ABSTRACT
Introduction
This systematic review and meta-analysis assessed the characteristics, types, and impact of interventions to improve adherence to attention-deficit hyperactivity disorder (ADHD) medications within the context of the three phases of adherence, namely, initiation, implementation, and discontinuation.
Methods
PubMed, Psychological Information Database, Embase, International Pharmaceutical Abstracts, and Google Scholar were systematically searched for relevant trials using appropriate search terms. Interventions were classified as educational, behavioural, affective, and multifaceted. Data was pooled using odds ratios and proportions.
Results
Seventeen studies were included in this review. In a pooled analysis of four RCTs, interventions did not significantly improve medication adherence (OR = 2.32; 95%-Confidence Interval=CI = 0.91–5.90; p = 0.08). In seven non-randomized trials, a pooled proportion of people who adhered to ADHD medication was considerably higher in the intervention group (85%, 95%CI = 78%-91%) than in the control group (47%, 95%CI = 33%–61%). Interventions varied in terms of study design, methods and their impact on different phases of adherence.
Conclusions
Despite some promising results, the lack of consideration of phase-specific adherence factors may limit the effectiveness and sustainability of interventions to improve adherence in clinical practice. Future interventions should be phase-specific, guided by factors which are pertinent to each phase. Meanwhile, clinicians should choose or tailor interventions based on individual needs and preferences.
1. Introduction
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity [Citation1]. ADHD is predominantly a childhood disorder; however, it often continues into adolescence and adulthood [Citation2]. ADHD is associated with reduced academic, occupational, and social functioning in affected patients, which can significantly impact their lives as well as their family members [Citation2]. The worldwide prevalence of ADHD in children [Citation3] and adults [Citation4] is 7.2% and 6.8%, respectively. ADHD can pose a huge economic burden at individual and societal levels, associated with higher healthcare costs. In the United States, the direct and indirect costs related to ADHD are between USD143 billion and USD266 billion [Citation5]. A review summarizing the global evidence suggested that the national estimates ranged between USD356 million to USD20.27 billion [Citation6].
Given the impact of ADHD on the health and well-being of the patients and their carers, it is important to manage ADHD to improve patient outcomes. Medications play an important role in managing ADHD [Citation7]. Various guidelines have recommended stimulants as a first-line treatment in children with ADHD [Citation8–10]. However, given the chronic nature of the condition, medications often need to be taken on a long-term basis to achieve the desired medication outcomes, highlighting the importance of adherence to ADHD medications [Citation7].
Several factors influence adherence to ADHD medications. However, there is limited information about the impact of those factors on different phases of adherence. According to the Ascertaining Barriers to Compliance (ABC) taxonomy, medication adherence is a dynamic process that can be defined as a medication-taking journey starting from the initiation phase when the patient begins taking the medication [Citation11]. The journey continues into the implementation phase, where the patient takes the medication. Finally, the journey ends at the discontinuation phase when the patient stops taking the medication [Citation11]. Literature suggests that adherence to ADHD medication is poor [Citation12,Citation13]. For example, a systematic review reported that adherence to ADHD medication in children and adolescents ranged between 9.4% and 64% [Citation12]. A recent study on adults with ADHD reported that only 42% of patients adhered to their medication [Citation14]. Several factors, such as the benefits of medication, side effects, stigma, etc., have been shown to influence medication adherence [Citation15]. Within the context of the three phases of adherence, it is suggested that some factors may be unique to each phase, while other factors may remain common across the three phases [Citation15].
The problem of nonadherence to ADHD medication has prompted researchers to design and implement interventions to improve medication adherence. Several trials including reviews have been conducted to assess the effectiveness of interventions intended to improve adherence to ADHD medication [Citation16–20]; however, to the best of our knowledge, no review has been conducted that has summarized the information about the interventions (type, delivery, settings, etc.) and assessed the impact of those interventions on medication adherence in the context of the three phases of adherence. The synthesis of the information surrounding the impact of interventions at different phases of adherence is essential as it can provide a useful guide for clinicians to know which interventions can be effectively applied in clinical settings and for researchers to design future interventions that are more effective and sustainable. Therefore, the objective of this systematic review and meta-analysis was to evaluate the impact of interventions intended to improve adherence to ADHD medication in the context of the three phases of adherence. The review also summarized the information related to the interventions, such as the type, component, delivery, settings, and the phase of adherence when the intervention was implemented, guided by the ABC taxonomy.
2. Methods
This systematic review, including a meta-analysis, was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix 1) [Citation21]. This review was registered in the Open Science Framework Registries (Registration code: osf.io/w8n6a).
2.1. Eligibility criteria
Studies that assessed an intervention to improve adherence to ADHD medication were included in this review. Studies had to describe the adherence phase in which the intervention was delivered. Studies were included regardless of their design as adherence studies utilize a wide range of designs, including randomized and non-randomized controlled trials. No restriction was applied in the criteria about the study population (children, adults, parents) as ADHD can impact people of all ages as well as their parents/carers. Studies that had evaluated adherence to non-pharmacological management or compared two or more treatment options for their impact on adherence without any intervention component were not included in this review.
