1. Introduction
During the last 30 years, hundreds of noninvasive brain stimulation (NIBS) demonstrated positive results and applicability for many neurological and psychological conditions, already synthesized in clinical practice guidelines [Citation1,Citation2]. Despite years of research, it is surprising that these techniques are less recognized than other medical devices, and are not yet included in clinical practice. Beyond the ongoing development of more sophisticated and accessible devices, the NIBS approaches have not been more widespread used because some evidence remains inconclusive due to a lack of proper double-blinded randomized clinical trials (RCT), lack of standardized mechanistic biomarkers, and understudied long-term effectiveness of NIBS approaches are most of the reasons for much inconclusive evidence of its effectiveness, which reduces its usability by clinicians [Citation3,Citation4]. Some authors also highlighted the challenges to designing a valid sham condition in RCTs due to device capabilities [Citation4]. On the other hand, the usability of NIBS devices should receive more attention and effort. Most of the studies have no assessments on usability; only a few of them pointed out aspects such as the appearance of the device, the patient’s scientific knowledge and experience, and the concerns about costs, which are relevant factors considered by patients before accepting the use of NIBS as a treatment [Citation5,Citation6]; therefore, the improvement in the device design has been slow [Citation3]. For instance, transcranial direct current stimulation (tDCS) devices have been developed following the same design principle (a box, wires, and electrodes) over the last 15 years [Citation7], with recent improvements related to new features that are important for the neuroscience researcher (such as coupling with neuroimaging techniques and new stimulation modalities); however, input from the end-users (clinicians with no research experience and patients) are not generally included in the design refinement. Similarly, transcranial magnetic stimulation (TMS) devices have been studied over the past 30 years [Citation8], with almost no design modification, apart from size reduction and added complexity with neuronavigation and robotic guidance. Again, usability feedback from end users has not been systematically collected to guide device improvement. One could argue that devices are still being tested for their efficacy, but the counterargument to this two-phase approach (efficacy and then usability) is to double the costs unnecessarily. And, in fact, although the process has already started, we are still looking for more significant design changes based on the user’s needs.
Design thinking is a method that prioritizes the human perspective by centering the user in the process of developing innovative solutions [Citation9]. Unlike traditional design processes, human-centered design focuses on individuals’ needs, aiming to make better devices and improve people’s lives. This approach requires empathy and creativity. Empathy – experiencing things from another person’s perspective – is pivotal throughout the design process [Citation9]. Conducting interviews, making observations, and even engaging in role-playing exercises, designers gain firsthand insights that humanize the problems they seek to solve [Citation10]. This empathetic connection not only fuels their passion for meaningful change but also drives them to create inclusive, accessible, and patient-centric solutions that resonate with the diverse needs of the community. The fusion of creativity with design thinking further amplifies the transformative potential of health design. This infusion of creativity sparks fresh ideas, allowing designers to challenge traditional norms and embrace more forward-thinking solutions to the ever-evolving demands of the healthcare industry [Citation10]. Design thinking has been proposed as a pillar of medical device development; however, its use is still suboptimal.
A graphic designer, Deborah Adler, exemplifies the application of design thinking to healthcare through her remarkable redesign of prescription drug packaging. Since 2004, her human-centered and creative ideas have significantly impacted pharmacies. One such innovation is Target’s ClearRx, which adopts the inventive flattened bottles with downward-facing caps, allowing more space to show critical dosage information [Citation11]. Additionally, Adler introduced a groundbreaking feature to CVS Pharmacy: timeline graphics on the bottles are precious for individuals using multiple medications; they help prevent harmful interactions and enhances understanding of the medication schedule. By incorporating clear labeling and visual timelines, patients are encouraged to adhere to their medication regimen, improving compliance and reducing unnecessary waste and lost revenue for pharmacies [Citation12]. These redesigning examples show the added value of a usable and human-centered device compared to a device that accomplished the task (drug packaging).
Design thinking principles, especially human-centered design, are needed to improve brain-stimulation device development and scalable therapeutic implementation in the real world. However, most of the effort is focused on the working principle and basic design of the device, without systematic and continuous work to promote usability (the degree to which a system may be utilized to fulfill the goals), and good user experience (person’s perceptions and responses from using the product). One alternative solution is applying design thinking principles to prioritize user needs, preferences, and safety during device development process. From the early stages of development, engaging end-users, such as patients and healthcare providers, can lead to more intuitive and user-friendly devices. Iterative prototyping and usability testing can help refine the design, making the devices more accessible and user-centric [Citation10]. However, several barriers are present in the medical devices field. For instance, the lack of investment in qualitative studies to explore patients’ needs due to the high pressure and tight timelines to secure a regulatory approval. Similarly, the disconnection between development teams and end-users due to the lack of collaboration agreements between academic or governmental institutions and medical device companies [Citation13].
