ABSTRACT
Introduction
Sialorrhea, also known as hypersalivation, ptyalis, or drooling, results in physical and psychosocial complications that may have a significant negative impact on quality of life for both the patient and their caregiver. The goal of pharmacological treatment is to reduce excessive salivary flow, while maintaining a moist and healthy oral cavity; until recently, however, few of the agents used to treat chronic sialorrhea have been approved in pediatric patients.
Areas covered
This article summarizes early evidence for the use of botulinum neurotoxin A formulations in the treatment of children/adolescents with chronic sialorrhea, and findings of the recently completed phase III trial of incobotulinumtoxinA in this indication. Alternative therapies are also briefly discussed.
Expert opinion
IncobotulinumtoxinA is the first botulinum neurotoxin A to be approved for the treatment of chronic sialorrhea in children and adults, following the results of phase III trials that demonstrate the efficacy and safety of the drug in these patients. The authors expect that the positive findings will result in updates to clinical guidelines for the treatment of children with chronic sialorrhea.
Abbreviations
AE, adverse event; AESI, adverse event of special interest; BoNT/A, botulinum neurotoxin A; CI, confidence interval; CP, cerebral palsy; DIS, drooling impact scale; DQ, drooling quotient; DSFS, Drooling Severity and Frequency Scale; GICS, Global Impression of Change Scale; LS, least squares; mTDS, modified Teacher’s drooling scale; NR, not reported; PD, Parkinson’s disease; SAE, serious adverse event; SE, standard error; SIAXI, Sialorrhea in Adults Xeomin Investigation; SIPEXI, Sialorrhea Pediatric Xeomin Investigation; SNAP-25, synaptosomal associated protein-25; TBI, traumatic brain injury; TDS, Teacher Drooling Scale; USA, United States of America; uSFR, unstimulated Salivary Flow Rate; VAS, visual analog scale
1. Introduction
Sialorrhea, also known as hypersalivation, ptyalis, or drooling, is excessive saliva beyond the lip margin, associated with neurological disorders or localized anatomical abnormalities in the oral cavity that inhibit complete closure of the laryngeal inlet [Citation1,Citation2]. Sialorrhea can be classified as anterior or dorsal pooling; anterior sialorrhea is the salivary incontinence or involuntary spillage of saliva over the lower lip that manifests as drooling, whereas dorsal sialorrhea is the flowing of saliva from the tongue to the pharynx [Citation3]. Drooling is common in normally developing infants, but is generally considered pathologic after 4 years of age [Citation4–6]. The most common cause of sialorrhea in children is cerebral palsy [Citation7,Citation8]; less common neurological disorders frequently associated with sialorrhea in children include Dravet, Rett, Goldenhar, and Angelman syndromes [Citation9]. In addition, malformations and traumatic defects can cause a lack of integrity of the mouth and jaw region, resulting in sialorrhea [Citation10].
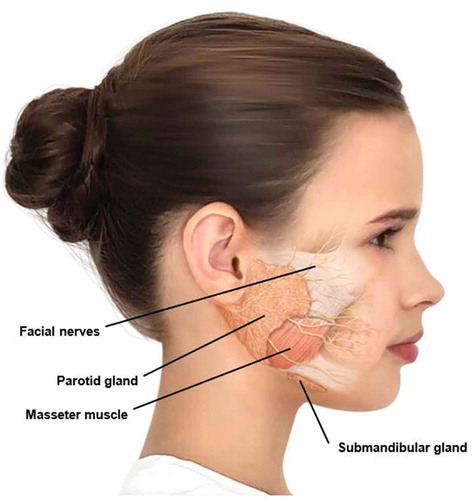
Sialorrhea occurs when there is excessive production of saliva (hypersalivation) or an inability to transport saliva from the oral cavity into the oropharynx and beyond due to reduced neuromuscular control of the tongue and oral tissues, and impairment of the swallowing mechanism [Citation2]. It is widely accepted that sialorrhea in children with cerebral palsy is caused by oral neuromuscular dysfunction, dysphagia, and/or intraoral sensitivity disorder rather than hypersalivation [Citation3]. The major salivary glands are the parotid, submandibular, and sublingual glands, the largest being the parotid gland (). Salivary secretion by these glands is controlled mainly by the parasympathetic nervous system, although sympathetic innervation has a minor influence [Citation1]. Parasympathetic nerves secrete acetylcholine, which stimulates the salivary glands.
Sialorrhea results in physical and psychosocial complications [Citation3,Citation11,Citation12]. Physical complications of anterior sialorrhea include maceration of the skin around the mouth with secondary infection, bad odor, dehydration, speech disturbance, and interference with feeding. Posterior sialorrhea can increase the risk of aspiration of saliva, food, or fluids into the lungs, particularly when normal reflex mechanisms such as gagging or coughing are impaired, which can result in pneumonia and chronic respiratory disorders. Psychosocial complications include isolation, barriers to education (such as an inability to share books or computer keyboards), increased dependency and level of care, damage to electronic devices, decreased self-esteem, and difficulty with social interaction. Thus, sialorrhea may have a significant negative impact on quality of life for both the patient and caregiver.
It is generally accepted that the management of sialorrhea is best accomplished with a multidisciplinary team approach [Citation3,Citation9,Citation10,Citation13]. However, there is a limited amount of reliable study data to support the use of the different therapies that are currently used for treating sialorrhea in children [Citation10,Citation13,Citation14]. Despite this lack of data, recommended treatment options include: improving patient positioning (e.g. improving posture and head positioning); swallowing exercises to reduce dysphagia; behavioral and bio-functional therapies (e.g. stimulating palatal plates) to improve and stimulate oral-motor activity and control; orthodontics; pharmacological therapy; and more aggressive methods, such as surgery or radiotherapy [Citation10,Citation13].
