ABSTRACT
Background
The following study evaluated the clinical outcomes of patients enrolled in the UK Medical Cannabis Registry, who were treated with inhaled dried flower (Adven® EMT2, Curaleaf International, Guernsey), and sublingual/oral medium-chain triglyceride-based oils (Adven, Curaleaf International, Guernsey) for chronic pain.
Methods
In this cohort study, the primary outcomes were changes in validated patient reported outcome measures (PROMs) at 1, 3, and 6 months compared to baseline, and adverse event analysis. Statistical significance was defined as p < 0.050.
Results
Three hundred and forty-eight (45.7%), 36 (4.7%), and 377 (49.5%) patients were treated with oils, dried flower, or both, respectively. Patients treated with oils or combination therapy recorded improvements within health-related quality of life, pain, and sleep-specific PROMs at 1, 3, and 6 months (p < 0.050). Patients treated with combination therapy recorded improvements in anxiety-specific PROMs at 1, 3, and 6 months (p < 0.050). 1,273 (167.3%) adverse events were recorded, with previously cannabis naïve users, ex-cannabis users, and females more likely to experience adverse events (p < 0.050).
Conclusions
This study observed an association between initiation of CBMP treatment and improved outcomes for chronic pain patients. Prior cannabis use and gender were associated with adverse event incidence. Placebo-controlled trials are still necessary to establish the efficacy and safety of CBMPs for chronic pain.
1. Introduction
Chronic pain is a condition characterized by physical pain persisting for longer than 3 months, as defined by the International Association for the Study of Pain [Citation1]. In the UK, chronic pain is estimated to affect between one-third to one-half of the adult population over the course of their lives [Citation2]. Existing treatments include non-steroidal anti-inflammatory drugs, opioids, and gabapentinoids [Citation1]. However, due to increasing evidence of the associated risk of long-term use and variable evidence of their efficacy across all causes of chronic pain, these medications are not suitable for every individual [Citation3,Citation4].
The endocannabinoid system, in particular cannabinoid type 1 (CB1R) and type 2 receptors (CB2R), has been implicated in pain sensation and perception pathways. CB1R, found most commonly within the nervous system, inhibits ascending nociceptive transmission from the dorsal horn in the spinal column, as well as stimulates descending analgesic pathways [Citation5,Citation6]. In contrast, CB2R predominantly modulates the release of inflammatory ligands and endogenous opioids in peripheral immune cells and keratinocytes [Citation7]. CB2R is also present in the central nervous system within glial cells, endothelial cells, and neurons, where they have been found to play a neuroprotective role in neurological disorders and mediate neuronal sensitization in chronic pain conditions [Citation6].
The (-)-trans-∆9-tetrahydrocannabinol (THC) and cannabidiol (CBD) compounds found within the cannabis plant are known to influence the endocannabinoid system [Citation8]. THC is a CB1R and CB2R partial agonist [Citation7]. CBD inhibits fatty acid–binding ligands responsible for transporting endocannabinoids intracellularly to be broken down by the enzyme fatty acid amino hydrolase [Citation7]. Thus, CBD primarily produces a reduction in pain by increasing the concentration of endocannabinoids present at synaptic junctions. CBD also acts as an agonist in serotonin 1A (5-HT1A) receptors and transient receptor potential subfamily V member 1 (TRPV1) cation channels, as well as inhibiting serotonin 3A (5-HT3A) receptors [Citation8]. THC, alternatively, produces a dose-dependent blockade of TRP cation channels [Citation8,Citation9]. Cannabis-based medicinal products (CBMPs) containing THC and CBD, have therefore been postulated as promising therapeutics for chronic pain considering their multifactorial role in reducing nociceptive signals in both the peripheral and central nervous system, as well as modulating the pathways in the primary somatosensory cortex and limbic system involved in the emotional and cognitive manifestations of pain via these receptors [Citation6].
Despite growing evidence implicating CBMPs as a viable chronic pain treatment, the results have been heterogenous, differing based on the type of CBMP administered, chronic pain type assessed, and study duration [Citation10,Citation11]. Additionally, there remains a paucity of evidence regarding the long-term safety of CBMPs [Citation10]. This was reflected in a recent systematic review, which investigated patient preferences and concerns regarding the use of CBMPs for chronic pain, finding that adverse effects were identified as a common concern, alongside medication composition, legal status, and cost [Citation12]. Moreover, factors such as route of administration, age, body mass index (BMI), and prior cannabis exposure influence CBMP safety have not been adequately investigated [Citation13].
