]. The superior efficacy of tolcapone to entacapone is well known [Citation90], but tolcapone’s safety profile means that it can be only considered after entacapone [Citation91]. Opicapone has no such restrictions and its once daily dosing together with data supportive of higher efficacy than entacapone has led to the suggestion that it is a good alternative in patients with motor fluctuations [Citation73].
5.4. Opicapone in early wearing-off
It is increasingly accepted that motor fluctuations start to emerge much earlier than once thought [Citation22], with wearing-off already common within the first few years from treatment, and underestimated by routine neurological clinical evaluation [Citation92]. Reasons for this underrecognition include a lack of appreciation of non-motor fluctuations as well as a general lack of patient, caregiver and physician awareness of the phenomenon [Citation5,Citation93]. With continued medical education these issues are slowly being addressed [Citation6], but there is still a particular tendency for neurologists to underestimate the presence of wearing-off in patients within the first few years of diagnosis (<2.5 years disease duration) perhaps as they wait to have a more objective picture of established symptom reemergence [Citation92]. Predictors of wearing-off (in addition to duration of treatment and disease severity) include a younger age, weight and female gender [Citation19,Citation92], with women showing an 80% increased risk for wearing-off [Citation94].
Taken overall, patients enrolled into the BIPARK studies had a disease duration of almost 8 years, motor fluctuations for almost 3 years and a mean daily OFF time of over 6 hours [Citation79]; the majority (83%) of patients received polypharmacy (levodopa plus at least one other PD medication) for their parkinsonian symptoms [Citation95]. While this might indicate a population with more chronic motor complications, the studies did include patients with lesser amounts of OFF time at baseline as well as patients who were not on other adjunct therapies. Recent post-hoc analyses of patients with wearing-off who were earlier in their PD and treatment journey indicate equivalent, and even enhanced, efficacy of opicapone 50 mg versus placebo when compared to the overall pooled population [Citation96]. For example, patients with a more recent onset of motor fluctuations (within the previous 2 years) had a placebo-adjusted reduction of OFF time of −68.5 minutes (p = 0.0003 vs. placebo) and patients taking less than 600 mg of levodopa per day had a placebo-adjusted reduction of OFF time of −75.5 minutes (p = 0.0005 vs. placebo) [Citation79,Citation96]. Such OFF time reductions are, again, likely to be clinically relevant as they exceed the estimated clinically relevant difference of 1 hour [Citation87,Citation97]. Although the sample size was relatively small (n = 67 in the opicapone 50 mg group and n = 59 in the placebo group), patients only taking levodopa at baseline (i.e. without dopamine agonists or MAO-B inhibitors as adjunct therapy) also showed a placebo-adjusted mean reduction of −65.6 minutes (p = 0.02 vs. placebo) with opicapone 50 mg, supporting its utility as soon as wearing-off symptoms appear.
Finally, there is some evidence that earlier versus later initiation of opicapone may be beneficial for patients with motor fluctuations. In a combined analysis of the BIPARK double-blind and open-label studies, OFF-time reductions at the end of the open-label phase were found to be numerically greater for the patients assigned to opicapone in the double-blind phase versus those who were originally assigned to placebo (change from baseline of −141.1 minutes in the group that received opicapone 50 mg throughout double-blind and open-label treatment vs. −114.7 minutes in the group that switched from placebo to opicapone) [Citation79]. A similar tendency for the earlier (vs. 6 months later) initiation of entacapone has previously been shown using data from another pooled analysis of placebo-controlled trials and open-label extensions [Citation98] and has led to the suggestion that there may be beneficial effects of earlier versus later initiation of COMT inhibitors in subjects with levodopa-related fluctuations. Whilst the participant numbers dropped by over 90% by the time a difference was detected at 4–5 years from baseline, this concept merits further prospective study.
As stated above, the standard treatment approach to wearing-off is to alter the dosing regimen of conventional levodopa formulations, either by increasing the size of each levodopa dose or by ‘fractionating’ the total daily levodopa dose into smaller, more frequent doses (Brooks DJ. 2008). Putting all pieces together, opicapone once-daily could be considered a potential first line adjunctive levodopa therapy to treat wearing-off that could, potentially, even limit the need to increase the amount of levodopa required in the long run. A randomized, parallel group, multicentre, multinational, prospective, open-label exploratory clinical study (eArly levoDopa with Opicapone in Parkinson’s paTients wIth motOr fluctuatioNs [ADOPTION] study; EudraCT number 2020–002754-24) is currently underway to evaluate the effect of opicapone 50 mg in PD patients with early wearing-off. In this study, patients (aged 30 years or older) with idiopathic PD, treated with 3–4 daily oral doses of up to 600 mg levodopa, with signs of treatable motor disability and experiencing wearing-off phenomenon for less than two years will be randomized (1:1 ratio) to receive opicapone 50 mg once-daily or an extra dose of 100 mg levodopa during a 1-month evaluation-period. Efficacy endpoints will be based on patients’ home diaries [Citation99], as well as the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [Citation100], the Movement Disorder Society-Non-Motor Rating Scale (MDS-NMS) [Citation101], the Parkinson’s Disease Questionnaire-8 (PDQ-8) [Citation102], the Clinical Global Impression of Improvement (CGI-I) and the Patient Global Impression of Change (PGI-C) [Citation103].
6. Efficacy in stable Parkinson’s disease
6.1. Is there a rationale for COMT inhibition in early ‘stable’ disease?
There are two potential reasons to consider COMT inhibition in stable disease, before the development of wearing-off. The first is to prevent or delay the development of motor fluctuations, and the second is to alleviate current symptoms in a stable patient. In this context, ‘stable’ disease refers to the period of time when patients are enjoying the benefits of their levodopa therapy without diagnosed motor complications. In other words, what has long been termed the ‘honeymoon’ period. It is not quite the same as (but often confused with) ‘early’ disease which is often used to refer to the first few years’ post-diagnosis. As previously mentioned, a proportion of patients develop motor complications fairly early on in the course of their disease.
