ABSTRACT
Objectives
Headache disorders are a common cause of disability and reduced health-related quality of life globally. Growing evidence supports the use of cannabis-based medicinal products (CBMPs) for chronic pain; however, a paucity of research specifically focuses on CBMPs’ efficacy and safety in headache disorders. This study aims to assess changes in validated patient-reported outcome measures (PROMs) in patients with headaches prescribed CBMPs and investigate the clinical safety in this population.
Methods
A case series of the UK Medical Cannabis Registry was conducted. Primary outcomes were changes from baseline in PROMs (Headache Impact Test-6 (HIT-6), Migraine Disability Assessment (MIDAS), EQ-5D-5L, Generalized Anxiety Disorder-7 (GAD-7) questionnaire and Single-Item Sleep Quality Scale (SQS)) at 1-, 3-, and 6-months follow-up. P-values <0.050 were deemed statistically significant.
Results
Ninety-seven patients were identified for inclusion. Improvements in HIT-6, MIDAS, EQ-5D-5L and SQS were observed at 1-, 3-, and 6-months (p < 0.005) follow-up. GAD-7 improved at 1- and 3-months (p < 0.050). Seventeen (17.5%) patients experienced a total of 113 (116.5%) adverse events.
Conclusion
Improvements in headache/migraine-specific PROMs and general health-related quality of life were associated with the initiation of CBMPs in patients with headache disorders. Cautious interpretation of results is necessary, and randomized control trials are required to ascertain causality.
1. Introduction
Headache disorders, such as migraine, tension-type headache, and cluster headache, are a common cause of disability and reduced quality of life globally [Citation1,Citation2]. Migraine is the 2nd most common neurological condition, affecting 15% of the United Kingdom (UK) population [Citation3], with females being more commonly affected [Citation4,Citation5]. The International Classification of Headache Disorders defines migraines as attacks lasting 4–72 hours which typically manifest as unilateral, pulsating, and severe headache pain often associated with nausea, vomiting, photophobia, and phonophobia [Citation6]. Symptoms of headache disorders can render patients physically, socially, and psychologically incapacitated [Citation7] and the World Health Organization ranks headaches in the top 10 global causes of disability [Citation8]. Additionally, headaches have a considerable burden on health services globally [Citation9,Citation10] and are responsible for high economic impact [Citation11].
Current migraine management includes a combination of acute and preventative pharmacological therapies [Citation12,Citation13]. Acute therapies include non-steroidal anti-inflammatory drugs, paracetamol and, for more severe attacks, triptans [Citation12]. Patients suffering from frequent migraine attacks may require additional preventative therapies, including repurposed medications such as beta-blockers, tricyclic antidepressants, and anticonvulsants [Citation12]. However, these regimes are associated with unpredictable effectiveness, with only one in three patients reporting symptom improvement [Citation14]. Moreover, side effects like fatigue, rashes, dizziness, weight gain, and constipation lend to their poor tolerability [Citation15]. Furthermore, due to frequent requirement for analgesia, patients may subsequently suffer from medication overuse headache (MOH) in the attempt to alleviate their migraine, resulting in a vicious circle of headache symptoms [Citation16,Citation17]. In recent years, new therapies have been introduced for migraine, including onabotulinumtoxin A injections, and monoclonal antibodies targeting calcitonin gene-related peptide (CGRP) or its receptor [Citation12]; whilst effective for many patients, these treatments are expensive, and access to them remains limited [Citation12]. Therefore, a clear demand remains to develop novel therapeutics.
The endocannabinoid system (ECS) influences a variety of physiological processes including pain signaling pathways and has been identified as a target for novel therapeutics for primary headache disorders [Citation18–20]. The main receptors of the ECS are cannabinoid receptor type 1 (CB1), a G-protein coupled receptor principally located in the brainstem, prefrontal cortex, cingulate cortex and amygdala, and cannabinoid receptor type 2 (CB2), a G-protein coupled receptor principally located in peripheral immune cells [Citation20]. Anandamide and 2-arachidonoylglycerol (2-AG) are two key endocannabinoids that act as retrograde or local neurotransmitters through interactions with CB receptors [Citation21]. Anandamide is a CB1 partial agonist and is degraded by fatty acid amide hydrolase (FAAH), whilst 2-AG is a CB1 and CB2 full agonist and is degraded by monoacylglycerol lipase [Citation18]. The binding of these endocannabinoids regulates γ-aminobutyric acid (GABA) and glutamate release in nociceptive pathways [Citation22]. Translational research findings suggest that modulation of the ECS may affect clinical outcomes in headache disorders [Citation23–25].
Cannabis-based medicinal products (CBMPs) contain over 100 phytocannabinoids which act as ligands for CB1/2 receptors [Citation21,Citation26]. (−)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the two most abundant phytocannabinoids within the cannabis plant [Citation26]. Δ9-THC is a partial agonist of CB1/2 receptors [Citation21]. Comparatively, CBD’s primary mechanism of action is to inhibit the breakdown of anandamide at synapses, therefore keeping anandamide constitutively active [Citation21].
There is growing evidence of the role of CBMPs in the setting of chronic pain, including headache disorders [Citation27]. Real-world evidence which has assessed pharmaceutical-grade and other preparations of cannabis, supports a potential benefit of using cannabis-based products for headache disorders [Citation28–30]. In 2012, the first single-centered, cross-over trial demonstrated that 30 patients with MOH experienced reduced headache pain duration and consumed less daily analgesia during an 8-week trial of nabilone, a synthetic Δ9-THC mimic, compared to ibuprofen [Citation31]. These findings are mechanistically corroborated by pre-clinical studies [Citation32–36]. Additionally, CBMPs have been deemed clinically safe, with few or no reports of severe and life-threatening adverse events (AEs) [Citation29,Citation30,Citation37]. Already, CBMPs are being utilized in an off-label manner for the treatment of severe psychiatric disorders such as post-traumatic stress disorder [Citation38]. Notably, unlike tightly controlled CBMPs, illicit cannabis, in particular high potency skunk, has been associated with negative effects on existing psychiatric disorders [Citation39–41].