2.2. Information sources
Five databases were used to search for the eligible studies: PubMed, Psychological Information Database (PsycINFO), Excerpta Medica dataBASE (Embase), International Pharmaceutical Abstracts (IPA), and Google Scholar. The databases were searched from their inception to the last update on 19 January 2024 and were limited to English language and human studies. Additional articles were also searched manually by screening the reference list of the relevant articles. The search was conducted by two authors independently.
2.3. Search strategy
The databases were systematically searched for eligible studies that tested an intervention to improve adherence to ADHD medication. Three broader concepts were used to search the literature; adherence, ADHD, and intervention, combined with the Boolean operator ‘AND.’ Several relevant terms (MeSH, free text words) were used within each concept, combined with the Boolean operator ‘OR.’ The detailed search strategy is attached as an appendix to this article (Appendix 2).
2.4. Study selection
The process of selecting studies was conducted in two steps. First, the studies were screened against the set eligibility criteria. The screening was performed by reading the title and the abstract of the potential articles. The articles that appeared to be eligible were selected for the second round of the process in which the full text of the articles was read to confirm their eligibility for this review. The articles that met the eligibility criteria were then processed for data extraction.
2.5. Data extraction
The relevant data were extracted from the selected studies in a pre-designed piloted form. The relevance of the data was decided based on the objectives of this review. The following information was extracted: authors, publication year, country, study design, sample size, study duration, adherence measure, adherence phase, medication type, intervention type, components of the intervention, delivery, and intervention outcomes. In cases where a study reported outcomes at more than one point, the last point (endpoint) data were extracted. The data were extracted by one author, a portion (20%) of which was counterchecked by another author for consistency.
2.6. Risk of bias
The risk of bias was assessed by one author, a portion (20%) of which was checked by another author for consistency. For randomized control trials, the risk of bias was assessed by using the revised Cochrane ‘risk-of-bias’ tool for randomized trials (RoB 2). The domains assessed were the risk of bias arising due to randomization, deviations from intervention, incomplete outcomes, measurement of results, and selective reporting of outcomes. For non-randomized studies, the risk of bias was assessed by using the ‘Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I)’ tool. The main sources of bias assessed were confounding bias, selection bias, classification of intervention bias, deviation from intervention bias, missing data bias, outcome measurement bias, and reporting bias. The risk of bias is summarized in Appendix 3.
2.7. Data synthesis
The outcome measure was the impact of an intervention to improve adherence to ADHD medication. The intervention and its components were evaluated for each study, including the person who delivered the intervention, the group to whom the intervention was delivered, the method for assessing the impact of adherence (adherence measure), and the adherence phase in which the intervention was delivered. We used two software programs for data analysis: RevMan for pooling data from RCTs and MetaXL for pooling data from non-randomized trials.
The number of people who adhered to medication in the intervention and the control group was extracted directly from each study. For RCTs, the odds ratio was calculated from the data provided in the study to determine the odds of people adhering to medication in the intervention and the control group with a 95% confidence interval (CI) for each study. We used the random effect model (due to the potential heterogeneity between studies) to pool the estimates. The heterogeneity between studies was assessed by calculating I2 statistics. I2 values with an upper limit of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively. An I2 statistic of more than 50% with p < 0.05 represented substantial heterogeneity. For non-randomized trials, the results were pooled by using the proportion of people who adhered to medication in the intervention and control groups.
The data from the RCTs and non-randomized trials were pooled separately because of the methodological heterogeneity and increased risk of bias in the summary effect. Studies that did not report the number of people who adhered to medication in the intervention and control groups were not included in the meta-analyses. Subgroup analysis was performed by pooling estimates from studies that looked at different phases of adherence separately for both RCTs and non-randomized studies where applicable. Publication bias was assessed using a funnel plot, where ratio measures of intervention effect (such as odds ratios) were plotted on a logarithmic scale and reported with a summary estimate extending 1.96 standard errors on either side, including about 95% of studies (in case of no bias).
2.8. Operational definitions
The following operational definitions have been used in this review:
2.8.1. Medication adherence
Medication adherence was defined as the extent to which patients take their medication as prescribed by the health professional. Adherence is composed of three distinct yet relevant phases; initiation, implementation, and discontinuation [Citation11].
Initiation was defined as the start of the medication process, that is when a patient takes the first dose of a medication [Citation11].
Implementation was defined as the extent to which a patient adheres to the prescribed regimen from the first dose of a medication until the last dose of the medication [Citation11].
Discontinuation was defined as the cessation of medication for any given reason [Citation11].
2.8.2. Types of intervention
Interventions were classified based on the classification (educational, behavioural, and affective) suggested by Roter et al. [Citation22]. This classification has been previously used in systematic reviews assessing the impact of adherence interventions in chronic disease conditions [Citation23,Citation24]. One additional category, multifaceted, was also used along with the other three categories.
Interventions were classified as educational if they provided verbal or written material with an emphasis on conveying knowledge or information to research participants. The delivery of intervention could be one-to-one and/or group teaching and may involve the use of written and audio-visual materials, mailed instructional materials, and telephone instructions (excluding reminders and prompts to comply). These interventions were based on the concept of making patients/carers well-informed about their medications and empowering them to improve their adherence to medications [Citation22].