Moreover, a digital health system, including a medical device and companion software, should continually gather data and deal with iterative adjustments, training, and changes, like an enclosed feedback system in which the environment, industry, researchers, regulators, clinicians, and patients work together to increase usability [Citation14]. When compared to ‘standard’ therapies, pharmacological treatments, are relatively simple to use (taking a tablet is something that can be done quickly), and some of them have an instantaneous effect, in contrast to neuromodulation, which may require several sessions to experience an effect [Citation15]. Therefore, any improvement in the user experience can affect adherence to the neuromodulatory treatment which is highly related to its efficacy. For example, tDCS has shown adequate efficacy and safety profile to treat chronic pain, but, there is still a lack of understanding and effort in making it more accessible and easier to use for patients [Citation16]
For instance, in one of our ongoing home-based supervised tDCS trials, even after a comprehensive training session on how to use the device at home, some participants still require refresher training sessions due to the non-intuitive user interfaces and multicomponent design of the stimulation cap (head-band and electrodes) [Citation17].
Appropriate designing of a noninvasive brain stimulation systems requires meticulous attention to ensure safety, efficacy, and usability (simple and intuitive). The most critical factor is how to clearly define specific target area of stimulation which usually requires a reliable holder apparatus to ensure target engagement [Citation15]. Therefore, it is essential to incorporate ergonomic design adjustments and solutions for anatomic heterogeneity of participants. In terms of user experience, it is important to reduce the ‘pain’ associated with device usage, namely all the potential barriers that could affect the real-world use of the device. For instance, well-designed equipment must be lightweight, fit comfortably on the scalp, do not cause interference due to excessive heat or other environmental factors, and allow integration with other modalities and activities improving daily use (requiring minimal behavioral change) [Citation16]. Other important elements to optimize NIBS protocols are state-dependent brain stimulation, EEG-triggered capabilities, spaced application of multiple NIBS protocols, and pharmacological neuromodulation [Citation18]. Future studies must test the usability of these optimizations. Furthermore, new brain stimulation devices would be required to be integrated into the concept of digital health; thus allowing options for remote usage and control, integration with wearables, and telehealth monitoring systems [Citation16,Citation19].
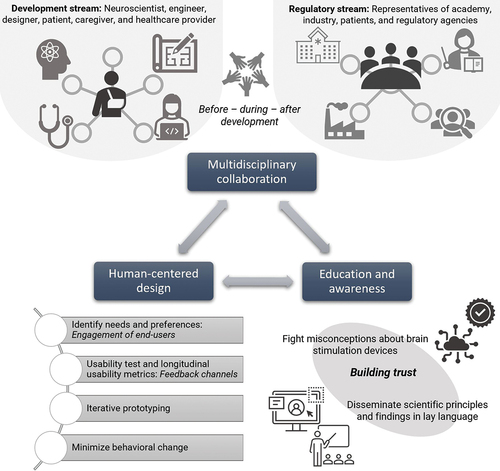
To ensure this sustainable development, we must promote multidisciplinary collaboration between researchers, engineers, clinicians, and caregivers in development teams. This approach can ensure a comprehensive understanding of both the medical and technical aspects of brain stimulation devices. Collaborative efforts can accelerate development, enabling innovations that consider real-world clinical needs and user experiences. Another crucial aspect is that multidisciplinary collaboration is needed to facilitate regulatory approval of the new technology. Therefore, constant conversations between representatives from academia, industry and regulatory agencies are recommended even before the piloting of the technology and clinical trial designs, as is being promoted with AI-based medical devices [Citation20].
Finally, increasing public awareness and understanding of noninvasive brain stimulation techniques and their potential benefits. Educating the public, healthcare professionals, and patients about the available brain stimulation devices can eliminate misconceptions and encourage broader adoption. Additionally, educational initiatives can promote responsible use and mitigate potential risks associated with home-based usage.
2. Conclusion
Improving the success of developing better brain stimulation devices requires a combination of strategies that address technical, regulatory, and user-related challenges. Given recent meta-analyses in the field, it is clear that noninvasive brain stimulation devices hold significant clinical effects [Citation1,Citation21–24]. In fact, for a few conditions, some of these techniques also have FDA approval. However, the progress to have its use clinically has been slow. We propose that addressing usability issues will also help to increase the adoption of these devices. Here we defined three critical approaches (): 1. Early multidisciplinary collaboration before, during, and after development and regulatory approval. 2. Promotion of human-centered design and constant usability tests and feedback. 3. Public awareness and education, disseminating brain stimulation principles and findings in lay language. The new era of the brain-stimulation field not only needs devices that ‘work’ but devices that are ease-of-use and intuitive, and can be integrated in the daily life of the target population without constant ‘usage pain.’