Existing studies investigating the use of CBMPs in headaches are primarily observational, small-scale, and retrospective [Citation42]. They frequently analyze outcomes from individuals who consume recreational cannabis which lack the regulations, and therefore consistency, of CBMPs [Citation28]. Furthermore, their outcomes have been inconclusive, owing to small sample sizes [Citation31] and significant heterogeneity due to variations in formulation, routes of administration and concentrations of constitute cannabinoids [Citation30]. Additionally, a paucity of research exists on the use of CBMPs in headache disorders specifically; instead, their use has been studied under the umbrella of chronic pain disorders [Citation27]. Lastly, studies often focus on headache-specific measures such as the number of days acute medications were required [Citation29,Citation30], which may not reflect the most valuable outcomes to patients, such as health-related quality of life (HRQoL) [Citation43].
In the UK, individuals with headache disorders may only be prescribed unlicensed CBMPs by consultant neurologists, if they have undertaken a satisfactory trial of previously licensed therapies and have failed to achieve sufficient clinical benefit [Citation44]. Each individual’s case must be reviewed by a multidisciplinary team to ensure that a prescription is suitable according to all physical and mental health requirements. Finally, all CBMPs must be manufactured to Good Manufacturing Practice criteria to ensure they meet the safety requirements outlined in the British Pharmacopeia [Citation44]. The UK Medical Cannabis Registry (UKMCR) was established in December 2019 and is privately owned by Sapphire Medical Clinics. The registry longitudinally captures data and outcomes of patients from the UK and the Channel Islands being treated with CBMPs for several medical conditions, including headache disorders.
This study aims to fill an evidence gap by examining real-world evidence from the largest prospectively enrolled case series of UK patients prescribed CBMPs for headache disorders. The primary aims of this study were to assess changes in validated patient-reported outcome measures (PROMs). Supplementary aims included analysis of AE frequency and dosage regimes.
2. Methods
2.1. Study design
This study reports a case series of patients diagnosed with a primary headache disorder, enrolled in the UKMCR. Participants were consecutively enrolled into the registry after they provided fully informed, written consent. Patients completed electronically administered questionnaires at baseline, 1-month, 3-months, and 6-months to record PROMs and AEs.
This study conforms to the STROBE statement for reporting observational studies [Citation45]. In line with NHS Health Research Authority and Research Ethics Committee’s guidance, this study was considered to not require formal ethical approval.
2.2. Settings and participants
The UKMCR was established in December 2019 and is a patient registry owned by Sapphire Medical Clinics which longitudinally captures pseudonymized data of patients prescribed CBMPs in the UK and the Channel Islands. The registry collates data on indication for CBMP treatment, patient demographics, PROMs, and AEs.
CBMPs in accordance with Good Manufacturing Practice were prescribed by specialists ensuring quality, consistency, and compliance with standards set by the Medicines and Healthcare products Regulatory Agency (MHRA) [Citation46]. It is unlikely patients were using medical grade cannabis/CBMP products prior to enrollment.
Patients were included if they were ≥18 years old and had been commenced on CBMP therapy with the intention to treat a headache disorder. Patients were excluded if they had not completed a baseline PROMs assessment or had been enrolled in the UKMCR <1-month prior to data extraction. No other reasons for exclusion were used. Patients who were consuming non-prescribed cannabis at baseline were additionally counseled to cease doing so. Data were extracted on 15 February 2022.
2.3. Outcomes of interest
Patient demographic details, including age, gender, and occupation were recorded. Additionally, patient body mass index (BMI) was calculated. The incidence of relevant co-morbidities was recorded, including anxiety/depression, arthritis, endocrine dysfunction, epilepsy, hypertension, and venous thromboembolic disease. Moreover, a Charlson comorbidity index score, a widely used prognostic scoring tool to measure health status in population studies [Citation47], was calculated for each patient.
Drug and alcohol data was captured and analyzed, including smoking status, smoking pack years, alcohol consumption, cannabis status and cannabis gram years. Gram years is a metric to assess lifetime cannabis consumption developed by our group. It is calculated as the average cannabis consumption per 24 hours, multiplied by years of use [Citation48].
Patients’ medication data was recorded, including drug names, drug dosages per 24 hours, and prescriptions’ start/end dates. Medication names were mapped to SNOMED CT codes to ensure consistent terminology [Citation49]. Opioid medications were converted to oral morphine equivalents (OMEs) using conversion factors from the British National Formulary [Citation50].
Primary, secondary, and tertiary indications for which CBMPs had been prescribed were recorded by the treating physician. Patient records were linked with prescription data, including manufacturing company, formulation, route of administration and dose of Δ9-THC and CBD. Dose of each cannabinoid was calculated as the concentration of cannabinoid (mg/g) multiplied by the prescribed dose of dried flower per 24 hours.
2.3.1. Patient-reported outcome measures
depicts and describes the PROMs which were measured at baseline, and at 1-, 3-, and 6-months follow-up, including the 6-item headache impact test (HIT-6) [Citation51], migraine disability assessment score (MIDAS) [Citation52], EQ-5D-5L [Citation53], generalized anxiety disorder-7 questionnaire (GAD)[Citation54] and single-item sleep quality scale (SQS) [Citation55]. Additionally, defines the patients’ global impression of change (PGIC-1/2) [Citation56].
Table 1. Displays and describes the patient-reported outcome measures (PROMs) measured at baseline, and at 1-, 3-, and 6-months follow-up.
2.3.2. Adverse events
AEs were self-reported by patients at follow-up assessments or inputted following a clinical encounter. They were reported and analyzed in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0[Citation57].
2.4. Outcome measures
Primary outcomes were changes from baseline in PROMs (HIT-6, MIDAS, EQ-5D-5L, GAD-7 questionnaire and SQS) at 1-, 3-, and 6-months compared to baseline. Sub-group analyses were also conducted according to prior cannabis exposure for these outcome measures. Median PGIC-1/2 scores at 1-, 3-, and 6-months were also analyzed. Secondary outcomes included analysis of the incidence and severity of reported AEs.
2.5. Statistical methods
Demographic variables, drug and alcohol use, medication data and AEs were analyzed using descriptive statistics. Data from PROMs were independently analyzed by comparing measurements recorded at 1-, 3-, and 6-months to the baseline independently to minimize the effects of missing data values during follow-up. Normality was assessed using the Shapiro–Wilk test. Thereafter, parametric data were presented as mean (±standard deviation (SD)), whilst non-parametric data were presented as median (interquartile range (IQR)). Statistical analysis was performed with a paired Student’s t-test or Wilcoxon rank-sum test for parametric and non-parametric data, respectively. Data were analyzed using SPSS (version: 28.0.0.0 SPSS Inc., [New York, IL], USA), and figures were created using GraphPad Prisma (version: 9.3.1(350) for macOS, GraphPad Software Inc., [San Diego, CA], USA). P-values <0.050 were deemed statistically significant.