Interventions were classified as behavioural if they were designed to influence behavior by shaping, reminding (cues), or reinforcing specific behavioural patterns (rewarding desired behavior). The strategies used to deliver these interventions could include skill building and practice activities led by health professionals (e.g. medication self-management skills), using adherence aids (e.g. medication boxes, calendars), a change in packaging or simplifying dosing regimen (e.g. once a day dose) to reduce behavioural demands, rewards and reinforcement through regular monitoring of adherence and providing feedback, and reminding patients to take medication through both mail and telephone reminders [Citation22].
Interventions were classified as affective if they were designed to influence medication adherence through appeals to feelings and emotions or social relationships and social supports. The support could be provided by family or another group. Examples of affective interventions could include family counseling, support group counseling, and supportive home visits. The concept of these interventions was to provide support to a patient to alleviate stress, increase self-efficacy, and improve medication adherence. Therefore, family or group sessions that were primarily didactic or educational in nature rather than supportive were considered educational interventions [Citation22].
Interventions were considered multifaceted if they had two or more distinct components of educational, behavioural, or affective intervention.
3. Results
3.1. Study selection
A total of 1,240 articles were identified from all databases. After removing the duplicates, 865 studies were screened for potential inclusion in this review. The screening was performed by scanning the title and abstract of the potential studies against the eligibility criteria set for this review. Eight hundred and forty articles were deemed ineligible, and the remaining 25 underwent full-text review. Eight articles did not meet the criteria. Hence, 17 articles were included in this review. For the meta-analyses, 12 studies were selected as the remaining 5 studies did not provide information on the number of people who adhered to medication in the intervention and control group. Of these 12 studies, 5 RCTs and 7 non-randomized studies were pooled separately. The study selection process is summarized as a PRISMA flow diagram in Appendix 4.
3.2. Study characteristics
Most studies were conducted in the US (n = 6) [Citation14,Citation16,Citation25–28] followed by China (n = 3) [Citation29–31], Canada (n = 2) [Citation17,Citation32], Spain (n = 2) [Citation33,Citation34], Israel (n = 1) [Citation20], United Kingdom (n = 1) [Citation35], Brazil (n = 1) [Citation36], and Japan (n = 1) [Citation37]. The median sample size was 348 participants (range: 35–2369) with a total of 5932 participants. The total number of months for which adherence was monitored ranged from 1.5 months to 24 months (Mean = 6.14 months; Median = 6 months). Eleven studies [Citation14,Citation16,Citation25–28,Citation30,Citation32–34,Citation37] were non-randomized while six studies [Citation17,Citation20,Citation29,Citation31,Citation35,Citation36] were randomized control studies. More than half of the studies (n = 9) [Citation17,Citation26,Citation28,Citation30,Citation31,Citation33–35,Citation37] used self-reported adherence measures, while the other eight studies [Citation14,Citation16,Citation20,Citation25,Citation27,Citation29,Citation32,Citation36] used objective measures. Differences were noted between studies that used self-reported measures; that is, some studies directly asked patients whether they took the medication, while other studies used questionnaires such as Family Therapy Adherence Scale [Citation26], Child Adherence Questionnaire [Citation37], Simplified Medication Adherence Questionnaire [Citation34], and Medication Adherence Rating Scale [Citation31]. Similarly, several objective measures were used, such as medication possession ratio (n = 4) [Citation27,Citation29,Citation32,Citation36], medication refill method (n = 3) [Citation14,Citation16,Citation25], and pill count (n = 1) [Citation20]. All studies but one [Citation30] measured adherence at a single phase. Ten studies [Citation14,Citation16,Citation20,Citation25,Citation27,Citation31,Citation32,Citation34,Citation36,Citation37] measured adherence at the implementation phase, five studies [Citation17,Citation28–30,Citation33] at the discontinuation phase, and three studies at the initiation phase [Citation26,Citation30,Citation35]. The study characteristics are summarized in .
Table 1. Study characteristics.
3.3. Risk of bias
Six randomized control trials were assessed using the RoB 2 assessment tool. Out of the six, one trial [Citation29] was at high risk of bias, two trials [Citation17,Citation35] were at low risk, and three trials [Citation20,Citation31,Citation36] were found to have some concerns (Appendix 5a). Eleven non-randomized studies were assessed using the ‘Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I)’ tool. Out of the eleven, two studies [Citation28,Citation33] were at serious risk of bias, eight studies [Citation14,Citation16,Citation25–27,Citation32,Citation34,Citation37] were at moderate risk, and one study [Citation30] could not be assessed because of a lack of information (Appendix 5b).
3.4. Characteristics of interventions
Seven studies [Citation14,Citation16,Citation20,Citation27,Citation33–35] investigated behavioural interventions, and four studies [Citation17,Citation29,Citation30,Citation37] investigated educational interventions while six studies [Citation25,Citation26,Citation28,Citation31,Citation32,Citation36] used a combination of educational and behavioural or affective (multifaceted) intervention. Multifaceted interventions used lectures, presentations, and written materials as educational components, text reminders [Citation25,Citation36] and management strategies as behavioural components [Citation32], and family/group support as affective components [Citation26,Citation28]. Interventions were delivered by healthcare professionals or by using digital platforms such as phone reminders. Thirteen studies targeted children (<18 years), while four studies targeted adult patients (≥18 years). In the case of children, most interventions [Citation17,Citation25,Citation28–30,Citation33] were delivered to parents (n = 6), and five interventions [Citation20,Citation25,Citation32,Citation35,Citation37] targeted both parents and children [Citation20,Citation26,Citation32,Citation35,Citation37], one intervention [Citation31] was delivered to parent and teacher while one intervention [Citation27] was delivered to children only. The characteristics of interventions are summarized in .
