ABSTRACT
Introduction
The true global burden of vascular cognitive impairment (VCI) is unknown. Reducing risk factors for stroke and cardiovascular disease would inevitably curtail VCI.
Areas Covered
The authors review current diagnosis, epidemiology, and risk factors for VCI. VCI increases in older age and by inheritance of known genetic traits. They emphasize modifiable risk factors identified by the 2020 Lancet Dementia Commission. The most profound risks for VCI also include lower education, cardiometabolic factors, and compromised cognitive reserve. Finally, they discuss pharmacological and non-pharmacological interventions.
Expert Opinion
By virtue of the high frequencies of stroke and cardiovascular disease the global prevalence of VCI is expectedly higher than prevalent neurodegenerative disorders causing dementia. Since ~ 90% of the global burden of stroke can be attributed to modifiable risk factors, a formidable opportunity arises to reduce the burden of not only stroke but VCI outcomes including progression from mild to the major in form of vascular dementia. Strict control of vascular risk factors and secondary prevention of cerebrovascular disease via pharmacological interventions will impact on burden of VCI. Non-pharmacological measures by adopting healthy diets and encouraging physical and cognitive activities and urging multidomain approaches are important for prevention of VCI and preservation of vascular brain health.
1. Introduction
Cardiovascular disease alters brain perfusion leading to strokes and other cerebrovascular events. These overt changes are associated with various dementias as well as depressive illness [Citation1–4]. Intracranial lesions described by cerebrovascular disease (CVD) are responsible for the second most common form of age-related dementia, namely vascular dementia (VaD). However, invariably in tandem with brain aging covert or sub-clinical changes are also evident. They include white matter disease, cerebral atrophy, silent lacunar infarcts, microinfarcts, microbleeds, arteriolosclerosis, and intracranial atherosclerosis, which are widely regarded as surrogates of underlying intracranial or cerebral small vessel disease (SVD). These cerebral lesions may accrue over time to impact on cognition, physical strength or ability, and cerebral reserve [Citation5–8].
Older age is the strongest risk factor for VaD or SVD-related dementia. The genome harbors another risk that constitutes monogenic or polygenic characteristics. However, modifiable risk factors for VaD or SVD including hypertension, diabetes mellitus (DM), dyslipidaemia, obesity, and metabolic syndrome can be reduced or prevented in reality to improve brain perfusion and substantially delay or curtail cognitive impairment and dementia. Prevention of cognitive impairment and dementia would require control of cardiovascular or cerebrovascular risks involving multi-faceted strategies at the individual, community, and population levels, perhaps more so in low- and middle-income countries (LMICs). Degree of risk may be further influenced by inherent factors such as sex and ethnicity. Thus, targeting prevention of stroke for example in high-risk ethnicities across the life course would be beneficial to reduce the burden of vascular cognitive impairment (VCI) [Citation9]. The key to prevention is, however, early detection of the type of risk and of apparent clinically silent disease, which often (~80%) progresses to frank dementia.
2. Scope of review
Our article incorporates clinical and pathological definitions of VCI, an estimation of the burden, accounts of the main modifiable risk factors and strategies for both non-pharmacological and pharmacological approaches to reduce or prevent VCI and promote vascular brain health. The narrative text is essentially compiled from papers published from original research performed by the authors as well as data derived from research papers in PubMed and Web of Science. We searched for all online narrative and systematic reviews on VCI, stroke epidemiology, and vascular disease risk and protective factors. We used the following combination of terms: Vascular cognitive impairment or vascular dementia and review and hypertension or diabetes or dyslipidaemia or obesity, or metabolic syndrome or diet or physical activity or tobacco or multidomain. We were also mindful to include comments on the 12 risk factors identified by the 2020 Lancet Dementia Commission. These, in order of most modifiable to the least perhaps at the individual level, are lower education, excessive alcohol, smoking, physical inactivity, hypertension, diabetes, obesity, depression, traumatic brain injury, hearing loss, social isolation, and air pollution. Similarly, to search for references on pharmacological treatments we used vascular cognitive impairment and various classes of individual drugs, e.g. aspirin, statins, etc. We also performed a search of systematic reviews and meta-analyses on vascular risk factors, inflammation, and dementia.
3. Current view of vascular cognitive impairment
VCI comprises all causes of cerebral vascular disease in relation to cognitive dysfunction. VCI involves degrees of impaired cognition in a continuum that will depend on the type and extent of vascular brain injury [Citation5,Citation10,Citation11]. Previously, vascular cognitive disorder [Citation12] was described and for all practical purposes is another interchangeable label with the more popular VCI that also incorporates a continuum comprising cognitive disorders of vascular etiology with diverse clinical manifestations associated with varied pathologies. In the most recently developed DSM-5 criteria incorporate the categories of mild and major vascular cognitive disorders [Citation13]. Major vascular neurocognitive disorder classification aligns with VaD, or frank dementia explained by largely cerebral vascular disease. This fits better with clinical practice, and more adapted to neurodegenerative cognitive disorders for which amnestic memory impairment is not superior but encompasses substantial pathologies in the frontal lobe [Citation14]. Large vessel disease is more often related to lateralized sensorimotor changes and aphasia whereas SVD is akin to more subtle signs, including extrapyramidal signs and gait disturbances (see below). Dementia resulting from large vessel obstruction or disease, also categorized as multi-infarct dementia, in preference to ‘cerebral atherosclerosis.’ It is caused by multiple infarcts and prominent in the neocortex (cortical VaD) and can be so in the gray-white borderzone. Cerebral SVD may entail degrees of pathological changes including hypertensive strategic infarcts leading to subcortical ischemic VaD. Dementia associated with strategic infarcts is diagnosed in patients who have lesion volume below the threshold for dementia but in whom ischemic injury is in regions e.g. thalamus, critical for normal cognitive function. Strategically located infarcts may also be large involving the deep gray matter including the basal ganglia and thalamus), white matter or the limbic system. However, it is recognized that overlap between the subtypes is common. For example, microinfarcts may affect cognition in both large vessel disease and SVD. VaD often comprises a combination of cortical and subcortical lesions, thereby referred to as cortico-subcortical VaD. It is rare for vascular lesions to be localized exclusively within the cortex.
To attain consensus on the diagnosis of VCI (), the vascular impairment of cognition classification consensus studies (VICCCS-1 and VICCCS-2) established key expressions in the understanding and language of cognitive impairment and dementia resulting from CVD [Citation15,Citation16]. Practical guidelines for the diagnosis of VCI were formulated via a Delphi type of iterative protocol. Given the various degrees of cognitive dysfunction could occur with varied lesions and location, VICCCS-1 study proposed the use of ‘Mild’ and ‘Major’ subdivisions of the severity of impairment, aligning with the recent nomenclature in DSM-5. VICCCS diagnosis guidelines specify deficits in at least 1 domain, with clinically significant cognitive deficits of sufficient severity e.g. moderate to severe, and severe deficits in daily living activities differentiating Mild and Major forms of VCI [Citation16]. It was then concluded it would be premature to further sub-classify Mild VCI but the Major forms of VCI, constituting frank dementia or VaD should be categorized into 4 main subtypes including post-stroke dementia (PSD), subcortical ischemic vascular dementia, multi-infarct (cortical) dementia and various mixed dementias, subclassed according to additional types of neurodegenerative pathologies. Thus, in practice, all the categories of VaD subtypes based upon current clinical and neuroimaging evidence and defined by the origin of vascular disease, arterial territory or size of the vessels involved, and lesion location are described under VCI. In considering this, it is of note that the DSM-5 and VICCCS criteria [Citation17] are comparable but the previous vascular behavioral and cognitive disorders (VASCOG) society criteria [Citation14] have greater sensitivity but modest concurrent and better predictive validity than the NINDS-AIREN criteria for VaD [Citation18].
Figure 1. Classification of VCI according to level of impairment into mild VCI and major VCI (VaD). Major VCI (VaD or frank dementia of vascular origin) is classified into four main subtypes as depicted. The estimated 6-month temporal basis for cognitive decline after stroke differentiates PSD from other forms of major VCI (VaD). We propose that ICH-VCI is included since cerebral hemorrhages cause hemorrhagic dementia, which is more common in Asia and Africa than in Europe and the U.S.A. Abbreviations: AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; ICH, intracerebral hemorrhage; PSD, post stroke dementia. PSD-# denotes other possible combinations when comorbid neuropathology is present in mixed dementias. Figure adapted from [Citation15] with permission of John Wiley & sons.
![Figure 1. Classification of VCI according to level of impairment into mild VCI and major VCI (VaD). Major VCI (VaD or frank dementia of vascular origin) is classified into four main subtypes as depicted. The estimated 6-month temporal basis for cognitive decline after stroke differentiates PSD from other forms of major VCI (VaD). We propose that ICH-VCI is included since cerebral hemorrhages cause hemorrhagic dementia, which is more common in Asia and Africa than in Europe and the U.S.A. Abbreviations: AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; ICH, intracerebral hemorrhage; PSD, post stroke dementia. PSD-# denotes other possible combinations when comorbid neuropathology is present in mixed dementias. Figure adapted from [Citation15] with permission of John Wiley & sons.](/cms/asset/0f20316d-6ca9-487b-a65f-7a7d82720201/iern_a_2273393_f0001_oc.jpg)
Subcortical ischemic VaD or dementia resulting from mostly SVD has been of much recent interest globally [Citation19–21] for two main reasons: 1) it results from the single most common cause of stroke injury and 2) survivors of lacunar strokes often survive long and at greater risk of cognitive impairment. Thus, cerebral SVD is probably the most common cause of cognitive impairment and dementia [Citation22]. Clinical features incorporate motor and executive slowing, forgetfulness, and dysarthria. A short-stepped gait is also common and can mimic that of Parkinsonism. These may be caused by disruption of pathways running from the prefrontal cortex to the basal ganglia and of thalamocortical pathways. The main vascular pathology relates to sclerotic changes or loss of medial vascular smooth cells in intracranial arteries and arterioles, most often evident in the basal ganglia. Evidence from pathological studies suggests there are two main presentations in subcortical ischemic VaD: 1) presence of multiple lacunar infarcts affecting subcortical gray matter and 2) diffuse or widespread rarefaction of the white matter.
Cognitive impairment or dementia following stroke or PSD [Citation23] is recognized to be relatively common in older age [Citation24,Citation25], ranging from a prevalence of 7.4% in population-based studies of first-ever stroke to 41% (30–53) in hospital-based studies of recurrent stroke. However, a most recent analysis of 5-year data from the Oxford Vascular Study [Citation26] found that either transient ischemic attack or stroke-related dementia at 1 year was 34% in patients with severe or major stroke, 8% in those with minor stroke, and 5% in those with transient ischemic attack. In comparison, systematic analysis involving 44 studies worldwide [Citation27] indicated at 1 year the prevalence of PSD as 18% and if pre-stroke dementia cases were included it was 20%. Most dementia occurred in the first year after major stroke, whereas onset was more gradual after minor stroke. PSD may develop within three months or after a stabilization period of a year or longer after stroke injury [Citation26,Citation28–30]. Multiple lesions over time and the characteristics and complications of the stroke were found to be most strongly associated with PSD. Thus, PSD has a complex etiology with varying combinations of large lesions and SVD as well as non-vascular pathology. However, it is also important to recognize that depression may confound VCI diagnosis particularly relating to white matter hyperintensities (WMHs) with the oft-debated presence of ‘vascular depression’ [Citation31,Citation32]. Previous studies have shown that the depressive syndrome is not the primary disease but can be considered as one of the clinical manifestations in the wide symptom spectrum of VCI [Citation33]. Mixed pathology is particularly common in the oldest old [Citation34] and the overlap of these may synergize risk of cognitive impairment [Citation5,Citation35,Citation36]. There should be sufficient evidence of clinical deterioration or progression [Citation37]. The most common form of mixed dementia comprises significant vascular lesions and Alzheimer type of pathology. Mixed dementia cases with coexisting vascular changes, including cerebral amyloid angiopathy and Lewy body pathology or other neurodegenerative inclusions appear less frequent.