3. Results
Preliminary data extraction from the UKMCR yielded 3546 patients. After exclusion of patients who had not completed a baseline PROM, not received CBMPs for >1-month or did not have a primary diagnosis of headache disorder, 97 patients remained for final analysis. Of these, PROMs had been recorded for 81 patients at 1-month, 63 patients at 3-months and 35 patients at 6-months.
3.1. Patient data
The mean age of patients was 37.9 (±11.1) years, and the female-to-male ratio was 1:1.3. The mean BMI of patients was 28.0 (±7.0) kg/m2. The most common occupation was ‘professional’ (n = 14; 14.4%), but more patients were unemployed (n = 23; 23.7%) ().
Table 2. Patient baseline demographic data (n = 97).
Baseline drug and alcohol data analysis revealed that 36.1% (n = 35), 41.2% (n = 40), and 22.7% (n = 22) of patients had never smoked, were ex-smokers, and were current smokers, respectively. The median smoking pack years was 6.0 (2.0–14.0). At the time of enrollment, most patients were current cannabis consumers (n = 54; 55.7%), nearly half of these patients consumed cannabis every day (n = 42; 43.3%). Fewer patients were ex-consumers of cannabis (n = 17; 17.5%) or were cannabis naïve (never consumed cannabis in the past) (n = 26; 26.8%). The cohort’s median cannabis gram years was 5.0 (1.0–20.0), with a median consumption of 1.0 (0.3–1.5) grams per day. The median alcohol consumption was 0.0 (0.0–3.0) units per week, and 88.5% (n = 86) of patients consumed ≤5 units per week.
Most patients had a primary indication of migraine (n = 82; 84.5%), followed by cluster headache (n = 9; 9.3%), and tension-type headache (n = 6; 6.2%). Anxiety was the most common secondary indication (n = 16; 16.5%), followed by insomnia and chronic pain (n = 7; 7.2%), and depression (n = 4; 4.1%) ().
Table 3. Primary, secondary, and tertiary indication for patients included in the analysis (n = 97).
The median Charlson comorbidity index was 0.0 (0.0–0.0), with 79.4% (n = 77) of patients scoring 0.0% and 8.3% (n = 8) of patients scoring >3.0. Arthritis was the most common comorbidity recorded (n = 7; 7.2%), followed by hypertension (n = 4; 4.1%), and uncomplicated diabetes (n = 4; 4.1%).
3.2. CBMP dosing and mode of administration
The median dose of CBD and THC per 24 hours was 20.0 (5.0–40.0) mg and 120.0 (53.6–204.1) mg, respectively. Twenty-five (25.8%) patients were prescribed vaporized flower (flos) only, 19 (19.6%) patients were prescribed oral/sublingual administration (oils) only and 51 (52.6%) patients were prescribed both. Two (2.1%) patients had missing CBMP data. In patients prescribed both oils and flos, the median CBD and THC dose of oils were 20.0 (4.5–30.0) mg and 20.0 (10.0–20.0) mg, respectively and for flos were 5.0 (0.0–5.0) mg and 100.0 (100.0–195.0) mg, respectively. The most common CBMP therapies were Adven® 20 THC oil, Adven® 50 CBD oil, Adven® EMT1 19% THC flower (Curaleaf International, Guernsey, UK).
3.3. PROMs analysis
Median HIT-6 scores decreased at 1- [n = 75; 66.0 (62.0–71.0) vs 62.0 (57.0–67.0); p < 0.001], 3- [n = 60; 66.5 (62.0–71.0) vs 59.5 (52.0–68.0); p < 0.001], and 6-months [n = 33; 66.0 (62.0–71.0) vs 59.0 (52.0–66.0); p < 0.001] follow-up, respectively (). Median MIDAS scores decreased at 1- [n = 75; 44.0 (12.0–118.0) vs 26.0 (6.0–102.0); p < 0.001], 3- [n = 60; 47.5 (14.3–121.0) vs 20.0 (4.0–70.0); p < 0.001], and 6-months [n = 33; 46.0 (9.0–146.0) vs 21.0 (3.5–69.5); p = 0.004] follow-up also ().
Figure 1. A) HIT-6 and b) MIDAS scores at baseline and follow-up measures at 1-, 3-, and 6-months in patients with headache disorders prescribed cannabis-based medicinal products. *<0.050; **<0.010; ***<0.001; HIT-6: 6-item headache impact test; MIDAS: migraine disability assessment score.
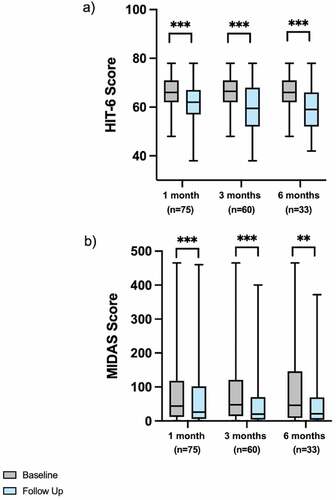
The proportion of patients’ reporting >2.5-point reduction in HIT-6 scores was 61.3% (n = 46/75), 71.7% (n = 43/60), and 66.7% (n = 22/33) at 1-, 3-, and 6-months, respectively. The proportion of patients’ who had >5.0-point reduction in MIDAS scores was 57.3% (n = 43/75), 66.7% (n = 40/60), and 63.6% (n = 21/33) at 1-, 3-, and 6-months, respectively.