Table 2. Type of interventions and their impact.
3.5. Impact of interventions
3.5.1. Meta-analysis results
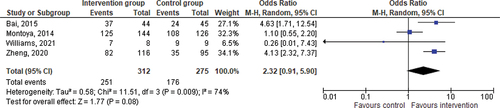
Initially, five RCTs were included for quantitative synthesis. One RCT [Citation36] had no events in both arms and did not affect the pooled estimates, and thus was excluded from the analysis. According to Cochrane, such studies do not have any impact on the direction or magnitude of the relative treatment effect, and therefore their exclusion is a standard practice in meta-analysis [Citation38]. In a pooled analysis of four RCTs including 587 participants, the delivery of an intervention, regardless of any other factor, improved medication adherence in people with ADHD; however the improvement in adherence was not statistically significant (Odds Ratio=OR = 2.32; Confidence Interval=CI = 0.91–5.90; p = 0.08) (). The ORs ranged from 0.26 to 4.63.
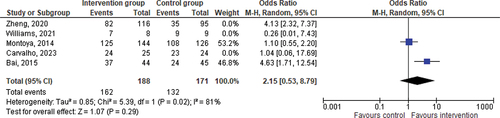
In the context of the three phases of adherence, we were able to evaluate improvement in adherence at the discontinuation phase. The impact of Interventions on the discontinuation phase of adherence was not significant (OR = 2.15; CI = 0.53–8.79; p = 0.29) ().
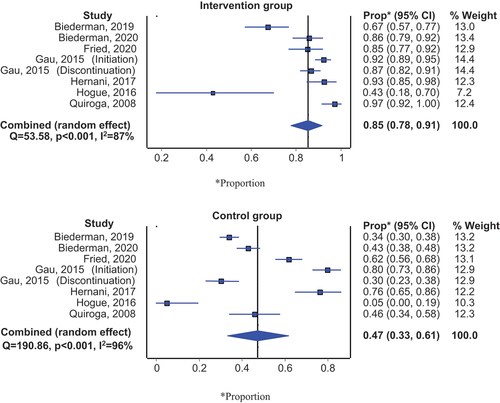
In addition, non-randomized studies were pooled based on the participants who adhered to medication in both the intervention and control groups. 85% of participants were adherent in the intervention group (95% CI: 78%–91%) and 47% of participants were adherent in the control group (95% CI: 33%–61%) (). Sub-group analysis showed that the difference in the effectiveness of intervention versus control at the implementation phase was higher (86% vs 46%) compared to the discontinuation (89% vs 54%) and initiation phase (72% vs 40%) (Appendix 6a & 6b).
3.5.2. Impact of different types of interventions at different phases of adherence
3.5.2.1. Behavioural interventions
Behavioural interventions delivered at the implementation phase of adherence showed considerable improvement in medication adherence. For example, Meyers et al. [Citation27] showed that the modified medication possession ratio increased from 0.68 to 0.87 due to therapy modification. Similarly, Quiroga et al. [Citation34] showed that the proportion of participants who adhered to medication was higher in the intervention group (therapy modification) (97.1%) compared to the control group (45.7%). Weisman et al. [Citation20] also reported better scoring of pill count (better adherence) in the intervention group (reminders) (Median = 0.92) compared to the control group (Median = 0.74) through medication reminders and increasing communication with the physician.
Hernani et al. [Citation33] targeted the discontinuation phase and reported a relative risk reduction of 0.67, meaning that the intervention (use of written informed consent) effectively reduced the risk of discontinuation by 67%.
3.5.2.2. Educational interventions
Education-only interventions were mainly implemented to prevent discontinuation of medication, except for one study [Citation37] that targeted the implementation phase of adherence. Gau et al. [Citation30] also implemented the intervention at the initiation phase of adherence in addition to the discontinuation phase. Bai et al. [Citation29] reported an 84.09% improvement in adherence by preventing discontinuation of medication, relatively similar to the findings of Gau et al. [Citation30] (85.39%) and Nagae et al. [Citation37] (86.80%). Bai et al. [Citation29] and Montoya et al. [Citation17] used a combination of educational interventions such as expert lectures, group sessions, community engagement, and homework) while Gau et al. [Citation30] focused on parental group education sessions. The educational intervention resulted in a slightly higher improvement in adherence when delivered at the initiation phase (92.24%), as reported by Gau et al. [Citation30].
3.5.2.3. Multifaceted interventions
Most studies that used multifaceted intervention also targeted the implementation phase of adherence. For example, Carvalho et al. [Citation36] showed that the use of a digital application (information, educational content, pill reminders) can improve medication adherence; however, it was not significantly different from the control group. Enns et al. [Citation32] showed that by providing education, parent support, and medication management services, adherence was improved in the intervention group (Adjusted rate ratio = 1.42 (1.03 to 1.96); p < 0.05). Fried et al. [Citation25] used educational material and text reminders to improve medication adherence (odds ratio = 3.46, p < 0.05). Zheng et al. [Citation31] combined educational content with behavioural strategies such as classroom management for teachers, which resulted in significant improvement in adherence in the intervention group compared to the control group (70.69% vs 36.84%).