Whereas the rationale for COMT inhibition in managing motor fluctuations is easy to understand, the rationale for preventing or delaying the emergence of motor fluctuations requires a deeper understanding of how the basal ganglia strives for equilibrium. At the turn of the century, there was an explosion of work to understand the impact of levodopa pharmacokinetics and the ‘pulsatile’ delivery associated with intermittent oral administration of levodopa/DDC inhibitor. In stable disease, a key reason for providing a more continuous drug delivery (CDD) is that it will provide a more continuous dopaminergic stimulation (CDS). The preclinical and clinical evidence for both concepts has been extensively reviewed elsewhere [Citation22,Citation72,Citation104–106]. While the concept of CDS is quite complex, the very basic premise is that, under normal physiological conditions dopaminergic neurons originating from the substantia nigra fire tonically (independently of movement), producing a steady baseline concentration of extracellular dopamine in the striatum. This sustains a background level of continuous stimulation of striatal dopamine receptors, with phasic dopamine release occurring in response to behavioral activity. In the normal brain, presynaptic vesicular storage of dopamine acts as a transmitter reservoir, thereby providing a natural buffer to ensure the constant stimulation the striatum expects. With nigrostriatal degeneration, this buffering capacity is progressively lost. In the short-term, this leads to abnormal patterns of striatal function, including the non-physiological modulation of corticostriatal glutamate release by dopamine. In the long-term, physiological consequences include an abnormal plasticity of corticostriatal synapses leading to a profound destabilization of striatal output, downstream molecular and neurophysiological changes in the rest of the basal ganglia (including changes in long-term potentiation [LTP] and depression [LTD]), and, ultimately, alters the way the basal ganglia processes motor information [Citation107].
Under these conditions, the way levodopa is delivered to replace the endogenous dopamine is believed to be important. Given its short half-life, oral administration is associated with peaks and troughs of levodopa and, therefore, exogenous dopamine – availability. This pattern of delivery does not reflect the physiological tonic stimulation that occurs in the normal brain and leads to a further perturbation of basal ganglia processing, ultimately manifesting as the motor complications of wearing-off and dyskinesia. Using a COMT inhibitor to smooth out the delivery of exogenous levodopa in early disease potentially avoids exacerbating the already destabilized basal ganglia processing thereby preventing or delaying the emergence of motor complications.
6.2. Clinical studies of COMT inhibition in ‘stable’ patients
The failure of the STRIDE (STalevo Reduction In Dyskinesia Evaluation) study to show a delay in dyskinesia development with early use of the levodopa/carbidopa/entacapone (Stalevo) combination initially suggested that there was no advantage to early COMT inhibition [Citation108]. However, The STRIDE study was flawed in a number of ways. A primary deficit was that the study was initiated on the basis of findings in MPTP marmosets who were dosed four times daily at 3.5 hourly intervals [Citation109]. Pharmacokinetic studies in man were only conducted later [Citation72,Citation110,Citation111] and the dosing interval was shown not to produce CDD [Citation19,Citation110,Citation111]. Furthermore, these pharmacokinetic studies showed that repeated entacapone dosing increased levodopa Cmax values in plasma which would increase the risk of dyskinesia development. Criticism can also be made of using levodopa dosing schedules that increased it up to 400 mg/day in the first year of treatment which contrasts with normal clinical practice in this period. A better understanding of these pharmacokinetic parameters at the time, would have led to the design of STRIDE being very different.
In contrast, treatment of patients with PD with once daily opicapone 50 mg increased systemic exposure to levodopa given every 3 and 4 hours, leading to both decreased peak-to-trough fluctuations in levodopa concentrations and to higher trough levodopa concentrations [Citation65]. This raises the obvious question as to whether opicapone could provide the level of CDD required to avoid dyskinesia if started in early disease. Pharmacokinetic evaluation of the effect of opicapone on different levodopa/carbidopa treatment regimens (given 4 or 5 times per day) is currently underway [Citation112]. However, the clinical translation into delayed or reduced dyskinesia is probably unlikely to be tested soon in a formal STRIDE-like study, which needs to be very large, at least 2–4 years long, and therefore difficult to recruit for and expensive to conduct.
Another important question is whether COMT inhibition would help to further alleviate motor symptoms in a stable patient. In an early tolcapone study, 6 months treatment with tolcapone at 100 or 200 mg three times daily produced significant reduction in the UPDRS Part II activities of daily living (ADL, −1.4 & −1.6 points, respectively) and motor (−2.0 & −2.3 points, respectively) scores in ‘stable’ patients. These improvements were maintained up to the 12-month assessment and fewer patients in the tolcapone groups than in the placebo group developed motor fluctuations during the trial [Citation113]. Likewise, in the FIRST-STEP study a significant difference in total UPDRS scores in favor of Stalevo was first observed at week 4 and was maintained through the 39-week observation period, with the greatest difference occurring at week 26 [Citation114]. Similar findings have also been hinted at in prior studies with separate entacapone, which showed that adding a COMT inhibitor to the levodopa regimen in subgroups of stable patients improved scores, while maintaining levodopa dose levels over 6 months (in contrast to increased levodopa dosing in the placebo group) [Citation115,Citation116]. These studies also showed that the benefit gained with entacapone in UPDRS scores was consistently lost when the drug was withdrawn [Citation115,Citation116]. However, these studies have not been viewed as unequivocally showing an effect in stable disease, and, as such, they have failed to make an impact on product labeling and on how COMT inhibitors are used in PD.