As further clinical trials addressing these unknowns are awaited, patient registries can assist by collecting prospective data from patients prescribed CBMPs in a real-world setting [Citation14]. The UK Medical Cannabis Registry (UKMCR) records longitudinal, long-term data collected from patients prescribed CBMPs to better assess the therapeutic benefits for different conditions. This study aimed to evaluate changes in general health-related quality of life (HRQoL), chronic pain-specific outcomes, and adverse events in patients treated exclusively with CBMPs. Changes in opioid prescriptions following CBMP treatment were also investigated.
2. Methods
2.1. Data collection
A prospective cohort study of patients from the UKMCR treated with an inhaled dried flower (Adven® EMT2, Curaleaf International, Guernsey, UK), sublingual/oral medium-chain triglyceride-based oil (Adven®, Curaleaf International, Guernsey, UK), or a combination of both for chronic pain was conducted. The UK Medical Cannabis Registry has received ethical approval from the South West – Central Bristol Research Ethics Committee (reference 22/SW/0145). All patients completed formal, written consent prior to enrollment. The study was reported in line with the STROBE statement for reporting observational studies [Citation15].
The UKMCR is a patient registry, set up in December 2019 to collect prospective, clinical data from patients prescribed CBMPs outside of the NHS. It is privately owned and managed by Sapphire Medical Clinics.
Patient demographics, medications, comorbidities, occupations, and drug and alcohol history were collected by clinicians. Clinicians recorded indications for CBMP therapy using primary, secondary, and tertiary diagnoses (Supplementary Table S1). Participants updated their concomitant medications using an online platform, or informed clinicians of any changes during follow-up appointments. Opioid medications were converted to oral morphine equivalents using conversion factors quoted by the British National Formulary and GP notebook [Citation16]. The Charlson comorbidity index, a model of underlying health status, was calculated for each patient in line with other longitudinal health-care registries [Citation17]. THC and CBD dosages (mg/24 h) were recorded for each CBMP prescription.
Occupations were classified according to the International Standard Classification of Occupations [Citation18]. Patients provided their cannabis history prior to receiving a CBMP prescription. Cannabis naïve users had never used cannabis before, ex-users had previously used cannabis but were not using it at the time of starting a CBMP prescription, and current users were using illicit cannabis up until the time of prescription. For ex- and current users, a novel metric, cannabis gram-years, was used to quantify cannabis use as previously described [Citation14]. Smoking status, smoking pack-years, and weekly alcohol consumption were also recorded. Patients were sent patient reported outcome measures (PROMs) and adverse event questionnaires electronically at baseline, 1, 3, and 6 months after CBMP treatment commenced.
2.2. Patient and public engagement
To develop the methodology of the present study, a concurrent patient evaluation of 600 patients enrolled on the UKMCR was performed [Citation19]. The majority found the electronic data collection platform easy to use when reporting PROMs (91.6%), adverse events (73.1%), and medication changes (90.4%). Over 90% of the patients agreed or strongly agreed that contribution to the UKMCR would impact the care of future patients [Citation19]. Finally, the highest priorities for future research were identified as ‘assessing the impact of medical cannabis on quality of life,’ ‘assessing the impact of medical cannabis on condition-specific outcomes,’ ‘collecting information about the side effects of medical cannabis,’ and ‘assessing the impact of medical cannabis on other prescribed medications’ [Citation19].
2.3. Patient and data selection
Patient data from those prescribed Adven® CBMPs for chronic pain conditions was extracted from the UKMCR on 9 January 2022. Individuals who had a primary indication for CBMP therapy other than chronic pain, or a condition for which treatment of chronic pain symptoms is the only reason CBMPs might be prescribed, were excluded from the analysis. Cohorts were identified according to the route of administration(s) of their prescribed product(s). Patients prescribed oil-based products (including reformulated oils in lozenges or capsules), inhaled dried flowers, and both products were distinguished into three separate cohorts. Full details of oil-based and dried flower products are detailed in Supplementary Table S2. Participants who had not completed all baseline PROMs were excluded.
2.4. PROMs
Primary outcomes of the study were changes in the following questionnaires: Pain Visual Analog Scale (P-VAS), Brief Pain Inventory Short-Form (BPI), Short-Form McGill Pain Questionnaire-2 (MPQ2), Single-Item Sleep Quality Scale (SQS), Generalized Anxiety Disorder-7 (GAD-7), EQ-5D-5L, and Patient Global Impression of Change (PGIC).
The P-VAS questionnaire asks patients to rate their pain from 0 to 10 where ‘0’ is ‘no pain at all’ and ‘10’ is ‘pain as bad as it could be’ on a 10 cm visual analog scale [Citation10].
BPI is a questionnaire where patients score their worst, strongest, current, and average pain intensity. Scores are combined to create a ‘pain severity score’ from 0 to 10 where ‘0: no pain’ and ‘10: pain as bad as you can imagine’ [Citation17]. Patients also rate the impact of their pain on daily activities, such as sleep, socializing, and work to create a ‘pain interference score’ from 0 to 10 with ‘0: no interference’ and ‘10: completely interferes’ [Citation20].