Hogue et al. [Citation26] and Monastra et al. [Citation28] targeted the initiation and discontinuation phases, respectively, and combined educational content with specialized protocols such as Medication Integration [Citation26] and Neurologic assessment [Citation28], which resulted in marked improvement in medication adherence. The Medication Integration Protocol was focused on delivering family-centered intervention by educating children and their caregivers about ADHD and its medication and integrating it with behavioural activities such as improving medication decision-making. The neuro-educational intervention consisted of a comprehensive neurological assessment followed by parent education about the causes of ADHD and the effects of ADHD medications. Monastra et al. [Citation28] showed an improvement of 95% after implementing the intervention.
Funnel plots revealed a gross asymmetry to either side, indicating that publication bias may be present (Appendix 7 & 8). In addition, studies were scattered asymmetrically around the summary effect; small or opposite-direction studies seem to be missing.
4. Discussion
This paper presents a systematic review and meta-analysis of interventions and their impact on adherence to ADHD medication. The findings of this review showed a modest impact of existing interventions in improving adherence to ADHD medication. In particular, it is challenging to predict the type of interventions that are most likely to work at a particular phase of adherence or if any intervention is better than other interventions. This is primarily because of wide heterogeneity in study design, intervention studied, delivery personnel, recipients, and how and when adherence was measured. Another important reason is that the interventions were not designed considering factors impacting different phases of adherence. Therefore, health professionals should be cautious of these factors in selecting the intervention in the absence of a gold standard. Rather, the choice of interventions should be based on the needs and preferences of the patients, the availability, feasibility, and acceptability of the intervention, as well as the consideration of the phase, and its associated factors, at which the intervention needs to be delivered.
The findings of this study suggest that there is considerable heterogeneity in study design and how adherence has been measured in the reviewed studies. This heterogeneity, in addition to the limited number of studies, makes it difficult to combine the studies and derive a meaningful conclusion statistically. The heterogeneity exists not only in studies that used different study designs but also among studies that used the same study design. For example, among Six RCTs, three studies used objective measures, while three used subjective measures. The objective and subjective measures also differed between the studies. The variation in the effectiveness of interventions might be, to some extent, related to the use of different measures. Available evidence suggests that there is no gold standard in measuring adherence as each method has its pros and cons [Citation39]. Therefore, it is important to develop a consensus among researchers to standardize the literature on ADHD to promote cross-study comparisons and synthesize evidence aimed at improving medication adherence. Another potential reason for heterogeneity could be a large variation in the sample sizes of studies included in this review. Studies with smaller sample sizes or underpowered studies are often associated with higher effect sizes, which in turn can increase heterogeneity [Citation40]. There is an ongoing debate on the inclusion of underpowered studies in meta-analyses. However, evidence suggests that meta-analysis containing many, even small, studies are more powerful than fewer large studies to estimate the treatment effect [Citation40,Citation41].
A majority of studies designed and tested interventions at the implementation phase of adherence. Although those interventions showed promising results, it is not clear if the same interventions will remain equally effective in the other phases of adherence. In addition, evidence suggests that factors that impact adherence at different phases might differ, implying that different interventions might be required based on the influencing factors [Citation42]. Therefore, interventions must be tailored to each phase, and a more targeted approach should be used to develop phase-specific interventions to improve medication adherence. There have been calls in the literature previously on developing phase-specific interventions that can be compared and contrasted to synthesize evidence that can aid clinical decision-making and inform health policy decisions [Citation43]. While developing phase-specific interventions, it is important to ensure that more standardized interventions and methodologies are used in future research to enhance comparability and reduce heterogeneity.
Medication adherence is a complex phenomenon influenced by various factors that may facilitate or prevent medication-taking. This complexity further increases in ADHD as it is a childhood disorder that involves parents and continues into adolescence and adulthood. Evidence suggests that factors influencing adherence during childhood are relatively different from those influencing medication adherence in adult patients [Citation42]. Similarly, factors affecting children might differ from those influencing their parents [Citation44]. Current evidence does not suggest any intervention specifically designed for parents, children, or adults with ADHD. Therefore, health professionals need to consider these important issues before choosing an intervention. Whilst we encourage and expect future research to focus on group-specific interventions, we suggest practitioners tackle this issue by using multifaceted interventions to address myriad factors that impact adherence in different groups as recommended in other mental health conditions [Citation45,Citation46]. In the case of a child, tailored interventions should also be extended to parents as they are the main decision-makers for their child’s health, including medication-taking. Parental beliefs about ADHD medications are an important determinant of medication adherence in children [Citation47,Citation48]. When evaluating the impact of an intervention on children, it is important to differentiate between younger and older children (adolescents) as during adolescence older children begin to explore their own identity and role in life and start taking autonomy about their healthcare including the use of medications. Evidence suggests that the impact of factors that affect adherence may vary between younger children and adolescents [Citation42]. Therefore, future interventions targeting children should consider the specific age group of children (such as younger children and older children/adolescents) and design interventions based on factors pertinent to that age group.
