ABSTRACT
Introduction
Meniere’s disease (MD) is a chronic disorder of inner ear, characterized by audial and vestibular symptoms. It presents a great variability among patients in terms of clinical features, etiology, pathology, and response to the same therapy. It’s challenging to diagnose and manage for its heterogeneity. Indeed, the consensus is reached that MD has subtypes. Identifying subtypes of MD is important for individualized therapy and further research.
Areas covered
In this review, we examined the heterogeneity of MD. We also included the valid subtyping solutions and updated data regarding the association among subtypes, disease progression, and management.
Expert opinion
MD is an etiologically multifactorial condition, and it might be a constellation of symptoms associated with endolymph hydrops, not a disease entity. So far, MD can be classified as distinct phenotypes and endotype, respectively based on symptoms, pathology, possible etiology, and co-existing condition. Patients in different subtypes present different clinical features and are suitable for different treatment. The identification of these subtypes will benefit both basic and clinical studies of MD, by helping achieve personalized therapy, accurate prognosis prediction and even disease screening in near future. Therefore, MD subtyping is the emerging direction of diagnosis and treatment in the future.
1. Introduction
Meniere’s disease (MD) represents a chronic disorder of inner ear and is characterized by recurrent vertigo, fluctuating sensorineural hearing loss, tinnitus, and aural fullness [Citation1]. MD is associated with the accumulation of endolymph in the cochlear duct and vestibular organs, known as idiopathic endolymphatic hydrops (EH) [Citation2]. The prevalence rate stands at 205/100,000 in Germany [Citation3], 190/100,000 in USA [Citation4], but only 34.5/100,0000 in Japan [Citation5].
It’s challenging for diagnosis and treatment of MD. MD accounted for 10% of the vertigo forms in vertigo clinics [Citation6] Only 38% of patients with MD had been initially diagnosed with MD [Citation7]. MD vertigo attacks will cause economic loss and decrease life-quality due to medical expenses, inferencing work and daily activity, triggering anxiety and social phobias [Citation8].
So far, researchers have preliminarily arrived at a consensus that MD is a heterogeneous disease and sub-types may exist, as proposed in the Barany criteria [Citation1, Citation9–11]. Epidemiological studies showed that multiple conditions develop concomitantly with MD, including autoimmune disorders (ADs), allergy, migraine, viral infection and autonomic dysfunction [Citation12–17]. The odds of MD are marginally higher in females, the older, and the obese [Citation12]. Till now, it has been hypothesized that migraine or ADs are extended phenotypes of MD [Citation12]. Forty-five percent of MD patients had at least one migraine episode during vertigo attack [Citation16] and 8% of them suffered from migraine-like headache during vertigo episode [Citation15].
Therefore, the purposes of the present study are: (1) examine the heterogeneity of MD in terms of symptom, natural course, etiology, of which effects on the clinical definition and efficacy evaluation; (2) summarize the valid subtyping solutions and updated data regarding the association among subtypes, disease progression, and management.
2. Heterogeneity of MD and its effects
2.1. Etiology
At present, it is believed that MD is a disease of unknown etiology with multiple contributing factors, including genetic factors, viral infection, allergy, and autoimmunity.
The development and progression of MD are genetically based as illustrated by its familial clustering and the higher prevalence in Caucasians [Citation18–20]. Although most families with familial MD (FMD) have an autosomal dominant inheritance pattern, an autosomal recessive inheritance and de novo mutations are observed [Citation19]. A wide array of genes are believed to be associated with FMD, such as FAM136A, DTNA [Citation21], SEMA3D, DPT [Citation22, Citation23], which are in autosomal dominant, OTOG and LSAMP which are in autosomal recessive [Citation24], and MYOA which is autosomal, either dominant, and/or recessive [Citation25]. Besides, some rare variants of putative candidate genes were found in sporadic MD (SMD) patients, such as PTPN22, NFKB1 [Citation23]. These findings suggest that multiallelic inheritance are implicated in the development of MD.
Many researchers believe that viral infection might be a cause of MD [Citation26, Citation27]. Viral structures have been observed in vestibular ganglion cells. MD-related vertigo could be relieved in 91% by acyclovir, antiviral agent [Citation28]. Recently, a systematic review reported that cytomegalovirus-infection was three-fold more associated with MD as compared to controls [Citation29].
Approximately one-third of MD cases seem to result from AD [Citation30, Citation31]. The most commonly reported in patients with MD was rheumatoid arthritis with a mean point prevalence of 4.3%. While others reported that thyroiditis is the most common autoimmune disease, accounting for 12.7% [Citation32,Citation33]. Several theories have been proposed to explain how autoimmune inner ear disease might arise: cross-reaction theory, intolerance theory [Citation31], genetic factors theory and bystander damage theory.
In 1923, Duke, for the first time, proposed that MD might be induced by an allergic reaction [Citation34]. Both inhalant and food allergies have been found to be linked with MD [Citation35]. When received specific allergy therapy, a significant percentage of Patients with MDs with allergic diseases show improvement in their symptoms of tinnitus and vertigo [Citation36].
Prosper, in his original description, suggested that migraine might be associated with MD. Lifetime prevalence of migraine increases in MD patients [Citation16]. Though some studies suggest that the occurrence and progression of MD is closely related to vascular disorders, these studies are relatively few [Citation37]. EH is connected to the changes in the microvasculature of the cochlea, including tiny ruptures of the membranous labyrinth [Citation38]. Both migraine and MD may be the result of trigeminovascular dysfunction.
A study reported that concomitant presence of allergy and migraine was 9 times more prevalent in the MD group when compared with a control group [Citation14]. Conspicuous similarities exist among MD, allergy, and migraines in terms of both symptom presentation and vascular changes, in a paroxysmal and recurrent manner, vasoconstriction, vasodilatation, and plasma extravasations, during the symptom attack [Citation14].
2.2. Pathology
EH is an event associated with a wide array of inner ear disorders [Citation39], but not the pathophysiological mechanism that directly mediating the disease process [Citation40]. Some researchers showed that every single living case of definite MD had signs of EH with magnetic resonance imaging (MRI) [Citation41,Citation42]. MD may be essentially a bilateral condition with EH and EH may be possibly fluctuated [Citation7]. EH was observed in 23.3% of the ‘asymptomatic ears’ [Citation43, Citation44]. Besides, some MD patients suffered from ES atrophy, hypoplasia of the vestibular aqueduct (VA), and narrowing of the lumen of the endolymphatic duct [Citation45]. A recent study exhibited that a discontinuous VA would predict the presence of saccular hydrops [Citation46].
2.3. Symptom
In terms of symptoms, vestibular and auditory symptoms may occur together or separately, at varying frequencies (e.g. on daily or monthly basis), and with some major symptoms (e.g. those that are of more vestibular than auditory nature) [Citation47, Citation48]. Symptoms may develop due to known inducing factors (e.g. high sodium intake, caffeine, stress) [Citation49] or without obvious triggers. In many cases, the condition is unilateral at the onset [Citation47, Citation50]. In the long-term, permanent hearing loss and vestibular hypofunction can stay mild to moderate or deteriorate [Citation47]. The incidence of bilateral involvement increased with the duration of the disease, from 9.1% (less than 1 year) to 41.5% (more than 20 years) [Citation50].
It also has two special variants. Drop attacks (DA), initially described as ‘otolithic catastrophes’ by Tumarkin in 1936 [Citation51], occurs without warning and without loss of consciousness but happens. It’s speculated that DAs tended to occur in the stage when the severity of EH worsened [Citation52]. Lermoyez syndrome, namely cochlear symptoms such as hearing loss and tinnitus occurred initially, after, during or immediately prior to sudden attack of vertigo, improvement of the hearing on the affected ear is noted [Citation53, Citation54]. It may be probably due to blockage in the ductus reunions caused by dislodged saccular otoconia and then the release of endolymph from the cochlea flooding into the semicircular canals. Lermoyez syndrome occurs most frequently in males and some elderly [Citation53]. MD can occur in two special population: the elderly and children. Ten percent of patients with MD had a disease onset after 65 years [Citation55]. And the elderly patients seem to have higher odds of drop attack [Citation56]. Pediatric MD is rare, accounting for 1%–2.3% prevalence in Patients with MD [Citation57, Citation58]. Bilateral affliction and symmetrical hearing levels on both ears and positive family history in pediatric MD are more prevalent than in adult MD [Citation58].
