ABSTRACT
Introduction
Deep brain stimulation (DBS) is an effective treatment for Parkinson’s disease (PD) motor symptoms that improves function and quality of life in appropriately selected patients. Because mild to moderate cognitive declines can follow DBS and impact quality of life in a minority of patients, an important consideration involves the cognitive deficit and its prediction.
Areas Covered
The author briefly summarizes cognitive outcomes from DBS and reviews in more detail the risks/predictors of post-DBS cognitive dysfunction by mainly focusing on work published between 2018 and 2024 and using comprehensive neuropsychological (NP) evaluations. Most publications concern bilateral subthalamic nucleus (STN) DBS. Comment is offered on challenges and potential avenues forward.
Expert opinion
STN DBS is relatively safe cognitively but declines occur especially in verbal fluency and executive function/working memory. Numerous predictors and risk factors for cognitive outcomes have been identified (age and pre-operative neuropsychological status appear the most robust) but precise risk estimates cannot yet be confidently offered. Future studies should employ study center consortia, follow uniform reporting criteria (to be developed), capitalize on advances in stimulation, biomarkers, and artificial intelligence, and address DBS in diverse groups. Advances offer an avenue to investigate the amelioration of cognitive deficits in PD using neuromodulation.
1. Introduction
Deep brain stimulation (DBS) for Parkinson’s disease (PD) is a symptomatic treatment that improves function and quality of life (QoL) [Citation1–8]. Indeed, randomized, controlled trials (RCT) comparing DBS to best medical therapy (BMT) show that DBS yields greater functional and QoL improvement than BMT, but carries a higher risk of serious adverse events [Citation9]. Several recent reviews and meta-analyses of cognitive outcome [Citation10–12], QoL [Citation13], and changes in personality, mood, and impulsivity after DBS [Citation14–16] support the relative neurobehavioral safety of DBS for PD, but the frequency, magnitude, clinical significance, and causes of neuropsychological (NP) alterations after DBS remain debated. A particular gap in the DBS neuropsychological literature is the identification of risk factors and predictors of cognitive deterioration after DBS. This area of inquiry has been neglected by prior reviews (perhaps due a paucity of quality literature) and is consequently a focus in this article.
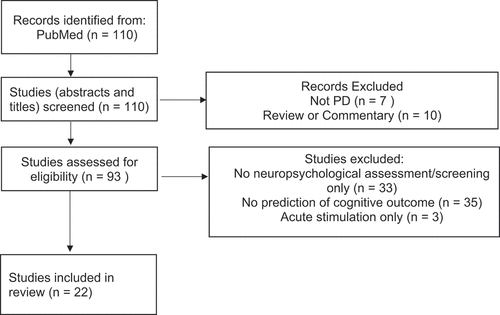
For the benefit of readers less familiar with the literature, and to provide a context for studies examining predictors of cognitive change after DBS, a brief summary of DBS cognitive outcomes is provided. This summary emphasizes subthalamic (STN) DBS, and especially recent meta-analytic studies, because the STN DBS literature is the most extensive. Thereafter this article reviews patient, surgical and stimulation characteristics implicated as risk factors or predictors of cognitive decline after DBS. To identify articles relevant to neuropsychological prediction and characterization of DBS cognitive outcomes a search was done on 6 February 2024 of PubMed using the terms NEUROPSYCHOL* AND (SUBTHAL* DBS) published between 2018 and 2024. depicts the PRISMA flowchart associated with the literature search. Of 110 papers identified, 22 were selected for qualitative review and citations to earlier work of relevance were also examined. The most attention and detail is devoted to RCT and larger sample studies using neuropsychological evaluations meeting or exceeding the MDS/CNS recommendations [Citation17], but studies using cognitive screening or evaluating a single cognitive domain with neuropsychological tests are mentioned when they provide findings meriting further investigation and replication.
2. A brief summary of cognitive outcomes after DBS
Historically, thalamic (Vim) DBS predated internal globus pallidus (GPi) DBS, which in turn predated STN DBS. Recent reviews and meta-analyses indicate that the majority of DBS studies evaluating cognitive changes have done so for STN DBS.
2.1. Thalamic (ventrointermediate nucleus; vim) DBS
Thalamic DBS is rarely done anymore for PD, but the small number of studies examining neuropsychological outcome of unilateral Vim DBS preliminarily supported the procedure’s cognitive safety [Citation18–20]. No significant declines were observed and small improvements variably observed in verbal memory, naming, and aspects of executive functioning (e.g. conceptualization and cognitive flexibility) perhaps reflect test-retest (practice) effects, especially since the studies had small sample sizes.
2.2. Globus pallidus pars interna (GPi) DBS
Three small sample studies suggest unilateral GPi DBS is cognitively safe [Citation21–23], but only one of these studies was a RCT. A RCT with 52 patients comparing unilateral GPi and STN DBS found cognitive outcomes to be comparable in the groups, with the GPi group showing a lesser phonemic verbal fluency decline [Citation24] when non-optimal stimulation (ventral or dorsal to the optimal stimulation site) or no stimulation was used.
Studies examining the NP effects of staged bilateral GPi DBS permit one to address whether bilateral surgery is riskier than unilateral intervention. A study of 6 patients evaluated before, 2 months after the first surgery and three months after the second surgery observed no significant declines but amelioration of anxiety symptoms and better delayed recall [Citation25]. In a trial of 42 patients randomly assigned to staged bilateral GPi or STN DBS (29 patients eventually underwent the contralateral surgery), verbal fluency and working memory declined after both unilateral and bilateral surgery, but verbal fluency declines were evident only after left-sided surgery, regardless of whether this side was operated first or second. Information processing speed and visual working memory (indexed by Digit Symbol test performance) declined more after STN than GPi DBS, but auditory working memory (digit span backward) declined more after GPi than STN DBS [Citation26].