Sub-group analysis according to prior cannabis exposure demonstrated that there were significant reductions in HIT-6 scores at 1- and 3-months follow-up for all sub-groups (p < 0.05), but only the sub-group of current cannabis consumers had a significant reduction in HIT-6 at 6-months follow-up (p < 0.001). There were significant reductions in MIDAS scores at 1-month for the cannabis naïve and current cannabis consumer sub-groups (p < 0.05), but only the sub-group of current cannabis consumers had a significant reduction in MIDAS at 3-months (p < 0.01). There was no significant change in MIDAS for ex-cannabis consumers at any follow-up point (p > 0.05). Full details of the sub-analysis are displayed in Supplementary Table 1.
depicts paired baseline and follow-up scores at 1-, 3-, and 6-months for EQ-5D-5L index values and EQ-5D-5L domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Median EQ-5D-5L index values increased at 1- [n = 79; 0.70 (0.34–0.80) vs 0.77 (0.57–0.84); p < 0.001], 3- [n = 62; 0.70 (0.34–0.80) vs 0.74 (0.53–0.84); p = 0.016], and 6-months [n = 34; 0.70 (0.48–0.82) vs 0.75 (0.65–0.85); p = 0.009] follow-up. Median EQ-5D-5L pain/discomfort domain scores decreased at 1- [n = 79; 3.0 (2.0–4.0) vs 2.0 (2.0–3.0); p < 0.001], 3- [n = 62; 3.0 (2.0–4.0) vs 2.0 (2.0–3.0); p < 0.001], and 6-months [n = 34; 3.0 (2.0–4.0) vs 2.0 (1.8–3.0); p = 0.006] follow-up. Additionally, statistically significant improvements were observed in the usual activity domain [n = 79; 2.0 (1.0–3.0) vs 2.0 (1.0–2.0); p = 0.013] and the anxiety/depression domain [n = 79; 2.0 (1.0–3.0) vs 2.0 (1.0–3.0); p < 0.001] at 1-month compared to baseline.scores decreased at 1- [n = 79; 3.0 (2.0–4.0) vs 2.0 (2.0–3.0); p < 0.001], 3- [n = 62; 3.0 (2.0–4.0) vs 2.0 (2.0–3.0); p < 0.001], and 6-months [n = 34; 3.0 (2.0–4.0) vs 2.0 (1.8–3.0); p = 0.006] follow-up. Additionally, statistically significant improvements were observed in the usual activity domain [n = 79; 2.0 (1.0–3.0) vs 2.0 (1.0–2.0); p = 0.013] and the anxiety/depression domain [n = 79; 2.0 (1.0–3.0) vs 2.0 (1.0–3.0); p < 0.001] at 1-month compared to baseline.
Table 4. Paired baseline and follow-up scores for EQ-5D-5L measures at 1-, 3-, and 6-months in patients with headache disorders prescribed cannabis-based medicinal products.
Sub-group analysis according to prior cannabis exposure demonstrated that there were significant improvements in EQ-5D-5L index value at 1-month follow-up for the cannabis naïve and current consumer of cannabis sub-groups (p < 0.01) and at 6-months for the current consumer of cannabis sub-group (p < 0.01). There were no other significant changes in the EQ-5D-5L index value according to prior cannabis use sub-groups (p > 0.05). Full details of the sub-analysis are displayed in Supplementary Table 1.
Median GAD-7 scores significantly improved at 1- [n = 81; 5.0 (2.0–10.5) vs 4.0 (1.0–7.0); p < 0.001] and 3-months [n = 63; 5.0 (2.0–9.0) vs 4.0 (1.0–6.0); p < 0.001] follow-up. At 6-months there was no change in GAD-7 score [n = 35; 4.0 (1.0–10.0) vs 4.0 (2.0–8.0); p = 0.239] (). Median SQS scores improved at 1- [n = 78; 4.0 (3.0–7.0) vs 7.0 (4.0–8.0); p < 0.001], 3- [n = 61; 4.0 (3.0–7.0) vs 7.0 (6.0–9.0); p < 0.001], and 6-months [n = 33; 4.0 (3.0–7.0) vs 6.0 (4.0–8.0); p = 0.002] follow-up ().
Figure 2. a) GAD-7 and b) SQS scores at baseline and follow-up measures at 1-, 3-, and 6-months in patients with headache disorders prescribed cannabis-based medicinal products. ns: no statistically significant difference; *<0.050; **<0.010; ***<0.00. GAD-7: generalised anxiety disorder-7 questionnaire; SQS: single-item sleep quality scale
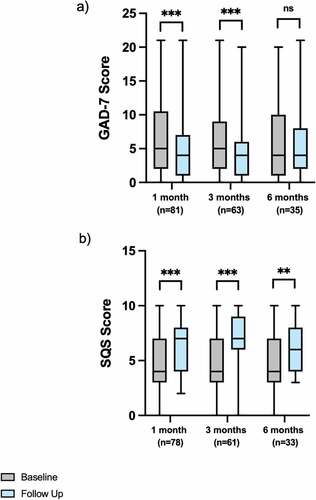
Sub-group analysis according to prior cannabis exposure demonstrated that there were significant reductions in GAD at 1-month for the cannabis naïve and current cannabis consumer sub-groups (p < 0.05). Additionally, there were significant reductions in GAD at 3-months follow-up for the cannabis naïve and ex-consumer of cannabis sub-groups (p < 0.05). There were no other significant changes in GAD in the sub-group analysis (p > 0.05). There were significant reductions in SQS at 1-month for the cannabis naïve and current cannabis consumer sub-groups (p < 0.05), and all three sub-groups had a significant decrease in SQS at 3-months follow-up (p < 0.05), but only the current cannabis consumers sub-group had significant improvements at 6-months (p < 0.01). Full details of the sub-analysis are displayed in Supplementary Table 1.
depicts the full statistical analysis of HIT-6, MIDAS, EQ-5D-5L, GAD-7, and SQS at 1-, 3-, and 6-months follow-up.
Table 5. Paired baseline and follow-up scores for HIT-6, MIDAS, GAD-7, SQS, and EQ-5D-5L measures at 1-, 3-, and 6-months in patients with headache disorders prescribed cannabis-based medicinal products.
At 1-,3- and 6-months follow-up median PGIC-1 scores were 6.0 (n = 78; 5.0–7.0), 6.0 (n = 63; 5.0–7.0), and 6.0 (n = 35; 6.0–7.0), respectively and PGIC-2 scores were 2.0 (n = 78; 1.0–3.0), 2.0 (n = 63; 1.0–3.0) and 2.0 (n = 35; 1.0–3.0), respectively. At 1- and 3-months 80.8% (n = 63/78) and 93.6% (n = 59/63) of patients rated PGIC-1 ≥ 5, respectively.