MPQ2 assesses the character and severity of pain. Patients rate different pain descriptors and symptoms from 0 to 10 with ‘0: none’ and ‘10: the worst pain possible’ [Citation21]. Questions are organized into four subscales: continuous pain, intermittent pain, neuropathic pain, and affective descriptors [Citation18]. An average from 0 to 10 is then calculated for each subscale and total score, with greater numbers indicating higher pain severity.
The SQS is a single validated efficacy measure whereby patients rate their sleep quality over the previous week from 0 to 10 with ‘0: terrible’ and ‘10: excellent’ [Citation22].
GAD-7 asks patients for the frequency of symptoms of generalized anxiety disorder over the past 2 weeks, generating a score from 0 to 21 [Citation20]. The score is classified according to severity, whereby mild, moderate, and severe anxiety are determined by scores of ≥5, ≥10, and ≥15, respectively [Citation23].
EQ-5D-5L is a global metric of HRQoL consisting of questions with 5 levels of severity across 5 domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression [Citation24,Citation25]. Respondents assign scores from 1 to 5 for each domain, where ‘1: no problems,’ ‘2: slight problems,’ ‘3: moderate problems,’ ‘4: severe problems’ and ‘5: extreme problems’ [Citation24,Citation25]. Collectively, these scores generate one of the 3125 health states, with each health state mapped to a UK-specific index value in line with the preferred methodology outlined by the National Institute of Health and Care Excellence [Citation25,Citation26]. An index value of ‘1’ represents ‘full health.’ An index value <0 represents a HRQoL ‘worse than death.’
PGIC uses a 7-point scale to determine if there has been an improvement or decline in a patient’s quality of life since treatment commenced. Scores range from ‘1: no change’ to ‘7: a great deal better’ [Citation27].
2.5. Adverse events
Adverse events were recorded in accordance with the common terminology criteria for adverse events version 4.0 [Citation28]. Adverse events could be recorded by patients ad hoc following their occurrence, contemporaneously with PROMs, or during a clinical consultation. Dried flower users were not included in adverse event analysis due to low numbers of adverse events (n = 1).
2.6. Statistical analysis
Shapiro–Wilk tests were performed to determine parametric/nonparametric data. Parametric data was presented as mean ± standard deviation (SD), and nonparametric data was presented as median and interquartile range (IQR). A Wilcoxon rank-sum test was performed to compare the paired differences for PROMs for each patient at 1, 3, and 6 months compared to baseline within each treatment category, as each dataset was confirmed to be nonparametric. PGIC was not included in this analysis as the questionnaire itself establishes health changes relative to baseline. Univariate binary logistic regression models were used to individually assess the effect of age, BMI, gender, treatment type, and prior cannabis exposure on the likelihood of experiencing an adverse event, an improvement in BPI severity score, or BPI interference score, after 6 months by calculating odds ratios (ORs) and 95% confidence intervals (CI). In each analysis, variables demonstrating statistically significant associations were included in a multivariate analysis, which determines the impact of each variable on the outcome measure, after adjusting for the other included variables. A paired samples t-test was used to investigate changes in opioid prescriptions after 6 months of treatment. Statistical significance was defined as p < 0.050. Analyses were conducted using RStudio version 4.1.2.
3. Results
3.1. Patient demographics, clinical history, and prescriptions
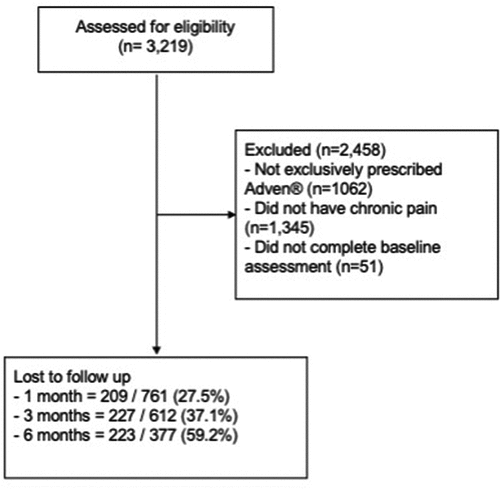
The data extraction included 812 patients prescribed Adven® CBMPs for chronic pain conditions within the UKMCR. Of those, 761 (93.7%) completed baseline PROMs and were included in the analysis (). presents patient demographics, clinical history, and prescription information in full.
Table 1. Patient demographics, clinical history, and prescriptions.