The goal of pharmacological treatment is to reduce excessive salivary flow, while maintaining a moist and healthy oral cavity [Citation1,Citation3,Citation9,Citation10,Citation13]. Avoidance of xerostomia (dry mouth) is essential. The main pharmacologic options for sialorrhea include anticholinergic drugs and botulinum neurotoxin A (BoNT/A) injections; neither of these types of agent act on the most common pathophysiology of the disorder in children.
The use of oral, transdermal, or sublingual anticholinergic drugs might be very helpful, but is limited by the fact that they are nonselective, and the blockage of muscarinic receptors expressed on tissues other than the salivary glands may cause numerous side effects, including irritability, mood, behavioral and cognitive changes, insomnia, visual disturbance, diarrhea, constipation, and urinary retention. Although these agents have generally been used off-label in children with sialorrhea, which affects reimbursement, glycopyrrolate has orphan-drug status in the USA for this indication and Europe-wide approval for the symptomatic treatment of severe hypersalivation in children and adolescents from the age of 3 years [Citation1,Citation3,Citation9,Citation10]. Unlike many other anticholinergics, glycopyrrolate, due to its structure, has lower blood–brain barrier penetration and therefore potentially fewer central nervous system side effects [Citation10].
Surgery to target salivation is usually indicated only in severe cases of sialorrhea because of the risk of permanent adverse consequences, such as xerostomia [Citation3], and radiotherapy is not appropriate for use in children because of the risk of malignancy.
2. Overview of the market
Commercial botulinum neurotoxins are based on the bacterium Clostridium botulinum serotypes A or B; however, BoNT/A formulations are the most widely used [Citation15]. Botulinum neurotoxin B is not used by some centers to treat sialorrhea in children because of the increased risk of developing neutralizing antibodies [Citation16], although rimabotulinumtoxinB is approved for the treatment of chronic sialorrhea in adults in the USA [Citation17].
BoNT/A injections have been used successfully to treat sialorrhea in children and adults for a number of years; however, specialist injection techniques are advisable and rarely patients may require sedation or anesthesia [Citation1,Citation3,Citation8,Citation9,Citation10,Citation18,Citation19]. Sonographic control of injections is useful, especially when treating the submandibular salivary gland [Citation16,Citation20,Citation21], as swallowing disorders caused by unintentional diffusion into the muscles of the oral floor can be avoided [Citation10].
Numerous studies conducted in adult and pediatric patients with sialorrhea of various etiologies have confirmed that BoNT/A injections (abobotulinumtoxinA, incobotulinumtoxinA, and onabotulinumtoxinA) effectively reduce salivary secretion for periods of up to 3–4 months [Citation1,Citation22]. To date, however, only one BoNT/A formulation has been approved for the treatment of sialorrhea based on the results of two phase III trials, Sialorrhea in Adults Xeomin Investigation (SIAXI, NCT02091739) [Citation23,Citation24] and Sialorrhea Pediatric Xeomin Investigation (SIPEXI, NCT02270736) [Citation25–27]. IncobotulinumtoxinA was approved by the United States Food and Drug Administration in 2018 for the treatment of chronic sialorrhea in adults, without restrictions, independent of the underlying disease, and the European Medicines Agency in 2019 for the treatment of adults with chronic sialorrhea due to neurological diseases [Citation1,Citation28,Citation29]. Subsequently, incobotulinumtoxinA was approved for the treatment of chronic sialorrhea in patients 2 years of age and older in the USA [Citation28], and is currently the only botulinum neurotoxin formulation to receive this indication.
3. Introduction to incobotulinumtoxinA
IncobotulinumtoxinA has been used for many years to treat conditions benefiting from the inhibition of acetylcholine release and neuromuscular blockade [Citation30].
3.1. Chemistry and mechanism of action
Commercial BoNT/A is produced by anaerobic fermentation of the Hall strain of C. botulinum serotype A [Citation15,Citation31]. BoNT/A consists of an active 150 kDa neurotoxin and pharmacologically inactive (on nerve terminals) neurotoxin-associated complexing proteins (up to 750 kDa), which together form high-molecular-weight progenitor complexes. BoNT/A is synthesized as a single chain protein (150 kDa) that is proteolytically cleaved into a 50 kDa light chain and a 100 kDa heavy chain, connected by a disulfide bond. These properties allow the neurotoxin to bind to cholinergic nerve endings, enter the nerve cell via endocytosis, and block release of acetylcholine into the synaptic cleft via inactivation of the 25-kDa synaptosome-associated protein (SNAP-25), a protein essential for the fusion and release of acetylcholine from vesicles [Citation31,Citation32]. It appears that the light chain is responsible for the duration of action of BoNT/A formulations, while both the light and heavy chain contribute to the onset of action [Citation33] and it is the heavy chain that binds to external neuronal receptors [Citation31,Citation32].
Depending on the target tissue, BoNT/A can block the cholinergic neuromuscular innervation of striated and smooth muscles or the cholinergic autonomic innervation of exocrine glands [Citation15]. Thus, injection of botulinum neurotoxin into the parotid and submandibular glands inhibits the release of acetylcholine from cholinergic nerve terminals, thereby reducing salivary secretion and sialorrhea. Injection into both sets of glands is required to reduce both resting and stimulated salivary secretion [Citation1,Citation10]. Impulse transmission is then reestablished by the degradation of injected neurotoxin [Citation34].