Now, the unique profile of opicapone makes it an excellent candidate to test for benefit in ‘stable’ patients. A randomized, double-blind, placebo-controlled, clinical study (Early ParkinSon wIth L-DOPA and OpicapoNe [EPSILON] study; EudraCT number 2020–005011-52) has been implemented to evaluate the effect of opicapone 50 mg in ‘stable’ PD patients. In this study, patients (aged 30–80 years) with idiopathic PD, treated with 3–4 daily oral doses of up to 500 mg levodopa, with signs of treatable motor disability but no motor complications will be randomized in a 1:1 ratio to receive opicapone 50 mg once-daily or placebo during a 6-month double-blind evaluation-period. The patients’ current levodopa/DDC inhibitor regimen should remain stable throughout the double-blind period. The primary endpoint is the change from baseline to end of double-blind period in MDS-UPDRS Part III (motor) scores, and secondary outcomes will assess non-motor symptoms, quality of life and global clinical impressions of change. At the end of the double-blind period, patients may enter an additional 1-year, open-label period of opicapone 50 mg treatment [Citation117].
7. Non-motor efficacy of opicapone
A relatively unexplored dimension of efficacy is the impact of adjunct opicapone on non-motor symptoms. In the BIPARK II study, non‐motor symptoms were assessed by the Non‐Motor Symptoms Scale (NMSS) at different time points, including baseline, end of the double-blind phase and end of the open-label phase. At the end of the double-blind period, NMSS scores slightly improved across the opicapone and placebo groups, with no significant differences between them. At the 1‐year open-label endpoint, a mean improvement of ‐4.2 in NMSS total score was still maintained [Citation77]. No deterioration of any particular domain was observed, and it is important to highlight that there was no worsening of dysautonomia, hallucinations or cognitive dysfunction.
Total NMSS scores are hard to interpret as the construct mixes together non-motor items that can be improved or worsened by dopaminergic agents at the same time. Another ongoing real-world study OPTI-ON (NCT04787965) [Citation118] is underway to describe real-world treatment patterns and will use the Non-Motor Fluctuation Assessment (NoMoFa) which better facilitates the identification and quantification of severity of both static and fluctuating non-motor symptoms [Citation119].
In the BIPARK II study, a significant signal seen for the sleep/fatigue domain where the 50 mg dose reduced the NMSS sleep/fatigue score by −1.2 points versus −0.5 points with placebo (p > 0.05). Such benefits in non-motor scores, including sleep/fatigue, were also seen in the OPTIPARK study, where the mean ± SD improvements of −6.8 ± 19.7 points for NMSS total score and − 1.3 ± 6.3 points for the sleep/fatigue score were statistically significant versus baseline (both p <0.0001) [Citation86]. Taken forward, the bedtime dosing of opicapone and the inferred efficacy against sleep/fatigue symptoms suggest that more work to understand which aspects of sleep might be improved with opicapone merits attention. For example, it can be hypothesized that optimization of the pharmacokinetic and pharmacodynamic profile of levodopa with opicapone is more likely to improve nighttime disabilities than sleep architecture. The OpicApone Sleep dISorder (OASIS) study (EudraCT number 2020–001176-15) is an open-label, single-arm, pilot study designed to evaluate the effect of opicapone 50 mg on PD patients with end-of-dose motor fluctuations and a Parkinson’s Disease Sleep Scale (PDSS-2) of ≥18. The primary endpoint is change from baseline to end of study in PDSS-2 Total scores, and secondary measures include the change from baseline in Parkinson’s Disease Fatigue Scale (PFS-16) and the change from baseline in Domain K (sleep and wakefulness) of the Movement Disorder Society-sponsored Non-motor Rating Scale (MDS-NMS) [Citation120].
Finally, pain, one of the most common and troublesome non-motor symptoms of PD, is another non-motor symptom which is often known to correlate with the motor OFF-state and be dopa-responsive [Citation121–123]. In particular, optimizing levodopa regimens may be advantageous in treating this symptom since levodopa (but not apomorphine) has been shown to normalize pain thresholds in PD patients. Obviously, any study examining the effects of an intervention on a particular non-motor symptom must ensure that the patient population is enriched with patients who experience that symptom. Therefore, in another randomized, double-blind, placebo-controlled, clinical study (the OpiCapone Effect on motor fluctuations and pAiN [OCEAN] study; EudraCT number 2020–001175-32) that is currently underway to evaluate the effect of opicapone 50 mg in PD patients with end-of-dose motor fluctuations and associated pain, eligible patients must not only have PD (Hoehn and Yahr stages I–III during ON), be on a stable levodopa regimen and experiencing at least 1.5 hours of daily OFF time despite optimal therapy, but also be experiencing PD associated pain for at least 4 weeks prior to screening as defined by a score of ≥12 on the King’s Parkinson’s Disease Pain Scale (KPPS). The primary efficacy measure is change from baseline to month 6 in Domain 3 (fluctuation-related pain) of the KPPS, and secondary efficacy measures will also assess anxiety and depression as well as sleep and wakefulness among other measures [Citation124].
8. Conclusions
Improving the efficacy of levodopa through the inhibition of peripheral COMT activity is an accepted option for the treatment of wearing-off in later stage PD. This has been comprehensively demonstrated in the clinical evaluation of entacapone, tolcapone and more recently, opicapone. While the significance of COMT in the periphery as a limiting factor in the perilous journey of levodopa to the brain is not contested, there is a good pharmacologic rationale to believe that earlier, effective COMT inhibition will bring clinical benefits in the management of long-term disease. The metabolism of levodopa is the same in early PD as it is in late-stage PD, and if the need to inhibit peripheral COMT exists at one stage of the illness, then it also exists at the other. To put this into context – nobody would dream of giving levodopa in large doses to a patient with early disease without adding a decarboxylase inhibitor and then adding carbidopa or benserazide at a later stage. The challenge is to understand whether the potential pharmacologic benefits of early dual inhibition with a DDC inhibitor plus a COMT inhibitor translate into a meaningful clinical benefit.