2.4. Treatment evaluation
The researchers have reached a consensus on the treatment of MD. Patients should change the lifestyle, receive vestibular rehabilitation during the intercortical period and if possible, psychotherapy. First-line conservative medications, i.e. diuretics and betahistine or local pressure therapy should be used. When medical treatment fails, a second-line treatment that is, the intratympanic injection of steroids, should be used. The third-line treatment, the endolymphatic sac surgery or the intratympanic injection of gentamicin should be used, depending on the hearing function. The last-ditch option is labyrinthectomy, a destructive surgery, without or in combination with cochlear implantation or vestibular nerve resection [Citation59, Citation60].
However, the efficacy evaluation of MD treatment is controversial. Most RCTs regarding MD were not able to confirm that diuretics or betahistine, intratympanic steroids and endolymphatic sac surgery were, in any way, effective or limited.
Betahistine is a structural analog of histamine that works as a weak partial agonist of postsynaptic histamine H1 receptors and antagonist of presynaptic H3 receptors [Citation61]. A Cochrane review in 2016 showed that betahistine might exert a positive effect on vertigo symptoms though the studies included were of low quality [Citation62]. But Adrion et al. [Citation63] suggested that in terms of vertigo attack control, the betahistine did not outperform the placebo, at either high or low dose.
Diuretics are suggested to change the electrolyte balance within the endolymph, leading to a decreased endolymph volume and pressure, either by increasing endolymph drainage or decreasing its production. Despite the widespread use of diuretics in MD treatment, literature reviews have consistently shown that evidence of effectiveness was lacking [Citation64–66].
Intratympanic steroid injection (ITST) could work directly on discrete inner ear tissue regions, thereby impacting local microhomeostasis [Citation67]. Garduno-Aaya et al. [Citation68] found ITST worked better than placebo (82 vs. 57%) in subjective hearing, subjective tinnitus loudness and aural fullness. But several studies failed to find any benefit of ITSI as compared with placebo [Citation69–72].
The putative goal of sac surgery is to promote the endolymph flow from the labyrinth to the endolymphatic sac, thereby relieving hydrops. Endolymphatic sac decompression surgery (ESDS) could control progressive bilateral hearing loss in BMD patients, and even decrease the incidence of MD development in contralateral silent EH within the first five postoperative years [Citation73,Citation74]. However, Thomsen et al. [Citation75] found only minor differences existed between the endolymphatic sac surgery and the placebo. Moreover, Jong et al. [Citation76] studied temporal bone and found that ES surgery does not relieve hydrops and the procedure failed in some cases. But the surgery could definitely relieve vertigo in some patients, the relieving mechanism remains poorly understood.
2.5. The existence of subtypes influences the clinical and basic research
Therefore, we can definitively conclude that MD is a heterogeneous disease, etiologically, pathologically and clinically. And then the efficacy of some treatments is still controversial. The main reason is that patients might vary greatly in terms of disease causes so they are capricious both in behavior and response to the same treatment; Identifying the subtypes is one step forward to solving these difficulties.
3. Trends and methods for subtyping
With the improvements in our knowledge and understanding of MD, the method of MD subtyping has been improved. At the early stage, many solutions were only based on MD symptoms (e.g. vertigo predominance, vestibular with cochlea dysfunction, disease laterality, etc.). Then, scholars focus on the comorbidities more and more, such as migraine and autoimmune disease. Later solutions do not include the factors mentioned above plus the interval for bilateral progression, age of onset and so on, and also use cluster analysis (CA), an advanced biostatistical method. Recently, there has been a further expansion in biomarker variables, e.g. cytokine, genetic, endolymphatic sac pathology, radical imaging. In this section, we will discuss further details, clinical appliances and research prospects of each subtyping approach. We have summarized MD subtypes in ().
Table 1. A summary of Meniere’s disease subtypes
3.1. The predominant symptom
In 1972 AAOO criteria, MD was defined as ‘a disease of the membranous inner ear characterized by deafness, vertigo, and usually tinnitus which has as its pathologic correlate hydropic distension of the endolymphatic system.’ Typical MD display fully the typical symptom triad. Atypical MD consists of two subvarieties of MD: cochlear MD and vestibular MD, which lacking the symptoms of either hearing loss or vertigo [Citation77]. Atypical MD has EH in both the cochlea and the vestibule, showing that atypical MD is a true variant of MD [Citation78]. More recently, this classification has been revived and applied to Japanese diagnostic criteria for MD [Citation79]. R Gürkov et al. [Citation80] proposed a new concept of inner ear disease with underlying EH: primary hydropic ear disease (PHED) and secondary hydropic ear disease (SHED). The PHED falls into 3 types: cochlea type, vestibular type and cochleovestibular type, which corresponds to 1972 criteria [Citation7, Citation48]. This classification attaches EH to clinical features briefly and clearly, which help doctors make personalized treatment plan and reduce the burden of medication for patients.
3.2. Disease laterality
Many researchers classify MD in light of disease laterality (unilateral vs. bilateral). Only a few differences were recognized between patients with unilateral MD (UMD) or bilateral MD (BMD). Patients with BMD tend to have initial episodes at a significantly younger age, more family history of MD and migraine, and personal history of migraines [Citation81, Citation82].
In many cases, they present unilateral disease at the onset, then can progress to bilateral involvement. The longer the disease lasts, the greater the possibility of bilateral involvement [Citation50, Citation83–85]. The audiometric curve configuration, EH in ‘asymptomatic ears’ may serve as indicators for UMD to future progress into BMD [Citation44, Citation86]. Patients with a long history of MD and severe hearing loss in the affected ear are more likely to exhibit endolymphatic hydrops in the asymptomatic contralateral ear [Citation44]. How to prevent or stop this progression is worthy of adequate attention.
3.3. Migraine
Lisheng Yu et al. [Citation87] suggested that the diagnosis of migraine should be included in the criteria for MD classification and it divided MD into ‘MD alone’ and ‘MD with migraine.’ They found patients with MD and migraine had larger retinal artery than their MD-alone counterparts. Besides, coexistent migraine affects relevant clinical features of patients with MD [Citation87, Citation88]. Patients with MD and migraine suffered from more and longer lasting vertigo attacks.
A previous study found the retinal artery diameter increases significantly on the headache side in ≥5 migraine attacks per month [Citation89]. These findings support that pathophysiologically migraine and MD attacks are intimately linked to the trigeminal vascular system (TVS) dysfunction. TVS activity and sensitization causes the secretion of vasoactive neuropeptides, which induces neurogenic inflammation and dilation of retinal blood vessels and the cochlear vasculature, which may worsen EH and speed up the outflow of EH [Citation87]. Some researchers hypothesis that MD is a variant of migraine, cochleovestibular migraine [Citation90].
Categorizing MD into ‘MD with migraine’ and ‘MD alone’ types may well improve the management of MD. Preventive treatment for migraine may partially prevent MD attacks in MD patients with migraine, such as lifestyle and diet changes, Nortriptyline [Citation91, Citation92]. If attacks are controlled, the previously inevitable progression to severe hearing loss may be preventable in some cases [Citation37].
3.4. Cardiovascular risk factors
Angela Reis Rego et al. [Citation93] first establish a new phenotype of MD in terms of cardiovascular risk factors. Cardiovascular risk factors include excessive BMI, dyslipidemia, type 2 diabetes mellitus, hypertension. MD patients are divided to groups with and without cardiovascular risk factors (74% vs 26%). Patients with risk factors show higher PTA threshold, lower speech discrimination, and more self-reported attacks. Patients with risk factors seem to meet a worse prognosis. The most frequent risk factor is hypertension. Further studies should shed on the relationship between MD and cardiovascular risk factors. If this relation is correct, evaluation and treatment of these risk factors should be approached in every MD patient with new treatment options. It also can build the basics to identify the pathological pathway of MD.