3. Expert opinion
In the field of NIBS devices, recent meta-analyses have underscored their significant clinical effects, and some techniques have gained FDA approval for specific conditions [Citation1,Citation2]. However, the slow progress in their clinical adoption suggests existing weaknesses in usability and integration into patients’ daily lives. Our expertise in medical devices and running neuromodulation trials for the past 15 years allows us to emphasize that while clinical efficacy is evident, the challenge lies in bridging the gap between technical functionality and seamless user experience.
The potential of integrating patient-centered design in neuromodulation is immense, with the ultimate goal being the development of brain stimulation devices that not only demonstrate clinical effectiveness but are also user-friendly and easily integrated into patients’ routines [Citation16]. To our knowledge, less than ten studies have studied the patients’ perceptions of noninvasive neuromodulation treatments [Citation5,Citation6]. Most of the studies examined the acceptability of using rTMS for psychiatric conditions [Citation6] and musculoskeletal pain [Citation5]. The common themes were costs and access barriers, familiarity with the technology, and safety concerns. However, there needs to be more exploration regarding other NIBS modalities, particularly more portable technologies that can be implemented at home (e.g. tDCS or transcutaneous vagus nerve stimulation). Therefore, the available knowledge of patients’ values and perspectives on NIBS interventions remains incomplete.
Further research must focus on multidisciplinary collaboration to address technical, medical, and user experience challenges holistically. Usability studies should be continuous and iterative, ensuring constant feedback loops to refine device interfaces [Citation25]. We recommend taking advantage of the exponential production of clinical trials on NIBS to include usability and patients’ preferences as part of the ‘core outcome set’ in all neuromodulatory devices [Citation26], together with important outcomes such as efficacy and safety. These assessments must include brief structured questionnaires with both closed and open questions [Citation27] that can be implemented without disrupting the clinical trial workflow. Using this strategy, we could gather standardized user-related data during the efficacy testing stage of NIBS device development. Furthermore, our expertise highlights another big challenge: the intricate nature of the human brain’s responses to stimulation [Citation1,Citation2]. Personalized approaches, grounded in neuro-engineering and neuroscientific knowledge, are necessary to navigate this complexity.
Additionally, evolving regulatory frameworks encompassing these innovative devices are paramount [Citation28]. For instance, regulatory agencies could include patients’ values and preferences data collected during qualitative exploration as part of the requirements for approval. In the context of NIBS application at home, the simplicity of the device and software are fundamental to assure an adequate adherence; however, this aspects are not considered as needed relevant information for regulatory approval. This will increase the awareness of the user-centered design methodology as a cornerstone of NIBS device development. Moreover, it will serve as an incentive for investors and companies to allocate longitudinal resources for qualitative studies assessing the users’ needs before, during, and after regulatory approval.
In the upcoming years, advancements are anticipated in miniaturization, portability, and integration of noninvasive brain stimulation devices with other technologies. Personalized treatment approaches, tailored to individual neural responses, are likely to become more prevalent. Future challenges and solutions in NIBS device design will include systematic finding of ‘dosage,’ data security, technology integration, remote monitoring, training, accessibility, clinical validation, patient acceptance, and regulatory approval innovations. We foresee a future where these devices seamlessly integrate into telemedicine platforms, enabling remote monitoring and personalized treatments, thereby enhancing their accessibility and impact. Achieving this goal holds the promise of revolutionizing healthcare by providing accessible, noninvasive treatments for various medical conditions.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Fregni F, El-Hagrassy MM, Pacheco-Barrios K, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24(4):256–313. doi: 10.1093/ijnp/pyaa051
- Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002
- Sanches C, Stengel C, Godard J, et al. Past, present, and future of non-invasive brain stimulation approaches to treat cognitive impairment in neurodegenerative diseases: time for a comprehensive critical review. Front Aging Neurosci. 2021;12:578339. doi: 10.3389/fnagi.2020.578339