9. Expert opinion
Some of the reasons for the current late positioning of COMT inhibitors in PD algorithms are historical, in that the decarboxylase inhibitors were developed in an era when levodopa was first being introduced into therapy when there were no barriers on the stage of illness where it could be used – and there were no alternatives. Other reasons relate to the process of drug development and regulatory approval for symptomatic treatments for PD. The standard practice has been to first trial drugs in an abundant population of later stage patients with wearing-off where improvements in ON time became a standardized end point accepted by regulatory authorities that then brought a product to the market. As a consequence, the late arrival of COMT inhibitors at the PD party resulted in them being pigeonholed for this more advanced treatment group.
Another reason is a fundamental lack of basic understanding of the metabolism of levodopa and the importance of COMT as a limiting factor in the availability of the drug to brain. In particular, the shunting of levodopa to the COMT pathway when used with a decarboxylase inhibitor. Why is this the case? The probable answer is that levodopa has been around for more than 60 years, and its efficacy is unchallenged, so there is little reason for physicians to think about how it works and how peripheral metabolism of the drug influences clinical outcome. There is a clear need for continued education if the timing of the introduction of COMT inhibitors in PD is to change.
A key question is how to widen the use of COMT inhibitors into all stages of PD and thus fully utilize their potential for treating motor, and potentially non-motor, symptoms. This is an opportunity that was not thought feasible until the introduction of opicapone because of the constraints of earlier generations of COMT inhibitors. The outcomes of the FIRST-STEP and STRIDE-PD studies showed why the short duration of effect of entacapone was a critical limitation in early use. There is now the potential for the expansion of COMT inhibitor use to be fully explored based on the once daily administration of opicapone, its long duration of effect and provision of more consistent levodopa levels, and its proven clinical efficacy in the classical later-stage indication. The case for early use based on the basic science surrounding opicapone and the relevance to the metabolism of levodopa is not easy to dismiss. What this requires is the appropriate clinical evaluation in an early patient population with outcome measures that encompass the potential of opicapone for the improvement of motor function, the delay of motor complications and the treatment of some non-motor symptoms of PD. This may become clearer with the outcome of the Early ParkinSon wIth L-DOPA and OpicapoNe (EPSILON) study.
9.1. Five-year view
The target for new clinical studies and the potential repositioning of COMT inhibitors is clear. In the recently initiated studies, opicapone is added once levodopa has been initiated and patients stabilized. If positive, these studies will support an earlier positioning of opicapone within the PD algorithm – in early wearing-off and in so called ‘stable’ disease, but there is also a pharmacological rationale to use COMT inhibitors in combination with levodopa and carbidopa/benserazide in a dual inhibitor preparation that is universally employed. Development of new dual inhibitor products may occur with the life-cycle management of modern COMT inhibitors (in the same way that Stalevo [Citation125], and more recently the levodopa/entacapone/carbidopa intestinal gel infusion for advanced patients [Citation126], developed from entacapone) and remains very much in the unknown future.
In the meantime, opicapone also offers the opportunity for once daily treatment, allowing levodopa to be administered in any desired regimen and frequency throughout the day. Several approaches to improve the pharmacokinetics and ways of administration of levodopa are in different stages of clinical development and commercialization, including novel formulations and nonoral routes of drug delivery [Citation127]. Whereas the need for frequent dosing limits the potential usefulness of entacapone, the once daily administration of opicapone may open doors to optimize the effectiveness of even these new products. For example, studies have shown that COMT inhibition with entacapone improves levodopa bioavailability when administered as a subcutaneous levodopa/carbidopa infusion [Citation128] and the use of adjunct opicapone is already reported to lower the daily levodopa dose (thereby reducing costs) of intestinal levodopa infusion [Citation129]. Finally, as already noted, opicapone does not interfere with the flexible dosing of levodopa and offers flexibility with potential future formulations (oral, infused etc.) of levodopa.
Article highlights
When levodopa is administered with a dopa decarboxylase inhibitor (DDCI), peripheral levodopa metabolism is shunted to the catechol O-methyl transferase (COMT) pathway, and <10% of levodopa crosses into the brain.
Opicapone is a third generation COMT inhibitor rationally designed to reduce the risk of toxicity and improve COMT inhibitory potency and peripheral tissue selectivity compared with other COMT inhibitors.
Opicapone has sub-picomolar binding affinity to S-COMT in peripheral tissues where its tight binding and slow complex dissociation characteristics mean that its COMT inhibitory activity is prolonged and outlasts the clearance of the drug from the systemic circulation.
Administration of opicapone as an adjunct to levodopa/DDCI increases levodopa plasma bioavailability (AUC) and trough levels (Cmin) with lesser effect on peak levels (Cmax).
The efficacy and safety of opicapone in reducing OFF time in patients with Parkinson’s disease and established motor fluctuations is well established in randomized clinical trials and observational studies.
This article sets out the clinical trial pathway for proving effectiveness in earlier ‘stable’ disease.
Reviewer disclosures
A reviewer on this paper is a scientific advisor and has received shares/financial compensation from and KeiferX (co-founder), Sun Pharmaceuaticals Research Industry, Neumentum and Jupiter Orphan Therapeutics. They are also an inventor on a number of Georgetown University Patents to use tyrosine kinase inhibitors and USP13 inhibitors to treat neurodegenerative diseases. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Declaration of interest
P Jenner has received honoraria for consultancy and advisory boards from AbbVie, Adamas, Bial, Britannia Pharmaceuticals, FP Pharmaceuticals, Kyowa Kirin, Roche, UCB, Worldwide Clinical Trials, Zambon, Chiesi Pharmaceuticals and Profile Pharma. J Ferreira and O Rascol have participated in clinical trials sponsored by Bial and have received honoraria for consultancy and advisory boards from Bial. In addition, J Ferreira has received grants from GlaxoSmithKline, Grunenthal, Fundação MSD (Portugal), TEVA, Allergan, Novartis, Medtronic and consultancy fees from GlaxoSmithKline, Novartis, TEVA, Lundbeck, Solvay, BIAL, Merck-Serono, Merz, Ipsen, Biogen, Acadia, Allergan, Abbvie, Sunovion Pharmaceuticals, Zambon, Neuroderm and Affiris. O Rascol has acted as a scientific advisor for drug companies developing antiparkinsonian medications (Abbott, Abbvie, Acorda, Adamas, Affiris, Biogen, Britannia, Cynapsus, Denali Pharmaceuticals, Impax, Lundbeck, Merck, Neuroderm, Novartis, Orian Pharma, Osmotica, Oxford-Biomedica, Prexton, Servier, Sunovion, TEVA, UCB, Zambon) and has received unrestricted scientific grants from academic non-profit entities (Toulouse University Hospital, French Health Ministry, MJFox Foundation, France-Parkinson, European Commission EU FP7 and Horizon 2020). JF Rocha and P Soares-da-Silva are employed by Bial. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Acknowledgments
The authors thank Anita Chadha-Patel and Elizabeth Hocking of ACP Clinical Communications Ltd (funded by BIAL) for medical writing support (literature searching, referencing and editing) in the development of this report.