Commercially available BoNT/A formulations contain different amounts of neurotoxin-associated complexing proteins, which results in variation in the molecular weight and three-dimensional structure of each formulation [Citation15]. BoNT/A formulations are associated with a low risk of antibody formation, but because incobotulinumtoxinA does not contain neurotoxin-associated complexing proteins, which have been implicated in immune responses [Citation15], it may have a very low risk of immunogenicity [Citation35–37].
3.2. Pharmacokinetics
It is not possible to detect incobotulinumtoxinA in the peripheral blood following intramuscular or intraglandular injection at the recommended doses using currently available analytical technology because the doses used are so small (picograms per injection) [Citation38].
4. Clinical efficacy
BoNT/A has been used for the treatment of pediatric sialorrhea for many years, following observed benefit in predominantly small studies evaluating a variety of doses and injection regimens [Citation19,Citation39–54] (Supplementary Table 1). These studies enrolled children and adolescents with sialorrhea associated with neurological deficits, most commonly cerebral palsy.
4.1. Uncontrolled studies
In uncontrolled studies, groups of 5–33 children and adolescents aged 2.5–24 years received injections of BoNT/A (formulation not specified or onabotulinumtoxinA) into each parotid gland, each submandibular gland or into both sets of glands, using a variety of dosage regimens, with efficacy measured both objectively and subjectively by parents/caregivers using a number of methods [Citation39–46,Citation55]. Responding patients showed reductions in drooling volume and frequency as early as one week after injections that were maintained for periods of 3–16 weeks in individual patients. Single studies found that injection into both the submandibular and parotid gland produced better results than submandibular injection only [Citation40,Citation52], and that repeated injections continued to inhibit saliva production [Citation44,Citation55].
4.2. Controlled studies
Controlled trials conducted in groups of up to 39 children/adolescents with sialorrhea further support the ability of BoNT/A injections (when specified in the publications into the submandibular glands, and in one study into both the submandibular and parotid glands [Citation56] using various dosage regimens (Supplementary Table 1)) to reduce salivary flow and drooling for periods of up to 12 months in individual patients [Citation19,Citation47–51,Citation56]. Reductions in various objective and subjective measures of drooling and saliva production were significant when compared with both baseline [Citation47,Citation48] and saline-treated/untreated controls [Citation49,Citation50,Citation57], and were generally similar to those achieved with scopolamine patches [Citation47,Citation48].
Submandibular duct ligation was more effective than submandibular injection of onabotulinumtoxinA for reducing measures of drooling in one randomized study in 53 children, although the BoNT/A injections were associated with fewer adverse events and complaints [Citation19]. Prospectively collected data from a single center study showed that drooling was reduced by both onabotulinumtoxinA (n = 5) and rimabotulinumtoxinB (n = 11) when injected into the submandibular and parotid glands, but reductions were greatest with the botulinum neurotoxin B formulation [Citation54]. However, in a randomized parallel-group trial comparing these botulinum formulations in 30 children with neurological disorders, both were similarly effective for reducing drooling at 4 weeks after the first injection and following repeated injections [Citation53].
4.3. Routine clinical practice
Findings from routine clinical practice have also been reported. Cohort studies (n = 45 to 160) evaluating the efficacy of onabotulinumtoxinA injections into the submandibular glands or submandibular and parotid glands in children with neurological deficits, most commonly cerebral palsy (71–90%), support the results of the smaller studies [Citation7,Citation8,Citation57–59] and also indicate that the effects of each injection may last for up to 8–12 months in some patients [Citation7,Citation57]. In these studies, BoNT/A was most frequently administered at a total dose of 50 U or using weight-dependent dosing (15 U/gland for children weighing <15 kg, 20 U/gland for children weighing 15–25 kg, and 25 U/gland for children weighing >25 kg). Experience from a large tertiary institution that utilized ultrasound-guided bilateral injections of BoNT/A (formulation not specified) into both the parotid and submandibular glands also showed a high clinical success rate and very low complication rate with their protocol in 111 children with any of 52 unique diagnoses that contributed to sialorrhea (most commonly cerebral palsy in 29%) [Citation20]. One group of investigators looked for factors predicting a response to BoNT/A injections into the submandibular gland, but none were identified [Citation58,Citation59].
4.4. Phase III trials
The only phase III trial conducted to date evaluating the efficacy and safety of a BoNT/A for the treatment of sialorrhea in children is the SIPEXI trial (). In this double-blind trial, children/adolescents aged 6–17 years with chronic troublesome sialorrhea associated with neurological disorders and/or intellectual disability, and severe drooling (investigator’s Modified Teacher’s Drooling Scale rating ≥6), were randomized to a single dose of incobotulinumtoxinA (n = 148) or placebo (n = 72), followed by up to three repeated doses of incobotulinumtoxinA at 16-week intervals; 35 children aged 2–5 years with similar clinical profiles to the older children received incobotulinumtoxinA only [Citation25]. IncobotulinumtoxinA was injected under ultrasound guidance into both the parotid and submandibular glands at weight-determined doses ().