3.2. PROMs
To assess the effect of CBMP treatment on HRQoL in chronic pain patients, patients were administered HRQoL questionnaires at baseline, 1, 3, and 6 months after CBMP treatment commenced. summarizes the paired results for pain-related PROMs. For patients only prescribed oils, statistically significant improvements in P-VAS, BPI, and MPQ2 were seen at 1, 3, and 6 months compared to the baseline (p < 0.010). For patients only prescribed dried flower CBMPs, statistically significant improvements were seen in P-VAS, BPI Severity Score, and MPQ2 after 1 and 3 months, respectively (p < 0.050). Improvements were seen after 1 month for the BPI Interference Score (p = 0.005). For patients prescribed both oils and dried flower CBMPs, improvements were found in P-VAS, BPI Interference Score, and MPQ2 after 1, 3, and 6 months, respectively (p < 0.010). Additionally, improvements were seen in the BPI Severity Score after 3 and 6 months (p < 0.01).
Table 2. Paired baseline and follow-up data for pain-specific patient-reported outcome measures (PROMs).
To determine the impact of a patient’s age, gender, BMI, prior cannabis experience, and CBMP treatment type on pain relief, a univariate binary logistic regression model was used to investigate the impact of these variables on recorded improvements in the BPI Interference and Severity scores after 6 months of treatment relative to baseline.
In univariate analysis (Supplementary Tables S3 & S4), no variables were found to be significantly associated with reduced pain severity or interference (p > 0.050). A multivariate analysis was conducted to confirm these findings, which similarly showed no significant difference (p > 0.050) ( and Supplementary Table S5).
Table 3. Multivariate analysis of variables affecting BPI Severity scores.
summarizes the paired results for non-pain-specific HRQoL questionnaires. For patients prescribed oils, statistically significant improvements were seen in SQS and the EQ-5D-5L index, usual activities, and pain and discomfort subscales after 1, 3, and 6 months (p < 0.050). Improvements in GAD-7 and the EQ-5D-5 L anxiety and depression subscales were found at 1 and 3 months compared to baseline (p < 0.001). For PGIC, the median remained constant at 5.00.
Table 4. Paired baseline and follow-up data for non-pain-specific patient-reported outcome measures (PROMs).
For dried flower users, improvements were seen after 1 and 3 months for SQS, and the EQ-5D-5L index, as well as the pain and discomfort subscale (p < 0.050). Improvements were also seen after 1 month for the EQ-5D-5L usual activities domain (p = 0.032).
For patients prescribed both oils and dried flower CBMPs, statistically significant improvements were found in SQS, GAD-7, as well as the EQ-5D-5L index, mobility, usual activities and pain and discomfort subscales after 1, 3, and 6 months, respectively (p < 0.050). Additionally, improvements were seen in the EQ-5D-5 L self-care and anxiety and depression subscales after 1 and 3 months (p < 0.050). The median PGIC increased from 5.00 at 1 month to 6.00 after 3 and 6 months.
3.3. Change in opioid medications
To investigate the impact of CBMP treatment on opioid prescriptions, a paired samples t-test was used to determine changes in patient opioid usage after 6 months of treatment relative to baseline. There was a reduction in opioid prescriptions after 6 months (40.7 mg/24 h ± 57.6 mg/24 h) compared to baseline (42.1 mg/24 h ± 58.3 mg/24 h; p = 0.018). This represents a 3.28% reduction in mean opioid dose.
3.4. Adverse events
Patients also reported adverse events to assess the safety profile of each CBMP treatment, as well as identify if prior cannabis exposure affected adverse event incidence.
For patients prescribed oils, 857 total adverse events were recorded, with 91 patients (26.1%) experiencing at least 1 adverse event. There were 387 (111.2%) mild, 360 (103.4%) moderate, 109 (31.3%) severe and 1 (0.3%) life-threatening adverse events recorded, respectively. For patients prescribed both oils and dried flowers, 415 total adverse events were recorded, with 56 (14.9%) patients experiencing at least 1 adverse event. There were 178 (47.2%) mild, 167 (44.3%) moderate, and 70 (18.6%) severe adverse events recorded. Supplementary Table S6 details the quantity and severity of adverse events in full for each treatment group, as well as patients’ prior cannabis exposure status. The most common recorded adverse events were fatigue (n = 114; 14.0%), somnolence (n = 88; 10.8%), and dry mouth (n = 84; 10.3%) (Supplementary Table S7).
On univariate analysis (Supplementary Table S8), the following were found to be associated with a higher incidence of adverse events; age between 71 and 80 years (OR = 2.294; 95% CI: 1.069–4.919; p = 0.033), ex-users of cannabis (OR = 2.316; 95% CI: 1.296–4.137; p = 0.005), and naïve cannabis users (OR = 3.697; 95% CI: 2.359–5.794; p < 0.001). Male patients (OR = 0.320; 95% CI: 0.213–0.481; p < 0.001) and those prescribed oils and dried flower (OR = 0.493; 95% CI: 0.340–0.714; p < 0.001) were less likely to experience and adverse events.