Additional information
Funding
References
- LeWitt PA. Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord. 2015;30(1):64–72.
- Olanow CW. Levodopa is the best symptomatic therapy for PD: nothing more, nothing less. Mov Disord. 2019;34(6):812–815.
- Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2015;30(1):80–89.
- Chaudhuri KR, Jenner P, Antonini A. Should there be less emphasis on levodopa-induced dyskinesia in Parkinson’s disease? Mov Disord. 2019;34(6):816–819.
- Matthews H, Stamford J, Saha R, et al. Exploring issues around wearing-off and quality of life: the OFF-PARK survey of people with Parkinson’s disease and their care partners. J Parkinsons Dis. 2015;5(3):533–539.
- Stacy M. The wearing-off phenomenon and the use of questionnaires to facilitate its recognition in Parkinson’s disease. J Neural Transm. 2010;117(7):837–846.
- Bjornestad A, Forsaa EB, Pedersen KF, et al. Risk and course of motor complications in a population-based incident Parkinson’s disease cohort. Parkinsonism Relat Disord. 2016;22:48–53.
- Scott NW, Macleod AD, Counsell CE. Motor complications in an incident Parkinson’s disease cohort. Eur J Neurol. 2016;23(2):304–312.
- Kim H-J, Mason S, Foltynie T, et al. Motor complications in Parkinson’s disease: 13-Year follow-up of the CamPaIGN Cohort. Mov Disord. 2020;35(1):185–190.
- LeWitt PA, Chaudhuri KR. Unmet needs in Parkinson disease: motor and non-motor. Parkinsonism Relat Disord. 2020;80(Suppl 1):S7–S12.
- Chapuis S, Ouchchane L, Metz O, et al. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov Disord. 2005;20(2):224–230.
- Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson’s disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord. 2014;20:969–974.
- Wu J, Lim E-C, Nadkarni NV, et al. The impact of levodopa therapy-induced complications on quality of life in Parkinson’s disease patients in Singapore. Sci Rep. 2019;9(1):9248.
- Soh S-E, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2011;17(1):1–9.
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123(Pt 11):2297–2305.
- Michael J Fox Foundation. Capturing and elevating the patient voice; 2014. [cited 2021 Aug]. Available from: https://www.michaeljfox.org/foundation/news-detail.php?capturing-and-elevating-the-patient-voice
- Pahwa R, Lyons KE. Levodopa-related wearing-off in Parkinson’s disease: identification and management. Curr Med Res Opin. 2009;25(4):841–849.
- Hauser RA, McDermott MP, Messing S. Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol. 2006;63(12):1756–1760.
- Olanow CW, Kieburtz K, Rascol O, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord. 2013;28(8):1064–1071.
- Kadastik-Eerme L, Taba N, Asser T, et al. Factors associated with motor complications in Parkinson’s disease. Brain Behav. 2017;7(10):e00837–e.
- Stocchi F. The levodopa wearing-off phenomenon in Parkinson’s disease: pharmacokinetic considerations. Expert Opin Pharmacother. 2006;7(10):1399–1407.
- Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol. 2010;63(5):257–266.
- Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149.
- Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–2073.
- Stocchi F, Giorgi L, Hunter B, et al. PREPARED: comparison of prolonged and immediate release ropinirole in advanced Parkinson’s disease. Mov Disord. 2011;26(7):1259–1265.
- Schapira AH, Barone P, Hauser RA, et al. Extended-release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology. 2011;77(8):767–774.
- Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–759.
- LeWitt PA, Lyons KE, Pahwa R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology. 2007;68(16):1262–1267.
- Antonini A, Tolosa E, Mizuno Y, et al. A reassessment of risks and benefits of dopamine agonists in Parkinson’s disease. Lancet Neurol. 2009;8(10):929–937.
- Jenner P, Mori A, Aradi SD, et al. Istradefylline – a first generation adenosine A2A antagonist for the treatment of Parkinson’s disease. Expert Rev Neurother. 2021;21:317–333.
- Elmer LW, Juncos JL, Singer C, et al. Pooled analyses of Phase III studies of ADS-5102 (Amantadine) extended-release capsules for Dyskinesia in Parkinson’s disease. CNS Drugs. 2018;32(4):387–398.
- Fox SH, Katzenschlager R, Lim SY, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–1266.
- Keating GM, Lyseng-Williamson KA. Tolcapone: a review of its use in the management of Parkinson’s disease. CNS Drugs. 2005;19(2):165–184.
- Kaakkola S. Problems with the present inhibitors and a relevance of new and improved COMT inhibitors in Parkinson’s disease. Int Rev Neurobiol. 2010;95:207–225.
- Kiss LE, Soares-da-Silva P. Medicinal chemistry of catechol O-methyltransferase (COMT) inhibitors and their therapeutic utility. J Med Chem. 2014;57(21):8692–8717.