Table 1. Phase III trials evaluating incobotulinumtoxinA in patients with sialorrhea [Citation23–27]
Table 2. Dosage regimen for incobotulinumtoxinA in children/adolescents with sialorrhea in the SIPEXI trial, where the concentration administered was 2.5 U/0.1 mL [Citation25] and in adults in the SIAXI trial, where the concentration administered was 5 U/0.1 mL [Citation23]
In older children/adolescents (aged 6–17 years), the first injection cycle of incobotulinumtoxinA was significantly more effective than placebo for reducing sialorrhea, as measured by the co-primary efficacy endpoints unstimulated Salivary Flow Rate (uSFR) change from baseline to week 4 and the carers’ Global Impression of Change Scale (GICS) score at week 4, with significant benefits maintained to week 16 (). The beneficial effects of incobotulinumtoxinA were maintained over up to three additional injection cycles, becoming slightly greater with each subsequent cycle [Citation25]. In younger patients aged 2–5 years, salivary flow rate was not assessed because the test procedure is not suitable for young children; however, changes in the carers’ GICS were consistently and incrementally improved at 4 and 16 weeks after each of the four incobotulinumtoxinA injection cycles () [Citation25]. In both age groups, changes in the modified Teacher’s drooling scale, drooling impact scale, and drooling quotient supported the findings of the primary endpoints [Citation25,Citation26,Citation27].
Figure 2. Results for the co-primary endpoints of the SIPEXI trial in children aged 6–17 years with sialorrhea associated with neurological disorders. Least squares mean results following the first injection cycle are presented for (a) the change in unstimulated salivary flow rate and (b) the carers’ global impression of change scale [Citation25]
![Figure 2. Results for the co-primary endpoints of the SIPEXI trial in children aged 6–17 years with sialorrhea associated with neurological disorders. Least squares mean results following the first injection cycle are presented for (a) the change in unstimulated salivary flow rate and (b) the carers’ global impression of change scale [Citation25]](/cms/asset/a8536016-ff33-4017-acd9-2ea5586b4035/iern_a_1979959_f0002_oc.jpg)
Results of the SIPEXI trial build on those of the phase III SIAXI trial (NCT02091739) conducted in adult patients with sialorrhea () [Citation23,Citation24]. In both trials, incobotulinumtoxinA was injected into both pairs of parotid and submandibular glands (the dose was divided 3:2 between each type of gland) for multiple cycles every 16 weeks. Similar patterns of maintained response to incobotulinumtoxinA were observed in both adults and children, despite the populations having sialorrhea due to different neurological disorders, as would be expected [Citation7,Citation8,Citation11].
5. Safety/post-marketing surveillance
In common with all BoNT/A formulations, adverse events occur infrequently with incobotulinumtoxinA, and this agent is generally better tolerated than anticholinergics, including glycopyrrolate ().
Table 3. Summary of the main adverse events associated with incobotulinumtoxinA [Citation28,Citation29], and glycopyrrolate [Citation60,Citation61] in the treatment of children with sialorrhea as reported in the product information documents
IncobotulinumtoxinA was well tolerated when injected into the salivary glands for the first time and after repeated injections, administered 16 weeks apart, in the SIPEXI trial () [Citation25]. The most frequently reported adverse events were respiratory infections, and no patients showed newly developed neutralizing antibodies during treatment. Few dental/periodontal adverse events were reported [Citation25,Citation62].
Table 4. Summary of adverse events during treatment with incobotulinumtoxinA in the pediatric sialorrhea SIPEXI trial [Citation25]
Adverse events of special interest (including dysphagia, aspiration, and aspiration pneumonia) were events possibly indicative of toxin spread and were actively questioned for at each study visit. Dysphagia was the only such event reported in the study, with one case (mild) after the first injection cycle and four cases (mild or moderate) during the remaining three injection cycles.
IncobotulinumtoxinA was also well tolerated in adult patients with sialorrhea in the SIAXI trial, the most common treatment-related adverse events being dry mouth (4.4% of patients receiving incobotulinumtoxinA 75 U and 11.1% of patients receiving incobotulinumtoxinA 100 U), dysphagia (1.5% and 4.2%, respectively), and speech disorder (2.9% and 0.0%, respectively) following repeated injections [Citation24]. Adverse events occurred with a similar pattern and frequency across injection cycles.
Clinicians continue to systematically study use of incobotulinumtoxinA in children for a variety of disorders, including sialorrhea. For example, pooled data from three phase III studies in lower limb, upper limb, and combined multipattern spasticity treatment also showed that incobotulinumtoxinA was well tolerated in children/adolescents when administered intramuscularly [Citation63]. Such research is further complemented by the ongoing monitoring of adverse events during on-label use of incobotulinumtoxinA in adults and children, which has confirmed the overall very good safety profile of this agent. Reported events include flu-like symptoms and hypersensitivity reactions (e.g. swelling, edema [also that occurring distant from the injection site], erythema, pruritus, local and generalized rash, and breathlessness). As these reactions were reported voluntarily from a population of uncertain size receiving incobotulinumtoxinA for a variety of indications, it was not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure [Citation28,Citation29].
6. Regulatory affairs
IncobotulinumtoxinA is approved for the treatment of children and adolescents with sialorrhea in the USA [Citation28], and this indication is currently under review with the European Medicines Agency. It is approved for the treatment of sialorrhea in adults in both the USA and Europe [Citation28,Citation29].
IncobotulinumtoxinA is also approved for use in a number of other indications in both the USA and European Union. These include upper limb spasticity, cervical dystonia, blepharospasm, and temporary improvement in the appearance of moderate to severe glabellar lines with corrugator and/or procerus muscle activity in adults, and upper limb spasticity in pediatric patients aged 2–17 years, excluding spasticity caused by cerebral palsy in the USA [Citation28], and blepharospasm and hemifacial spasm, cervical dystonia of a predominantly rotational form (spasmodic torticollis), and spasticity of the upper limb in adults in Europe [Citation29].