3.5. Data-driven cluster analysis
In 2011, Lourdes et al. [Citation94] firstly employed CA to classify patients with definite UMD according to their clinical data. The parameters they used inclusively encompassed audiometric and vestibular test results, posturographic findings and disability severities. Their results revealed that the patients could be divided into four clusters with unique disease profiles.
Cluster 1 patients (13.1%), known as ‘mildly active elderly,’ were the eldest and had a low frequency of attacks. It’s a special subset for that MD in the elderly can occur as a de novo disease or as the reactivation of a previous disorder. They had good vestibular function and slight disability but with a significant balance dysfunction and the worst hearing in the asymptomatic ear mainly due to age. Different from a previous study [Citation95], elderly patients in this study didn’t have a higher incidence of Tumarkin attacks. This cluster was defined most clearly by the hearing level in both ears and the result of sensory organization test condition 6. Cluster 2 patients (41.2%) were the least affected and all their parameters were close to normal, called ‘mildly active young.’ Patients in Cluster 3 (34.6%), or ‘active compensated,’ were the most affected, suffering from frequent and serious vertigo attacks, but they were well compensated with visually dependent. Cluster 4 patients (11.1%) had strong asymmetric hearing between two ears and had the most uncompensated vestibular deficit. They were moderately disabled, and were dubbed ‘active uncompensated.’
Patients in cluster 1,2 had lower frequency of vertigo attacks than cluster 3,4 (active vs. mildly active). Patients in cluster 2,3,4 were the young. Clusters 2,3,4 represent the natural courses of MD and could transfer from one to another. On basis of this, we can make efforts to build a predictive model for MD to develop tailored or personalized therapeutic strategies.
In 2016, Frejo et al. [Citation96, Citation97] used laterality (unilateral vs. bilateral) as a primary classifier, and they further employed CA to identify the clinical features that distinguish 5 discrete subgroups of UMD and BMD patients.
They defined BMD patients in light of four clinical predictors: FMD, autoimmune history, migraine symptom, and the types of onsets of hearing loss.
Type 1 is the most frequent type, involving 46% of the patients, and is defined in terms of metachronic hearing loss (a transition interval more than 1 month), without migraine and without AD. Since it is not associated with any particular clinical feature or etiological factor, further research is needed to determine contributing factors. Type 2 occurs in 17% of the patients, and it is defined as synchronic hearing loss (a transition interval no more than 1 month), without migraine or AD. Of note, BMD type 2 patients bore high vascular risks and high prevalence of headache. However, the better hearing stage was mostly observed in type 2 patients other than type 1, although the two groups are not significantly different in terms of the age of onset, disease duration or gender ratio. So further studies should examine the part the vascular risk factors play in the labyrinthine microcirculation in MD patients. We cannot definitively know the causes for this phenomenon now. Type 3, FMD, could be further classified into two subgroups (3a with migraine, 82%, and 3b BMD without migraine 18%). These findings confirm the previous description on MD families comorbid with migraine or not [Citation19]. They reflect the genetic heterogeneity in FMD. Type 4 is associated with the presence of migraine in all cases, whose mean age of onset is younger than others. This group may overlap with VM and they may have common pathological mechanisms [Citation98]. Type 5 is defined by AD.
In 2018, Frejo et al. [Citation97] performed a two-step CA in UMD patients to define clinical subgroups. In terms of the four clinical predictors: i.e. delayed MD (DMD, hearing loss antedates the vertigo episodes by months or years), FMD, migraine and AD, they identified five subgroups with strong etiological significance.
Type 1 is the most frequent type, including 53% of the patients, and is defined in terms of the sporadic, classic MD without migraine and without AD. Like the type 1 in BMD, it has no definitive characteristics and is not associated with any particular clinical feature or etiological factor. Type 2, found in 8% of patients, is dubbed DMD. The mean disease course of DMD is shorter than their non-DMD counterparts, but the hearing loss is more severe. This type tends to have more serious cochlear impairment. Type 3 is seen as FMD. They also can be divided into two subgroups (type 3a without migraine, accounting for 78%, and 3b with migraine, making up 22%). The mean hearing threshold at diagnosis in FMD was about 30 dB HL, worse than that of SMD, suggesting that subclinical fluctuating hearing loss may go unnoticed in familial recurrent vertigo. And the AD prevalence was higher in sporadic cases, which is not consistent with the finding previous studies [Citation99]. Type 4 is associated with the presence of migraine in all cases. About 41% of UMD type 4 had AD. These individuals with SMD plus migraine and AD pose a great treatment challenge. Type 5 is found in 11% of patients and is defined in terms of a concomitant AD. UMD type 3 and type 5 overlap in 10% of cases (FMD with AD but without migraine). SMD with AD has an earlier-age onset, more severe hearing loss, and higher prevalence of headache and migraine than SMD without AD. This suggests AD may affect the MD symptoms.
This approach makes us to recognize the differences between BMD and UMD that not found previously. Between patients with uni- and bilateral MD in type 3, 4, significant differences existed in the concomitant AD distribution. In UMD, part of type 3 type 4 had AD, while none of their BMD counterparts had AD. BMD group 5 is heterogeneous and includes patients with sporadic MD, FMD, migraine, however UMD type 5 are all sporadic and without FMD and migraine. These clinical variants observed in UMD and BMD suggest genetics and autoimmunity, as separate factors, contribute to the development of MD. Moreover, the CA using the aggregated data of the unilateral and bilateral patients was not able to yield the fully identical groups. This is might be attributed to increased heterogeneity, indicating that unilateral and bilateral MD might be different disorders.
A study investigated the distribution of the 5 MD subtypes in 81 patients with definite MD in USA [Citation100]. We are led to reach a conclusion that UMD cases in USA had a characteristically different phenotypic distribution from the European ones. This might be ascribed to differences in environment and ethnicities.
This cohort of UMD was significantly different, in distribution, from European cohort (): 1) There were more type 2 cases in US population than in European subjects, suggesting that US patients might be more subject to the homeostatic changes in cochlear organ than in the vestibular organ; 2) fewer FMD cases were found in US, coincident with a previous study, the Spanish and Portuguese cohorts might be more genetically homogenous than their US counterparts [Citation19].
Figure 1. The comparison of MD subtypes distribution between European and USA cohorts. Bar chart comparing percentages of Ménière’s disease (MD) subtypes reported by Crossley J et al [Citation96] in US population and Frejo et al [Citation92, Citation93] in a European population, respectively. a, unilateral Meniere’s disease subtypes distribution. Type 1, sporadic MD without migraine and autoimmune disease; type1 delayed MD; type 3, familial MD; type 4, MD with migraine; type 5, MD with autoimmune disease. b, bilateral Meniere’s disease subtypes distribution. Type 1, bilateral MD with metachronic hearing loss; type 2, bilateral MD with synchronic hearing loss; type 3, familial MD; type 4, MD with migraine; type 5, MD with autoimmune disease.
![Figure 1. The comparison of MD subtypes distribution between European and USA cohorts. Bar chart comparing percentages of Ménière’s disease (MD) subtypes reported by Crossley J et al [Citation96] in US population and Frejo et al [Citation92, Citation93] in a European population, respectively. a, unilateral Meniere’s disease subtypes distribution. Type 1, sporadic MD without migraine and autoimmune disease; type1 delayed MD; type 3, familial MD; type 4, MD with migraine; type 5, MD with autoimmune disease. b, bilateral Meniere’s disease subtypes distribution. Type 1, bilateral MD with metachronic hearing loss; type 2, bilateral MD with synchronic hearing loss; type 3, familial MD; type 4, MD with migraine; type 5, MD with autoimmune disease.](/cms/asset/fda16722-52de-4b6b-af92-53dbabb74020/iern_a_2030221_f0001_oc.jpg)
When it comes to clinical features, a vascular mechanism may weigh more in US cohort for that, sporadic UMD population in US had a higher frequency of headache and a significantly higher rate of smoking than the European sporadic UMD cohort. So far, whether smoking is a positive factor for the development of MD remains controversial [Citation101, Citation102]. In US population, the sporadic UMD cases had a low incidence of fall attacks but there was no difference in the mean age between the two studies. The age might not be associated with high incidence of drop attacks.