4. Epidemiology of VCI
Given that the worldwide prevalence of stroke-related cerebrovascular diseases and cardiovascular disorders are higher than any common neurodegenerative disorder, it is expected that there will proportionally be high burden of cognitive impairment due to vascular causes. Stroke patients may develop cognitive dysfunction in the form of mild cognitive impairment, possibly some involving amnestic memory deficits [Citation38]. However, there are no large prospective population-based longitudinal studies to determine the true prevalence of VCI [Citation20]. Global frequency of VCI may be estimated reliably from the current cases of stroke. The recent report from the global burden of disease (GBD) collaborators showed that in 2019 worldwide there were 12·2 million incident cases of all strokes [Citation39] whereas 7.6 million cases of these comprised ischemic stroke [Citation40]. This analysis reported 101 million prevalent cases of stroke [Citation39]. Annual total numbers of all strokes increased substantially from 1990 to 2019 although they remained stable in many high-income countries and there were reductions in age-standardized rates, particularly among elderly who were over 70 years of age. The incidence of ischemic strokes increased by 88% from 1990 to 2019 [Citation40]. Interestingly, the most rapidly growing risk factor for stroke between 1990 and 2019 was body-mass index of > 30 (). Previous meta-analyses [Citation25–27] showed that on average 20–30% of stroke survivors of the first stroke and recurrent strokes develop dementia. Thus taking these numbers and computing against the 12.2 incident cases of all strokes in 2019, a conservative estimate calculates to 2.44 million, who will have developed VCI or VaD. This number compares with recent GBD data on four common causes of dementias including Down’s Syndrome, Parkinson’s disease, traumatic brain injury and stroke [Citation41]. The analysis estimated 3.7 million stroke-related dementia cases occurred globally and for every region, stroke accounted for the largest number of dementia cases compared to the other causes. Given that there might be a number of mild VCI cases occurring after stroke, these numbers are likely gross underestimates. The GBD data showed that worldwide in 2019 there were 7.2 million dementia cases including those resulting from stroke. Taken together, the global prevalence of VCI in 2019 could be approximately 4 million and that number is likely to be much higher in 2023. Irrespective, since nearly 90% of the global burden of stroke can be attributed to modifiable risk factors, a formidable opportunity arises to reduce the burden of not only stroke but VCI outcomes.
Table 1. Unmodifiable and modifiable risk factors for VCI*.
Cardiovascular diseases also remain the leading cause of disease burden in the world. In 2019, prevalent cases of total cardiovascular disease were estimated to be 523 million, increased by nearly 100% since 1990 [Citation42]. These estimates also suggest that at least 1 in 5 individuals with cardiovascular disease had a stroke. In addition, meta-analysis of data from 28 million individuals suggests that the global prevalence varies from 13% to 31% according to the definitions incorporated in high blood glucose, hypertension, waist-to-hip ratio, and high-density lipoprotein-cholesterol values [Citation43]. The prevalence was significantly higher in the Eastern Mediterranean Region and the Americas and increased per level of country income. This suggests frequencies of VCI may vary widely in some global regions. The disparities and their drivers call for diversity, equity and inclusion in global VCI research and in the allocation of resources for research, prevention, treatment and rehabilitation by allocating resources where the burden is large. In addition, recent studies have revealed sex-differences in some cognitive domains and functional status of those diagnosed with post-stroke VCI [Citation44] or mild VCI [Citation45]. In the latter study [Citation45], men performing worse in some cognitive domains was also reflected by differences in intracortical and cortico-spinal excitability to multimodal transcranial magnetic stimulation. These findings have prognostic and diagnostic implications as well as strategies for neuromodulation.
The ongoing calculations do not consider that many individuals will have covert lesions including WMHs, which occur at high frequencies in older age and denote presence of cerebral SVD. In addition to WMHs, silent infarcts mostly subcortical lesions were shown to increase the risk of all cause dementia including Alzheimer’s disease (AD) in the earlier longitudinal Rotterdam study [Citation10]. Evidence from the US Atherosclerosis Risk in Communities study showed that subclinical cerebrovascular disease may be up to ten times more common than clinically evident stroke in the general population [Citation46]. Similarly, in the multicentre European Leukoariosis and Disability study (LADIS) the presence of WMHs was shown to independently predict functional decline [Citation47] and contribute to vascular cognitive disorders and physical instability [Citation48].
WM attenuation or WMHs may progress to precipitate mild VCI and dementia. Recent meta-analysis of longitudinal studies [Citation49] suggests that mean WMH increases by 1.74 ml over time of 2–4 years but there are wide ranging changes. Various factors and mechanisms could be associated with regression of volume in asymptomatic stroke and neurocognitive disorders. There is also a significant association between WMHs, both deep WMHs and periventricular WMHs and post-stroke depression during the chronic post-stroke phase [Citation50]. Periventricular lesions were more strongly related to post-stroke depression in each period after stroke than deep WMHs.
Carotid artery disease is a risk for ipsilateral strokes especially in patients with 70–99% stenosis compared to those with 50–69% stenosis [Citation51] but high degrees of stenosis may affect cognition in the absence of strokes. In the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis-2 or CREST-2 trial patients showed lower levels of baseline cognition particularly memory impairment compared to a population-based cohort, controlled for demographic as well as cardiovascular risk factors [Citation52]. Irrespective these estimates are just the ‘tip of the iceberg’ and likely gross underestimates. They importantly implicate the urgent implementation of cost-effective primary prevention strategies to reduce stroke and CVD globally and hence prevent VCI.
5. Pathophysiology and inflammation in VCI
Several mechanisms including genetic, biological, and behavioral are likely involved in the link between risk factors causing vascular diseases and cognitive impairment or dementia or depressive illness. Ageing per se causes irreversible structural changes in the brain vasculature. Brain perfusion may not only be influenced by intracranial atherosclerotic disease but also arteriolosclerosis which increases exponentially with age [Citation53,Citation54]. In addition, blood supply via the carotid and vertebral arteries may be affected by degrees of age-related atherosclerotic disease and arterial stiffness within the cardiovascular system and a variety of heart conditions such as atrial fibrillation. Ageing also impacts on autonomic regulation, neurovascular uncoupling, and blood-brain barrier (BBB) functions, which will all dictate the ultimate dynamics of brain blood flow and local perfusion during different periods to initiate stroke injury and VCI [Citation54]. It is not unlikely that venous diseases in form of varicose veins, venous insufficiency and deep vein thrombosis as well as cerebral venous collagenosis invariably play a part in vascular cognitive impairment [Citation55]. Some evidence also suggests that age-related venous pathology is associated with WMHs [Citation56]. Thus, age-related changes in brain blood vessels may be attributed to several structural and pathological alterations including cerebral atrophy, white matter attenuation, endothelial or blood-brain barrier (BBB) damage, oxidative stress, covert accumulation of neurodegenerative proteins such as amyloid and hyperphosphorylated tau and failure of intramural periarterial drainage (IPAD) inducing hypoperfusion or hypoxic events in the brain [Citation57].
Moreover, complex physiological interactions between markers of metabolic syndrome (e.g. hypertension) and arteries occur to produce arteriolar inflammation that impacts on perfusion [Citation58]. Brain inflammation is now considered as an early event and an important contributor in the progression of VCI. For example, systemic levels of the proinflammatory cytokine interleukin-1β have been reported to be increased in VCI. Experimental studies suggest that the inflammasome signaling pathways mediated by the NLR-pyrin domain containing 3 (NLRP3) and absent in melanoma 2 (AIM2) inflammasomes regulate interleukin-1β production. Furthermore, recent meta-analysis showed that three inflammatory markers interleukin-6 (IL-6), C-reactive protein, tumor necrosis factor-α were found to be higher in the blood and CSF samples from VaD patients and high IL-6 and tumor necrosis factor-α levels were also associated with increased risk of incident VaD [Citation59]. These findings pave the way for future disease-modifying treatments to reduce inflammation induced damage and cognitive deficits observed in VCI.
6. Common risk factors for VCI
Various factors comprising inflexible and modifiable risks may alter cognitive status during the life course (). The association between vascular disease and cognitive dysfunction is compounded by hormonal, inflammatory, dietary and lifestyle factors such as physical inactivity. In addition, cognitively impaired subjects may be prone to unhealthy habits including ingestion of more processed foods, tobacco use and alcohol abuse resulting in obesity and insulin resistance. The combined potentially modifiable risk for dementia globally, estimated by the Lancet Commission for 12 risk factors was estimated to be 40% compared to 41% for the US population. While comparisons of unweighted population attributable fractions for dementia appear to be highly variable for some risk factors (), there is great potential to reduce the burden of dementia and therefore VCI globally.
The strongest risk factor for VCI is older age. Several genetic traits have also been associated with VCI, but the two of the common genes are APOE and NOTCH3 (). We have previously reported on the association of APOE alleles ε4 (OR = 1.85) and ε2 (OR = 0.67) in VCI [Citation60]. This is consistent with our previous study in elderly stroke survivors with early cognitive impairment, where the presence of an APOE ε4 allele was associated with greater progression of cognitive decline [Citation61]. With respect to NOTCH3 while over 280 distinct gene mutations are causal in typical CADASIL. However, large exome analysis has indicated that the frequency of archetypal cysteine altering NOTCH3 mutations are remarkably 100-fold higher than expected and cause SVD disease phenotype with less severe typical CADASIL symptoms [Citation62]. In a recent UK Biobank study [Citation63], NOTCH3 mutation carriers were found to have 5-fold greater risk of VaD, adding to the global burden of SVD and risk of VCI. Other genes of interest were the high- temperature requirement A serine peptidase 1 (HTRA1) carriers, who have two-fold increased risk of all-cause dementia and Collagen 4 (COL4) A1/2 carriers with nearly 4-fold risk of intracerebral hemorrhage [Citation63]. The Biobank data emphasize that cardiovascular risk is associated with increased risk of stroke in NOTCH3 and HTRA1 variant carriers; control of cardiovascular risk factors could still improve disease prognosis in individuals with monogenic cerebral SVD variants. There is no doubt that a variety of mitochondrial mutations involving mitochondrial dysfunction modify risk and promote early onset of VCI [Citation64]. Genetic factors, therefore, add to the risk for dementia beyond the identified 40% attributed to modifiable factors (). This 40% attribution was also estimated in another important prospective real-world study which looked at modifiable risk factors including low education, obesity, hypertension, diabetes, depression, smoking, physical inactivity, hearing loss, loneliness, heart disease, stroke, head injury, and delirium and inflexible traits such as the APOE ε4 allele [Citation65].
Figure 2. Scheme illustrates how various risk factors may influence the cerebral as well as systemic vasculature at distinct levels to induce chronic brain or cerebral hypoperfusion and result in variable degrees of cognitive impairment over time. Several genetic and environmental factors may modify the intensity of vascular damage.