- Cotzias GC. L-Dopa for Parkinsonism. N Engl J Med. 1968;278(11):630.
- Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–2508.
- Verschuur CVM, Suwijn SR, Boel JA, et al. Randomized delayed-start trial of levodopa in Parkinson’s disease. N Engl J Med. 2019;380(4):315–324.
- Hauser RA. Levodopa: past, present, and future. Eur Neurol. 2009;62(1):1–8.
- Calne DB, Reid JL, Vakil SD, et al. Idiopathic Parkinsonism treated with an extracerebral decarboxylase inhibitor in combination with levodopa. Br Med J. 1971;3(5777):729–732.
- Nutt JG, Woodward WR, Anderson JL. The effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: the mechanism of action in the treatment of parkinsonism. Ann Neurol. 1985;18(5):537–543.
- Lieberman A, Goodgold A, Jonas S, et al. Comparison of dopa decarboxylase inhibitor (carbidopa) combined with levodopa and levodopa alone in Parkinson’s disease. Neurology. 1975;25(10):911–916.
- Markham C, Diamond SG, Treciokas LJ. Carbidopa in Parkinson disease and in nausea and vomiting of levodopa. Arch Neurol. 1974;31(2):128–133.
- Gershanik OS. Improving l-dopa therapy: the development of enzyme inhibitors. Mov Disord. 2015;30(1):103–113.
- Deleu D, Northway MG, Hanssens Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson’s disease. Clin Pharmacokinet. 2002;41(4):261–309.
- Karhunen T, Tilgmann C, Ulmanen I, et al. Distribution of catechol-O-methyltransferase enzyme in rat tissues. J Histochem Cytochem. 1994;42(8):1079–1090.
- Nutt JG, Woodward WR, Gancher ST, et al. 3-0-methyldopa and the response to levodopa in Parkinson’s disease. Ann Neurol. 1987;21:584–588.
- Tohgi H, Abe T, Kikuchi T, et al. The significance of 3-O-methyldopa concentrations in the cerebrospinal fluid in the pathogenesis of wearing-off phenomenon in Parkinson’s disease. Neurosci Lett. 1991;132(1):19–22.
- Mena MA, Muradas V, Bazan E, et al. Pharmacokinetics of L-dopa in patients with Parkinson’s disease. Adv Neurol. 1987;45:481–486.
- Müller T, van Laar T, Cornblath DR, et al. Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19(5):501–507.
- Kaakkola S. Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs. 2000;59(6):1233–1250.
- Bonifácio MJ, Palma PN, Almeida L, et al. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev. 2007;13(3):352–379.
- Kaakkola S, Gordin A, Mannisto PT. General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase. Gen Pharmacol. 1994;25(5):813–824.
- Artusi CA, Sarro L, Imbalzano G, et al. Safety and efficacy of tolcapone in Parkinson’s disease: systematic review. Eur J Clin Pharmacol. 2021;77(6):817–829.
- Olanow CW, Watkins PB. Tolcapone: an efficacy and safety review (2007). Clin Neuropharmacol. 2007;30(5):287–294.
- Kiss LE, Ferreira HS, Torrao L, et al. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem. 2010;53(8):3396–3411.
- Palma PN, Bonifacio MJ, Loureiro AI, et al. Computation of the binding affinities of catechol-O-methyltransferase inhibitors: multisubstate relative free energy calculations. J Comput Chem. 2012;33(9):970–986.
- Bonifacio MC, Torrao M, Loureiro AI, et al. Opicapone: characterization of a novel peripheral long-acting catechol-O-methyltransferase inhibitor. Parkinsonism Relat Disord. 2012;18(suppl 2):S125.
- Bonifacio MJ, Torrao L, Loureiro AI, et al. Pharmacological profile of opicapone, a third-generation nitrocatechol catechol-O-methyl transferase inhibitor, in the rat. Br J Pharmacol. 2015;172(7):1739–1752.
- Bonifacio MJ, Sutcliffe JS, Torrao L, et al. Brain and peripheral pharmacokinetics of levodopa in the cynomolgus monkey following administration of opicapone, a third generation nitrocatechol COMT inhibitor. Neuropharmacology. 2014;77:334–341.
- Bonifacio MJ, Sousa F, Soares-da-Silva P. Opicapone enhances the reversal of MPTP-induced Parkinson-like syndrome by levodopa in cynomolgus monkeys. Eur J Pharmacol. 2021;892:173742.
- Tissot R, Bartholini G, Pletscher A. Drug-induced changes of extracerebral dopa metabolism in man. Arch Neurol. 1969;20(2):187–190.
- Rocha JF, Falcao A, Santos A, et al. Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur J Clin Pharmacol. 2014;70(9):1059–1071.
- Almeida L, Rocha JF, Falcao A, et al. Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel catechol-O-methyltransferase inhibitor, in healthy subjects: prediction of slow enzyme-inhibitor complex dissociation of a short-living and very long-acting inhibitor. Clin Pharmacokinet. 2013;52(2):139–151.
- Loewen G, Lewitt P, Olanow CW, et al. Pharmacokinetics of opicapone and effect on COMT and levodopa pharmacokinetics in patients with Parkinson’s disease [abstract]. Mov Disord. 2019;34(suppl 2); [cited 2021 Aug]. Available from: https://www.mdsabstracts.org/abstract/pharmacokinetics-of-opicapone-and-effect-on-comt-and-levodopa-pharmacokinetics-in-patients-with-parkinsons-disease/
- Stocchi F, Vacca L, Ruggieri S, et al. Intermittent vs continuous levodopa administration in patients with advanced Parkinson disease: a clinical and pharmacokinetic study. Arch Neurol. 2005;62(6):905–910.
- Brusa L, Pierantozzi M, Bassi A, et al. Temporal administration of entacapone with slow release L-dopa: pharmacokinetic profile and clinical outcome. Neurol Sci. 2004;25(2):53–56.