7. Conclusion
BoNT/A has been used for many years for the treatment of sialorrhea, both in adults and children; however, published data to support its use have been limited to predominantly small clinical trials and cohort studies. It is therefore of great interest and value to pediatric patients with sialorrhea and their treating physicians that incobotulinumtoxinA has confirmed efficacy and safety in this indication, as shown by the results of the only phase III randomized controlled trial of a BoNT/A in pediatric sialorrhea to date (SIPEXI), and that it is approved for such use in the USA, with approval expected shortly in the European Union. Findings from the SIPEXI trial complement those of the SIAXI trial, conducted in adults with chronic sialorrhea, suggesting the potential for continued treatment with incobotulinumtoxinA from childhood through to adulthood.
Physicians can be reassured that the findings of the SIPEXI trial will likely be applicable to the pediatric patients with sialorrhea they see in clinical practice, since the underlying causes of sialorrhea in the pediatric SIPEXI trial population reflect those seen in general practice and cohort studies of other BoNT/A formulations [Citation7,Citation8,Citation57–59], with most patients affected by cerebral palsy. In addition, as expected of a BoNT/A formulation [Citation9], incobotulinumtoxinA is well tolerated over multiple injection cycles.
8. Expert opinion
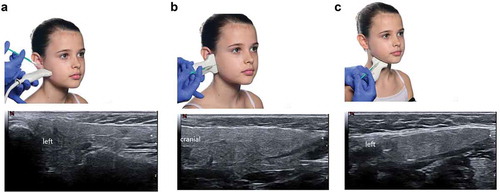
As the efficacy and safety of incobotulinumtoxinA in the treatment of children/adolescents with chronic sialorrhea have been confirmed in a large phase III trial (SIPEXI), BoNT/A should become a first-line treatment option in the management of this distressing disorder. Although BoNT/A is more complicated to administer than anticholinergic drugs, in a vulnerable group of patients sensitive to the adverse effects of anticholinergics, the purely local effect, and the excellent safety profile outweigh the challenges of the injection procedure. In our opinion, injection of BoNT/A is best achieved using ultrasound to guide needle insertion, a strategy that minimizes the risk of unnecessary adverse events [Citation16,Citation21] (). We also suggest initiating a fixed injection schedule every 4 months or more, rather than treating patients when sialorrhea returns. As shown in the open-label phase of SIPEXI, saliva reduction is maintained with repeated injections, and possibly even increases with each subsequent injection () when incobotulinumtoxinA is administered using the dosing regimen specified in the trial protocol (). It is therefore possible that, to achieve a constant effect, the required dose of BoNT/A could be decreased over time, potentially as a result of atrophy of the injected gland(s), as we have observed in clinical practice. Repeated ultrasound can be used to determine whether such atrophy has occurred in individual patients, allowing dose reduction or prolongation of the interval between doses.
Figure 3. Insertion sites for the ultrasound probe and needle for injection of incobotulinumtoxinA into the parotid gland (a) vertical and (b) horizontal, and (c) the submandibular gland. Resulting ultrasound images are also shown for each injection site
IncobotulinumtoxinA may have advantages over other BoNT/A formulations when considering treatment of children. It has a very low risk of immunogenicity and has proven efficacy and safety from phase III trials in both children/adolescents and adults with chronic sialorrhea. Thus, the transition of treating children with sialorrhea, which is a long-term condition, to treatment as adults can occur seamlessly. Minor dose adjustments may be required, based on individual requirements and the extent of gland atrophy, but the general incobotulinumtoxinA treatment schedule is the same for both children and adults.
Further research is to be encouraged in a number of areas. The value of combined therapy with BoNT/A and glycopyrrolate in specific patient groups is worthy of investigation. Such a combination, with glycopyrrolate administered in specific saliva-stimulating situations may minimize the risk of adverse effects. It is possible that the baseline effects of BoNT/A could help to overcome excess saliva production at rest, while caregivers could administer glycopyrrolate before play meetings, speech therapies, or other activities to prevent further saliva flow. Additional research into predictive factors for a good (or bad) response to BoNT/A would also be of value, since the effect of treatment is not the same in every patient. Finally, we also suggest further study to investigate the effect of incobotulinumtoxinA on posterior drooling, as most published data concerning the use of BoNT/A in pediatric sialorrhea focus on anterior drooling.
Completion of the phase III SIPEXI trial should result in the updating of information guides and formal guidelines for the management of pediatric sialorrhea. IncobotulinumtoxinA is now an approved, effective, and safe treatment option for children and adolescents with chronic sialorrhea.
Article highlights
Sialorrhea occurs when there is excessive production of saliva or an inability to transport saliva out of the oral cavity; it can have a significant negative impact on quality of life for both the patient and their caregiver
It is generally accepted that the management of sialorrhea is best accomplished with a multidisciplinary team approach, but there are limited reliable study data to support use of the different therapies that are currently used for treating sialorrhea in children
IncobotulinumtoxinA has shown good efficacy and safety after single and repeated injection cycles in a relatively large phase III trial of children/adolescents with chronic sialorrhea, resulting in its approval for pediatric sialorrhea in the USA
Injection of botulinum neurotoxin A is best achieved using ultrasound to guide needle insertion
IncobotulinumtoxinA may be a valuable treatment option for chronic sialorrhea because it has a similar treatment schedule in affected children and adults, and has demonstrated ongoing efficacy and safety after multiple injection cycles in phase III trials in both populations
Further research to evaluate the combination of incobotulinumtoxinA with glycopyrrolate for specific saliva stimulating situations would be of interest, as would determination of which patients will likely obtain the greatest benefit from incobotulinumtoxinA, and investigation of incobotulinumtoxinA treatment in patients with posterior drooling.