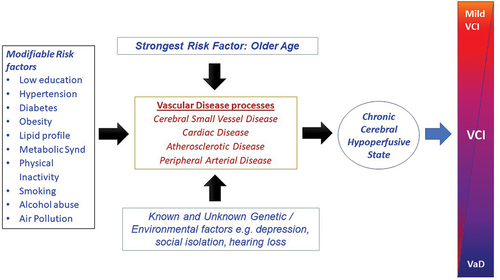
Projected estimates indicate that by 2025, 3 of every 4 persons will be living with high blood pressure and may incur co-morbidities such as diabetes, high body-mass index or obesity and individual features of metabolic syndrome risks including high fasting plasma glucose, high total cholesterol and low glomerular filtration rate [Citation66,Citation67]. Current evidence also shows cumulative vascular disease risk factors worsen neurocognitive deficits [Citation68] and promote depression [Citation32] as well as severe frailty [Citation69,Citation70]. These realizations are a major alarm call for appraisal of vascular brain and mental health, work capacity and socioeconomic development particularly in the peoples of Africa, Asia and Latin America [Citation71].
In addition to modifiable risk factors for VCI, cognitive reserve, both biological and cognitive aspects, is an important factor in the resilience of the brain to stroke or other injury [Citation72]. This may be modulated by levels of education and type of occupation. Findings from a recent meta-analysis showed that besides the risk of stroke in less educated individuals, childhood socioeconomic background and intelligence are also associated with albeit modest risk of stroke and by extension VCI [Citation73].
6.1. High blood pressure and variability
By 2025, it is estimated that the global prevalence of hypertension, diagnosed as blood pressure ≥ 140/90 mm Hg, will increase by 30%. Remarkably, on average 1 in 3 adults is hypertensive or will develop hypertension not only in high-income but also in low- and middle-income countries [Citation74]. Long-term blood pressure variability also plays a major role in determining the outcome of stroke and is associated with cardiovascular and mortality suggesting serious implications for VCI [Citation75]. However, improvements in the detection, treatment, and strict control of hypertension vary substantially across countries, with some middle-income nations now outperforming most high-income countries. Reducing the global burden of hypertension prevalence through primary prevention and enhancing its treatment and control is still attainable globally in all settings [Citation76,Citation77]
Hypertension is the most common risk factor and the most modifiable for VCI. Several studies over the past 25 years have collectively shown that sustained hypertension is a major vascular risk factor for cognitive dysfunction [Citation78]. Hypertension particularly in midlife is associated with later-life cognitive impairment and the development of dementia [Citation78]. Young-to-midlife higher blood pressure exposure is further associated with midlife cognitive impairment. The duration of hypertension during the life-time course may also impact on cognition differently. The association between hypertension and cognitive outcomes in very late-life is complex and less consistent but it seems hypotension is of greater importance in the oldest old [Citation78]. Thus, distinct patterns of blood pressure changes over the whole life-course appear important predictors of late-life cognitive impairment. The cognitive deficits associated with hypertension include several domains including impairment of planning, reasoning and executive dysfunction. Older age and increased body weight are consistent predictors of hypertension, given that prevalence estimates of hypertension were substantially higher in the elderly, compared with younger adults, and in overweight/obese persons, compared with normal weight persons. Like dementia prevalence, lower educational status was also found to be largely associated with a higher prevalence of hypertension.
Previous inconsistent findings on certain effects of raised blood pressure on cognition during the life course have raised the concerns that there are other factors beyond determining the mean blood pressure [Citation79]. This could be important for VCI prevention and early intervention. In a recent meta-analysis, that included 20 studies for the primary outcome, both a higher mean level of blood pressure as well as a higher degree of blood pressure variability were associated with cognitive impairment [Citation80]. Thus, high blood pressure variability may be a predictor for the risk of VCI meaning that the relative contribution of variability in blood pressure appears to be more notable than the mean blood pressure. Variability might be a novel blood pressure-derived parameter to be considered in hypertension management as a future target to prevent VCI. In another meta-analysis of longitudinal studies [Citation81], 13 studies reported visit-to-visit systolic blood pressure variability significantly increased risk of cognitive impairment and dementia. Both visit-to-visit and day-to-day diastolic blood pressure variability also increased risk of dementia and cognitive decline. While the biological mechanisms involved in how variability increases risk are not known it is plausible that even slight fluctuations in flow may exaggerate the dynamics of perfusion and exchange across the BBB as well as affect the glymphatic pathway [Citation57]. However, these studies collectively suggest that long-term blood pressure variability is an independent risk factor for cognitive impairment or dementia. Therefore, an intervention plan for reducing blood pressure variability could be an important target for prevention of VCI.
Hypertension is associated with greater WMHs volumes and progression of white matter changes [Citation82,Citation83], which are known to cause cognitive decline. These observations suggest that high blood pressure promotes white matter damage. In addition, diffusion-tensor MR has indicated that there are more microstructural changes within the white matter in hypertensives than in normotensives. Consistent with this the CARDIA cohort study showed that higher systolic blood pressure from young adulthood to midlife was associated with greater changes in fluidity of normal appearing white matter in middle aged adults [Citation84]. Thus, integrity of the white matter and therefore brain connectivity is an important substrate of hypertension and cardiovascular disease [Citation85] related brain injury and potential VCI. Consistent with this is a recent postmortem study [Citation86], which showed that use of antihypertensives in life by older individuals was associated with a reduction in white matter perivascular dilation and rarefaction and edema.
Still hypertension probably remains the most modifiable among causes of cardiovascular and cerebral vascular diseases leading to VCI (). Hypertension can be reduced by 10–15% if controlled via safe medication and modification of behaviors [Citation87]. While none of the recent trials have incorporated VCI as the primary cognitive outcome, current meta-analyses of randomized controlled trials suggest lowering blood pressure provides at best modest or no benefits on declining cognitive function (). However, several randomized controlled trials have shown marginally beneficial or neutral results in relation to blood pressure treatment or lowering and cognitive outcomes (). While cognition was not worsened, none of the blood pressure medications (e.g. candersartan, telmisartan, atenolol, ramipril) or combination of two agents showed consistent effects in blood pressure lowering on cognition [Citation78]. Irrespective, majority of the meta-analyses (90%) show a clear association between blood pressure reduction and lower risk of incident dementia or stabilization of cognitive decline ().
Table 2. Pharmacological approaches to control VRFs and prevent VCI progression.
Of increased interest is that as conveyed by the SPRINT MIND trials, beneficial effects were apparent not only on cognition but other markers of VCI (e.g. brain volume, WMHs), depending on whether the target treatment included intensive systolic blood pressure (<120 mm Hg) or standard treatment (<140 mm Hg). Upon secondary analysis of data from a SPRINT trial [Citation119], intensive systolic blood pressure control in 80-year-olds resulted in lower risk of major cardiovascular events, mild cognitive impairment and death, with no between-group differences in the rate of injurious falls. However, the benefits did not extend to older adults with lower baseline cognitive function [Citation119]. Encouraging results were also apparent in the most recent SPRINT study on intensive blood pressure control in mild cognitive impairment patients [Citation120]. These results not only suggest that intensive treatment decreased risk for amnestic and multi-domain subtypes of mild cognitive impairment but highlight the relevance of using cognitive impairment as a primary outcome measure in individuals in prodromal stages of dementia or who progress to frank dementia. Taken together, these meta-analyses suggest there is a measure of benefit in slowing cognitive decline. In terms of classes of antihypertensives, angiotensin-2 receptor blockers (ARBs) seem better than other agents although diuretics were also effective in a limited way (). However, a number of variables seem to govern these trials including the type of eligibility criteria including patients with high enough cardiovascular risk, aim to preserve cognition or slow impairment, adequate sample size, follow-up time, and utilization of relevant neuropsychological tests for primary outcomes of cognitive impairment or dementia [Citation78].
6.2. Diabetes mellitus and glucose-lowering strategy
Diabetes mellitus (DM) or T2DM is a considerable risk for VCI largely because it is involved in SVD [Citation121]. It increases risk of VaD by 2.5 fold and of AD by 1.5 fold [Citation122]. Recent analysis of the UK Biobank records [Citation123] indicated that T2DM was associated with marked cognitive deficits, particularly in executive functioning and processing speed, which are key cognitive domains of VCI. Alarmingly, in U.S.A. approximately one-third of the adult population has prediabetes or diabetes [Citation124]. Conversely, about one-half of persons aged 60 years and older, who are most at risk for cognitive impairment may have prediabetes or diabetes. The UK Biobank data also suggest there is marked acceleration of normal brain aging in individuals with long standing T2DM. Imaging records suggest gray matter atrophy occurs, particularly in subcortical regions involved in fronto-subcortical circuits, at a ~ 26% faster rate in diabetics compared to those aging normally whereas disease duration was associated with increased neurodegeneration [Citation123].
The prevalence of T2DM tends to occur most often with hypertension, particularly in certain ethnic groups. For example, hypertension and T2DM frequencies were almost two times and five times higher in the Boston Hispanic community adults than in Alzheimer’s Disease Neuroimaging Initiative (ADNI) non-Hispanic White participants. Diffusion-tensor MRI showed more white matter damage and smaller hippocampal volumes and larger brain aging deviations consistent with lower executive function and global cognitive scores in individuals with both hypertension and T2DM [Citation125] Consistent with these findings, a large RCT reported that intensive blood pressure control (systolic BP target <120 mm Hg versus <140 mm Hg) may reduce death and cardiovascular events among patients with T2DM receiving standard glycemic treatment and without cognitive impairment [Citation126].
Irrespective, it is clear that comorbidities promote greater brain structural disruptions and probably more cognitive domains than one risk such as hypertension alone. However, there is conflicting evidence from clinical trials of T2DM interventions evaluating the effects of greater glucose control on cognitive decline. Lifestyle interventions in people with diabetes and prediabetes do not seem to be related to better cognitive outcomes either. Collectively, these findings argue for targeting multiple domains or deploying polypill approaches to prevention of VCI. It is not entirely clear how diabetes causes SVD but there is increasing interest in use of glucose-lowering strategies [Citation127]. Hyperglycaemia may inflict direct effects on brain tissue, or it is likely that there is deleterious accumulation of glycated protein conjugates and oxidants in blood vessel walls of smaller arteries causing reduced pulsatility and vascular tone to cause hypoperfusion. In addition, there may be effects of one or more diabetes-linked comorbidities including hypertension, dyslipidaemia and hyperinsulinemia. Among glucose-lowering agents, metformin was associated with better cognitive function, particularly executive tasks whereas injectable insulin was largely ineffective in improving or stabilizing cognitive decline (). Metformin is considered a first-line anti-diabetic medication for T2DM. It has several effects including promoting neurogenesis, reduce oxidative stress, enhance spatial memory deficits and more probably acts as an antidepressant [Citation128]. Adding thiazolidinedione, or dipeptidyl peptidase IV (DPP-4) instead of sulphonylureas as a second-line anti-diabetic treatment may be considered for delaying or preventing impairment although recommended clinical guidelines point at further investigation. Additionally, thiazolidinedione users relative to non-users on dual oral therapy were significantly associated with lower risk of various types of dementia [Citation106]. Pooled results from large registries also suggest that glucagon-like pepetide-1 (GLP-1) receptor agonists provide another option to reduce VCI burden [Citation110]. All cause dementia rate was lower in T2DM patients taking GLP-1 receptor agonists by 11–33% ().
Newer glucose lowering drugs were associated with a decreased risk of all-cause dementia in people with T2DM. However, the observational nature and significant heterogeneity between studies, necessitates that the results should be interpreted with caution. Further research is warranted to confirm our findings [Citation111]. Sodium glucose co-transporter-2 (SGLT2) inhibitors showed an association with lower dementia risk in older people with T2DM. Randomized controlled trials are warranted [Citation114].