Statistically significant variables were taken forward to a multivariate model (). Cannabis naïve (OR = 2.515; 95% CI: 1.470–4.301) and ex-users (OR = 2.286; 95% CI: 1.246–4.195) were more likely to experience adverse events relative to current users, to a statistically significant extent. Additionally, males (OR = 0.403; 95% CI: 0.262–0.618) were less likely to experience adverse events to a statistically significant extent. No statistically significant difference in adverse event incidence was found between patients prescribed oils or both CBMPs (OR = 1.005; 95% CI: 0.639–1.581), or between age groups.
Table 5. Multivariate analysis of factors increasing the risk of adverse events from cannabis-based medicinal products.
4. Discussion
The findings in this study demonstrate treatment with oil-based, dried flowers, or a combination of both CBMPs are associated with statistically significant improvements in pain relief and sleep quality after 6 months in chronic pain patients. Additionally, patients prescribed oils or both types of CBMPs experienced reduced anxiety and an improvement in their ability to perform daily activities. Patients prescribed a combination of both CBMPs recorded improvements in their self-care and mobility abilities. Collectively, this evidence signals that initiation of CBMP treatment is associated with improved HRQoL. Improvements in pain measured by BPI scores were also independent of patient age, gender, BMI, prior cannabis experience, and CBMP treatment type. Additionally, cannabis naïve and ex-users were more likely to experience adverse events relative to current users, whereas males were less likely to experience adverse events.
For patients prescribed oil-based or both CBMP types, statistically significant improvements in pain-specific PROMs were recorded at all time periods. This is supported by a recent observational study, which investigated the impact of oil-based CBMPs on HRQoL in 71 chronic pain patients [Citation29]. The study found reductions in pain impact scores after an average treatment time of 133 days. Additionally, a recent meta-analysis of randomized clinical trials found chronic pain patients treated with CBMPs experienced an average P-VAS reduction of −0.5 cm [Citation11]. This is lower than in the present study, which reported between −1 cm and −2 cm reductions in P-VAS after 6 months of CBMP treatment. This discrepancy may have arisen from the different study durations, as the median study duration in the meta-analysis was 50 days. Further, the P-VAS reductions recorded in this study are greater than the P-VAS minimum clinically significant difference, which is a 1 cm reduction, having been previously identified as beneficial enough to warrant CBMP treatment [Citation11].
Previous studies have not investigated the effects of age, gender, BMI, prior cannabis exposure, or CBMP treatment type on cannabis-induced analgesia. In the present study, none of the aforementioned factors were found to influence improvements in BPI scores after 6 months. This suggests that cannabis-based analgesia is independent of these factors. However, this study only considered the dichotomous outcome of whether patient’s BPI scores improved and did not quantify the magnitude of improvement. As such, some factors may have correlated with a greater improvement in pain relief, which would not have been captured in the present analysis. Additionally, the impact of various CBMP dosages was not considered. These represent important avenues for future research.
Patients prescribed both CBMPs reported improvements in SQS, GAD-7, and all EQ-5D-5L metrics. This is consistent with an audit of 400 patients prescribed CBD oil, which found a significant reduction in all EQ-5D-5L subscales in chronic non-cancer pain patients and patients with anxiety, depression, and insomnia after 3 weeks of treatment [Citation30]. In contrast, this study found no difference in the EQ-5D-5L mobility or self-care subscales for those only prescribed oils. Vaporized CBMPs allow for a quicker onset of action due to bypassing first-pass metabolism and are absorbed straight into the bloodstream [Citation31]. In contrast, oil-based CBMPs take longer to reach peak serum levels, thus the onset of action is observed later. Patients prescribed both types of CBMPs may therefore experience benefits from both administrative routes, leading to the differences observed.
A 3.28% reduction in daily opiate consumption was reported in the present study. A recent study determined that a 28.2% reduction was necessary for a clinically significant reduction [Citation32]. Research on the effect of CBMPs on opioid usage remains limited as existing clinical trials investigating the use of CBMPs for chronic pain maintained opioid medications at constant doses [Citation33]. However, pooled results from observational studies have reported a reduction of 22.5 mg/24 h oral morphine equivalents, higher than reported here [Citation33]. There are several reasons as to why there may have under-reported the reduction in opioid usage. About 42.8% of the patients were utilizing illicit cannabis prior to enrollment. As such, these patients may have already reduced their opioid medications. Second, patient medications taken as required may have contributed to an underestimation in opioid reduction as patients may have reduced their regular opioid medications after CBMP treatment commenced, yet this change may not have been captured. Refining the data collection platform to include this consideration will be important for improving the accuracy of UKMCR medication data.