Of them, the bilateral cases were too small to draw any definitive conclusion about subtype distribution.
The new classification of BMD and UMD has potential etiological implications. It can facilitate patient selection in genetic and clinical research. For example, research on type 3 patients can lead to identification of genetic factors and then develop genetic therapy in the future. It might help improve the diagnostic workflow and the management of MD. Previous studies on BMD patients mainly focused on the diagnosis by means of electrocochleography or MRI, the classification shift our attention to the common comorbidities of MD, such as migraine or AD.
CA is one of data-driven hypothesis-free approaches, which can group sets of objects that share similar characteristics without delineating a priori assumptions. It can avoid over-weighing a specific variable and ensure better subgrouping results, but a comparison among different CA studies is challenging since these studies methologically varies a lot and their patient selection and testing methods might be flawed.
3.6. The interval for bilateral progression
Although not publishing as subtype, a study provides that the interval for progression to BMD is associated with prognostic. The BMD patients fall into an early bilateral involvement group (EBIG, the interval<18 months) and a late bilateral involvement group (LBIG, the interval>18 months). Patients with synchronous BMD and those in EBIG had poorer prognosis when compared with patients with metachronous BMD and those in LBIG. Different pathological mechanisms might be implicated in the two disease entities [Citation103].
Receiver operating characteristic curve showed that an interval<18-months, serving as a cutoff value. However, in 12 of 25 cases (48.0%), the second ear was affected within 12 months, and no patient had an interval ranging from 12 to 24 months. The result was consistent with a previous study [Citation104]. It’s more reasonable to hypothesize that the development of bilateral involvement within 12 months can serve as a cutoff value for predicting poor prognosis in patients.
None of the patients developed systemic autoimmune disease in this cohort, which was different from the result of the European study [Citation96]. The low incidence of autoimmune disorders in BMD in Asians may reflect that their autoimmune, autoinflammatory, or genetic pathological states are different from Caucasians.
3.7. The level of cytokines
In a follow-up study, Frejo et al. [Citation105] identified two subgroups in terms of cytokine profile in the supernatant of peripheral blood mononuclear macrophages (PBMCs). Patients with MD exhibited elevated basal levels of proinflammatory cytokines, compared to healthy controls. The basal levels of IL-1β in MD patients displayed a bimodal distribution pattern. Nineteen percent patients had low basal level of IL-1β and 81% patients had high basal levels of IL-1β. Moreover, Aspergillus and Penicillium trigger the release of TNF-α in MD patients and initiate or worsen the inflammatory response in the inner ear. The gene expression profile in PBMC of patients with high level of IL-1β is different from patients with low levels of IL-1β
In fact, none of the MD patients with higher levels of proinflammatory cytokines had an acute infection or another autoimmune condition. This subset may have an autoinflammation-related inner ear condition.
The two subgroups of MD patients with intrinsic differences in the immune response or two functional states of the immune system in patients with MD. Most of the patients with increased IL-1β were categorized as MD type 1. Future study could examine the levels of cytokines and gene express to further characterize MD patients.
Moleon et al. [Citation106] further categorized MD patients into early-onset MD (EOMD, <35 years old), and late-onset MD (LOMD, >50 years old). No differences in cytokines and chemokine were found between EOMD and LOMD, MD with and without migraine, respectively. However, patients with EOMD were more likely to develop migraine. The levels of some of chemokines, such as CCL18, CCL22, and CCL4, were higher in MD or migraine patients than in controls. And MD patients presented a methylation profile in mononuclear cells different from controls, and such difference might be ascribed to an increased activation level of immune cells in MD [Citation107]. These findings suggested that MD and migraine are associated with autoinflammation.
This etiology-based approach also has differentiated and therapeutic implications. It can help differentiate MD from other balance disorder, such as vestibular migraine. Patients with higher level inflammatory factors are more suitable for intratympanic injection of glucocorticoid. IL‐1 inhibitors may be effective to treat MD.
This etiology-based approach also has differentiated and therapeutic implications. It can help differentiate MD from other balance disorder, such as vestibular migraine. Patients with higher level inflammatory factors are more suitable for intratympanic injection of glucocorticoid. IL-1 inhibitors may be effective to treat MD.
3.8. Genes
Requena T et al. [Citation19] divided MD into SMD and FMD. They found although most of the families shared an autosomal dominant pattern, five pedigrees seemed to possess an autosomal recessive inheritance and another two families with monozygotic twins might be caused by de novo mutations. Apart from an early onset in familial cases, no differences were revealed in clinical features between sporadic and familial MD. Hence, SMD could be caused by rare or de novo mutations with moderate penetrance.
Frejo et al. and Gallego-Martinez et al. [Citation108, Citation109] reviewed the genetic evidence of SMD and FMD. The genes or loci of FMD included DTNA, FAM136A etc. The FMD showing incomplete penetrance and variable expressivity [Citation22]. So far, multiple other genes involved in FMD have been identified, as listed in [Citation21, Citation22, Citation25, Citation108–111]. DTNA encodes alpha-dystrobrevin, which interacts with transmembrane proteins and actin in the basolateral membranes of epithelial cells. FAM136A is a mitochondrial protein of unknown function.
Table 2. Genes involved in familial Meniere’s disease
The genes or loci associated with SMD included MICA, TLR10, NFKB1, AQP1-5, KCNE1, KCNE3, ADD1 etc., listed as [Citation23, Citation109, Citation112, Citation113]. A great many genes are not identified and of unknown function. A few hereditary and syndromic deafness genes were contributed to susceptivity to hearing loss of SMD, such as GJB2, USH1G. Several allelic variants in immune innate response genes, i.e. MICA, TLR10 and NFKB1, influence the hearing function in SMD. KCNE1 and KCNE3 are connected to allelic variants of two voltage-gated potassium channel genes. There are hypothesis supporting that aquaporins (AQP) serves as a molecular target in MD. AQPs contribute to regulate volume homeostasis in the inner ear fluid, especially AQP2,5 [Citation113] Genotyping studies on AQP yielded conflicting findings about AQP2 and AQP4 genes. Moreover, a homozygous synonymous variant in AQP3 was found in Japanese. Vertigo attacks might originate from interplay between exaggerated vasopressin release and allelic variants in AQP genes. Roberto Teggi et al. [Citation112] found that an ionic dysregulation predisposing to MD. ADD1 gene encodes a cytoskeletal protein, which interacts with the Na+-K+-ATPase transporters. Nonetheless, they found that ADD 1 mutation was associated with a subgroup of MD subjects with migraine, but no difference in the mutation was found between MD and control. They also found SLC8A1 and SIK1 might be triggering factors of MD. SLC8A1 gene encodes Na+-Ca2+ exchanger protein. SIK1 gene encodes Salt Inducible Kinase 1, an enzyme associated with Na+-K+ ATPase function and might interact with AQP4 [Citation112].
Table 3. Genes involved in sporadic Meniere’s disease
MD is mechanistically complicated and its development involves multiple genes and environmental factors. The AD-FMD showing incomplete penetrance and variable expressivity [Citation22]. The aforementioned two studies principally focused on the genetic basis of MD. The improvement in the classification of MD families, in combination with exome sequencing, will help to further find rare variants and genes implicated in FMD.
3.9. ES pathology
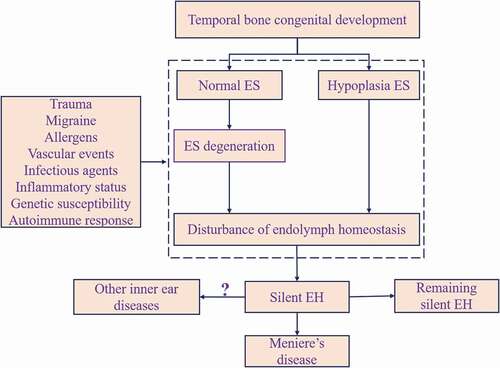
In 2018, Eckhard et al. [Citation114] revealed, for the first time, that iEH and MD idiopathic EH and clinical MD can be subdivided according to ES pathology: degeneration and hypoplasia. In degenerative ES pathology, the extraosseous portion of the endolymphatic sac (eES) epithelium showed i.e. pycnotic nuclei, expelled/missing cells, and fibrotic replacement. In hypoplastic ES pathology, the ES did not develop properly and had no extraosseous portion. Both of them have lost or absence of Aldosterone (ALDO) regulated Na+ transport proteins, which are perform as the primary mediator of extracellular Na+ and fluid homeostasis. As a result, they presumably impair the inner ear’s overall (Na+) homeostatic capacity, resulting in osmotic changes and ultimately to the development of EH. Therefore, it is believed that the loss or absence of the eES and its ion transport function is critical the etiology of idiopathic EH and MD symptoms.