The use of a theoretical framework has been highly recommended in adherence research to identify the factors that impact medication adherence as well as to design interventions [Citation49]. Interventions grounded in a theoretical framework are more likely to be effective and sustainable than those without any theoretical support [Citation50]. This might explain the limited success of adherence interventions in the past, as they were not supported by theoretical underpinning [Citation51]. Similarly, the findings of this review suggest that most adherence interventions in ADHD are not guided by any theoretical framework. Whilst we cannot conclude due to a limited number of studies, we did find certain indications that might suggest that theory-driven interventions are relatively more effective. For example, one of the RCTs [Citation29] in this review that used the theory of planned behavior (TPB) to guide their interventions reported higher effect size (odds ratio = 4.63) compared to another RCT [Citation17] (odds ratio = 1.10) that did not have any theoretical support. This finding is in line with other mental health studies and wider health conditions supporting the use of theory-based interventions to improve medication adherence [Citation52,Citation53].
Another area that needs more attention in adherence research in ADHD is the consideration of drug holidays (planned suspension of medication for a defined period of time). Drug holidays in ADHD are common as patients/parents are often advised or may decide not to take medications over a holiday period or vacation [Citation54]. Current literature does not take into account drug holidays when assessing the impact of an intervention to improve medication adherence. This may result in an overestimation of non-adherence to medication and may not reflect the true impact of an intervention. Future research should consider drug holidays when assessing the impact of interventions to improve medication adherence.
The findings of this study should be interpreted in light of some limitations. First, despite using a thorough search strategy, we cannot ignore the possibility of missing out on potential studies. Second, due to the heterogeneity of the study design, we were not able to pool all the studies together. Rather, we pooled randomized trials and non-randomized studies separately, which limited the number of studies in each pool. Studies were limited in number and heterogeneous in terms of the definition of adherence and type of interventions (educational, behavioural, affective), which further warrants cautious interpretation of the results. For example, educational and behavioural interventions were pooled together that may vary in their mechanism of action, delivery, and effectiveness, which could potentially influence the overall effect estimates. The percentage of variation across studies that is due to heterogeneity (I2) should be interpreted with caution due to the potential inclusion of studies with a lower sample size. Due to a limited number of studies in each pool, we were not able to conduct sub-group analyses, which may limit the generalizability of the findings. Third, it is common to exclude studies from meta-analysis due to a lack of required data, as in this study. For example, studies that did not report the number of people who adhered to medication in the intervention and control group were excluded from the meta-analysis.
We categorized the interventions broadly into four groups; educational, behavioural, affective, and multifaceted, as discussed in the methods section. Although we used a set definition for each type of intervention, there is a possibility that elements of one type of intervention (such as educational intervention) might have been used to deliver the other types of interventions (such as behavioural and affective) and were not explicitly mentioned in the reviewed studies. The cross-over of intervention components that were not explicitly mentioned in the reviewed studies was not accounted for in this review. Most studies included in this review did not provide adherence data based on the type of medication. Therefore, we could not analyze adherence and the impact of intervention based on the type of medication. Lastly, we also observed publication bias that could mainly result from reporting bias as researchers tend to publish statistically significant positive results and ignore studies with negative results (or smaller studies) as the nature and direction of results influence them [Citation55]. However, publication bias is only one of several possible explanations for funnel-plot asymmetry [Citation56].
5. Conclusions
The present review identified a range of interventions aimed at improving adherence to ADHD medications. However, due to wide heterogeneity and lack of consideration of phase-specific factors, it is difficult to predict if any intervention is better than the other interventions. Our findings highlight the need for designing interventions focusing on different phases of adherence (initiation/implementation/discontinuation) and targeting a specific group of people (younger children, adolescents, adults, parents), allowing better comparisons between interventions and their outcomes. Future research should also strive to minimize methodological heterogeneity by developing a consensus on study design and the use of adherence measures. Meanwhile, clinicians may consider individual needs and preferences and choose interventions that are multifaceted and delivered over a longer period considering phase-specific factors to improve the effectiveness and sustainability of the intervention.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Availability of data
Data supporting the findings of this study are available within the article and its supplementary materials.
Author contributions
MU Khan conceived the research idea. MU Khan and SS Hasan designed the study and were involved in screening articles, data extraction, analysis, and interpretation. MU Khan wrote the first draft. MU Khan and SS Hasan revised and approved the manuscript for final submission. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (180.7 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14737175.2024.2360118
Additional information
Funding
References
- Mirabella G. Inhibitory control and impulsive responses in neurodevelopmental disorders. Dev Med Child Neurol. 2021;63(5):520–526. doi: 10.1111/dmcn.14778
- Leffa DT, Caye A, Rohde LA. ADHD in children and adults: diagnosis and prognosis. New Discov Behav Neurosci Attention-Deficit Hyper Dis. 2022;57:1–18.