6.3. Dyslipidaemia and obesity
The imbalance of lipids, particularly triglycerides and LDL-cholesterol in the blood, has been of much interest both in the context of stroke and dementia incidence [Citation129]. Recent meta-analysis involving records of some 1.2 million subjects strongly suggests midlife hypercholesterolemia is associated with increased incidence of MCI as well as all cause dementia. Furthermore, each 1 mmol/L increase in low-density lipoprotein was associated with an 8% increase in incidence of all-cause dementia [Citation130] Similarly, a longitudinal cohort study reported that elevated triglycerides as well as total cholesterol were associated with greater 20-year decline in executive function, attention and processing speed, all features of VCI. Higher total cholesterol and triglycerides were further associated with greater decline in memory [Citation131]. Thus, in keeping with risk of stroke, dyslipidaemia even as component of the metabolic syndrome is invariably associated with higher risk of VCI.
Obesity defined as body mass index of greater than 30 is associated with decreased blood supply to the brain likely causing rarefaction of the white matter [Citation132,Citation133]. Obesity induces sustained release of the adipocyte-secreted proteins and a repertoire of inflammatory cytokines, which explain the association between obesity and increased risk of dementia. In chronic phase these changes may alter neuronal function and induce cerebral atrophy [Citation134,Citation135].
6.4. Other risks both acquired and environmental
Several other risk factors may directly or indirectly be implicated in the causation of VCI by enhancing covert changes or even overt vascular disease (). The well known among these with a clear vascular basis include hyperhomocysteinaemia and heart disease that may influence cognitive function. It is widely known that excessive alcohol and tobacco use increase vascular damage and therefore can affects cognitive function by compromised brain perfusion. Peripheral arterial disease is also regarded as a risk for cognitive impairment.
While there is not much information on hearing loss and VCI, there is now good evidence that depressive illness is strongly associated with cardiovascular disease as well as dementia. People with cardiovascular disease are at higher risk for dementia but also mental disorders including depression [Citation1]. Major depressive disorder is associated with the progression of a range of vascular disease comorbidities and mortality [Citation2]. In fact, there is a bidirectional relationship between depression and stroke risk. Depression increases the risk of stroke, there is high prevalence of depression post-stroke, and this appears to worsen post-stroke, outcomes possibly promoted by inflammatory mechanisms. In addition, the ‘vascular depression’ hypothesis is characterized by executive dysfunction and memory impairment and SVD in the frontal lobe [Citation31], which is challenging to treat clinically, and where cognitive impairment does not full resolve with effective depression treatment, resulting in a high-risk state for progression. These observations, however, collectively suggest reducing risk for vascular disease and treating depression will impact on VCI. Among the least investigated risk factors associated with stroke incidence, air pollution is gaining prominence as an important risk to vascular brain health. Accumulating evidence suggests that exposure to several air pollutants containing particulate matter is associated with reduced white matter volumes and integrity [Citation136,Citation137]. Short-term and long-term particulate matter (e.g. at PM2.5) may also cause vascular damage and lead to neurodegeneration [Citation138].
Remarkably, there is increased risk of VCI compared with Alzheimer type of dementia in countries yet to undergo demographic transition [Citation139]. This is because of the links between stroke and cerebrovascular diseases due to several infectious diseases including HIV [Citation140]. In addition, adverse childhood experiences in LMICs including both psychosocial such as neglect and abuse but also displacement, and early malnutrition are recognized to be associated with increased cardiovascular risk in later life [Citation141,Citation142]. Moreover, there is an association between mental health conditions such as post-traumatic stress disorder and increased cardiovascular risk [Citation143]. This may be due to more risky lifestyles but also long-term inflammation [Citation140]. The mechanism is not well understood, but it’s important to explore as a psychotherapy and addressing post-traumatic stress disorder may be protective against later CVD.
7. View on pharmacological interventions
Prevention of VCI may also be implemented by pharmacological management of blood pressure, T2DM, dyslipidaemia, and antiplatelet therapy for secondary prevention of stroke and relevant long-term outcomes. Interventions for specific causes of stroke including anticoagulation could also benefit patients at risk of VCI (). The recent European Stroke Organisation guidelines [Citation144] suggest treatments for long-term risk of recurrent ischemic stroke should involve lowering blood pressure lowering to < 130/80 mmHg, use of statins (HMGCoA-reductase inhibitors) and reducing low density lipoprotein level to <1.8 mmol/l (70 mg/dl) as well as avoiding dual antiplatelet therapy with aspirin and clopidogrel after first 90 days, rule out direct oral anticoagulant drugs for embolic stroke of undetermined source and consider pioglitazone for T2DM or insulin resistance upon careful consideration of possible risks. However, encouragingly most current meta-analyses involving several thousands of patients have indicated mostly favorable effects of pharmacological agents on stabilizing, reducing or delaying VCI ().
8. Protective factors
To keep age-related vascular disease at bay and maintain cognitive function and vascular brain health a number of protective factors could prevent or substantially delay VCI. These include adopting healthy diets and, increasing physical and cognitive activities even in older age and promoting multidomain approaches globally in all settings at the population level are priority ().
Table 3. Lifestyle and non-pharmacological approaches to prevent VCI.
8.1. Dietary measures and food ingredients
Over the past 20 years various efforts have been made to promote healthy eating habits in midlife and late-life (). There are several studies showing limited benefits of the Medi, DASH and MIND diets and from consumption of berries, leafy green vegetables, fresh food ingredients and nuts. Foods with high fiber content are also advocated but evidence for beneficial effects from consumption of these in large quantities is derived from experimental studies. Among Indigenous Africans, consumption of plant-based diets including green leafy vegetables has demonstrated dose-dependent protection against occurrence of stroke, hypertension giving some protection against post-stroke VCI [Citation164,Citation165]. These are largely thought to impact on oxidative and nitrosative stresses, mitophagy processes and inflammation. Thus diets rich in antioxidants, vitamins B, D and K can contribute to better vascular brain health [Citation166]. The invention of the Mediterranean (medi) diet has been major advancement. Adherence to the medi diet indicates a positive trajectory with excellent potential benefits for mental and cognitive health among Southern Italian elderly [Citation167]. Indeed, its implementation as an intervention as well as prevention has overall been perceived as a positive step forward although the benefits have not always been substantial. In recent years, the DASH and MIND diets have been invented particularly to reduce risk of AD [Citation168] and related disorders () and therefore have promise for VCI. Moreover, studies have also suggested intake of total saturated fatty acids intake appeared to be inversely associated with cognitive impairment and that specific subtypes of fatty acids including short- and middle-chain saturated fatty acids are more beneficial [Citation169].
The benefits of coffee consumption have also been of interest in the context of dietary lifestyles. For example, moderate daily mocha coffee drinking was associated with higher cognitive and mood status among individuals at risk of mild VCI and age-related depression [Citation170,Citation171]. Other approaches have been to target the gut microbiome [Citation172]. The production status of equol, a metabolite of soy isoflavone, is dependent on the gut microbiome, and equol-producing status was found to be associated with delayed burden of WMH some 6–9 years later, suggesting that the gut microbiome plays a key role in the development of SVD via the gut-brain axis. Overall, these measures have produced moderately positive results in either stabilizing or reducing cognitive decline but certainly show no harmful effects.
8.2.1. Physical Activity
Current meta-analyses suggest there are some effects on prevention of cognitive decline and dementia (). Just over 50% of all the studies show beneficial effects of exercise on cognition. Multiple activity component protocols involving aerobic exercise or integrity training seem to be more beneficial. For example, isometric exercise training improves vascular integrity and elicits blood pressure reductions in hypertensives greater than those seen with dynamic aerobic and resistance exercises. Such training may stimulate reactive hyperemia to trigger a cascade of vascular, neurotrophic and neuro-endocrine events leading to improvement in cognitive function [Citation173]. Similarly, regimes that promote cardiorespiratory fitness and increase cerebral vascular function incorporating cerebral vasoreactivity and cerebral pulsatility are more likely to impact on cognitive function even with increasing age-related arterial stiffness [Citation174]. Consistent with experimental studies, the beneficial effects of enriched environment and intense physical activity not only reduce WM abnormalities but also recurrent stroke lesions [Citation175,Citation176]. However, more research is needed in human trials with greater optimization in methods of assessment, types of training to test, physiological limitations of the participants and outcome measures (). The presence of vascular pathology in the brain of would-be participants in such trials would also be factor in how they might benefit or improve. In a unique pathological study [Citation177], cystic infarcts and microinfarcts, but not pathological markers of dementia were associated with physical performance levels five years prior to death. These observations suggest degrees of physical function in the years prior to death are affected by vascular brain pathologies, which include substrates of VCI such as WMHs, microbleeds and microinfarcts. Thus, an evident implication from these studies is that irrespective of the robust findings it is imperative to do all possible to reduce vascular disease risk and promote vascular protection.
9. Multidomain approaches
Multi-domain rather than single domain approaches is likely to be beneficial in prevention (). Interventions need to ensure factors such as frailty or co-morbid conditions, current medications and potential lifestyles could impede interpretation of the outcomes. Muti-domain trials that particularly target cardiovascular risk reduction or vascular prevention [Citation178] would benefit not only VCI but all cause dementias. In the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial involving 60–77 year-olds and with high Cardiovascular Risk Factors, Aging and Dementia Risk scores showed a positive change in global cognition with effects on executive function and remarkably in processing speed [Citation160]. Other large multidomain trials (FINGER, MAPT, and PreDIVA) have shown that a multidomain lifestyle intervention can benefit cognition in elderly people with an elevated risk of dementia (). Overall, results from these three trials suggest that targeting of preventive interventions to at-risk individuals is an effective strategy. There is further promise from more ongoing multidomain trials such as Medex-UK [Citation179] with feasible protocols and strong encouragement to adherence. The Japan-Multimodal Intervention Trial for Prevention of Dementia PRIME Tamba (J-MINT PRIME Tamba) randomized clinical trials involving participants aged 65–85 years living in a rural Japan is also expected to deliver beneficial effects on those at risk of VCI and dementia [Citation162].
Other non-pharmacological approaches may include the use of transcranial magnetic stimulation, as a noninvasive tool to evaluate in vivo cortical excitability and relate to the pathophysiological process and VCI progression. It can enable responders to benefit from specific pharmacological or other agents in the attempt to restore neural plasticity [Citation180,Citation181].