- Brunoni AR, Fregni F. Clinical trial design in non‐invasive brain stimulation psychiatric research. Int J Methods Psychiatr Res. 2011;20(2):e19–e30. doi:10.1002/mpr.338
- Stillianesis G, Cavaleri R, Summers SJ, et al. Exploring patient perceptions of repetitive transcranial magnetic stimulation as a treatment for chronic musculoskeletal pain: a qualitative study. BMJ Open. 2022;12(8):e058928. doi:10.1136/bmjopen-2021-058928
- Stillianesis G, Cavaleri R, Tang CY, et al. Exploring patient perceptions of noninvasive brain stimulation: a systematic review. Neuromodulation: Technol Neural Interface. 2022;25(4):487–493. doi:10.1111/ner.13461
- Woods AJ, Antal A, Bikson M, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–1048. doi: 10.1016/j.clinph.2015.11.012
- Boes AD, Kelly MS, Trapp NT, et al. Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatry Clin Neurosci. 2018;30(3):173–179. doi:10.1176/appi.neuropsych.17110262
- Saidi T, Mutswangwa CT, Douglas TS. Design thinking as a complement to human factors engineering for enhancing medical device usability. Engineering Studies. 2019;11(1):34–50. doi:10.1080/19378629.2019.1567521
- Oliveira M, Zancul E, Fleury AL. Design thinking as an approach for innovation in healthcare: systematic review and research avenues. BMJ Innovations. 2021;7(2):491–498. doi: 10.1136/bmjinnov-2020-000428
- McDonagh D, Thomas J. Rethinking design thinking: empathy supporting innovation. Australas Med J. 2010;3(8):458–464. doi:10.4066/AMJ.2010.391
- Soller RW, Lightwood JM. Comparison of the packaging and labeling of target ClearRx with conventional prescription drug packaging and labeling. J Am Pharm Assoc. 2007;47(4):484–490. doi:10.1331/JAPhA.2007.06089
- Lyon AR, Koerner K. User‐centered design for psychosocial intervention development and implementation. Clin Psychol: Sci Pract. 2016;23(2):180. doi:10.1111/cpsp.12154
- Bitkina OV, Kim HK, Park J. Usability and user experience of medical devices: an overview of the current state, analysis methodologies, and future challenges. Inter J Ind Ergon. 2020;76:102932. doi:10.1016/j.ergon.2020.102932
- Pacheco-Barrios K, Carvalho S, Leite J, et al. Optimization strategies for pain management with neuromodulation. Front Pain Res. 2022;3:1012790. doi:10.3389/fpain.2022.1012790
- Brunoni AR, Ekhtiari H, Antal A, et al. Digitalized transcranial electrical stimulation: A consensus statement. Clin Neurophysiol. 2022;143:154–165. doi: 10.1016/j.clinph.2022.08.018
- Pacheco-Barrios K, Cardenas-Rojas A, de Melo PS, et al. Home-based transcranial direct current stimulation (tDCS) and motor imagery for phantom limb pain using statistical learning to predict treatment response: an open-label study protocol. Principles And Practice Of Clinical Research (2015). 2021;7(4):8–22. 8. doi: 10.21801/ppcrj.2020.74.2
- Terranova C, Rizzo V, Cacciola A, et al. Is there a future for non-invasive brain stimulation as a therapeutic tool? Front Neurol. 2019;9:1146. doi: 10.3389/fneur.2018.01146
- Pacheco-Barrios K, Cardenas-Rojas A, Thibaut A, et al. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Rev Med Devices. 2020;17(9):879–898. doi: 10.1080/17434440.2020.1816168
- Benjamens S, Dhunnoo P, Meskó B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: an online database. npj Digital Med. 2020;3(1):118. doi:10.1038/s41746-020-00324-0
- Teixeira PEP, Pacheco-Barrios K, Branco LC, et al. The analgesic effect of transcranial direct Current stimulation in fibromyalgia: a systematic review, meta-analysis, and meta-regression of potential influencers of clinical effect. Neuromodulation: Technol Neural Interface. 2022;26(4):715–727. doi: 10.1016/j.neurom.2022.10.044
- Zeng H, Pacheco-Barrios K, Cao Y, et al. Non-invasive neuromodulation effects on painful diabetic peripheral neuropathy: a systematic review and meta-analysis. Sci Rep. 2020;10(1):19184. doi: 10.1038/s41598-020-75922-9
- Moffa AH, Martin D, Alonzo A, et al. Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: an individual patient data meta-analysis. Prog Neuro Psychopharmacol Biol Psychiatry. 2020;99:109836. doi: 10.1016/j.pnpbp.2019.109836
- Pacheco-Barrios K, Meng X, Fregni F. Neuromodulation techniques in phantom limb pain: a systematic review and meta-analysis. Pain Med. 2020;21(10):2310–2322. doi:10.1093/pm/pnaa039
- Borsci S, Macredie RD, Martin JL, et al. How many testers are needed to assure the usability of medical devices? Expert Rev Med Devices. 2014;11(5):513–525. doi:10.1586/17434440.2014.940312
- Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):1–8. doi: 10.1186/1745-6215-13-132
- Bastien JMC. Usability testing: a review of some methodological and technical aspects of the method. Int J Med Inform. 2010;79(4):e18–e23. doi:10.1016/j.ijmedinf.2008.12.004
- Darrow JJ, Avorn J, Kesselheim AS. FDA regulation and approval of medical devices: 1976-2020. JAMA. 2021;326(5):420–432. doi:10.1001/jama.2021.11171