3.4. Oral morphine equivalent (OME) analysis
Ten (10.3%) patients had records of regularly prescribed opioid-containing medications, but 2 (20.0%) of these patients were no longer prescribed these medications at the time CBMP therapy was commenced. Post CBMP treatment commencement, 1 (12.5%) patient had a reduction in OME from 24.0 mg to 6.0 mg, but all other patients (n = 7; 87.5%) did not have a change in OME doses. There was no change in OME doses between baseline and end of follow-up after patients were commenced on CBMP [n = 8; 24.0 (24.0–36.0) mg vs 24.0 (24.0–36.0) mg; p = 0.317].
3.5. Adverse events
Seventeen (17.5%) patients experienced a total of 113 (116.5%) AEs (). Of these 63 (64.9%) were classified as mild, 39 (40.2%) as moderate and 11 (11.3%) as severe. The most common AEs were dry mouth (n = 11; 11.3%), headache (n = 11; 11.3%), fatigue (n = 8; 8.2%), and concentration impairment (n = 8; 8.2%). Two (2.1%) patients reported life-threatening/disabling AEs of fatigue (n = 1; 1.0%) and headache (n = 1; 1.0%). However, in accordance with CTCAE version 4.0[Citation57], these AEs were downgraded to ‘severe.’
Table 6. Adverse Events reported by patients prescribed cannabis-based medicinal products for headache disorders (n = 97).
4. Discussion
This case series examined PROMs, general HRQoL metrics and AEs to evaluate the efficacy and safety of prescribed CBMPs for patients in the UK with headache disorders. Overall, results suggest that initiation of CBMP therapy was associated with improvements in validated headache- and migraine-specific PROMs and certain general HRQoL metrics, demonstrated by sustained improvements at 6-months in HIT-6, MIDAS and EQ-5D-5L index values.
There were statistically significant improvements in HIT-6 and MIDAS scores at all follow-up points compared to baseline. These results echoed previous findings by Cuttler et al.’s retrospective observational study, whereby Canadian participants with headache disorders reported a decrease in headache and migraine severity of nearly 50% after consuming inhaled medical cannabis [Citation30]. Results in the present analysis were further corroborated in a retrospective study which investigated 121 patients with migraine across two medical cannabis specialty clinics in Colorado [Citation29] where patients reported a decrease in migraine frequency from 10.4 to 4.6 per month at follow-up assessment. However, unlike this study, these analyses did not assess changes in clinical outcomes by using validated PROMs; therefore, it is difficult to compare the magnitude of efficacy between these cohorts. Mirroring results reported here, over half of the patient cohort in Rhyne et al.’s study were lost to follow-up, which may have contributed to an attrition bias and have led to an overestimation of the effect size in both studies [Citation29]. However, individuals in Rhyne et al.’s study consumed medical cannabis via inconsistent routes of administration, varying product formulae, and the daily frequency of consumption was not recorded, which potentially confounded the results [Citation29]. In contrast, in this study, healthcare and CBMP prescriptions were delivered in compliance with standards set by MHRA and other regulatory bodies [Citation46].
More recently, Baraldi et al. conducted a retrospective study of 32 Italian patients with chronic migraine where three oral cannabinoid preparations (Bediol, Bedrocan, and FM2) were prescribed [Citation42]. They reported a reduction of headache pain intensity at 3- and 6-months follow-up compared to baseline; however, the number of migraines per month did not decrease. From the present study, it is unclear if the improvements in HIT-6 and MIDAS were uniquely a result of a decreased pain intensity or is attributed to a reduced migraine frequency. Regardless, headache-specific PROMs are a gold-standard measure of headache/migraine burden, which encompass many different components; thus, this study builds on previous studies by providing a more holistic and validated summary of CBMPs’ effect on HRQoL [Citation58].
The associated changes in headache- and migraine-specific PROMs reached clinically important thresholds. At all follow-up points, PGIC-1 scores reflected ‘a definite improvement’ that had made a ‘real and worthwhile difference’ and PGIC-2 scores indicated a ‘good’ degree of change since beginning care at the clinic. Furthermore, the minimal clinically important changes in HIT-6 and MIDAS are −2.5[Citation59] and −5.0[Citation60] points, respectively. At 3-months follow-up, 71.7% and 66.7% of patients had exceeded the minimally clinically important decrease in HIT-6 and MIDAS, respectively. This proportion was higher than a study which previously reported that 53% of patients receiving lasmiditan for migraines had a minimally clinically important decrease in MIDAS at 3-months follow-up [Citation61]. Furthermore, a recent study reported that only 58.1% of the patient cohort would continue taking triptans for acute relief of their migraines, with the leading reason for patient dissatisfaction being that patients did not consider the regimes to be working well [Citation62]. In this study, a higher proportion of clinical response and patient satisfaction is notable, especially considering that CBMPs may only be prescribed in the UK for patients refractory to first-line therapies, such as triptans. These encouraging results mandate further evaluation with randomized controlled trials to assess if CBMPs are the causative agent for the associated change rather than regression to the mean.
Previous studies have reported that many patients with headache disorders are already self-treating with cannabis products [Citation63,Citation64]. A similar trend was identified in the baseline demographic data from the present study where, in comparison to the general population [Citation65], a larger number of patients were current and daily cannabis consumers. Sub-group analysis according to prior cannabis exposure demonstrated that there were differences in reported PROMs at 1-, 3-, and 6-months follow-up between sub-groups compared to baseline. However, these differences were inconsistent, and no specific trend was identified. Importantly, the present study demonstrated that even when patients had consumed cannabis regularly, they still benefited from switching to or addition of CBMP therapy. This implies that access to consistent, safe CBMPs under the supervision of medical care may provide additional benefits. However, in the present study, the large proportion of participants who were consuming cannabis at the time of enrollment potentially confounded results due to influences such as tolerance [Citation30]. Moreover, the cohort is self-selected introducing a selection bias because patients may have anticipated the same beneficial experience of illicit cannabis with CBMP therapy, thus, improvements have perhaps been overstated. This theoretical limitation is supported by the results as cannabis naïve patients had less pronounced reductions in headache/migraine-specific PROMs.