10. Expert opinion
VCI represents a huge burden in the older population. The true prevalence or incidence of VCI is not known but it is certainly more common than any of the dementias by virtue of the prevalence of both stroke and cardiovascular disease. The high global burden of VCI estimated to be greater than 4 million in 2019 compared to 7.2 million cases of dementia reported by the GBD collaborators predict proportionally high rates of morbidity, cognitive and behavioral disorders and mortality. While major VCI or VaD is recognized as the second most common cause of dementia worldwide, the prevalence of mild VCI which involving executive dysfunction different from memory deficits is grossly underestimated. Thus, we need better prevalence and incident data on VCI to more confidently address strategies for prevention and protection. VCI can also be different in LMICs. It is clear that both strategies for primary and secondary stroke prevention strategies will impact on VCI. The key modifiable risk factors for vascular disease include hypertension, T2DM, dyslipidaemia, obesity and metabolic syndrome. These factors clearly increased risk of stroke and impair vascular brain health. Indeed, there is now stroke epidemiological and experimental evidence suggesting that lifestyle factors and dietary habits influence cerebrovascular regulation and impact on VCI [Citation182,Citation183]. Hypertension is the most common modifiable risk factor for VCI, but emerging data suggest that blood pressure variability may be even more important. Recent meta-analyses on use of specific pharmacological approaches to control vascular risk suggest there are definite albeit small to moderate benefits in either preservation or improvement of cognitive function. This especially in those studies where there is good adherence to treatment. However, better designed randomized clinical trials to particularly assess efficacy of medications are needed. Universally standardized eligibility criteria, adequate sample size and length of follow-up time are key factors in obtaining more reliable data. In terms of eligibility for example the selection of patients with high enough cardiovascular risk should be considered [Citation78]. The selection of primary outcomes would also be important. For VCI, trials should ensure either mild VCI or severe VCI (VaD) criteria is fulfilled. Mild VCI target is reasonable as it is defined to include impairment in at least one cognitive domain and mild to no impairment in activities of daily living or instrumental activities of daily living. This would be independent of any motor or sensory sequelae of the vascular event [Citation16].
Other risk factors delineated by the 2020 Lancet Dementia Commission that negatively impact on vascular function and promote cognitive decline include lifestyle habits of smoking, alcohol use and physical inactivity. Non-pharmacological approaches including cessation of smoking even later in life and sustaining physical and cognitive activities and encouraging multidomain approaches are good for the prevention of stroke and cognitive decline. Thus, future trials should always consider multi-domain intervention rather than a single area of intervention. Current data suggest that physical exercise to a moderate level is beneficial. However, more vigorous or extreme training does not necessarily add to the benefit. Protocols encouraging cardiorespiratory fitness and enhance cerebral vascular function including cerebral vasoreactivity and pulse will have greater impact on cognitive function. Diet is an important player in maintaining vascular brain health. Several studies show reasonable benefits of the Medi, DASH and MIND diets and regular or even daily consumption of particularly berries, leafy green vegetables, nuts and high fiber are advocated for sustaining low blood pressure, better gut microbiome environment, reducing stroke risk and protection against other risks.
Exposure to degrees of air pollution no doubt enhances VCI and more vigorous control at the individual level in homes or within communities would promote better vascular functioning and cerebral perfusion. However, clear mechanistic studies are needed to fully understand how particulate matter affects vascular function. There is now a remarkable understanding on the association of cardiovascular disease and mental health, depression and behavioral disorders in both causal directions. In controlling cardiovascular risk there would be a dual advantage in reducing the burden of mental illness as well as VCI. There are still several other factors which may potentially affect VCI. These are not specifically addressed in this review. For example, factors such as stress, general wellness, sleep (or sleep apnea), poverty or satisfactory occupation could have implications for vascular brain health. However, all measures and policy changes that address control of vascular disease risk at the individual, community and population levels have the potential to protect vascular brain health and reduce the burden of VCI.
Article highlights
The worldwide prevalence of VCI is not known but is estimated to be high, particularly in the Global South.
Vascular risk factors including hypertension, type 2 diabetes and dyslipidaemia evident in midlife are key modifiable factors.
Current meta-analyses overall reveal control of the key vascular disease risks provide small to moderate benefits in reducing incident dementia but are not wholly detrimental.
Prevention via control of risk factors, protective factors including adherence to diet and greater physical activity are key to reducing burden of VCI.
All measures and drives at the individual and population levels to reduce stroke injury and protect vascular brain health would reduce the burden of VCI.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Karami N, Kazeminia M, Karami A, et al. Global prevalence of depression, anxiety, and stress in cardiac patients: a systematic review and meta-analysis. J Affective Disorders. 2023;324:175–189. doi:10.1016/j.jad.2022.12.055
- Warriach ZI, Patel S, Khan F, et al. Association of depression with cardiovascular diseases. Cureus. 2022;14(6):e26296. doi:10.7759/cureus.26296
- Goldfarb M, De Hert M, Detraux J, et al. Severe mental illness and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80(9):918–933. doi: 10.1016/j.jacc.2022.06.017
- Arnaud AM, Brister TS, Duckworth K, et al. Impact of major depressive disorder on comorbidities: a systematic literature review. J Clin Psychiatry. 2022;83(6). doi: 10.4088/JCP.21r14328
- Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496
- Akinyemi RO, Mukaetova-Ladinska EB, Attems J, et al. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Curr Alzheimer Res. 2013;10(6):642–653. doi:10.2174/15672050113109990037
- Howlett SE, Rockwood K. Ageing: develop models of frailty. Nature. 2014;512(7514):253. doi:10.1038/512253d
- Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age Ageing. 2015;44(4):545–547. doi:10.1093/ageing/afv043
- Wang X, Carcel C, Woodward M, et al. Blood pressure and stroke: a review of sex- and Ethnic/Racial-specific attributes to the epidemiology, pathophysiology, and management of raised blood pressure. Stroke. 2022;53(4):1114–1133. doi:10.1161/STROKEAHA.121.035852
- O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/S1474-4422(03)00305-3
- Hachinski V, Iadecola C, Petersen RC, et al. National Institute of neurological disorders and stroke–Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47
- Sachdev P. Vascular cognitive disorder. Int J Geriat Psychiatry. 1999;14(5):402–403. doi:10.1002/(SICI)1099-1166(199905)14:5<402:AID-GPS958>3.0.CO;2-H
- American Psychiatric Association D, Association AP. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American psychiatric association; 2013. p. 591–644. doi: 10.1176/appi.books.9780890425596
- Sachdev P, Kalaria R, O’Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034
- Skrobot OA, O’Brien J, Black S, et al. The vascular impairment of cognition classification consensus study. Alzheimer’s Dementia. 2017;13(6):624–633. doi: 10.1016/j.jalz.2016.10.007
- Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the vascular impairment of cognition classification consensus study. Alzheimer’s Dementia. 2018;14(3):280–292. doi: 10.1016/j.jalz.2017.09.007
- Sachdev PS, Lipnicki DM, Crawford JD, et al. The vascular behavioral and cognitive disorders criteria for vascular cognitive disorders: a validation study. Eur J Neurol. 2019;26(9):1161–1167. doi:10.1111/ene.13960
- Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/WNL.43.2.250
- Akinyemi RO, Owolabi MO, Ihara M, et al. Stroke, cerebrovascular diseases and vascular cognitive impairment in Africa. Brain Res Bull. 2019;145:97–108. doi: 10.1016/j.brainresbull.2018.05.018
- Lam BYK, Cai Y, Akinyemi R, et al. The global burden of cerebral small vessel disease in low- and middle-income countries: a systematic review and meta-analysis. Int J Stroke. 2023;18(1):15–27. doi: 10.1177/17474930221137019
- Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(7):684–696. doi:10.1016/S1474-4422(19)30079-1
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6
- Mijajlovic MD, Pavlovic A, Brainin M, et al. Post-stroke dementia – a comprehensive review. BMC Med. 2017;15(1):11. doi: 10.1186/s12916-017-0779-7
- Leys D, Hénon H, Mackowiak-Cordoliani M-A, et al. Poststroke dementia. Lancet Neurol. 2005;4(11):752–759. doi:10.1016/S1474-4422(05)70221-0
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–1018. doi:10.1016/S1474-4422(09)70236-4
- Pendlebury ST, Rothwell PM, Oxford Vascular S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford vascular study. Lancet Neurol. 2019;18(3):248–258. doi:10.1016/S1474-4422(18)30442-3
- Craig L, Hoo ZL, Yan TZ, et al. Prevalence of dementia in ischaemic or mixed stroke populations: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2022;93(2):180–187. doi:10.1136/jnnp-2020-325796
- Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2012;134(Pt 12):3716–3727. doi: 10.1093/brain/awr273
- Bejot Y, Aboa-Eboule C, Durier J, et al. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke. 2011;42(3):607–612. doi: 10.1161/STROKEAHA.110.595553
- Pohjasvaara T, Erkinjuntti T, Vataja R, et al. Dementia three months after stroke. Baseline frequency and effect of different definitions of dementia in the helsinki stroke aging memory study (SAM) cohort. Stroke. 1997;28(4):785–792. doi:10.1161/01.STR.28.4.785
- Thomas AJ, Kalaria RN, O’Brien JT. Depression and vascular disease: what is the relationship? J Affective Disorders. 2004;79(1–3):81–95. doi:10.1016/S0165-0327(02)00349-X
- Allan LM, Rowan EN, Thomas AJ, et al. Long-term incidence of depression and predictors of depressive symptoms in older stroke survivors. Br J Psychiatry. 2013;203(6):453–460. doi:10.1192/bjp.bp.113.128355
- Bella R, Ferri R, Cantone M, et al. Motor cortex excitability in vascular depression. Int J Psychophysiol. 2011;82(3):248–253. doi: 10.1016/j.ijpsycho.2011.09.006
- Polvikoski TM, van Straaten EC, Barkhof F, et al. Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology. 2010;75(23):2071–2078. doi: 10.1212/WNL.0b013e318200d6f9
- Zekry D, Duyckaerts C, Moulias R, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol. 2002;103(5):481–487. doi: 10.1007/s00401-001-0493-5
- Schneider JA, Wilson RS, Bienias JL, et al. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148–1155. doi:10.1212/01.WNL.0000118211.78503.F5
- Kalaria RN, Kenny RA, Ballard CG, et al. Towards defining the neuropathological substrates of vascular dementia. J Neurolog Sci. 2004;226(1–2):75–80. doi:10.1016/j.jns.2004.09.019
- Kalaria RN, Ihara M. Medial temporal lobe atrophy is the norm in cerebrovascular dementias. Eur J Neurol. 2017;24(4):539–540. doi:10.1111/ene.13243
- Collaborators GBDS, Stark BA, Johnson CO. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0
- Ding Q, Liu S, Yao Y, et al. Global, regional, and National burden of ischemic stroke, 1990–2019. Neurology. 2022;98(3):e279–e290. doi:10.1212/WNL.0000000000013115
- Collaborators GBDD. The burden of dementia due to down syndrome, Parkinson’s disease, stroke, and traumatic brain injury: a systematic analysis for the global burden of disease study 2019. Neuroepidemiology. 2021;55(4):286–296
- Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
- Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabet Res Clin Pract. 2022;188:109924. doi: 10.1016/j.diabres.2022.109924
- Exalto LG, Weaver NA, Kuijf HJ, et al. Sex differences in Poststroke cognitive impairment: a multicenter study in 2343 patients with acute ischemic stroke. Stroke. 2023;54(9):2296–2303. doi: 10.1161/STROKEAHA.123.042507
- Cantone M, Fisicaro F, Ferri R, et al. Sex differences in mild vascular cognitive impairment: a multimodal transcranial magnetic stimulation study. PLoS One. 2023;18(3):e0282751. doi: 10.1371/journal.pone.0282751
- Bryan RN, Cai J, Burke G, et al. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the atherosclerosis risk in communities study. AJNR Am J Neuroradiol. 1999;20(7):1273–1280.
- Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339(jul06 1):b2477. doi: 10.1136/bmj.b2477
- Del Brutto OH, Mera RM, Cagino K, et al. Neuroimaging signatures of frailty: a population-based study in community-dwelling older adults (the atahualpa project). Geriatrics Gerontol Int. 2016;17(2):270–276. doi: 10.1111/ggi.12708
- Jochems ACC, Arteaga C, Chappell F, et al. Longitudinal changes of white matter hyperintensities in sporadic small vessel disease: a systematic review and meta-analysis. Neurology. 2022;99(22):e2454–e2463. doi: 10.1212/WNL.0000000000201205
- Zhang F, Ping Y, Jin X, et al. White matter hyperintensities and post-stroke depression: a systematic review and meta-analysis. J Affective Disorders. 2023;320:370–380. doi:10.1016/j.jad.2022.09.166
- Howard DPJ, Gaziano L, Rothwell PM, et al. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021;20(3):193–202. doi:10.1016/S1474-4422(20)30484-1
- Lazar RM, Wadley VG, Myers T, et al. Baseline cognitive impairment in patients with asymptomatic carotid stenosis in the CREST-2 trial. Stroke. 2021;52(12):3855–3863. doi: 10.1161/STROKEAHA.120.032972
- Blevins BL, Vinters HV, Love S, et al. Brain arteriolosclerosis. Acta Neuropathol. 2021;141(1):1–24. doi: 10.1007/s00401-020-02235-6
- Kalaria RN, Hase Y. Neurovascular Ageing and age-related diseases. Subcell Biochem. 2019;91:477–499.
- Molnar AA, Nadasy GL, Dornyei G, et al. The aging venous system: from varicosities to vascular cognitive impairment. Geroscience. 2021;43(6):2761–2784. doi: 10.1007/s11357-021-00475-2
- Kapadia A, Dmytriw AA. Venous dysfunction plays a critical role in “normal” white matter disease of aging. Med Hypotheses. 2021;146:110457. doi:10.1016/j.mehy.2020.110457
- Carare RO, Aldea R, Agarwal N, et al. Clearance of interstitial fluid (ISF) and CSF (CLIC) group-part of vascular professional interest area (PIA): cerebrovascular disease and the failure of elimination of amyloid-beta from the brain and retina with age and Alzheimer’s disease-opportunities for therapy. Alzheimer’s Dementia. 2020;12(1):e12053.
- Poh L, Sim WL, Jo DG, et al. The role of inflammasomes in vascular cognitive impairment. Mol Neurodegener. 2022;17(1):4. doi: 10.1186/s13024-021-00506-8
- Custodero C, Ciavarella A, Panza F, et al. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: a systematic review and meta-analysis. Geroscience. 2022;44(3):1373–1392. doi: 10.1007/s11357-022-00556-w
- Skrobot OA, McKnight AJ, Passmore PA, et al. A validation study of vascular cognitive impairment genetics meta-analysis findings in an independent collaborative cohort. J Alzheimer’s Disease: JAD. 2016;53(3):981–989. doi: 10.3233/JAD-150862
- Ballard CG, Morris CM, Rao H, et al. APOE epsilon4 and cognitive decline in older stroke patients with early cognitive impairment. Neurology. 2004;63(8):1399–1402. doi: 10.1212/01.WNL.0000141851.93193.17
- Kalaria RN, Kittner SJ. Top-NOTCH3 variants in the population at large. Stroke. 2020;51(12):3482–3484. doi:10.1161/STROKEAHA.120.031609
- Cho BPH, Harshfield EL, Al-Thani M, et al. Association of vascular risk factors and genetic factors with penetrance of variants causing monogenic stroke. JAMA Neurol. 2022;79(12):1303–1311. doi:10.1001/jamaneurol.2022.3832
- Rajeev V, Chai YL, Poh L, et al. Chronic cerebral hypoperfusion: a critical feature in unravelling the etiology of vascular cognitive impairment. Acta Neuropathol Commun. 2023;11(1):93. doi: 10.1186/s40478-023-01590-1
- Rolandi E, Zaccaria D, Vaccaro R, et al. Estimating the potential for dementia prevention through modifiable risk factors elimination in the real-world setting: a population-based study. Alzheimer’s Res Ther. 2020;12(1):94. doi: 10.1186/s13195-020-00661-y
- Ingaramo RA. Obesity, diabetes, and other cardiovascular risk factors in native populations of South America. Curr Hypertens Rep. 2016;18(1):9. doi:10.1007/s11906-015-0613-6
- Noale M, Limongi F, Maggi S. Epidemiology of cardiovascular diseases in the elderly. Adv Exp Med Biol. 2020;1216:29–38.
- Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134(Pt 12):3716–3727. doi: 10.1093/brain/awr273
- Afilalo J, Karunananthan S, Eisenberg MJ, et al. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103(11):1616–1621. doi:10.1016/j.amjcard.2009.01.375
- Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35(26):1726–1731. doi:10.1093/eurheartj/ehu197
- Gheorghe A, Griffiths U, Murphy A, et al. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: a systematic review. BMC Public Health. 2018;18(1):975. doi:10.1186/s12889-018-5806-x
- Delgado J, Masoli J, Hase Y, et al. Trajectories of cognitive change following stroke: stepwise decline towards dementia in the elderly. Brain Commun. 2022;4(3):fcac129. doi: 10.1093/braincomms/fcac129
- McHutchison CA, Backhouse EV, Cvoro V, et al. Education, socioeconomic status, and intelligence in childhood and stroke risk in later life: a meta-analysis. Epidemiology. 2017;28(4):608–618. doi:10.1097/EDE.0000000000000675
- Sarki AM, Nduka CU, Stranges S, et al. Prevalence of hypertension in low- and middle-income countries: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(50):e1959. doi:10.1097/MD.0000000000001959
- Cantone M, Lanza G, Puglisi V, et al. Hypertensive crisis in acute cerebrovascular diseases presenting at the emergency department: a narrative review. Brain Sci. 2021;11(1):70. doi: 10.3390/brainsci11010070
- Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990-2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043
- Zhou B, Carrillo-Larco RM, Danaei G, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1
- Mahinrad S, Sorond FA, Gorelick PB. Hypertension and cognitive dysfunction: a review of mechanisms, life-course observational studies and clinical trial results. Rev Cardiovasc Med. 2021;22(4):1429–1449. doi:10.31083/j.rcm2204148
- Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375(9718):938–948. doi:10.1016/S0140-6736(10)60309-1
- de Heus RAA, Tzourio C, Lee EJL, et al. Association between blood pressure variability with dementia and cognitive impairment: a systematic review and meta-analysis. Hypertension. 2021;78(5):1478–1489. doi: 10.1161/HYPERTENSIONAHA.121.17797
- Jia P, Lee HWY, Chan JYC, et al. Long-term blood pressure variability increases risks of dementia and cognitive decline: a meta-analysis of longitudinal studies. Hypertension. 2021;78(4):996–1004. doi:10.1161/HYPERTENSIONAHA.121.17788
- Aribisala BS, Morris Z, Eadie E, et al. Blood pressure, internal carotid artery flow parameters, and age-related white matter hyperintensities. Hypertension. 2014;63(5):1011–1018. doi: 10.1161/HYPERTENSIONAHA.113.02735
- Markus HS, Erik de Leeuw F. Cerebral small vessel disease: recent advances and future directions. Int J Stroke. 2023;18(1):4–14. doi:10.1177/17474930221144911
- Jiang X, Lewis CE, Allen NB, et al. Premature cardiovascular disease and brain health in midlife: the CARDIA study. Neurology. 2023;100(14):e1454–e1463. doi:10.1212/WNL.0000000000206825
- Kim WSH, Luciw NJ, Atwi S, et al. Associations of white matter hyperintensities with networks of gray matter blood flow and volume in midlife adults: a coronary artery risk development in young adults magnetic resonance imaging substudy. Human Brain Mapp. 2022;43(12):3680–3693. doi: 10.1002/hbm.25876
- Affleck AJ, Sachdev PS, Halliday GM. Past antihypertensive medication use is associated with lower levels of small vessel disease and lower Aβ plaque stage in the brains of older individuals. Neuropathol Appl Neurobiol. 2023;49(4):e12922. doi: 10.1111/nan.12922
- Kayima J, Wanyenze RK, Katamba A, et al. Hypertension awareness, treatment and control in Africa: a systematic review. BMC Cardiovasc Disord. 2013;13(1):54. doi: 10.1186/1471-2261-13-54
- Chang-Quan H, Hui W, Chao-Min W, et al. The association of antihypertensive medication use with risk of cognitive decline and dementia: a meta-analysis of longitudinal studies. Int J Clin Pract. 2011;65(12):1295–1305. doi: 10.1111/j.1742-1241.2011.02810.x
- Levi Marpillat N, Macquin-Mavier I, Tropeano AI, et al. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J Hypertens. 2013;31(6):1073–1082. doi:10.1097/HJH.0b013e3283603f53
- Tully PJ, Hanon O, Cosh S, et al. Diuretic antihypertensive drugs and incident dementia risk: a systematic review, meta-analysis and meta-regression of prospective studies. J Hypertens. 2016;34(6):1027–1035. doi:10.1097/HJH.0000000000000868
- Gupta A, Perdomo S, Billinger S, et al. Treatment of hypertension reduces cognitive decline in older adults: a systematic review and meta-analysis. BMJ Open. 2020;10(11):e038971. doi:10.1136/bmjopen-2020-038971
- Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323(19):1934–1944. doi: 10.1001/jama.2020.4249
- Ou YN, Tan CC, Shen XN, et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020;76(1):217–225. doi: 10.1161/HYPERTENSIONAHA.120.14993
- Peters R, Yasar S, Anderson CS, et al. Investigation of antihypertensive class, dementia, and cognitive decline: a meta-analysis. Neurology. 2020;94(3):e267–e281. doi: 10.1212/WNL.0000000000008732
- den Brok M, van Dalen JW, Abdulrahman H, et al. Antihypertensive medication classes and the risk of dementia: a systematic review and network meta-analysis. J Am Med Dir Assoc. 2021;22(7):1386–1395 e1315. doi: 10.1016/j.jamda.2020.12.019
- Ho JK, Moriarty F, Manly JJ, et al. Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: a meta-analysis. Hypertension. 2021;78(3):629–643. doi: 10.1161/HYPERTENSIONAHA.121.17049
- Peters R, Xu Y, Fitzgerald O, et al. Blood pressure lowering and prevention of dementia: an individual patient data meta-analysis. Eur Heart J. 2022;43(48):4980–4990. doi: 10.1093/eurheartj/ehac584
- van Rijssel AE, Stins BC, Beishon LC, et al. Effect of antihypertensive treatment on cerebral blood flow in older adults: a systematic review and meta-analysis. Hypertension. 2022;79(5):1067–1078. doi: 10.1161/HYPERTENSIONAHA.121.18255
- Dallaire-Theroux C, Quesnel-Olivo MH, Brochu K, et al. Evaluation of intensive vs standard blood pressure reduction and Association with cognitive decline and dementia: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(11):e2134553. doi: 10.1001/jamanetworkopen.2021.34553
- Cunningham EL, Todd SA, Passmore P, et al. Pharmacological treatment of hypertension in people without prior cerebrovascular disease for the prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2021;5(5):CD004034. doi:10.1002/14651858.CD004034.pub4
- Jordan F, Quinn TJ, McGuinness B, et al. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst Rev. 2020;4(4):CD011459. doi: 10.1002/14651858.CD011459.pub2
- Kwan J, Hafdi M, Chiang LLW, et al. Antithrombotic therapy to prevent cognitive decline in people with small vessel disease on neuroimaging but without dementia. Cochrane Database Syst Rev. 2022;7(7):CD012269. doi:10.1002/14651858.CD012269.pub2
- Ryan J, Storey E, Murray AM, et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 2020;95(3):e320–e331. doi: 10.1212/WNL.0000000000009277