- Myllyla VV, Sotaniemi KA, Illi A, et al. Effect of entacapone, a COMT inhibitor, on the pharmacokinetics of levodopa and on cardiovascular responses in patients with Parkinson’s disease. European Journal Clinical Pharmacology. 1993;45:419–423.
- Falcão A, Santos A, Ferreira JJ, et al. Decision-making process for opicapone’s bedtime regimen [abstract]. Mov Disord. 2017;32(Suppl 2); [cited 2021 Aug]. Available from: https://www.mdsabstracts.org/abstract/decision-making-process-for-opicapones-bedtime-regimen/
- NICE. Parkinson’s disease with end-of-dose motor fluctuations: opicapone. Evidence summary [ES9] Published date; 2017 Mar 21. [cited 2021 Aug]. Available from: https://www.nice.org.uk/advice/es9/chapter/Estimated-impact-for-the-NHS
- de la Fuente-Fernandez R, Lu JQ, Sossi V, et al. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol. 2001;49(3):298–303.
- Stocchi F, Olanow CW. Continuous dopaminergic stimulation in early and advanced Parkinson’s disease. Neurology. 2004;62(1 Suppl 1):S56–63.
- Fabbri M, Ferreira JJ, Lees A, et al. Opicapone for the treatment of Parkinson’s disease: a review of a new licensed medicine. Mov Disord. 2018;33(10):1528–1539.
- Lees AJ, Ferreira J, Rascol O, et al. Opicapone for the management of end-of-dose motor fluctuations in patients with Parkinson’s disease treated with L-DOPA. Expert Rev Neurother. 2017;17(7):649–659.
- Scott LJ. Opicapone: a review in Parkinson’s disease. CNS Drugs. 2021;35(1):121–131.
- Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154–165.
- Lees AJ, Ferreira J, Rascol O, et al. Opicapone as adjunct to levodopa therapy in patients with parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74(2):197–206.
- Takeda A, Takahashi R, Tsuboi Y, et al. Randomized, controlled study of opicapone in Japanese Parkinson’s patients with motor fluctuations. Mov Disord. 2021;36(2):415–423.
- Ferreira JJ, Lees A, Rocha JF, et al. Long-term efficacy of opicapone in fluctuating Parkinson’s disease patients: a pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol. 2019;26(7):953–960.
- Deane KH, Spieker S, Clarke CE. Catechol-O-methyltransferase inhibitors versus active comparators for levodopa-induced complications in Parkinson’s disease. Cochrane Database Syst Rev. 2004;4:CD004553.
- Schade S, Mollenhauer B, Trenkwalder C. Levodopa equivalent dose conversion factors: an updated proposal including opicapone and safinamide. Mov Disord Clin Pract. 2020;7(3):343–345.
- Ferreira JJ, Lees AJ, Poewe W, et al. Effectiveness of opicapone and switching from entacapone in fluctuating Parkinson disease. Neurology. 2018;90(21):e1849–e57.
- Hansen RN, Suh K, Serbin M, et al. Cost-effectiveness of opicapone and entacapone in reducing OFF-time in Parkinson’s disease patients treated with levodopa/carbidopa. J Med Econ. 2021;24(1):563–569.
- Findley LJ, Wood E, Lowin J, et al. The economic burden of advanced Parkinson’s disease: an analysis of a UK patient dataset. J Med Econ. 2011;14(1):130–139.
- Thach A, Jones E, Pappert E, et al. Real-world assessment of “OFF” episode-related healthcare resource utilization among patients with Parkinson’s disease in the United States. J Med Econ. 2021;24(1):540–549.
- Reichmann H, Lees A, Rocha JF, et al., investigators O. Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: the OPTIPARK open-label study. Transl Neurodegener. 2020;9(1):9.
- Hauser RA, Auinger P. Determination of minimal clinically important change in early and advanced Parkinson’s disease. Mov Disord. 2011;26(5):813–818.
- Lees A, Ferreira JJ, Rocha JF, et al. Safety profile of opicapone in the management of Parkinson’s disease. J Parkinsons Dis. 2019;9:733–740.
- Vokurka P, Barron A, Sumaria S, et al. Opicapone efficacy and tolerability in Parkinson’s disease patients reporting insufficient benefit/failure of entacapone. Mov Disord Clin Pract. 2020;7(8):955–960.
- Lees AJ. Evidence-based efficacy comparison of tolcapone and entacapone as adjunctive therapy in Parkinson’s disease. CNS Neurosci Ther. 2008;14(1):83–93.
- Muller T. Catechol-o-methyltransferase inhibitors in Parkinson’s disease. Drugs. 2015;75(2):157–174.
- Stocchi F, Antonini A, Barone P, et al. Early DEtection of wEaring off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord. 2014;20(2):204–211.
- Stacy M, Bowron A, Guttman M, et al. Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord. 2005;20(6):726–733.
- Colombo D, Abbruzzese G, Antonini A, et al. The “gender factor” in wearing-off among patients with Parkinson’s disease: a post hoc analysis of DEEP study. ScientificWorldJournal. 2015;2015:787451.
- Lees A, Ferreira JJ, Rocha JF, et al. Safety profile of opicapone in the management of Parkinson’s disease. J Parkinsons Dis. 2019;9(4):733–740.
- Ebersbach G, Ferreira J, Antonini A, et al. Opicapone’s added benefit as a first-line adjunctive therapy to levodopa and when used promptly in the motor fluctuations spectrum of Parkinson’s disease: a post-hoc analysis of BIPARK-I and II [abstract]. Mov Disord. 2020;35(suppl 1); [cited 2021 Aug]. Available from: https://www.mdsabstracts.org/abstract/opicapones-added-benefit-as-a-first-line-adjunctive-therapy-to-levodopa-and-when-used-promptly-in-the-motor-fluctuations-spectrum-of-parkinsons-disease-a-post-hoc-analysis-of-bipark/
- Hauser RA, Gordon MF, Mizuno Y, et al. Minimal clinically important difference in Parkinson’s disease as assessed in pivotal trials of pramipexole extended release. Parkinsons Dis. 2014;2014:467131.