Interestingly, cannabis naïve and ex-users were more likely to experience adverse events compared to current users. This agrees with a year-long prospective cohort study of patients prescribed a 12.5% THC CBMP formulation [Citation34]. Three hundred and sixteen adverse events were recorded from 74 cannabis naïve or ex-users, with an incidence rate ratio of 2.15 (95% CI: 1.69–2.74). In comparison, 141 current users recorded 502 adverse events (incidence rate ratio = 1.64; 95% CI: 1.35–1.99). Additionally, the present study found males were less likely to experience adverse events. A few studies have investigated the sex-dependent effects of cannabinoids [Citation35], and further assessment in randomized controlled trial settings is required to assess if there are gender- or sex-dependent effects of CBMPs.
There are several limitations to this study. First, although follow-up scores decreased for all pain-specific PROMs, so too did the baseline scores. This may be because patients with higher pain scores may not have achieved clinical benefit and dropped out, raising the attrition bias. Second, there was a limited sample size of patients prescribed dried flowers. A similar issue occurred during a previous UKMCR study, which found improved pain relief in chronic pain patients treated with oil-based CBMPs after 1 and 3 months, but not 6 months (n = 12) [Citation10]. In the current study and with increased numbers (n = 68), the improvements were statistically significant after 6 months of treatment. Thus, it would be beneficial to repeat this analysis as more patients are enrolled in the UKMCR and prescribed dried flower and would also provide sufficient data for adverse event analysis. Third, although this study attempted to limit heterogeneity by subgrouping patients based on route of administration, a variety of treatments remained within the oils category (Supplementary Table S2). This is an inherent drawback of observational studies, unable to control for the diversity in prescriptions associated with clinical settings. Due to this, one could argue differences in HRQoL or adverse events may be due to different THC/CBD concentrations, not the route of administration, or patients’ gender or prior cannabis use. However, whilst this compromises the internal validity of the study, it does help to elucidate outcomes in a real-world setting, outside of the stringent criteria of randomized controlled trials. Moreover, there are challenges in patients reporting the impact of symptom severity or adverse events accurately. The future incorporation of wearables would be beneficial in providing objective data. In this way, quantifiable metrics, such as step counts, sleep duration, and heart rate can be measured to accompany other outcomes. Additionally, the efficacy of CBMPs may be different according to the underlying cause of pain. Consequently, PROM responses may have been influenced by the type of chronic pain patients were treated for, which was not accounted for in this study. Future analyses with sufficient sample size should aim to perform separate analyses according to chronic pain etiology. Finally, due to the lack of an active comparator group, it is not possible to conclude that the associations observed in this study are directly caused by the CBMPs themselves. This also extends to adverse events, which were not individually assessed by clinicians as to whether they were treatment-related, which could lead to overreporting.
4.1. Conclusions
In summary, these results suggest that both oil-based and dried flower CBMPs are associated with long-term improved HRQoL in chronic pain patients, in agreement with both our hypothesis and existing literature investigating short-term outcomes. Furthermore, it details the scarcity of severe/disabling adverse events associated with long-term CBMP use and reveals increased adverse event incidence for females, cannabis naïve and ex-users. Thus, future studies and clinicians should consider the impact of gender and prior cannabis use when prescribing CBMPs. Additionally, whether these factors influence the extent of HRQoL improvements should be investigated in active comparator trials. Due to the limitations outlined, concrete conclusions regarding the efficacy and safety of individual CBMP prescriptions cannot be drawn. Hence, future analyses of the UKMCR should investigate individual CBMP products and their safety and efficacy for chronic pain treatment when controlled for confounding factors such as gender and prior cannabis use.