They also found that both pathologies were linked with distinct clinical traits. ES-dg was associated with unilateral disease significantly more than the bilateral condition. On the contrast, ES-hp was related to BMD. Moreover, onset of MD was earlier and EH was more severe in ES-hp patients. Another notable finding was that only the patients who had family history of MD developed hypoplasia. They hypothesize that hypoplastic pathological change might be of hereditary nature.
On the basis of this, a group of researchers stablished the vestibular aqueduct angular trajectory (ATVA), in particular, the CT-imaging-based angle αexit, as a surrogate marker of ES pathologies (histopathological endotypes) in MD [Citation115]. ES-hp was found to be associated with abnormally short, straight VA (αexit>140°), as found at the fetal developmental stage (gestational weeks 6–38). This premature VA morphology, together with a hypoplastic ES, supports the hypothesis that the endotype is of genetic/developmental etiology, as Eckhard et al. proposed [Citation114]. In contrast, the normal (mature) VA morphology in MD patients with degenerative (αexit<120°) suggests that a certain etiology presents in adult life and triggers progressive ES degeneration, but does not affect the VA.
Then, they measured αexit on the basis of gadolinium-enhanced magnetic resonance imaging (Gd-MRI) and successfully subgrouped a series of MD patients in terms of their ES pathologies [Citation116]. In their cohort, ES hypoplasia was present in 23.6%, and ES degeneration was found in 76.4% of their subjects. Only several variables showed very strong evidence of difference between groups (MD-dg vs. MD-hp).
There was a strong preponderance of male patients in the MD-hp group (male-to-female ratio = 1:0.06) and a virtually balanced male-to-female ratio in the MD-dg group (1:1.2). Although frequencies in headache differed between the two groups, it’s believed that gender distribution was a confounding factor, since generally prevalence of migraine is higher in women than in men [Citation117]
The radiological signs of superior semicircular canal (SCC) dehiscence were more common in the MD-hp group (29.4%) than in the MD-dg group (3.6%), as a result, MD-hp patients may be on an increased risk of concomitant audiovestibular symptoms caused by SCC dehiscence syndrome. This may be compounded by the abnormal development at the fetal stage.
Generally speaking, MD-dg was associated with a higher frequency of vertigo episodes and a more gravely impaired vestibular (caloric) function. These findings may be explained by a presumably more abrupt manifestation of ES degeneration in the matured inner ear. ES degeneration is likely more disruptive to vestibular function due to the adult inner ears are less capable of developing compensating mechanisms for homeostasis [Citation116].
Bilateral ES-hp tend to have bilateral EH and MD, but unilateral ES-hp not. Only MD-dg was linked to contralateral ‘clinically silent’ EH. Since clinically silent hydrops reportedly progressed to MD in 33% of the cases [Citation118], MD-dg patients with contralateral ‘clinically silent’ EH also have a high risk of developing BMD.
This pathology-based method will improve treatment strategies. Patients with unilateral ES-hp may be suitable candidates for ablative therapies (e.g. gentamicin injections into the affected ear) if needed. Since a bilaterally impaired audio-vestibular function (bilateral MD) plus a significantly affected quality of life is very likely, caution should be taken in considering ablative therapies for bilateral ES-hp and MD-dg with contralateral hydrops. Caution should be exercised in the decision-making of if to subject MD-hp patient to ES operation, including decompression, shunting, or endolymphatic duct blockage. Since the eES epithelium in the dura of the posterior cranial fossa is either inaccessible due to ES hypoplasia or functionally compromised due to degenerative changes, the surgical procedures for MD, either ES decompression procedure or ES shunting procedure, will not attain their goals as assumed.
They demonstrated that CT/MRI-imaging-based determination of the ATVA enables endotyping of MD patients in terms of ES pathology. This will also further improve the current diagnostic criteria Clinically, MRI and Gd-CT can be employed to identify the respective ES-hp in MD patients. Therefore, the specific radiological feature of the endotype (VA bending angle ≥140◦) may be of value for predictive clinical screening (e.g. in family members of MD-hp patients) for this potentially inheritable inner ear pathology and to predict the risk for MD development. The association between different eES pathologies and different ‘clinical phenotypes’ of MD may render them promising surrogate indicators for distinguishing prognostic subgroups of MD patients in terms of treatment efficacy and the course of the disease.
At this point, we are led to hypothesize the link between the EH and MD as schematically depicted in . Briefly, at the fetal stage, genetic or developmental factors exert their effects on the temporal bone development, resulting in hypoplasia of ES. After birth, intrinsic and extrinsic factors (autoimmune response, allergens, trauma, among others) work, structurally and functionally, on ES, either normal or hypoplastic. Susceptible individuals happen to have disordered endolymph homeostasis and then silent EH results. Silent EH may represent limited or still progressing ES pathological change. The silent EH might precure the emergence of MD or other ear disorders with EH or might remain unchanged.
4. Conclusion
Our review confirms the heterogeneity of MD in terms of symptom, natural course, pathology, and etiology. And its heterogeneity challenges its diagnosis, prognosis and treatment, especially evaluation of the efficacy of treatment. It’s necessary to identify different clinical subgroups with different potential pathogenesis or clinical features. If so, long-term multicenter, randomized, placebo-controlled clinical trials would work on different clinical subgroups of MD.
Successful subtyping of people with MD has important practical implications for clinicians. This work provided a basis for the development of tailored or personalized therapeutic strategies. In particular, knowing the specific subtype of each MD patient at baseline allows one to estimate prognosis and the spectrum of clinical manifestations likely to appear. This can be used to plan treatment protocol, avoid medications with high chance of side effects, and more accurately counsel patients and families. Future clinical trials might also wish to investigate the effects of interventions on each subtype through subgroup analysis, to provide evidence for a more efficient personalized treatment approach. Similarly, it is one step forward in elucidating the underlying mechanisms of the disease by allowing researchers to focus on clinical specific subgroups of the patients.
5. Expert opinion
Meniere’s disease represents one of the most complex and puzzling conditions that presents a great challenge to otolaryngologists worldwide. Clinical data and imaging evidence both indicate that MD is a continuum from mono-symptomatic cases to full-blown disorders [Citation7]. It financially poses a huge burden on both the patients and the society at large and physically impairs the quality of life of the victims. Although a myriad of papers about it are published, so far, its pathogenesis has been poorly understood and no specific and definitively efficacious treatment is available. Its diagnosis, management and the evaluation of therapeutic efficacy are still challenges to clinicians since its clinical features and underlying etiology vary substantially.
On the one hand, our review collects the main findings among the etiology hypothesis, pathology present and clinical features of MD. We also outlined many treatments for MD, including lifestyle change, medical treatment, intratympanic injection medical and surgical therapy. Unfortunately, mainly because the patients are heterogeneous, the evaluation of treatment efficacy is controversial. Recently, accurate diagnosis and personalized treatment have been more and more important. There is sufficient evidence to support subtyping of MD patients. Therefore, it’s rational and necessary to stratify the MD.