Declaration of interests
WH Jost, A Steffen, and S Berweck are advisors and speakers for Merz Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this article has participated in research funded by the Parkinson Foundation, Tourette Association, Dystonia Coalition, AbbVie, Boston Scientific, Eli Lilly, Neuroderm, Prilenia, Revance, Teva but has no owner interest in any pharmaceutical company. They have received travel compensation or honoraria from the Tourette Association of America, Parkinson Foundation, International Association of Parkinsonism and Related Disorders, Medscape, and Cleveland Clinic, and royalties for writing a book with Robert Rose publishers. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Information resources
The following website provides support and resources for pediatric patients with sialorrhea and their caregivers: https://www.rch.org.au/uploadedFiles/Main/Content/plastic/salivabook.pdf.
The following website provides guidance for clinicians treating children/youth with cerebral palsy and sialorrhea, and provides an evidence-based care pathway for those affected: https://www.aacpdm.org/publications/care-pathways/sialorrhea.
The National Institute for Health and Care Excellence published an evidence summary for severe sialorrhea in children and young people with chronic neurological disorders with a focus on oral glycopyrrolate in 2017: https://www.nice.org.uk/advice/es5/resources/severe-sialorrhoea-drooling-in-children-and-young-people-with-chronic-neurological-disorders-oral-glycopyrronium-bromide-pdf-32176358341.
Updated German guidelines for the treatment of hypersalivation have been published [9].
The following book provides a comprehensive overview of the proper use of botulinum toxin in a number of indications: Wolfgang Jost. Atlas of Botulinum Toxin Injection, Dosage, Localization, Application, 3rd Edition. 2019. Quintessence Publishing, United Kingdom.
Supplemental Material
Download MS Word (14.9 KB)Acknowledgments
The authors would like to thank Clara Jost, who was available as a model for the photos, and Mr. David Kühn, KVM Verlag, who supported the adaptation of the anatomy photo, and acknowledge Caroline Spencer and Dr. Sue Chambers (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Merz Pharmaceuticals.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Jost WH, Bäumer T, Laskawi R, et al. Therapy of sialorrhea with botulinum neurotoxin. Neurol Ther. 2019;8(2):273–288. Epub 2019 Sep 21. PMID: 31542879; PMCID: PMC6858891.
- Lakraj AA, Moghimi N, Jabbari B. Sialorrhea: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins (Basel). 2013;5(5):1010–1031. PMID: 23698357; PMCID: PMC3709276.
- Dias BL, Fernandes AR, Maia Filho HS. Sialorrhea in children with cerebral palsy. J Pediatr (Rio J). 2016;92(6):549–558. Epub 2016 Jun 6. PMID: 27281791.
- Crysdale WS. Management options for the drooling patient. Ear Nose Throat J. 1989;68(820):825–826. 829–30. PMID: 2612390.
- Blasco PA, Allaire JH. Drooling in the developmentally disabled: management practices and recommendations. Consortium on drooling. Dev Med Child Neurol. 1992;34(10):849–862. PMID: 1397726.
- van Hulst K, van den Engel-Hoek L, Geurts ACH, et al. Development of the Drooling Infants and Preschoolers Scale (DRIPS) and reference charts for monitoring saliva control in children aged 0-4 years. Infant Behav Dev. 2018;50:247–256. Epub 2018 Feb 12. PMID: 29448187.
- Khan WU, Campisi P, Nadarajah S, et al. Botulinum toxin A for treatment of sialorrhea in children: an effective, minimally invasive approach. Arch Otolaryngol Head Neck Surg. 2011;137(4):339–344. Epub 2011 Jan 17. PMID: 21242533.
- Van Hulst K, Van Der Burg JJ, Jongerius PH, et al. Changes in severity and impact of drooling after submandibular gland botulinum neurotoxin A injections in children with neurodevelopmental disabilities. Dev Med Child Neurol. 2020;62(3):354–362. Epub 2019 Nov 14. PMID: 31729034; PMCID: PMC7028146.
- Riva A, Federici C, Piccolo G, et al. Exploring treatments for drooling in children with neurological disorders. Expert Rev Neurother. 2021;21(2):179–187. Epub 2020 Dec 6. PMID: 33222543.
- Steffen A, Jost W, Bäumer T, et al. Hypersalivation: update of the German S2k guideline (AWMF) in short form. J Neural Transm (Vienna). 2019;126(7):853–862. Epub 2019 Apr 10. PMID: 30972507.
- Hockstein NG, Samadi DS, Gendron K, et al. Sialorrhea: a management challenge. Am Fam Physician. 2004;69:2628–2634. PMID: 15202698.
- Chang SC, Lin CK, Tung LC, et al. The association of drooling and health-related quality of life in children with cerebral palsy. Neuropsychiatr Dis Treat. 2012;8:599–604. Epub 2012 Dec 11. PMID: 23251093; PMCID: PMC3523561.
- Glader L, Delsing C, Hughes A, et al. Sialorrhea in cerebral palsy. 2018. Available at: https://www.aacpdm.org/publications/care-pathways/sialorrhea[Lastaccessed03May2021]
- National Institute for Health and Care Excellence. Severe sialorrhoea (drooling) in children and young people with chronic neurological disorders: oral glycopyrronium bromide. Evidence summary. 2017 Feb 14. [cited 2021 May 17]. Available at: https://www.nice.org.uk/advice/es5/chapter/Introduction-and-current-guidance.
- Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15(1):1–9. PMID: 25559581; PMCID: PMC4359186.
- Jost WH. The option of sonographic guidance in Botulinum toxin injection for drooling in Parkinson’s disease. J Neural Transm (Vienna). 2016;123(1):51–55. Epub 2015 Jul 3. PMID: 26138438.