Declaration of interest
S. Erridge is a junior doctor and Head of Research at Sapphire Medical Clinics. He is also a research fellow at Imperial College London. C Holvey is chief clinical pharmacist at Sapphire Medical Clinics. R. Coomber is a consultant orthopedic surgeon at St George’s Hospital, London, and Operations Director at Sapphire Medical Clinics. A. Usmani is a pain specialist at Sapphire Medical Clinics (London) and a consultant at Dartford and Gravesham NHS Trust. M. Sajad is a pain specialist at Sapphire Medical Clinics (London) and a consultant at Dudley Group of Hospitals NHS Trust. J Hoare is a consultant in gastroenterology at Sapphire Medical Clinics (London) and Imperial College NHS Healthcare Trust. S. Khan is a consultant in palliative medicine at Sapphire Medical Clinics (London) and Guy’s and St Thomas’ NHS Foundation Trust. M.W. Weatherall is a consultant in neurology at Buckinghamshire Healthcare NHS Trust and Sapphire Medical Clinics (London). JJ Rucker is a consultant psychiatrist, a director and a shareholder at Sapphire Medical Clinics (London). He is also an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR Clinician Scientist Fellow at the Center for Affective Disorders at King’s College London. J. Rucker is further funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR). J Rucker leads the Psychedelic Trials Group at King’s College London. King’s College London receives grant funding from COMPASS Pathways PLC to undertake phase 1 and phase 2 trials with psilocybin. COMPASS Pathways PLC has paid J. Rucker to attend trial-related meetings and conferences to present the results of research using psilocybin. J. Rucker has undertaken paid consultancy work for Beckley PsyTech and Clerkenwell Health. Payments for consultancy work are received and managed by King’s College London and J. Rucker does not benefit personally. M. Platt is a consultant in pain services at Sapphire Medical Clinics (London). M.H. Sodergren is a consultant hepatopancreatobiliary surgeon at Imperial College NHS Trust, London. He is a senior clinical lecturer at Imperial College London. He is also the Managing Director of Sapphire Medical Clinics (London) and the Chief Medical Officer of Curaleaf International. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One reviewer declares that they have conducted cannabis research and have consulted for two companies regarding cannabis: Schedule 1 Therapeutics and Vectura Fertin. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
J Tait, S Erridge, C Holvey, R Coomber, JJ Rucker and MH Sodergren declare responsibility for study conception and design. J Tait, S Erridge, C Holvey, A Usmani, M Sajad, J Hoare, S Khan, M Weatherall, JJ Rucker, M Platt acquired the data. J Tait, S Erridge, MH Sodergren are responsible for the analysis and interpretation of data while J Tait, S Erridge, MH Sodergren are responsible for drafting the manuscript. J Tait, S Erridge, C Holvey, R Coomber, A Usmani, M Sajad, J Hoare, S Khan, M Weatherall, JJ Rucker, M Platt, MH Sodergren all critically revised the manuscript, and all authors agree to be accountable for all aspects of the work.
Ethical approval
Approval from South West – Central Bristol Research Ethics Committee (reference 22/SW/0145)
Supplemental Material
Download MS Word (47.3 KB)Acknowledgments
The data have been presented as a poster at the International Cannabinoid Research Society Conference, 2022. The authors confirm that the PI for this paper is Mikael H. Sodergren and that he had direct clinical responsibility for patients.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737175.2023.2195551
Additional information
Funding
References
- Nicholas M, Vlaeyen JWS, Rief W, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28–37. Cited: in: PMID: 30586068. DOI:10.1097/j.pain.0000000000001390
- Fayaz A, Croft P, Langford RM, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. Cited: in: PMID: 27324708. DOI:10.1136/bmjopen-2015-010364.
- Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021 May 29;397(10289):2082–2097.
- Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2016 [[cited 2021 Oct 13]];Internet 37(1):29–42. DOI:10.1007/S00296-016-3481-8.
- Huang W-J, Chen W-W, Zhang X. Endocannabinoid system: role in depression, reward and pain control (Review). Mol Med Rep. 2016 [[cited 2021 Oct 12]];InternetCited: in: PMID: 27484193 14(4):2899–2903. DOI:10.3892/MMR.2016.5585.
- Maldonado R, Baños JE, Cabañero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157(Supplement 1):S23–32. Cited in: PMID: 26785153.inoids DOI:10.1097/j.pain.0000000000000428.
- Vučković S, Srebro D, Vujović KS, et al. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;0:1259.
- Pertwee RG. Handbook of cannabis. USA: Oxford University Press; 2014.
- Anand U, Pacchetti B, Anand P, et al. Cannabis-based medicines and pain: a review of potential synergistic and entourage effects. Pain Manag. 2021 Jul;11(4):395–403.
- Kawka M, Erridge S, Holvey C, et al. Clinical outcome data of first cohort of chronic pain patients treated with cannabis-based sublingual oils in the United Kingdom: analysis from the UK Medical Cannabis Registry. J Clin Pharmacol. 2021 [[cited 2021 Oct 15]];2021:1–10. Internet DOI:10.1002/JCPH.1961
- Wang L, Hong PJ, May C, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ. Cited: in: PMID: 34497047 2021;374:n1034. DOI:10.1136/bmj.n1034
- Zeng L, Lytvyn L, Wang X, et al. Values and preferences towards medical cannabis among people living with chronic pain: a mixed-methods systematic review. BMJ Open. 2021 [[cited 2022 Jan 29]];11(9):e050831. InternetCited: in: PMID: 34493521. DOI:10.1136/BMJOPEN-2021-050831
- Wilson P, Pangarkar S Cannabis in Medicine. In: Finn K, editor. Cannabis Med [Internet]. 1st ed. Springer International Publishing; 2020. p. 172–173,176. Available from: https://www.springer.com/gp/book/9783030459673.