On the other hand, this review summarizes the subtyping methods of MD published till now. Because the cohorts of existing studies are different, it is difficult for us to compare and pool these subtypes. So far, MD patients can be divided in terms of clinical features or etiologically (‘bottom-up’ method). Some phenotypes are identified in light of the predominant symptom, laterality of involvement, concomitant migraine, or cardiovascular risk. Clinicians can obtain the information conveniently and quickly by careful history-taking and ordering a few biochemical tests. Several phenotypes are different for they are identified by comprehensive indices using cluster analysis. These phenotypes provide adequate information but some patients might defy categorization. Besides, endotypes are identified by cytokine, genes and ES pathology, for which specific biochemical tests or imaging tests are needed. Identifying endotypes are too expensive and may not be affordable in clinical practice. Researchers are also making efforts to identify the biomarkers of MD. All of these approaches may help select more clinically and genetically homogeneous patients for future clinical and etiological studies, work out better diagnostic tools, facilitate clinical decision-making, predict the prognosis of MD patients. However, some subgroups, as defined by different approaches, may overlap one other and the potential mechanism remains poorly understood. For instance, the patients with increased IL-1β were classified as MD type 1 (83.4%), but type 1 has no specific or unique features. As to migraine, some researchers use migraine diagnosis as a parameter, nevertheless, some use migraine headache.
In order for this to happen, statistical clusters need to be translated into rules to assign each patient to their subtype. Finally, it should be remembered that information in office settings is often relatively limited. Therefore, for clinical application, subtyping solutions need not only be valid but also feasible, cost-efficient, noninvasive, and user-friendly.
5.1. New insights: where are we going?
MD is not a single disease, but a syndrome that involves overlapping auditory, vestibular, and neurovascular symptoms. What seems certain is that all MD patients with MD are more amenable to conservative treatment, such as life-style change, oral medication, and vestibular rehabilitation than to disruptive treatments, including intratympanic gentamicin and surgery. MD subtyping is the emerging direction of diagnosis and treatment in the future. Currently, we are at a stage to definitively subtype the patients with MD. Researchers still have a long way to go in determining which approach works best. Further studies that combine the described subgrouping strategies will determine whether the subgroups defined by the different methods truly overlap and why. Moreover, several factors are worthy of research endeavor, such as allergy, anxiety and depression, sleep quality, and vestibular ossification [Citation8, Citation14, Citation35, Citation46,Citation119–122].
Article highlights
Meniere’s disease is an inner ear disease of unknown etiology with multiple contributing factors, including genetic factor, viral infection, allergy and autoimmunity.
The therapies for MD are various, whereas, their efficacies are controversial. Most RCTs regarding MD were not able to confirm that diuretics, betahistine, intratympanic steroids were, in any way, effective or limited.
There is enough evidence to support a subtype of patients with MD.
So far, patient subtypes in MD can be divided into the clinical features-based method, and etiology-based ‘bottom-up’ method.
MD subtyping is the emerging direction of diagnosis and treatment in the future.
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Menière’s disease. J Vestib Res Equilib Orientat. 2015;25:1–7.
- Hallpike CS, Cairns H. Observations on the pathology of Ménière’s syndrome: (section of otology). Proc R Soc Med. 1938;31:1317–1336.
- Hülse R, Biesdorf A, Hörmann K, et al. Peripheral vestibular disorders: an epidemiologic survey in 70 million individuals. Otol Neurotol. 2019;40:88–95.
- Harris JP, Alexander TH. Current-day prevalence of Ménière’s syndrome. Audiol Neurotol. 2010;15:318–322.
- Shojaku H, Watanabe Y, Fujisaka M, et al. Epidemiologic characteristics of definite Ménière’s disease in Japan. ORL; J Oto-Rhino-Laryngol Relat Specialties. 2005;67:305–309.
- Brandt T, DIeterich M. The dizzy patient: don’t forget disorders of the central vestibular system. Nat Rev Neurol. 2017;13:352–362.
- Pyykkö I, Nakashima T, Yoshida T, et al. Ménière’s disease: a reappraisal supported by a variable latency of symptoms and the MRI visualisation of endolymphatic hydrops. BMJ Open. 2013;3:e001555.
- Kim SY, Lee CH, Min C, et al. Bidirectional analysis of the association between Ménière’s disease and depression: two longitudinal follow‐up studies using a national sample cohort. Clin Otolaryngol. 2020;45:687–694.
- Pearson BW, Brackmann DE. Committee on hearing and equilibrium guidelines for reporting treatment results in Meniere’s disease. Otolaryngol Neck Surg. 1985;93:579–581.
- Surgery N, Monsell M, Balkany TA, et al. Committee on hearing and equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. Otolaryngol Neck Surg. 1995;113:181–185.
- Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Society of Otorhinolaryngology Head and Neck Surgery Chinese Medical Association. Guideline of diagnosis and treatment of Meniere disease (2017). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52(3):167–172.
- Tyrrell JS, Whinney DJD, Ukoumunne OC, et al. Prevalence, associated factors, and comorbid conditions for Ménière’s disease. Ear Hear. 2014;35:e162–e169.
- Greco A, Gallo A, Fusconi M, et al. Meniere’s disease might be an autoimmune condition?. Autoimmun Rev. 2012;11:731–738.
- Derebery MJ. Allergic and immunologic features of Ménière’s disease. Otolaryngol Clin North Am. 2011;44:655–666.
- Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, et al. Accompanying symptoms overlap during attacks in Meniere’s disease and vestibular migraine. Front Neurol. 2014;5:265.
- Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link?. Neurology. 2002;59:1700–1704.
- Von Brevern M, Neuhauser H. Epidemiological evidence for a link between vertigo and migraine. J Vestib Res. 2011;21:299–304. cited 2020 Dec 11.
- Ohmen JD, White CH, Li X, et al. Genetic evidence for an ethnic diversity in the susceptibility to Ménière’s disease. Otol Neurotol. 2013;34:1336–1341.
- Requena T, Espinosa‐Sanchez JM, Cabrera S, et al. Familial clustering and genetic heterogeneity in Meniere’s disease. Clin Genet. 2014;85:245–252.
- Lee JM, Kim MJ, Jung J, et al. Genetic aspects and clinical characteristics of familial meniere’s disease in a South Korean population. Laryngoscope. 2015;125:2175–2180.
- Requena T, Cabrera S, Martin-Sierra C, et al. Identification of two novel mutations in FAM136A and DTNA genes in autosomal-dominant familial Meniere’s disease. Hum Mol Genet. 2015;24:1119–1126.
- Martín-Sierra C, Gallego-Martinez A, Requena T, et al. Variable expressivity and genetic heterogeneity involving DPT and SEMA3D genes in autosomal dominant familial Meniere’s disease. Eur J Hum Genet. 2017;25:200–207.
- Oh EH, Shin J-H, Kim H-S, et al. Rare variants of putative candidate genes associated with sporadic Meniere’s disease in East Asian population. Front Neurol. 2020;10:1424.
- Roman-Naranjo P, Gallego-Martinez A, Soto-Varela A, et al. Burden of rare variants in the OTOG gene in familial Meniere’s disease. Ear Hear. 2020;41:1598–1605.
- Roman-Naranjo P, Moleon MDC, Aran I, et al. Rare coding variants involving MYO7A and other genes encoding stereocilia link proteins in familial meniere disease. Hear Res. 2021;409:108329.
- Williams LL, Lowery HW, Shannon BT. Evidence of persistent viral infection in Meniere’s disease. Arch Otolaryngol - Head Neck Surg. 1987;113:397–400.
- Cotter CS, Singleton GT, Corman LC. Immune-mediated inner ear disease and Parvovirus B19. Laryngoscope. 1994;104:1235–1239.
- Gacek RR. Ménière’s disease is a viral neuropathy. ORL J Otorhinolaryngol Relat Spec. 2009;71(2):78–86.
- Dean NJ, Pastras C, Brown D, et al. Are viral-infections associated with Ménière’s disease? A systematic review and meta-analysis of molecular-markers of viral-infection in case-controlled observational studies of MD. PLoS One. 2019;14:e0225650.
- Dornhoffer JL, Kaufman Arenberg I. Immune mechanisms in Meniere’s syndrome. Otolaryngol Clin North Am. 1997;30:1017–1026.
- Gloddek B, Arnold W. Clinical and Experimental Studies of Autoimmune Inner Ear Disease. Acta Otolaryngol. 2002;122:10–14.
- Teggi R, Battista RA, Di Berardino F, et al. Evaluation of a large cohort of adult patients with ménière’s disease: bedside and clinical history. Acta Otorhinolaryngol Ital. 2020;40:444–449.
- Kim SY, Song YS, Wee JH, et al. Association between Ménière’s disease and thyroid diseases: a nested case–control study. Sci Rep. 2020;10:18224.