- Solstice Neurosciences LLC. MYOBLOC® (rimabotulinumtoxinB) injection, for intramuscular or intraglandular use. 2000. Prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103846s5190lbl.pdf[Lastaccessed17May2021]
- Jost WH. Treatment of drooling in Parkinson’s disease with botulinum toxin. Mov Disord. 1999;14(6):1057. PMID: 10584695.
- Bekkers S, Delsing CP, Kok SE, et al. Randomized controlled trial comparing botulinum vs surgery for drooling in neurodisabilities. Neurology. 2019;92:e1195–e1204. Epub 2019 Feb 6. PMID: 30728311.
- Lungren MP, Halula S, Coyne S, et al. Ultrasound-guided botulinum toxin type A salivary gland injection in children for refractory sialorrhea: 10-year experience at a large tertiary children’s hospital. Pediatr Neurol. 2016;54:70–75. Epub 2015 Sep 28. PMID: 26706481.
- Loens S, Brüggemann N, Steffen A, et al. Localization of salivary glands for botulinum toxin treatment: ultrasound versus landmark guidance. Mov Disord Clin Pract. 2020;7(2):194–198. PMID: 32071939; PMCID: PMC7011800.
- Vashishta R, Nguyen SA, White DR, et al. Botulinum toxin for the treatment of sialorrhea: a meta-analysis. Otolaryngol Head Neck Surg. 2013;148(2):191–196. Epub 2012 Oct 30. PMID: 23112272.
- Jost WH, Friedman A, Michel O, et al. SIAXI: placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology. 2019;92(17):e1982–e1991. Epub 2019 Mar 27. PMID: 30918101; PMCID: PMC6511076.
- Jost WH, Friedman A, Michel O, et al. Long-term incobotulinumtoxinA treatment for chronic sialorrhea: efficacy and safety over 64 weeks. Parkinsonism Relat Disord. 2020;70:23–30. Epub 2019 Nov 26. PMID: 31794936.
- Berweck S, Bonikowski M, Kim H, et al. Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children: SIPEXI. Neurology. 2021 Aug 2:10.1212/WNL.0000000000012573. Epub ahead of print. PMID: 34341153.
- Berweck S, Kim H, Banach M, et al. Efficacy of incobotulinumtoxinA in the treatment of 6–17-year-old children and adolescents with chronic sialorrhea associated with neurological disorders and/or intellectual disability. Poster presented at TOXINS 2021 Virtual Conference; 16–17 January 2021.
- Berweck S, Bonikowski M, Banach M, et al. Efficacy and safety of incobotulinumtoxinA in the treatment of 2–5-year-old children with chronic sialorrhea associated with neurological disorders and/or intellectual disability. Poster presented at TOXINS 2021 Virtual Conference; 16–17 January 2021.
- Merz Pharmaceutical, LLC. XEOMIN- incobotulinumtoxina injection, powder, lyophilized, for solution. 2020. Prescribing information. Available at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=ccdc3aae-6e2d-4cd0-a51c-8375bfee9458&type=display[Lastaccessed26April2021]
- Merz Pharma UK Ltd. Xeomin 100 units powder for solution for injection. Summary of prescribing information. 2020. [cited 2021 Apr 26]. Available at: https://www.medicines.org.uk/emc/product/6202/smpc
- Jost WH, Benecke R, Hauschke D, et al. Clinical and pharmacological properties of incobotulinumtoxinA and its use in neurological disorders. Drug Des Devel Ther. 2015;9:1913–1926. PMID: 25897202; PMCID: PMC4389813.
- Lorenc ZP, Kenkel JM, Fagien S, et al. IncobotulinumtoxinA (Xeomin): background, mechanism of action, and manufacturing. Aesthet Surg J. 2013;33(1_Supplement):S18–S22. PMID: 23515195.
- Ferrari A. Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal. Funct Neurol. 2018Jan/Mar;33(1):7–18. PMID: 29633692; PMCID: PMC5901944.
- Pellett S, Bradshaw M, Tepp WH, et al. The light chain defines the duration of action of botulinum toxin serotype A subtypes. mBio. 2018;9(2):e00089–18. PMID: 29588398; PMCID: PMC5874905.
- Meunier FA, Schiavo G, Molgó J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris. 2002;96(1–2):105–113. PMID: 11755789.
- Hefter H, Brauns R, Ürer B, et al. Effective long-term treatment with incobotulinumtoxin (Xeomin®) without neutralizing antibody induction: a monocentric, cross-sectional study. J Neurol. 2020;267(5):1340–1347. Epub 2020 Jan 20. PMID: 31960136; PMCID: PMC7184051.
- Walter U, Mühlenhoff C, Benecke R, et al. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology. 2020;94(20):e2109–e2120. Epub 2020 Apr 24. Erratum in: Neurology. 2020;95:802. PMID: 32332130.
- Albrecht P, Jansen A, Lee JI, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. 2019;92(1):e48–e54. Epub 2018 Nov 21. PMID: 30464031.
- Jost WH, Blümel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (XEOMIN) in focal dystonia. Drugs. 2007;67(5):669–683. PMID: 17385940.
- Bothwell JE, Clarke K, Dooley JM, et al. Botulinum toxin A as a treatment for excessive drooling in children. Pediatr Neurol. 2002;27(1):18–22. PMID: 12160968.
- Suskind DL, Tilton A. Clinical study of botulinum-A toxin in the treatment of sialorrhea in children with cerebral palsy. Laryngoscope. 2002;112(1):73–81. PMID: 11802042.
- Ellies M, Rohrbach-Volland S, Arglebe C, et al. Successful management of drooling with botulinum toxin A in neurologically disabled children. Neuropediatrics. 2002;33(6):327–330. PMID: 12571790.