- Erridge S, Salazar O, Kawka M, et al. An initial analysis of the UK Medical Cannabis Registry: outcomes analysis of first 129 patients. Neuropsychopharmacol Reports. 2021;41(3):362–370. Cited: in: PMID: 33988306. DOI:10.1002/npr2.12183. .
- Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies*. Bull World Health Organ. 2007 [[accessed 2022 Mar 29]];InternetCited: in: PMID: 18038077 85(11):867. DOI:10.2471/BLT.07.045120.
- BNF British National Formulary - NICE [Internet]. [accessed 2022 Mar 8]. Available from: https://bnf.nice.org.uk/?utm_source=evidencebnfredirect&utm_medium=other&utm_campaign=old_site_redirect.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis [Internet]. 1987 [cited 2022 Mar 8]; 40(5):373–383. Cited: in: PMID: 3558716. DOI:10.1016/0021-9681(87)90171-8.
- ISCO - International Standard Classification of Occupations [Internet]. [cited 2022 Mar 11]. Available from: https://www.ilo.org/public/english/bureau/stat/isco/isco08/index.htm.
- Tait J, Erridge S, Sodergren MH. UK Medical Cannabis Registry: a Patient Evaluation. J Pain Palliat Care Pharmacother. 2023 Jan 27;1–8. DOI:10.1080/15360288.2023.2174633.
- Im DD, Jambaulikar GD, Kikut A, et al. Brief pain inventory–short form: a new method for assessing pain in the emergency department. Pain Med. 2020 [[cited 2022 Mar 10]];InternetCited: in: PMID: 32918473 21(12):3263–3269. DOI:10.1093/PM/PNAA269.
- Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144(1):35–42. Cited: in: PMID: 19356853. DOI:10.1016/j.pain.2009.02.007
- Snyder E, Cai B, DeMuro C, et al. A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018 [[cited 2022 Mar 10]];InternetCited: in: PMID: 30373688 14(11):1849. DOI:10.5664/JCSM.7478.
- Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008 [[cited 2022 Mar 10]];InternetCited: in: PMID: 18388841 46(3):266–274. DOI:10.1097/MLR.0B013E318160D093.
- Van Hout B, Janssen MF, Feng YS, et al. Interim Scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L Value Sets. Value Heal. 2012 [[cited 2022 Mar 10]];InternetCited: in: PMID: 22867780 15(5):708–715. DOI:10.1016/J.JVAL.2012.02.008.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011 [[cited 2022 Mar 10]];InternetCited: in: PMID: 21479777 20(10):1727. DOI:10.1007/S11136-011-9903-X.
- Position statement on use of the EQ-5D-5L value set for England (updated October 2019) | Technology appraisal guidance | NICE guidance | Our programmes | What we do | About | NICE [Internet]. [accessed 2022 Mar 10]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l.
- Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004 Jan 1;27(1):26–35.
- Trotti A, Colevas AD, Setser A, et al. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–5127. Cited: in: PMID: 17991931. DOI:10.1200/JCO.2007.12.4784.
- Abelev S, Warne LN, Benson M, et al. Medicinal cannabis for the treatment of chronic refractory pain: an investigation of the adverse event profile and health-related quality of life impact of an oral formulation. Med Cannabis Cannabinoids. 2022;5(1):1–12.
- Gulbransen G, Xu W, Arroll B. Cannabidiol prescription in clinical practice: an audit on the first 400 patients in New Zealand. BJGP Open. 2020;4(1):1–8.
- Romero-Sandoval EA, Kolano AL, Alvarado-Vázquez PA. Cannabis and cannabinoids for chronic pain. Curr Rheumatol Rep. 2017;19(11). Cited: in: PMID: 28983880. DOI:10.1007/s11926-017-0693-1
- Goudman L, De Smedt A, Forget P, et al. Determining the minimal clinical important difference for medication quantification scale III and morphine milligram equivalents in patients with failed back surgery syndrome. J Clin Med. 2020 [[cited 2022 Feb 12]];Internet 99(11):3747–3747. DOI:10.3390/JCM9113747.
- Noori A, Miroshnychenko A, Shergill Y, et al. Opioid-sparing effects of medical cannabis or cannabinoids for chronic pain: a systematic review and meta-analysis of randomised and observational studies. BMJ Open. 2021 [[cited 2022 Mar 8]];11(7):47717. Internet. DOI:10.1136/bmjopen-2020-047717
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182(14):E694–701. Cited: in: PMID: 20805210. DOI:10.1503/cmaj.091414.
- Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacol Rev. 2018 [[cited 2022 Mar 31]];Internet 43(1):34–51. DOI:10.1038/npp.2017.140.