- Nissinen H, Kuoppamaki M, Leinonen M, et al. Early versus delayed initiation of entacapone in levodopa-treated patients with Parkinson’s disease: a long-term, retrospective analysis. Eur J Neurol. 2009;16(12):1305–1311.
- Hauser RA, Deckers F, Lehert P. Parkinson’s disease home diary: further validation and implications for clinical trials. Mov Disord. 2004;19(12):1409–1413.
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement disorder society-sponsored revision of the Unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170.
- Chaudhuri KR, Schrag A, Weintraub D, et al. The movement disorder society nonmotor rating scale: initial validation study. Mov Disord. 2020;35(1):116–133.
- Jenkinson C, Fitzpatrick R, Peto V, et al. The PDQ-8: development and validation of a short-form parkinson’s disease questionnaire. Psychol Health. 1997;12(6):805–814.
- Guy W. Clinical global impressions. ECDEU assessment manual for psychopharmacology. Rockville, MD, Washington, DC: Department of Health, Education, and Welfare; 1976. p. 218–222.
- Jenner P, McCreary AC, Scheller DK. Continuous drug delivery in early- and late-stage Parkinson’s disease as a strategy for avoiding dyskinesia induction and expression. J Neural Transm. 2011;118(12):1691–1702.
- Olanow CW, Obeso JA, Stocchi F. Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5(8):677–687.
- Olanow CW, Obeso JA, Stocchi F. Drug Insight: continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Nat Clin Pract Neurol. 2006;2(7):382–392.
- Picconi B, Piccoli G, Calabresi P. Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol. 2012;970:553–572.
- Stocchi F, Rascol O, Kieburtz K, et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol. 2010;68(1):18–27.
- Smith LA, Jackson MJ, Al-Barghouthy G, et al. Multiple small doses of levodopa plus entacapone produce continuous dopaminergic stimulation and reduce dyskinesia induction in MPTP-treated drug-naive primates. Mov Disord. 2005;20(3):306–314.
- Muhlack S, Herrmann L, Salmen S, et al. Fewer fluctuations, higher maximum concentration and better motor response of levodopa with catechol-O-methyltransferase inhibition. J Neural Transm. 2014;121(11):1357–1366.
- Kuoppamaki M, Korpela K, Marttila R, et al. Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four or five times daily. Eur J Clin Pharmacol. 2009;65(5):443–455.
- Ferreira JJ, Poewe W, Rascol O, et al. Study-design to assess the effect of opicapone on levodopa PK at different levodopa-optimized treatment regimens [Abstract]. Eur J Neurol. 2021;28(1):718.
- Waters CH, Kurth M, Bailey P, et al. Tolcapone in stable Parkinson’s disease: efficacy and safety of long-term treatment. The Tolcapone Stable Study Group. Neurology. 1997;49(3):665–671.
- Hauser RA, Panisset M, Abbruzzese G, et al. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson’s disease. Mov Disord. 2009;24(4):541–550.
- Brooks DJ, Sagar H. Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry. 2003;74(8):1071–1079.
- Poewe WH, Deuschl G, Gordin A, et al. Efficacy and safety of entacapone in Parkinson’s disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand. 2002;105(4):245–255.
- Early ParkinSon wIth L-DOPA and OpicapoNe [EPSILON] study. EudraCT number 2020-005011-52. [ cited 2021 Jul 25]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-005011-52/CZ
- Opicapone Treatment Initiation Open-Label Study (OPTI-ON). NCT number NCT04787965. [ cited 2021 Jul 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT04787965
- Kleiner G, Fernandez HH, Chou KL, et al. Non-Motor Fluctuations in Parkinson’s Disease: validation of the Non-Motor Fluctuation Assessment Questionnaire. Mov Disord. 2021;36(6):1392–1400.
- OpicApone Sleep dISorder (OASIS) study. EudraCT number 2020-001176-15. [ cited 2021 Jul 25]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001176-15/DE
- Storch A, Schneider CB, Wolz M, et al. Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology. 2013;80(9):800–809.
- Antonini A, Tinazzi M, Abbruzzese G, et al. Pain in Parkinson’s disease: facts and uncertainties. Eur J Neurol. 2018;25(7):917–e69.
- Nebe A, Ebersbach G. Pain intensity on and off levodopa in patients with Parkinson’s disease. Mov Disord. 2009;24(8):1233–1237.
- OpiCapone Effect on motor fluctuations and pAiN [OCEAN] study. EudraCT number 2020-001175-32. [ cited 2021 Jul 25]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001175-32/PT
- Hauser RA. Levodopa/carbidopa/entacapone (Stalevo). Neurology. 2004;62(1 Suppl 1):S64–71.
- Öthman M, Widman E, Nygren I, et al. Initial Experience of the Levodopa-Entacapone-Carbidopa Intestinal Gel in Clinical Practice. J Pers Med. 2021;11(4):254.
- Poewe W, Antonini A. Novel formulations and modes of delivery of levodopa. Mov Disord. 2015;30(1):114–120.
- Giladi N, Caraco Y, Gurevich T, et al. ND0612 (levodopa/carbidopa for subcutaneous infusion) achieves stable levodopa plasma levels when administered in low and high doses in patients with PD [abstract]. Mov Disord. 2017;32(suppl 2). [cited Aug 2021]. http://www.mdsabstracts.org/abstract/nd0612-levodopacarbidopa-for-subcutaneous-infusion-achieves-stable-levodopa-plasma-levels-when-administered-in-low-and-high-doses-in-patients-with-pd/.
- Leta V, Van Wamelen DJ, Sauerbier A, et al. Opicapone and levodopa-carbidopa intestinal gel infusion: the way forward towards cost savings for healthcare systems? J Parkinsons Dis. 2020;10(4):1535–1539.