- Duke WW. Meniere’s syndrome caused by allergy. AMA. 1923;81:20–22.
- Powers WH. Allergic factors in Meniere’s disease. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:ORL22–9.
- Derebery MJ. Allergic management of Meniere’s disease: an outcome study. Otolaryngol Neck Surg. 2000;122:174–182.
- Foster CA, Breeze RE. The Meniere attack: an ischemia/reperfusion disorder of inner ear sensory tissues. Med Hypotheses. 2013;81:1108–1115. [Internet].
- Yazawa Y, Kitano H, Suzuki M, et al. Studies of cochlear blood flow in Guinea pigs with endolymphatic hydrops. Orl. 1998;60:4–11.
- Lundquist PG, Kimura R, Wersaell J. Experiments in endolymph circulation. Acta Otolaryngol Suppl. 1964;188(SUPPL 188):198+.
- Baloh RW. Harold schuknecht and pathology of the ear. Otol Neurotol. 2001;22:113–122.
- Fiorino F, Pizzini FB, Beltramello A, et al. Reliability of magnetic resonance imaging performed after intratympanic administration of gadolinium in the identification of endolymphatic hydrops in patients with Ménière’s disease. Otol Neurotol. 2011;32:472–477.
- Nakashima T, Naganawa S, Teranishi M, et al. Endolymphatic hydrops revealed by intravenous gadolinium injection in patients with Ménière’s disease. Acta oto-laryngologica. 2010;130(3):338–343.
- Yazawa Y, Kitahara M. Bilateral endolymphatic hydrops in Meniere’s disease: review of temporal bone autopsies. Ann Otol Rhinol Laryngol. 1990;99:524–528.
- Wu H. Endolymphatic hydrops detected by 3-dimensional fluid-attenuated inversion recovery MRI following intratympanic injection of gadolinium in the asymptomatic contralateral ears of patients with unilateral Ménière’s disease. Med Sci Monit. 2015;21:701–707.
- Clemis JD, Valvassori GE. Recent radiographic and clinical observations on the vestibular aqueduct: (A preliminary report). Otolaryngol Clin North Am. 1968;1:339–352.
- Mainnemarre J, Hautefort C, Toupet M, et al. The vestibular aqueduct ossification on temporal bone CT: an old sign revisited to rule out the presence of endolymphatic hydrops in Menière’s disease patients. Eur Radiol. 2020;30:6331–6338.
- Friberg U, Stahle J, Svedberg A. The natural course of Meniere’s disease. Acta Otolaryngol. 1983;96:72–77.
- Gürkov R, Jerin C, Flatz W, et al. Clinical manifestations of hydropic ear disease (Menière’s). Eur Arch Otorhinolaryngol. 2019;276:27–40.
- Rauch SD. Clinical hints and precipitating factors in patients suffering from Meniere’s disease. Otolaryngol Clin North Am. 2010;43:1011–1017.
- Kitahara M. Bilateral aspects of Meniere’s disease: meniere’s disease with bilateral fluctuant hearing loss. Acta Otolaryngol. 1991;111:74–77.
- Tumarkin A. the otolithic catastrophe: a new syndrome. BMJ. 1936;2:175–177.
- Wu Q, Li X, Sha Y, et al. Clinical features and management of Meniere’s disease patients with drop attacks. Eur Arch Oto-Rhino-Laryngology. 2019;276:665–672.
- Shen KC, Young YH. Lermoyez syndrome revisited: 100-year mystery. Acta Otolaryngol. 2018;138:981–986.
- Schmidt PH, Schoonhoven R. Lermoyez’s syndrome a follow-up study in 12 patients. Acta Otolaryngol. 1989;107:467–473.
- Espinosa-Sanchez JM, Lopez-Escamez JA. Menière’s disease. Handb Clin Neurol. 2016;137:257-77. doi: https://doi.org/10.1016/B978-0-444-63437-5.00019-4.
- Ballester M, Liard P, Vibert D, et al. Meniere’s Disease in the Elderly. Otol Neurotol. 2002;23:73–78.
- Hausler R, Toupet M, Guidetti G, et al. Ménière’s disease in children. Am J Otolaryngol. 1987;8:187–193.
- Wang C, Wu C, Cheng P, et al. Pediatric Meniere’s disease. Int J Pediatr Otorhinolaryngol. 2018;105:16–19.
- Espinosa-Sanchez JM, Lopez-Escamez JA. The pharmacological management of vertigo in Meniere disease. Expert Opin Pharmacother. 2020;21:1753–1763.
- Nevoux J, Barbara M, Dornhoffer J, et al. International consensus (ICON) on treatment of Ménière’s disease. Eur Ann Otorhinolaryngol Head Neck Dis [Internet]. 2018;135:S29–S32.
- Gbahou F, Davenas E, Morisset S, et al. Effects of betahistine at histamine H 3 receptors: mixed inverse agonism/agonism in vitro and partial inverse agonism in vivo. J Pharmacol Exp Ther. 2010;334:945–954.
- Murdin L, Hussain K, and Schilder AGM. Betahistine for symptoms of vertigo. Cochrane Database Syst Rev. 2016;(6): CD010696.
- Adrion C, Fischer CS, Wagner J, et al. Efficacy and safety of betahistine treatment in patients with Meniere’s disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. 2016:h6816. DOI:https://doi.org/10.1136/bmj.h6816.
- Crowson MG, Patki A, Tucci DL. A systematic review of diuretics in the medical management of Ménière’s disease. Otolaryngol Neck Surg. 2016;154:824–834.
- Ralli G, Celestino D, Fabbricatore M, et al. Effect of Acetazolamide on Menière’s disease. Acta Otorhinolaryngol Ital. 1989;9(5):503–509.
- Brookes GB, Booth JB. Oral Acetazolamide in Menière’s disease. J Laryngol Otol. 1984;98:1087–1095.
- Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996;115:38–41.
- Garduño-Anaya MA, De Toledo HC, Hinojosa-González R, et al. Dexamethasone inner ear perfusion by intratympanic injection in unilateral Ménière’s disease: a two-year prospective, placebo-controlled, double-blind, randomized trial. Otolaryngol Neck Surg. 2005;133:285–294.
- Silverstein H, Isaacson JE, Olds MJ, et al. Dexamethasone inner ear perfusion for the treatment of Meniere’s disease: a prospective, randomized, double-blind, crossover trial. Am J Otol. 1998;19:196–201.
- Paragache G, Panda NK, Ragunathan M, et al. Intratympanic dexamethasone application in Meniere’s disease - Is it superior to conventional therapy?. Indian J Otolaryngol Head Neck Surg. 2005;57:21–23.
- Lambert PR, Nguyen S, Maxwell KS, et al. A randomized, double-blind, placebo-controlled clinical study to assess safety and clinical activity of OTO-104 given as a single intratympanic injection in patients with unilateral ménière’s disease. Otol Neurotol. 2012;33:1257–1265.
- Lavigne P, Lavigne F, Saliba I. Intratympanic corticosteroids injections: a systematic review of literature. Eur Arch Oto-Rhino-Laryngology. 2016;273:2271–2278.
- Horii A, Kitahara T, Uno A, et al. Vestibular function and vasopressin. Acta Otolaryngol Suppl. 2004;124:50–53.
- Kitahara T, Horii A, Imai T, et al. Does endolymphatic sac decompression surgery prevent bilateral development of unilateral Ménière disease?. Laryngoscope. 2014;124:1932–1936.
- Thomsen J, Per Bonding BB. The Non-specific effect of endolymphatic sac surgery in treatment of Meniere’s disease: a prospective, randomized controlled study comparing “classic” endolymphatic sac surgery with the insertion of a ventilating tube in the tympanic membrane. Acta Otolaryngol. 1998;118:769–773.
- Chung JW, Fayad J, Linthicum F, et al. Histopathology after endolymphatic sac surgery for Meniere’s syndrome. Otol Neurotol. 2011;32:1–7.
- Committee on Hearing and Equilibrium. Report of Subcommittee on Equilibrium and its Measurement. Meniere’s disease: criteria for diagnosis and evaluation of therapy for reporting. Trans - Am Acad Ophthalmol Otolaryngol. 1972;76:1462–1464.