Results also indicated associated improvements in other HRQoL metrics, including sleep-quality, anxiety, and EQ-5D-5L index values. These findings are consistent with other clinical studies [Citation37,Citation66,Citation67] and the longer follow-up reported in this study elucidates a sustained association with improved HRQoL. However, in this study, GAD-7 scores were ≤5 at baseline, signifying ‘mild’ anxiety symptoms, and the median GAD-7 change did not reach the estimated minimal clinically important difference of −3.3[Citation68] which collectively suggests changes were not meaningful. Moreover, extensive loss to follow-up reported here could disguise potential worsening anxiety and depression symptoms, which have been reported especially with high-dose cannabinoid consumption [Citation69]. Likewise, continual consumption of cannabis products has been associated with habituation to its sleep-inducing properties and other harmful impacts on sleep [Citation70]. Thus, it is necessary to assess the longer-term effect as well as the consequences of withdrawal from CBMPs to clarify the benefits reported here.
The present study revealed no change in OME consumption after patients commenced CBMP treatment. This contradicts findings in Baraldi et al.’s [Citation42] retrospective study of patients with chronic migraine. However, this may be because the database did not capture data from ‘pro re nata’ prescriptions and so OMEs were only available for 11 patients which does not represent the true proportion of patients taking acute medications. Importantly, other studies which report significant changes in medication consumption specifically measure acute medication utilization.
AEs were experienced by 17 patients (17.5%), totaling 113 incidences (116.5%), with the majority being classified as mild. This patient proportion is marginally smaller than has been reported in a similar study [Citation48]. It is understood that the proportions of THC, CBD, and other active pharmaceutical ingredients can influence the AEs profile of CBMPs [Citation5] and so it is possible that these discrepancies are explained by variations in formulations and dosages between studies. Regardless, the true incidence of AEs may be overstated because they may have occurred in periods where patients’ CBMP therapies were being increased to identify the optimal dose as opposed to occurring when a patient had been on a sustained dose. Thus, reported AEs may not be a true reflection of maintenance therapies. Moreover, long follow-up periods with repeated prompting to report such events during clinical assessment may have introduced a nocebo effect. Notably, higher rates of headache were reported here compared to similar studies [Citation48,Citation66]. This may be because cannabinoids have been shown to decrease headache stress threshold in migraine patients [Citation71]; however, it is also possible that these reports were associated with the underlying headache pathology and not an effect of the patient’s CBMP therapy. Overall, in accordance with literature [Citation72], there were no life-threatening or disabling AEs suggesting relative clinical safety.
4.1. Limitations
Several study limitations must be noted. Crucially, a case series can only draw associations and no cause-and-effect relationship can be established. Additionally, the lack of blinding or control group, which reduces the internal validity because self-reported outcomes may have been overstated. This bias was potentially amplified because patients did not discontinue their preventative and acute medication for their headaches which may have confounded the effects of prescribed CBMPs. However, this effect may have been minimal because it has previously been demonstrated that preventative medication does not affect the outcome of changes in headache impact during CBMP therapy [Citation42]. Moreover, PROMs are subjective scores that hold different values for each patient, reducing comparability and subject to recall bias. The follow-up period was 6-months due to insufficient data collection after this time point. This prevented the assessment of long-term efficacy and any tolerability resulting from sustained CBMP use, which may be significant because tolerability has been established both pre-clinically [Citation73] and clinically [Citation30]. Furthermore, loss to follow-up during these 6-months resulted in missing data which reduced statistical power and introduced an attrition bias. Similarly, the clinic involved in prescribing the CBMPs is private which not only limits external validity because results might not be generalizable to a low-middle socioeconomic group but also because patients may have been more likely to overstate clinical improvements, and the financial burden of therapies may have exacerbated loss to follow-up. However, 23.7% of patients were unemployed, implying that socioeconomic status may not have precluded access to therapy. Furthermore, in this study, males were overrepresented which is not representative of migraine patients as females have a higher incidence of migraines. However, this may be a reflection that males are more likely to access illicit cannabis and therefore transition to medical cannabis [Citation74]. Lastly, only a small number of patients experienced cluster or tension-type headache; therefore, any conclusions may only apply to migraines and not be valid for the other headache disorders included in the analysis.
Despite these limitations, this study has many strengths. Being the largest prospective UK case series of its kind, it fills an evidence gap. Moreover, real-world data were collected from routine clinical practice without interfering with a patient’s usual treatment process. Therefore, it is an inexpensive, quick, and resource-efficient method of analysis with high external validity. The study also assessed changes in PROMs which are holistic and gold-standard measures for pain conditions. Meanwhile, STROBE guidelines for observational studies were followed to minimize bias [Citation45]. Overall, results from this study could aid in generating a hypothesis for future clinical trials.
In the future, randomized control trials (RCT) are necessary to establish causality. Importantly, trials should assess CBMP’s efficacy and safety for individual primary headache disorders and should establish the place of cannabis therapy in optimal headache treatments. There is potential for linkage of the UKMCR with clinical practice data sets to create matched controls for future comparisons. Additionally, the long-term (>6-months) efficacy and safety should be assessed to determine any tolerability, and statistical adjustments should be made to ensure other medications used, such as acute medications, do not confound findings. Wearable technology, such as smart watches, could allow for more accurate prospective and contemporary recording of head pain levels, reducing recall bias and subjectivity [Citation75]. Studies should distinguish the effects of CBMPs according to underlying clinico-pathological differences. Furthermore, full-spectrum products should be compared with isolated cannabinoids to elucidate if other compounds derived from the Cannabis plant may be contributing to an overall therapeutic effect [Citation76].
5. Conclusion
Whilst these results provide promise with respect to the changes in health-related quality of life experienced by those with primary headache disorders, there is still a requirement for further RCTs to be conducted to understand the true efficacy of CBMPs for this indication. However, whilst these are awaited, the present study outcomes with respect to safety and efficacy provide useful insights to inform current clinical practice.
Data Availability
Data that support the findings of this study are available from the UK Medical Cannabis Registry. Restrictions apply to the availability of these data. Data specifications and applications are available from the corresponding author.
Ethical approval
In the UK, formal ethics approval is not required for research database analysis as detailed by the UK Health Research Authority.
Principal Investigator
The authors confirm that the PI for this paper is Mikael H Sodergren and that he had direct clinical responsibility for patients.