- Alexander P, Visagan S, Jawhar S, et al. Antiplatelets and vascular dementia: a systematic review. J Aging Res. 2022;2022:9780067. doi: 10.1155/2022/9780067
- Wong KS, Wang Y, Leng X, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis. Circulation. 2013;128(15):1656–1666. doi: 10.1161/CIRCULATIONAHA.113.003187
- Kim WJ, Noh JH, Han K, et al. The Association between second-line oral antihyperglycemic medication on types of dementia in type 2 diabetes: a nationwide real-world longitudinal study. J Alzheimer’s Disease: JAD. 2021;81(3):1263–1272. doi:10.3233/JAD-201535
- Dai J, Ports KD, Corrada MM, et al. Metformin and dementia risk: a systematic review with respect to time related Biases. J Alzheimers Dis Rep. 2022;6(1):443–459. doi:10.3233/ADR-220002
- Zhang JH, Zhang XY, Sun YQ, et al. Metformin use is associated with a reduced risk of cognitive impairment in adults with diabetes mellitus: a systematic review and meta-analysis. Front Neurosci. 2022;16:984559. doi:10.3389/fnins.2022.984559
- Kim YG, Jeon J, Kim HJ, et al. Risk of dementia in older patients with type 2 diabetes on dipeptidyl-peptidase IV inhibitors versus sulfonylureas: a real-world population-based cohort study. J Clin Med. 2018;8(1):28. doi: 10.3390/jcm8010028
- Norgaard CH, Friedrich S, Hansen CT, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement (N Y). 2022;8(1):e12268. doi: 10.1002/trc2.12268
- Tang H, Shao H, Shaaban CE, et al. Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J American Geriatrics Society. 2023;71(7):2096–2106. doi: 10.1111/jgs.18306
- Luan S, Cheng W, Wang C, et al. Impact of glucagon-like peptide 1 analogs on cognitive function among patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol. 2022;13:1047883. doi:10.3389/fendo.2022.1047883
- Jin Y, Zhao H, Hou Y, et al. The effects of dipeptidyl peptidase-4 inhibitors and glucagon-like peptide 1 receptor agonists on cognitive functions in adults with type 2 diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2020;57(10):1129–1144. doi:10.1007/s00592-020-01529-1
- Wu CY, Iskander C, Wang C, et al. Association of Sodium–glucose cotransporter 2 inhibitors with time to dementia: a population-based cohort study. Diabetes Care. 2023;46(2):297–304. doi: 10.2337/dc22-1705
- Yang Z, Wang H, Edwards D, et al. Association of blood lipids, atherosclerosis and statin use with dementia and cognitive impairment after stroke: a systematic review and meta-analysis. Ageing Res Rev. 2020;57:100962. doi: 10.1016/j.arr.2019.100962
- McGuinness B, Craig D, Bullock R, et al. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;2016(1):CD003160. doi:10.1002/14651858.CD003160.pub3
- Wardlaw JM, Woodhouse LJ, Mhlanga II, et al. Isosorbide mononitrate and Cilostazol treatment in patients with symptomatic cerebral small vessel disease: the lacunar intervention trial-2 (LACI-2) randomized clinical trial. JAMA Neurol. 2023;80(7):682. doi: 10.1001/jamaneurol.2023.1526
- Tai SY, Chien CY, Chang YH, et al. Cilostazol use is associated with reduced risk of dementia: a nationwide cohort study. Neurotherapeutics. 2017;14(3):784–791. doi:10.1007/s13311-017-0512-4
- Pajewski NM, Berlowitz DR, Bress AP, et al. Intensive vs standard blood pressure control in adults 80 years or older: a secondary analysis of the systolic blood pressure intervention trial. J American Geriatrics Society. 2020;68(3):496–504. doi: 10.1111/jgs.16272
- Gaussoin SA, Pajewski NM, Chelune G, et al. Effect of intensive blood pressure control on subtypes of mild cognitive impairment and risk of progression from SPRINT study. J American Geriatrics Society. 2022;70(5):1384–1393. doi: 10.1111/jgs.17583
- Bello-Chavolla OY, Antonio-Villa NE, Vargas-Vazquez A, et al. Pathophysiological mechanisms linking type 2 diabetes and dementia: review of evidence from clinical, translational and epidemiological Research. Curr Diabetes Rev. 2019;15(6):456–470. doi:10.2174/1573399815666190129155654
- Cheng G, Huang C, Deng H, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–491. doi:10.1111/j.1445-5994.2012.02758.x
- Antal B, McMahon LP, Sultan SF, et al. Type 2 diabetes mellitus accelerates brain aging and cognitive decline: complementary findings from UK Biobank and meta-analyses. Elife. 2022;11: doi: 10.7554/eLife.73138
- Luchsinger JA, Ryan C, Launer LJ. Diabetes and cognitive impairment. In: Cowie C, Casagrande S, and Menke A, et al. Diabetes in America. 3rd ed. Chapter 24, Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018. PMID: 33651563.
- Guan Y, Ebrahimzadeh SA, Cheng CH, et al. Association of diabetes and hypertension with brain structural integrity and cognition in the Boston Puerto Rican health study cohort. Neurology. 2022;98(15):e1534–e1544. doi: 10.1212/WNL.0000000000200120
- Shi S, Gouskova N, Najafzadeh M, et al. Intensive versus standard blood pressure control in type 2 diabetes: a restricted mean survival time analysis of a randomised controlled trial. BMJ Open. 2021;11(9):e050335. doi:10.1136/bmjopen-2021-050335
- Wu CY, Shapiro L, Ouk M, et al. Glucose-lowering drugs, cognition, and dementia: the clinical evidence. Neurosci Biobehav Rev. 2022;137:104654. doi: 10.1016/j.neubiorev.2022.104654
- Hamal C, Velugoti L, Tabowei G, et al. Metformin for the improvement of comorbid depression symptoms in diabetic patients: a systematic review. Cureus. 2022;14(8):e28609. doi: 10.7759/cureus.28609
- Sergi D, Zauli E, Tisato V, et al. Lipids at the nexus between cerebrovascular disease and vascular dementia: the impact of HDL-Cholesterol and ceramides. Int J Mol Sci. 2023;24(5):4403. doi: 10.3390/ijms24054403
- Wee J, Sukudom S, Bhat S, et al. The relationship between midlife dyslipidemia and lifetime incidence of dementia: a systematic review and meta-analysis of cohort studies. Alzheimer’s Dementia. 2023;15(1):e12395. doi: 10.1002/dad2.12395
- Power MC, Rawlings A, Sharrett AR, et al. Association of midlife lipids with 20-year cognitive change: a cohort study. Alzheimer’s Dementia. 2018;14(2):167–177. doi: 10.1016/j.jalz.2017.07.757
- Seixas AA, Turner AD, Bubu OM, et al. Obesity and race may explain differential burden of white matter hyperintensity load. Clin Interventions Aging. 2021;16:1563–1571. doi: 10.2147/CIA.S316064
- Yu J, Morys F, Dagher A, et al. Associations between sleep-related symptoms, obesity, cardiometabolic conditions, brain structural alterations and cognition in the UK biobank. Sleep Med. 2023;103:41–50. doi: 10.1016/j.sleep.2023.01.023
- Ishii M, Iadecola C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2016;1862(5):966–974. doi:10.1016/j.bbadis.2015.10.029
- Anjum I, Fayyaz M, Wajid A, et al. Does obesity increase the risk of dementia: a literature review. Cureus. 2018;10(5):e2660. doi:10.7759/cureus.2660
- Erickson LD, Gale SD, Anderson JE, et al. Association between exposure to air pollution and total gray matter and total white matter volumes in adults: a cross-sectional study. Brain Sci. 2020;10(3):164. doi: 10.3390/brainsci10030164
- Delgado-Saborit JM, Guercio V, Gowers AM, et al. A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci Total Environ. 2021;757:143734. doi:10.1016/j.scitotenv.2020.143734
- Shaffer RM, Sheppard L, Peskind ER, et al. Fine particulate matter exposure and cerebrospinal fluid markers of vascular injury. J Alzheimer’s Disease: JAD. 2019;71(3):1015–1025. doi:10.3233/JAD-190563
- Perales-Puchalt J, Vidoni ML, Llibre Rodriguez J, et al. Cardiovascular health and dementia incidence among older adults in Latin America: results from the 10/66 study. Int J Geriat Psychiatry. 2019;34(7):1041–1049. doi: 10.1002/gps.5107
- Fernandes BFS, Caramelli P. Ischemic stroke and infectious diseases in low-income and middle-income countries. Curr Opin Neurol. 2019;32(1):43–48. doi:10.1097/WCO.0000000000000641
- Obi IE, McPherson KC, Pollock JS. Childhood adversity and mechanistic links to hypertension risk in adulthood. Br J Pharmacol. 2019;176(12):1932–1950. doi:10.1111/bph.14576
- Godoy LC, Frankfurter C, Cooper M, et al. Association of adverse childhood experiences with cardiovascular disease later in life: a review. JAMA Cardiol. 2021;6(2):228–235. doi:10.1001/jamacardio.2020.6050
- Seligowski AV, Webber TK, Marvar PJ, et al. Involvement of the brain–heart axis in the link between PTSD and cardiovascular disease. Depress Anxiety. 2022;39(10–11):663–674. doi:10.1002/da.23271
- Dawson J, Bejot Y, Christensen LM, et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur Stroke J. 2022;7(3):I–XLI. doi: 10.1177/23969873221100032
- van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, et al. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH intervention for neurodegenerative delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—a review. Adv Nutr. 2019;10(6):1040–1065. doi:10.1093/advances/nmz054
- Andrews V, Zammit G, O’Leary F. Dietary pattern, food, and nutritional supplement effects on cognitive outcomes in mild cognitive impairment: a systematic review of previous reviews. Nutr Rev. 2023;81(11):1462–1489. doi: 10.1093/nutrit/nuad013
- Malek Rivan NF, Shahar S, Fakhruddin N, et al. The effect of dietary patterns on mild cognitive impairment and dementia incidence among community-dwelling older adults. Front Nutr. 2022;9:901750. doi:10.3389/fnut.2022.901750
- Chen H, Dhana K, Huang Y, et al. Association of the mediterranean dietary approaches to stop hypertension intervention for neurodegenerative delay (MIND) diet with the risk of dementia. JAMA Psychiatry. 2023;80(6):630–638. doi: 10.1001/jamapsychiatry.2023.0800
- Gil Martinez V, Avedillo Salas A, Santander Ballestin S. Vitamin supplementation and dementia: a systematic review. Nutrients. 2022;14(5):1033. doi: 10.3390/nu14051033
- Bonyadi N, Dolatkhah N, Salekzamani Y, et al. Effect of berry-based supplements and foods on cognitive function: a systematic review. Sci Rep. 2022;12(1):3239. doi:10.1038/s41598-022-07302-4
- Bermejo PE, Dorado R, Zea-Sevilla MA. Role of Citicoline in patients with mild cognitive impairment. J Exp Neurosci. 2023;18:26331055231152496. doi:10.1177/26331055231152496
- Davinelli S, Ali S, Solfrizzi V, et al. Carotenoids and cognitive outcomes: a meta-analysis of randomized intervention trials. Antioxidants. 2021;10(2):223. doi: 10.3390/antiox10020223
- Iso-Markku P, Kujala UM, Knittle K, et al. Physical activity as a protective factor for dementia and Alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case–control studies. Br J Sports Med. 2022;56(12):701–709. doi:10.1136/bjsports-2021-104981
- Balbim GM, Falck RS, Barha CK, et al. Effects of exercise training on the cognitive function of older adults with different types of dementia: a systematic review and meta-analysis. Br J Sports Med. 2022;56(16):933–940. doi: 10.1136/bjsports-2021-104955
- Yan J, Li X, Guo X, et al. Effect of multicomponent exercise on cognition, physical function and activities of daily life in older adults with dementia or mild cognitive impairment: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2023. doi:10.1016/j.apmr.2023.04.011.