- Savarese R, Diamond M, Elovic E, et al. Intraparotid injection of botulinum toxin A as a treatment to control sialorrhea in children with cerebral palsy. Am J Phys Med Rehabil. 2004;83(4):304–311; quiz 312–314, 336. PMID: 15024333;
- Banerjee KJ, Glasson C, O’Flaherty SJ. Parotid and submandibular botulinum toxin A injections for sialorrhoea in children with cerebral palsy. Dev Med Child Neurol. 2006;48(11):883–887. PMID: 17044954.
- Gerlinger I, Szalai G, Hollódy K, et al. Ultrasound-guided, intraglandular injection of botulinum toxin A in children suffering from excessive salivation. J Laryngol Otol. 2007;121(10):947–951. Epub 2007 Mar 29. PMID: 17391573.
- Ong LC, Wong SW, Hamid HA. Treatment of drooling in children with cerebral palsy using ultrasound guided intraglandular injections of botulinum toxin A. J Pediatr Neurol. 2009;7:141–145.
- Hassin-Baer S, Scheuer E, Buchman AS, et al. Botulinum toxin injections for children with excessive drooling. J Child Neurol. 2005;20(120–3):120–123. PMID: 15794177.
- Jongerius PH, Rotteveel JJ, van Limbeek J, et al. Botulinum toxin effect on salivary flow rate in children with cerebral palsy. Neurology. 2004;63(8):1371–1375. PMID: 15505151.
- Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620–627. PMID: 15342830.
- Lin YC, Shieh JY, Cheng ML, et al. Botulinum toxin type A for control of drooling in Asian patients with cerebral palsy. Neurology. 2008;70(4):316–318. PMID: 18209205.
- Reid SM, Johnstone BR, Westbury C, et al. Randomized trial of botulinum toxin injections into the salivary glands to reduce drooling in children with neurological disorders. Dev Med Child Neurol. 2008;50(2):123–128. PMID: 18201301.
- Alrefai AH, Aburahma SK, Khader YS. Treatment of sialorrhea in children with cerebral palsy: a double-blind placebo controlled trial. Clin Neurol Neurosurg. 2009;111(1):79–82. Epub 2008 Nov 1. PMID: 18977585.
- Møller E, Pedersen SA, Vinicoff PG, et al. Onabotulinumtoxin A treatment of drooling in children with cerebral palsy: a prospective, longitudinal open-label study. Toxins (Basel). 2015;7(7):2481–2493. PMID: 26134257; PMCID: PMC4516924.
- Wilken B, Aslami B, Backes H. Successful treatment of drooling in children with neurological disorders with botulinum toxin A or B. Neuropediatrics. 2008;39(4):200–204. Epub 2009 Jan 22. PMID: 19165707.
- Schroeder AS, Kling T, Huss K, et al. Botulinum toxin type A and B for the reduction of hypersalivation in children with neurological disorders: a focus on effectiveness and therapy adherence. Neuropediatrics. 2012;43(1):27–36. Epub 2012 Mar 19. PMID: 22430158.
- Taib BG, Williams SP, Sood S, et al. Treatment of sialorrhoea with repeated ultrasound-guided injections of botulinum toxin A into the parotid and submandibular glands. Br J Oral Maxillofac Surg. 2019;57(5):442–448. Epub 2019 Apr 19. PMID: 31010597.
- Wu KP, Ke JY, Chen CY, et al. Botulinum toxin type A on oral health in treating sialorrhea in children with cerebral palsy: a randomized, double-blind, placebo-controlled study. J Child Neurol. 2011;26(7):838–843. Epub 2011 May 6. PMID: 21551374.
- Scheffer AR, Erasmus C, van Hulst K, et al. Efficacy and duration of botulinum toxin treatment for drooling in 131 children. Arch Otolaryngol Head Neck Surg. 2010;136(9):873–877. PMID: 208556Ne79.
- Erasmus CE, Scheffer AR, van Hulst K, et al. Does motor performance matter in botulinum toxin efficacy for drooling? Pediatr Neurol. 2011;45(2):95–99. PMID: 21763949.
- Erasmus CE, van Hulst K, Scheffer AR, et al. What could predict effectiveness of Botulinum Toxin to treat drooling: a search for evidence of discriminatory factors on the level of body functions or structures. Eur J Paediatr Neurol. 2012;16(2):126–131. Epub 2011 Jul 23. PMID: 21783393.
- Proveca Limited. Sialanar 320 micrograms/ml Glycopyrronium (400 micrograms/ml Glycopyrronium Bromide) Oral Solution. Summary of prescribing information. 2020. [cited 2021 May 19]. Available at: https://www.medicines.org.uk/emc/medicine/32715]
- Merz Pharmaceutical LLC. CUVPOSA (glycopyrrolate) oral solution. 2018. Prescribing information. [cited 2021 May 27]. Available at: https://www.cuvposa.com/wp-content/themes/cuvposa/assets/cuvposa-prescribing-information.pdf.
- Berweck S, Bonikowski M, Kim H, et al. Safety of incobotulinumtoxinA in the treatment of 6-17-year-old children and adolescents with chronic sialorrhea associated with neurological disorders and/or intellectual disability. Poster presented at TOXINS 2021 Virtual Conference; 16–17 January 2021.
- Banach M, Kaňovský P, Schroeder AS, et al. Safety of incobotulinumtoxinA in multipattern treatment of upper- and lower-limb spasticity in children/adolescents with cerebral palsy: a pooled analysis of 3 large phase 3 studies. Toxicon. 2021;190(Suppl 1):S7.