- Kato M, Sugiura M, Shimono M, et al. Endolymphatic hydrops revealed by magnetic resonance imaging in patients with atypical Meniere’s disease. Acta Otolaryngol. 2013;133:123–129.
- Iwasaki S, Shojaku H, Murofushi T, et al. Diagnostic and therapeutic strategies for Meniere’s disease of the Japan Society for Equilibrium Research. Auris Nasus Larynx. 2021;48:15–22.
- Gürkov R, Hornibrook J. On the classification of hydropic ear disease (Menière’s disease). HNO. 2018;66:455–463.
- Chaves AG, Boari L, Munhoz MSL. The outcome of patients with ménière’s disease. Braz J Otorhinolaryngol. 2007;73:346–350.
- Clemmens C, Ruckenstein M. Characteristics of patients with unilateral and Bilateral Ménière’s Disease. Otol Neurotol. 2012;33:1266–1269.
- Orchik DJ, Shea JJ Jr, and Ge NN. Summating potential and action potential ratio in Meniere’s disease before and after treatment. Am J Otol. 1998;19(4):478–482.
- Havia M, Kentala E. Progression of symptoms of dizziness in Ménière’s Disease. Arch Otolaryngol Neck Surg. 2004;130:431.
- Stahle J, Friberg U, Svedberg A. Long-term Progression of Meniere’s Disease. Acta Otolaryngol. 1991;111:78–83.
- Belinchon A, Perez- Garrigues H, Tenias JM, et al. Hearing assessment in Menière’s disease. Laryngoscope. 2011;121:622–626.
- Wang Y, Diao T, Han L, et al. Association of Meniere’s disease and retinal vascular calibre: a prospective observational study in China. BMJ Open. 2018;8:e022069.
- Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Ménière’s disease. Acta Otolaryngol. 2007;127:1241–1245.
- Unlu M, Sevim DG, Gultekin M, et al. Changes in retinal vessel diameters in migraine patients during attack-free period. Int J Ophthalmol. 2017;10:439–444.
- Sarna B, Abouzari M, Lin HW, et al. A hypothetical proposal for association between migraine and Meniere’s disease. Med Hypotheses. 2020;134:1–5.
- Ghavami Y, Haidar YM, Moshtaghi O, et al. Evaluating quality of life in patients with Meniere’s disease treated as migraine. Ann Otol Rhinol Laryngol. 2018;127:877–887.
- Abouzari M, Abiri A, Djalilian HR, et al. Successful treatment of a child with definite Meniere’s disease with the migraine regimen. Am J Otolaryngol. 2019;40:440–442.
- Rego ÂR, Dias D, Pinto A, et al. The cardiovascular aspects of a Ménière’s disease population - A pilot study. J Otol. 2019;14:51–56.
- Montes-Jovellar L, Guillen-Grima F, Perez-Fernandez N. Cluster analysis of auditory and vestibular test results in definite menière’s disease. Laryngoscope. 2011;121:1810–1817.
- Teggi R, Meli A, Trimarchi M, et al. Does Ménière’s disease in the elderly present some peculiar features?. J Aging Res. 2012;2012:1–5.
- Frejo L, Soto-Varela A, Santos-Perez S, et al. Clinical subgroups in bilateral Meniere disease. Front Neurol. 2016;7:1–10.
- Frejo L, Martin-Sanz E, Teggi R, et al. Extended phenotype and clinical subgroups in unilateral Meniere disease: a cross-sectional study with cluster analysis. Clin Otolaryngol. 2017;42:1172–1180.
- Espinosa-Sanchez JM, Lopez-Escamez JA. New insights into pathophysiology of vestibular migraine. Front Neurol. 2015;6:10–15.
- Vergara-Jimenez M, Missimer A, DiMarco DM, et al. Higher prevalence of autoimmune diseases and longer spells of vertigo in patients affected with familial Ménière’s disease: a clinical comparison of familial and sporadic Ménière’s disease. EC Nutr. 2014;1:164–173.
- Crossley J, Hussaini AS, Kim HJ, et al. Ménière’s disease clinical subtypes in a population from the USA. J Laryngol Otol. 2020;134:24–28.
- Kim H-J, Lee H-J, An S-Y, et al. Analysis of the prevalence and associated risk factors of tinnitus in adults. Chen L, editor. PLoS One. 2015;10:e0127578.
- Bruderer SG, Bodmer D, Stohler NA, et al. Population-based study on the epidemiology of Ménière’s disease. Audiol Neurotol. 2017;22:74–82.
- Lee HJ, Lee JM, Shim DB, et al. Is early progression to bilateral involvement in Menière’s disease a poor prognostic indicator?. Otol Neurotol. 2019;40:1333–1338.
- Enander A, Stahle J. Hearing in Menière’s disease: a study of pure-tone audiograms in 334 patients. Acta Otolaryngol. 1967;64:543–556.
- Frejo L, Gallego-Martinez A, Requena T, et al. Proinflammatory cytokines and response to molds in mononuclear cells of patients with Meniere disease. Sci Rep. 2018;8:5974.
- Moleon MDC, Martinez-Gomez E, Flook M, et al. Clinical and cytokine profile in patients with early and late onset meniere disease. J Clin Med. 2021;10:4052.
- Flook M, Escalera-Balsera A, Gallego-Martinez A, et al. Dna methylation signature in mononuclear cells and proinflammatory cytokines may define molecular subtypes in sporadic meniere disease. Biomedicines. 2021;9:1530.
- Frejo L, Giegling I, Teggi R, et al. Genetics of vestibular disorders: pathophysiological insights. J Neurol. 2016;263:45–53.
- Gallego-Martinez A, Lopez-Escamez JA. Genetic architecture of Meniere’s disease. Hear Res. 2020;397:107872.
- Martín-Sierra C, Requena T, Frejo L, et al. A novel missense variant in PRKCB segregates low-frequency hearing loss in an autosomal dominant family with Meniere’s disease. Hum Mol Genet. 2016;25:3407–3415.
- Mehrjoo Z, Kahrizi K, Mohseni M, et al. Limbic system associated membrane protein mutation in an Iranian family diagnosed with ménière’s disease. Arch Iran Med. 2020;23:319–325.
- Teggi R, Zagato L, Delli Carpini S, et al. Genetics of ion homeostasis in Ménière’s Disease. Eur Arch Oto-Rhino-Laryngology. 2017;274:757–763.
- Eckhard A, Gleiser C, Arnold H, et al. Water channel proteins in the inner ear and their link to hearing impairment and deafness. Mol Aspects Med. 2012;33:612–637.
- Eckhard AH, Zhu M, O’Malley JT, et al. Inner ear pathologies impair sodium-regulated ion transport in Meniere’s disease. Acta Neuropathol. 2019;137:343–357.
- Bächinger D, Luu N, Kempfle JS, et al. Vestibular aqueduct morphology correlates with endolymphatic sac pathologies in Menière’s disease—A correlative histology and computed tomography study. Otol Neurotol. 2019;40:e548–e555.
- Bächinger D, Brühlmann C, Honegger T, et al. Endotype-phenotype patterns in Meniere’s disease based on gadolinium-enhanced MRI of the vestibular aqueduct. Front Neurol. 2019;10:1–11.
- Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache J Head Face Pain. 2013;53:427–436.
- House JW, Doherty JK, Fisher LM, et al. Meniere’s disease: prevalence of contralateral ear involvement. Otol Neurotol. 2006;27:355–361.
- Di Berardino F, Cesarani A. Gluten sensitivity in Meniere’s disease. Laryngoscope. 2012;122:700–702.
- Sen P, Georgalas C, Papesch M. Co-morbidity of migraine and Ménière’s disease – is allergy the link?. J Laryngol Otol. 2005;119:455–460.
- Derebery MJ, Berliner KI. Prevalence of allergy in Meniere’s disease. Otolaryngol Neck Surg. 2000;123:69–75.
- Kim SK, Kim JH, Jeon SS, et al. Relationship between sleep quality and dizziness. Veauthier C, editor. PLoS One. 2018;13:e0192705.