- Thomas R, Sanders S, Doust J, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–e1001. doi: 10.1542/peds.2014-3482
- Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi: 10.7189/jogh.11.04009
- Sayal K, Prasad V, Daley D, et al. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175–186. doi: 10.1016/S2215-0366(17)30167-0
- Chhibber A, Watanabe AH, Chaisai C, et al. Global economic burden of attention-deficit/hyperactivity disorder: a systematic review. PharmacoEconomics. 2021;39(4):399–420. doi: 10.1007/s40273-020-00998-0
- Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. doi: 10.1542/peds.2019-2528
- Australian guidelines on attention-deficit-hyperactivity/disorder. Royal Australasian college of physicians. 2009 [cited 2024 Jan]. Available from: https://www.racp.edu.au/docs/default-source/advocacy-library/pa-australian-guidelines-on-adhd-draft.pdf?sfvrsn=c905321a_8
- NICE guideline: attention deficit hyperactivity disorder: diagnosis and management. 2018 [cited 2024 Jan]. Available from: https://www.nice.org.uk/guidance/ng87
- Canadian ADHD Practice Guidelines. Canadian ADHD resource alliance (CADDRA). Available at 2018 [cited 2024 Jan]. Available from: https://adhdlearn.caddra.ca/wp-content/uploads/2022/08/Canadian-ADHD-Practice-Guidelines-4.1-January-6-2021.pdf
- Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x
- Gajria K, Lu M, Sikirica V, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder–a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543. doi: 10.2147/NDT.S65721
- Schein J, Childress A, Adams J, et al. Treatment patterns among children and adolescents with attention-deficit/hyperactivity disorder in the United States - a retrospective claims analysis. BMC Psychiatry. 2022;22(1):555. doi: 10.1186/s12888-022-04188-4
- Biederman J, Fried R, DiSalvo M, et al. Further evidence of low adherence to stimulant treatment in adult ADHD: an electronic medical record study examining timely renewal of a stimulant prescription. Psychopharmacology. 2020;237(9):2835–2843. doi: 10.1007/s00213-020-05576-y
- Khan MU, Aslani P. Exploring factors influencing medication adherence from initiation to discontinuation in parents and adolescents with attention deficit hyperactivity disorder. Clin Pediatr. 2020;59(3):285–296. doi: 10.1177/0009922819900973
- Biederman J, Fried R, DiSalvo M, et al. Perlis RHA novel text message intervention to improve adherence to stimulants in adults with attention deficit/hyperactivity disorder. J Clin Psychopharmacol. 2019;39(4):351–356. doi: 10.1097/JCP.0000000000001055
- Montoya A, Hervas A, Fuentes J, et al. Cluster-randomized, controlled 12-month trial to evaluate the effect of a parental psychoeducation program on medication persistence in children with attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. 2014;10:1081. doi: 10.2147/NDT.S62487
- Parkin R, Mc Nicholas F, Hayden JC. A systematic review of interventions to enhance adherence and persistence with ADHD pharmacotherapy. J Psychiatr Res. 2022;152(2022):201–218. doi: 10.1016/j.jpsychires.2022.05.044
- Thongseiratch T, Chalermphol K, Traipidok P, et al. Promoting medication adherence in children with attention deficit hyperactivity disorder: a mixed-methods systematic review with meta-analysis and qualitative comparative analysis. JAtten Disord. 2024;28(2):139–150. doi: 10.1177/10870547231211021
- Weisman O, Schonherz Y, Harel T, et al. Testing the efficacy of a smartphone application in improving medication adherence, among children with ADHD. Isr J Psychiatry. 2018;55(2):59–67.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
- Roter DL, Hall JA, Merisca R, et al. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med care. 1998;36(8):1138–1161. doi: 10.1097/00005650-199808000-00004
- Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–549. doi: 10.1001/archinte.167.6.540
- Sapkota S, Brien JAE, Greenfield JR, et al. A systematic review of interventions addressing adherence to anti-diabetic medications in patients with type 2 diabetes—components of interventions. PLoS One. 2015;10(6):e0128581. doi: 10.1371/journal.pone.0128581
- Fried R, DiSalvo M, Kelberman C, et al. An innovative SMS intervention to improve adherence to stimulants in children with ADHD: preliminary findings. J Psychopharmacol. 2020;34(8):883–890. doi: 10.1177/0269881120908014
- Hogue A, Lichvar E, Bobek M. Pilot evaluation of the medication integration protocol for adolescents with ADHD in behavioural care: treatment fidelity and medication uptake. J Emot Behav Disord. 2016;24(4):223–234. doi: 10.1177/1063426615611648
- Meyers J, Gajria K, Candrilli SD, et al. The impact of adjunctive guanfacine extended release on stimulant adherence in children/adolescents with attention-deficit/hyperactivity disorder. J Comp Eff Res. 2017;6(2):109–125. doi: 10.2217/cer-2016-0039
- Monastra VJ. Overcoming the barriers to effective treatment for attention-deficit/hyperactivity disorder: a neuro-educational approach. Int J Psychophysiol. 2005;58(1):71–80. doi: 10.1016/j.ijpsycho.2005.03.010
- Bai GN, Wang YF, Yang L, et al. Effectiveness of a focused, brief psychoeducation program for parents of ADHD children: improvement of medication adherence and symptoms. Neuropsychiatr Dis Treat. 2015;11:2721. doi: 10.2147/NDT.S88625
- Gau H, Zhu D. The role of group parent education on medication adherence in children with Attention-Deficit/Hyperactivity Disorder. Conference: 5th world congress on ADHD: from child to adult disorder, Glasgow United Kingdom, 2015. Conference Publication. 2015;7(SUPPL. 1):S41–S42.