Declaration of Interest
S Erridge, C Holvey, R Coomber, JJ Rucker, MW Weatherall & MH Sodergren are the founding clinicians of Sapphire Medical Clinics, which is the first clinic registered with the CQC to evaluate patients for medical cannabis in England. S Erridge is a junior doctor and undertakes paid consultancy work at Sapphire Medical Clinics. He is also an honorary clinical research fellow at Imperial College London. C Holvey is chief clinical pharmacist at Sapphire Medical Clinics. R Coomber is a consultant orthopedic surgeon at St George’s Hospital, London, and Head of Operations at Sapphire Medical Clinics. JJ Rucker is a consultant psychiatrist at Sapphire Medical Clinics (London). He is an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King’s College London. JJ Rucker is funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR). He also leads the Psychedelic Trials Group at King’s College London. King’s College London receives grant funding from COMPASS Pathways PLC to undertake phase 1 and phase 2 trials with psilocybin. COMPASS Pathways PLC has paid for JJ Rucker to attend trial-related meetings and conferences to present the results of research using psilocybin. JJ Rucker has also undertaken paid consultancy work for Beckley PsyTech and Clerkenwell Health. Payments for consultancy work are received and managed by King’s College London. M Weatherall is a consultant in neurology and a director at Sapphire Medical Clinics (London). MH Sodergren is a consultant hepatopancreatobiliary surgeon, a director at Sapphire Medical Clinics and a consultant at Imperial College NHS Trust, London. He is senior clinical lecturer at Imperial College London and Chief Medical Officer at Curaleaf International. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author Contributions
Study conception and design: M Nicholas, S Erridge, C Holvey, R Coomber, MW Weatherall, JJ Rucker and MH Sodergren
Acquisition of data: M Nicholas, S Erridge, L Bapir, M Pillai, N Dalavaye, C Holvey, MW Weatherall, JJ Rucker
Analysis and interpretation of data: M Nicholas, S Erridge, JJ Rucker, MW Weatherall, MH Sodergren
Drafting of manuscript: M Nicholas, S Erridge, MH Sodergren
Critical revision: M Nicholas, S Erridge, L Bapir, M Pillai, N Dalavaye, C Holvey, R Coomber, MW Weatherall, JJ Rucker, MH Sodergren.
All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (25.8 KB)Acknowledgments
The data has been presented as a poster at the International Cannabinoid Research Society Conference, 2022.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737175.2023.2174017
Additional information
Funding
References
- Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the global burden of disease study 2010. Lancet. 2012;380(9859):2129–2143.
- Ahmed F. Headache disorders: differentiating and managing the common subtypes. Br J Pain. 2012;6(3):124–132.
- Steiner TJ, Stovner LJ, Jensen R, et al. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137.
- Fuller G, Kaye C. Headaches. BMJ. 2007;334(7587):254–256.
- Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1278–1299.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629–808.
- Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain. 2013;14:1.
- World health report. WHO, Geneva. World Health Organisation. 2001
- Latinovic R. Headache and migraine in primary care: consultation, prescription, and referral rates in a large population. J Neurol Neurosurg Psychiatry. 2005;77:385–387.
- Goldstein J, Camargo C, Pelletier A, et al. Headache in United States Emergency Departments. Cephalalgia. 2006;26:684–690.
- Negro A, RD S. Cost of chronic and episodic migraine patients in continuous treatment for two years in a tertiary level headache Centre. J Headache Pain. 2019;20:120.
- Ferrari MD, Goadsby PJ, Burstein R, et al. * Migraine. Nat Rev Dis Primers. 2022;8:1.
- National Institute of Health and Care Excellence (NICE, 2021). Guidelines/guidance Migraine. 2021
- Evers S, Áfra J, Frese A, et al. EFNS guideline on the drug treatment of migraine - revised report of an EFNS task force. Eur J Neurol. 2009;16:968–981.
- Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478–488.
- Vikelis M, Spingos KC, Rapoport AM. A new era in headache treatment. Neurol Sci. 2018;39:47–58.
- Diener H-C, Dodick D, Evers S, et al. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019;18:891–902.
- Greco R, Demartini C, Zanaboni AM, et al. The endocannabinoid system and related lipids as potential targets for the treatment of migraine‐related pain. Headache. 2022;62:227–240.
- Greco R, Demartini C, Zanaboni AM, et al. * Endocannabinoid system and migraine pain: an update. Front Neurosci. 2018;12:172.
- Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29.
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215.
- Finn D, Haroutounian S, Hohmann AG;, et al. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain. 2021;162:s5–25.
- Russo E. Clinical Endocannabinoid Deficiency (CECD): can this concept explain therapeutic benefits of cannabis. Neuro Endocrinol Lett. 2004;25:39.
- Christiansen IM, Edvinsson JCA, Reducha PV, et al. Dual action of the cannabinoid receptor 1 ligand arachidonyl-2′-chloroethylamide on calcitonin gene-related peptide release. J Headache Pain. 2022;23:30.
- Cupini L, Bari M, Battista N, et al. Biochemical Changes in Endocannabinoid System are Expressed in Platelets of Female but not Male Migraineurs. Cephalalgia. 2006;26:277–281.
- Hanuš LO, Meyer SM, Muñoz E, et al. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–1392.
- Wang L, Hong PJ, May C, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ. 2021;374(8305):n1034.
- Lintzeris N, Mills L, Suraev A, et al. Medical cannabis use in the Australian community following introduction of legal access: the 2018–2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18). Harm Reduct J. 2020;17:37.
- Rhyne DN, Anderson SL, Gedde M, et al. Effects of medical marijuana on migraine headache frequency in an adult population. Pharmacother J Human Pharmacol Drug Ther. 2016;36:505–510.
- Cuttler C, Spradlin A, Cleveland MJ, et al. Short- and long-term effects of cannabis on headache and migraine. J Pain. 2020;21:722–730.
- Pini LA, Guerzoni S, Cainazzo MM, et al. Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial. J Headache Pain. 2012;13:677–684.
- Kilinc E, Ankarali S, Torun IE, et al. Receptor mechanisms mediating the anti‐neuroinflammatory effects of endocannabinoid system modulation in a rat model of migraine. Eur J Neurosci. 2022;55:1015–1031.
- Akerman S, Holland PR, Lasalandra MP, et al. Endocannabinoids in the brainstem modulate dural trigeminovascular nociceptive traffic via CB1 and “Triptan” receptors: implications in migraine. J Neurosci. 2013;33:14869–14877.