- Rivas-Campo Y, Garcia-Garro PA, Aibar-Almazan A, et al. The effects of high-intensity functional training on cognition in older adults with cognitive impairment: a systematic review. Healthcare. 2022;10(4):670. doi: 10.3390/healthcare10040670
- Liu L, Dong H, Jin X, et al. Tackling dementia: a systematic review of interventions based on physical activity. J Geriatr Phys Ther. 2022;45(4):E169–E180. doi:10.1519/JPT.0000000000000332
- Xu W, Wang HF, Wan Y, et al. Leisure time physical activity and dementia risk: a dose-response meta-analysis of prospective studies. BMJ Open. 2017;7(10):e014706. doi:10.1136/bmjopen-2016-014706
- Venegas-Sanabria LC, Cavero-Redondo I, Martinez-Vizcaino V, et al. Effect of multicomponent exercise in cognitive impairment: a systematic review and meta-analysis. BMC Geriatr. 2022;22(1):617. doi:10.1186/s12877-022-03302-1
- Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653–666. doi:10.1038/s41582-018-0070-3
- Gomez-Soria I, Marin-Puyalto J, Peralta-Marrupe P, et al. Effects of multi-component non-pharmacological interventions on cognition in participants with mild cognitive impairment: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2022;103:104751. doi:10.1016/j.archger.2022.104751
- Kumagai R, Osaki T, Oki Y, et al. The Japan-multimodal intervention trial for prevention of dementia PRIME Tamba (J-MINT PRIME Tamba): study protocol of a randomised controlled multi-domain intervention trial. Arch Gerontol Geriatr. 2023;104:104803. doi: 10.1016/j.archger.2022.104803
- Solomon A, Stephen R, Altomare D, et al. Multidomain interventions: state-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for brain health services—part 4 of 6. Alzheimer’s Res Ther. 2021;13(1):171. doi: 10.1186/s13195-021-00875-8
- Akpa OM, Okekunle AP, Asowata OJ, et al. Frequent vegetable consumption is inversely associated with hypertension among indigenous Africans. Eur J Prev Cardiol. 2022;29(18):2359–2371. doi: 10.1093/eurjpc/zwac208
- Okekunle AP, Asowata O, Akpa OM, et al. Dietary patterns associated with stroke among West Africans: a case–control study. Int J Stroke. 2023;18(2):193–200. doi: 10.1177/17474930221094933
- Ding H, Reiss AB, Pinkhasov A, et al. Plants, plants, and more plants: plant-derived Nutrients and their protective roles in cognitive function, Alzheimer’s disease, and other dementias. Medicina (Kaunas). 2022;58(8):1025. doi: 10.3390/medicina58081025
- Godos J, Grosso G, Ferri R, et al. Mediterranean diet, mental health, cognitive status, quality of life, and successful aging in southern Italian older adults. Exp Gerontol. 2023;175:112143. doi: 10.1016/j.exger.2023.112143
- Saito S, Yamamoto Y, Ihara M. Development of a multicomponent intervention to prevent Alzheimer’s disease. Front Neurol. 2019;10:490. doi:10.3389/fneur.2019.00490
- Currenti W, Godos J, Alanazi AM, et al. Dietary fats and cognitive status in Italian middle-old adults. Nutrients. 2023;15(6):1429. doi: 10.3390/nu15061429
- Fisicaro F, Lanza G, Pennisi M, et al. Daily mocha coffee intake and psycho-cognitive status in non-demented non-smokers subjects with subcortical ischaemic vascular disease. Int J Food Sci Nutr. 2022;73(6):821–828. doi: 10.1080/09637486.2022.2050999
- Fisicaro F, Lanza G, Pennisi M, et al. Moderate mocha coffee consumption is associated with higher cognitive and mood status in a non-demented elderly population with subcortical ischemic vascular disease. Nutrients. 2021;13(2):536. doi: 10.3390/nu13020536
- Sekikawa A, Higashiyama A, Lopresti BJ, et al. Associations of equol-producing status with white matter lesion and amyloid-β deposition in cognitively normal elderly Japanese. Alzheimers Dement (N Y). 2020;6(1):e12089. doi: 10.1002/trc2.12089
- Hess NC, Smart NA. Isometric exercise training for managing vascular risk factors in mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci. 2017;9:48. doi:10.3389/fnagi.2017.00048
- Barnes JN, Pearson AG, Corkery AT, et al. Exercise, arterial stiffness, and cerebral vascular function: potential impact on brain health. J Int Neuropsychol Soc. 2021;27(8):761–775. doi:10.1017/S1355617721000394
- Stevenson W, Hase Y, Wilson E, et al. Long-term effects of experimental carotid stenosis on hippocampal infarct pathology, neurons and glia and amelioration by environmental enrichment. Brain Res Bull. 2020;163:72–83. doi: 10.1016/j.brainresbull.2020.07.014
- Hase Y, Polvikoski TM, Ihara M, et al. Carotid artery disease in post-stroke survivors and effects of enriched environment on stroke pathology in a mouse model of carotid artery stenosis. Neuropathol Appl Neurobiol. 2019;45(7):681–697. doi: 10.1111/nan.12550
- LaCroix AZ, Hubbard RA, Gray SL, et al. Trajectories of physical function prior to death and brain neuropathology in a community-based cohort: the act study. BMC Geriatr. 2017;17(1):258. doi: 10.1186/s12877-017-0637-7
- Frisoni GB, Altomare D, Ribaldi F, et al. Dementia prevention in memory clinics: recommendations from the European task force for brain health services. Lancet Reg Health Eur. 2023;26:100576. doi: 10.1016/j.lanepe.2022.100576
- Shannon OM, Lee V, Bundy R, et al. Feasibility and acceptability of a multi-domain intervention to increase Mediterranean diet adherence and physical activity in older UK adults at risk of dementia: protocol for the MedEx-UK randomised controlled trial. BMJ Open. 2021;11(2):e042823. doi: 10.1136/bmjopen-2020-042823
- Cantone M, Lanza G, Fisicaro F, et al. Evaluation and treatment of vascular cognitive impairment by transcranial magnetic stimulation. Neural Plast. 2020;2020:8820881. doi: 10.1155/2020/8820881
- Lanza G, Bella R, Giuffrida S, et al. Preserved transcallosal inhibition to transcranial magnetic stimulation in nondemented elderly patients with leukoaraiosis. Bio Med Res Int. 2013;2013:351680. doi: 10.1155/2013/351680
- Balasubramanian P, DelFavero J, Ungvari A, et al. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res Rev. 2020;64:101189. doi: 10.1016/j.arr.2020.101189
- Forte M, Rodolico D, Ameri P, et al. Molecular mechanisms underlying the beneficial effects of exercise and dietary interventions in the prevention of cardiometabolic diseases. J Cardiovasc Med (Hagerstown). 2023;24(Supplement 1):e3–e14. doi: 10.2459/JCM.0000000000001397
- Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–826. doi: 10.1016/S1474-4422(08)70169-8
- O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–775. doi: 10.1016/S0140-6736(16)30506-2
- Owolabi MO, Sarfo F, Akinyemi R, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health. 2018;6(4):e436–e446. doi: 10.1016/S2214-109X(18)30002-0
- Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2
- Schwarzinger M, Pollock BG, Hasan OSM, et al. Contribution of alcohol use disorders to the burden of dementia in France 2008-13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3(3):e124–e132. doi:10.1016/S2468-2667(18)30022-7
- Price BR, Wilcock DM, Weekman EM. Hyperhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front Aging Neurosci. 2018;10:350. doi:10.3389/fnagi.2018.00350
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6
- Kivimaki M, Singh-Manoux A, Batty GD, et al. Association of alcohol-induced loss of consciousness and overall alcohol consumption with risk for dementia. JAMA Netw Open. 2020;3(9):e2016084. doi: 10.1001/jamanetworkopen.2020.16084
- Piras F, Banaj N, Porcari DE, et al. Later life depression as risk factor for developing dementia: epidemiological evidence, predictive models, preventive strategies and future trends. Minerva Med. 2021;112(4):456–466. doi:10.23736/S0026-4806.21.07571-6
- Huuskonen MT, Liu Q, Lamorie-Foote K, et al. Air pollution particulate matter amplifies white matter vascular pathology and demyelination caused by Hypoperfusion. Front Immunol. 2021;12:785519. doi: 10.3389/fimmu.2021.785519
- Joyce DP, Gracias CS, Murphy F, et al. Potentially undiagnosed cognitive impairment in patients with peripheral arterial disease: a systematic review of the literature. Surgeon. 2022;20(4):e134–e143. doi:10.1016/j.surge.2021.06.007
- Xu YY, Xie J, Yin H, et al. The global burden of disease attributable to low physical activity and its trends from 1990 to 2019: an analysis of the global burden of disease study. Front Public Health. 2022;10:1018866. doi: 10.3389/fpubh.2022.1018866
- Mooldijk SS, Ikram MK, Ikram MA. Adiponectin, leptin, and Resistin and the risk of dementia. J Gerontol. 2022;77(6):1245–1249. doi:10.1093/gerona/glab267
- Wang S, Molassiotis A, Guo C, et al. Association between social integration and risk of dementia: a systematic review and meta-analysis of longitudinal studies. J American Geriatrics Society. 2023;71(2):632–645. doi:10.1111/jgs.18094
- Serafin P, Zaremba M, Sulejczak D, et al. Air pollution: a silent key driver of dementia. Biomedicines. 2023;11(5):1477. doi: 10.3390/biomedicines11051477
- Fu X, Eikelboom RH, Tian R, et al. The relationship of age-related hearing loss with cognitive decline and dementia in a Sinitic language-speaking adult population: a systematic review and meta-analysis. Innov Aging. 2023;7(1):igac078. doi:10.1093/geroni/igac078
- Wilker EH, Osman M, Weisskopf MG. Ambient air pollution and clinical dementia: systematic review and meta-analysis. BMJ. 2023;381:e071620. doi:10.1136/bmj-2022-071620
- Mukadam N, Sommerlad A, Huntley J, et al. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019;7(5):e596–e603. doi:10.1016/S2214-109X(19)30074-9
- Mulligan MD, Murphy R, Reddin C, et al. Population attributable fraction of hypertension for dementia: global, regional, and national estimates for 186 countries. EClinicalMedicine. 2023;60:102012. doi: 10.1016/j.eclinm.2023.102012
- Yu JT, Xu W, Tan CC, et al. Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(11):1201–1209. doi: 10.1136/jnnp-2019-321913
- Oscanoa TJ, Amado J, Vidal X, et al. Angiotensin-receptor blockers and the risk of Alzheimer s disease: a meta-analysis. Curr Rev Clin Exp Pharmacol. 2021;16(1):73–78. doi:10.2174/1574884715666200131120224
- Adesuyan M, Jani YH, Alsugeir D, et al. Antihypertensive agents and incident Alzheimer’s disease: a systematic review and meta-analysis of observational studies. J Prev Alzheimers Dis. 2022;9(4):715–724. doi: 10.14283/jpad.2022.77
- Battle CE, Abdul-Rahim AH, Shenkin SD, et al. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst Rev. 2021;2(2):CD013306. doi:10.1002/14651858.CD013306.pub2