- Zheng X, Shen L, Jiang L, et al. Parent and teacher training increases medication adherence for primary school children with attention-deficit/hyperactivity disorder. Front Pediatr. 2020;8:486353. doi: 10.3389/fped.2020.486353
- Enns JE, Randall JR, Smith M, et al. A multimodal intervention for children with ADHD reduces inequity in health and education outcomes. Can J Psychiatry. 2017;62(6):403–412. doi: 10.1177/0706743717692301
- Naenen-Hernani K, Palazón-Bru A, Colomina-Climent F, et al. Influence of written informed consent for methylphenidate on medicine persistence rates in children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2017;38(8):603–610. doi: 10.1097/DBP.0000000000000495
- Ramos-Quiroga JA, Bosch R, Castells X, et al. Effect of switching drug formulations from immediate-release to extended-release OROS methylphenidate: a chart review of Spanish adults with attention-deficit hyperactivity disorder. CNS Drugs. 2008;22(7):603–611. doi: 10.2165/00023210-200822070-00005
- Williams L, Hall CL, Brown S, et al. Optimising medication management in children and young people with ADHD using a computerised test (QbTest): a feasibility randomised controlled trial. Pilot And Feasibility Stud. 2021;7(68):1–18. doi: 10.1186/s40814-021-00788-1
- Carvalho LR, Haas LM, Zeni G, et al. Evaluation of the effectiveness of the FOCUS ADHD app in monitoring adults with attention-deficit/hyperactivity disorder. Eur Psychiatry. 2023;66(1):e53. doi: 10.1192/j.eurpsy.2023.2422
- Nagae M, Tokunaga A, Morifuji K, et al. Efficacy of a group psychoeducation program focusing on the attitudes towards medication of children and adolescents with ADHD and their parents: a pilot study. Acta Medica Nagasakiensia. 2019;62(3):77–86.
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M Welch V, editors. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane; 2023. Available from: www.training.cochrane.org/handbook
- IntHout J, Ioannidis JP, Borm GF, et al. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol. 2015;68(8):860–869.
- Khan MU, Aslani P. A review of measures used to examine medication adherence in people with ADHD at initiation, implementation and discontinuation of pharmacotherapy. Res Social Adm Pharm. 2020;16(3):277–289. doi: 10.1016/j.sapharm.2019.06.001
- Roloff V, Higgins JP, Sutton AJ. Planning future studies based on the conditional power of a meta‐analysis. Stat Med. 2013;32(1):11–24. doi: 10.1002/sim.5524
- Khan MU, Aslani P. A review of factors influencing the three phases of medication adherence in people with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2019;29(6):398–418. doi: 10.1089/cap.2018.0153
- Khan MU, Kohn M, Aslani P. The need for a paradigm shift in adherence research: the case of ADHD. Res Social Adm Pharm. 2019;15(3):318–320. doi: 10.1016/j.sapharm.2018.04.033
- Charach A, Yeung E, Volpe T, et al. Exploring stimulant treatment in ADHD: narratives of young adolescents and their parents. BMC Psychiatry. 2014;14(1):1–11. doi: 10.1186/1471-244X-14-110
- Chong WW, Aslani P, Chen TF. Effectiveness of interventions to improve antidepressant medication adherence: a systematic review. Int J Clin Pract. 2011;65(9):954–975. doi: 10.1111/j.1742-1241.2011.02746.x
- Pakpour AH, Modabbernia A, Lin CY, et al. Promoting medication adherence among patients with bipolar disorder: a multicenter randomized controlled trial of a multifaceted intervention. Psychol Med. 2017;47(14):2528–2539. doi: 10.1017/S003329171700109X
- Barnard-Brak L, Roberts B, Valenzuela E. Examining breaks and resistance in medication adherence among adolescents with ADHD as associated with school outcomes. J Atten Disord. 2020;24(8):1148–1155. doi: 10.1177/1087054718763738
- DosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135–141. doi: 10.1080/09540260801933084
- Ribaut J, Leppla L, Teynor A, et al. Theory-driven development of a medication adherence intervention delivered by eHealth and transplant team in allogeneic stem cell transplantation: the SMILe implementation science project. BMC Health Serv Res. 2020;20(1):1–22. doi: 10.1186/s12913-020-05636-1
- Easthall C, Barnett N. Using theory to explore the determinants of medication adherence; moving away from a one-size-fits-all approach. Pharmacy. 2017;5(3):50. doi: 10.3390/pharmacy5030050
- Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11(11):CD000011. doi: 10.1002/14651858.CD000011.pub4
- Sirey JA, Bruce ML, Kales HC. Improving antidepressant adherence and depression outcomes in primary care: the treatment initiation and participation (TIP) program. Am J Geriatr Psychiatry. 2010;18(6):554–562. doi: 10.1097/JGP.0b013e3181cdeb7d
- Nili M, Mohamed R, Kelly KM. A systematic review of interventions using health behavioural theories to improve medication adherence among patients with hypertension. Transl Behav Med. 2020;10(5):1177–1186. doi: 10.1093/tbm/ibaa020
- Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551–568. doi: 10.1177/1087054714548035
- Hopewell S, Clarke MJ, Stewart L, et al. Time to publication for results of clinical trials. Cochrane Database Syst Rev. 2005;2:MR000011.
- Sterne JA, Harbord RM. Funnel plots in meta-analysis. Stata J. 2004;4(2):127–141. doi: 10.1177/1536867X0400400204