- Yamamoto T, Mulpuri Y, Izraylev M, et al. Selective targeting of peripheral cannabinoid receptors prevents behavioral symptoms and sensitization of trigeminal neurons in mouse models of migraine and medication overuse headache. Pain. 2021;162:2246–2262.
- Kandasamy R, Dawson CT, Craft RM, et al. Anti-migraine effect of ∆9-tetrahydrocannabinol in the female rat. Eur J Pharmacol. 2018;818:271–277.
- Akerman S, Kaube H, Goadsby PJ. Anandamide is able to inhibit trigeminal neurons using an in vivo model of trigeminovascular-mediated nociception. J Pharmacol Exp Ther. 2004;309:56–63.
- Ergisi M, Erridge S, Harris M, et al. An updated analysis of clinical outcome measures across patients from the UK medical cannabis registry. Cannabis Cannabinoid Res. 2022. Epub. Doi:10.1089/can.2021.0145.
- Orsolini L, Chiappini S, Volpe U, et al. Use of medicinal cannabis and synthetic cannabinoids in Post-Traumatic Stress Disorder (PTSD): a systematic review. Medicina (B Aires). 2019;55:525.
- Martinotti G, Di Iorio G, Sepede G, et al. Cannabis use and psychosis: theme introduction. Curr Pharm Des. 2012;18:4991–4998.
- Ricci V, Martinotti G, Ceci F, et al. Duration of untreated disorder and cannabis use: an observational study on a cohort of young Italian patients experiencing psychotic experiences and dissociative symptoms. Int J Environ Res Public Health. 2021;18:12632.
- Martinotti G, Di Iorio G, Tedeschi D, et al. Prevalence and intensity of basic symptoms among cannabis users: an observational study. Am J Drug Alcohol Abuse. 2011;37:111–116.
- Baraldi C, Lo Castro F, Negro A, et al. Oral cannabinoid preparations for the treatment of chronic migraine: a retrospective study. Pain Med. 2022;23:396–402.
- Ojelabi AO, Graham Y, Haighton C, et al. A systematic review of the application of Wilson and Cleary health-related quality of life model in chronic diseases. Health Qual Life Outcomes. 2017;15:241.
- The CP. NICE guideline on medicinal cannabis: keeping pandora’s box shut tight? Med Law Rev. 2020;28:401–411.
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457.
- Medicines and Healthcare products Regulatory Agency. The supply of unlicensed cannabis-based products for medicinal use in humans. 2020
- Brusselaers N, Lagergren J. The Charlson comorbidity index in registry-based research. Methods Inf Med. 2017;56:401–406.
- Erridge S, Salazar O, Kawka M, et al. An initial analysis of the UK medical cannabis registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021;41:362–370.
- Lee D, de Keizer N, Lau F, et al. Literature review of SNOMED CT use. J Am Med Inf Assoc. 2014;21:e11–9.
- National Institute of Health and Care Excellence (NICE, 2022). Medicines guidance: prescribing in palliative care. British National Formulary. 2022. [cited 2022 Aug 01]. Available from: https://bnf.nice.org.uk/medicines-guidance/prescribing-in-palliative-care/
- Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6TM. Qual Life Res. 2003;12:963–974.
- Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56:S20–8.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–1736.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. 2006;166:1092.
- Snyder E, Cai B, DeMuro C, et al. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14:1849–1857.
- Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10:S73.
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009
- Luedtke K, Basener A, Bedei S, et al. Outcome measures for assessing the effectiveness of non-pharmacological interventions in frequent episodic or chronic migraine: a Delphi study. BMJ Open. 2020;10:e029855.
- Coeytaux RR, Kaufman JS, Chao R, et al. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol. 2006;59:374–380.
- Carvalho GF, Luedtke K, Braun T. Minimal important change and responsiveness of the Migraine Disability Assessment Score (MIDAS) questionnaire. J Headache Pain. 2021;22:126.
- Lipton RB, Lombard L, Ruff DD, et al. Trajectory of migraine-related disability following long-term treatment with lasmiditan: results of the GLADIATOR study. J Headache Pain. 2020;21:20.
- Lombard L, Farrar M, Ye W, et al. A global real-world assessment of the impact on health-related quality of life and work productivity of migraine in patients with insufficient versus good response to triptan medication. J Headache Pain. 2020;21:41.
- Sexton M, Cuttler C, Finnell JS, et al. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1:131–138.
- Azcarate PM, Zhang AJ, Keyhani S, et al. Medical reasons for marijuana use, forms of use, and patient perception of physician attitudes among the US population. J Gen Intern Med. 2020;35:1979–1986.
- Census 2020. Drug misuse in England and Wales: year ending March 2020. United Kingdom; 2020
- Cahill SP, Lunn SE, Diaz P, et al. Evaluation of patient reported safety and efficacy of cannabis from a survey of medical cannabis patients in Canada. Front Public Health. 2021;9:626853.
- Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19:23.
- Bauer-Staeb C, Kounali D-Z, Welton NJ, et al. Effective dose 50 method as the minimal clinically important difference: evidence from depression trials. J Clin Epidemiol. 2021;137:200–208.
- Lev-Ran S, Roerecke M, le Foll B, et al. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44:797–810.
- Walsh JH, Maddison KJ, Rankin T, et al. Treating insomnia symptoms with medicinal cannabis: a randomized, crossover trial of the efficacy of a cannabinoid medicine compared with placebo. Sleep. 2021;44:11.
- Zhang N, Woldeamanuel Y. Medication Overuse Headache in chronic migraine patients using cannabis: a case-referent study. 2021;61(8):1234–1244.
- Wang T, Collet J-P, Shapiro S, et al. Adverse effects of medical cannabinoids: a systematic review. Can Med Assoc J. 2008;178:1669–1678.
- Kandasamy R, Dawson CT, Hilgendorf TN, et al. Medication overuse headache following repeated morphine, but not ∆9-tetrahydrocannabinol administration in the female rat. Behav Pharmacol. 2018;29:469–472.
- Couch D. Left behind: the scale of illegal cannabis use for medicinal intent in the UK. 2020
- Smuck M, Odonkor CA, Wilt JK, et al. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digit Med. 2021;4:45.
- Worth T. Cannabis’s chemical synergies. Nature. 2019;572:S12–3.