An early study of bilateral STN (n = 49) and GPi DBS (n = 13) failed to disclose significant cognitive alterations except in verbal fluency [Citation27] as did a study of cognition on and off stimulation in 48 STN and 8 GPi patients at 3 and 12 months after surgery [Citation28]. Two large RCTs comparing bilateral GPi and STN DBS (56 STN, 58 GPi and 84 STN, 80GPi, respectively) found minimal differences in cognitive outcomes between the two targets [Citation29,Citation30]. A smaller randomized, albeit uncontrolled trial of STN (n = 22) and GPi DBS (n = 23) that only looked at phonemic and semantic verbal fluency, found no difference between the groups at optimal stimulation settings [Citation24].
Generally, statistically significant declines that are unlikely to be clinically meaningful are observed in processing speed and verbal fluency, but they might slightly favor GPi DBS over STN DBS. One meta-analysis that separately calculated effect sizes of cognitive declines after STN and GPi DBS (n = 1,622) concluded that GPi was slightly safer, but both procedures produced mild, circumscribed, and well-tolerated cognitive declines [Citation31]. A more recent meta-analysis (2,039 DBS and 271 PD non-surgery patients) reported a Stroop task decline only after STN DBS, and a slightly larger decline in phonemic verbal fluency after STN DBS. The semantic verbal fluency decline was comparable in the STN and GPi DBS groups [Citation12].
Meta-analyses that extract effect sizes separately for the pre- to post-surgical differences in GPi and STN studies and then compare these effect sizes need to be interpreted with great caution and a comparison of the cognitive safety of GPi and STN DBS should be avoided. Specifically, patient groups in different GPi and STN DBS studies are likely to differ in demographic and disease characteristics already before surgery, and there is likely subject selection bias. For example, patients may be assigned to GPi rather than STN DBS because they are deemed more cognitively impaired and GPi DBS may be perceived as cognitively safer [Citation32,Citation33].
Meta-analyses of RCTs involving random assignment are likely to more accurately adjudicate the relative safety of GPi and STN DBS. Two meta-analyses included results from 4 RCTs. Whereas one study included 4 reports from the 4 RCT (n = 435) and reported effect sizes for differences in change between STN and GPi DBS on individual neuropsychological tests [Citation34], the other analysis included 7 reports from the same 4 RCTs and examined group differences in cognitive domains [Citation35]. The analysis reporting on individual tests reported a statistical difference on only 1 of 10 test scores, namely Stroop color naming (a test of processing speed) favoring GPi over STN DBS (standardized mean difference [SMD] = 0.31) [Citation34]. The other meta-analysis [Citation35] reported statistically significant differences in attention/processing speed, verbal fluency, and learning and memory favoring GPi DBS, but the standardized mean differences (SMD) were modest, ranging from 0.16 to 0.24. Overall, GPi and STN DBS probably produce more similar than dissimilar cognitive outcomes, with GPi perhaps resulting in slightly milder verbal fluency and processing speed declines.
2.3. Subthalamic (STN) DBS
Because reviews of early STN DBS studies (many uncontrolled and with small samples) exist [Citation36], as does a more voluminous current literature, the focus here is on meta-analyses. The first meta-analysis of the cognitive effects of STN DBS [Citation37] found small declines in executive function and verbal memory, but moderate declines in lexical and semantic verbal fluency. This study was limited in that early studies often had small samples and were case series, and the meta-analysis also included studies of acute stimulation effects. Nonetheless, the findings were consistent with earlier qualitative reviews’ conclusions that verbal fluency declines occurred commonly after STN DBS, but that declines in global cognitive functioning, executive functions, memory, and attention were only inconsistently observed, and when observed, were typically nominal and transient. Furthermore, patients undergoing DBS earlier in the disease course may show verbal fluency declines only in the presence of serious adverse events, such as infection or stroke [Citation38].
Subsequent meta-analyses [Citation10,Citation12,Citation31,Citation34,Citation35,Citation39–41], despite varying in quality, comprehensiveness, analytic focus, study inclusion criteria, the number of studies and patients included, extent and quality of neuropsychological test batteries, and the assignment of various neuropsychological tests to specific cognitive domains, have typically yielded similar conclusions. The effect sizes reported by many of these meta-analyses have been reviewed and presented in tabular form [Citation42]. The most consistent and largest declines after bilateral STN DBS are seen in verbal fluency, with suggestive evidence that semantic fluency may decline more than phonemic fluency. Furthermore, a recent meta-analysis of only studies including a BMT control group [Citation10], shows verbal fluency declines to be greater after STN DBS than BMT. Declines in other cognitive domains occur variably and are only of modest effect size.
It might be noted that uncontrolled studies sometimes show more noteworthy declines in cognitive function after bilateral STN DBS than do RCT. Because a recent meta-analysis [Citation12] found uncontrolled studies of cognitive outcomes after DBS to generally be of low quality per a GRADE criteria checklist [Citation43], one possibility is that the rates of impairment are inaccurate due to low study quality. As has been commented elsewhere, however, the cognitive adverse event rates may be higher in historically later, uncontrolled studies because such studies are less apt to use the strict inclusion and exclusion criteria used in initial regulatory approval trials [Citation42].
2.4. Other targets
Cognitive effects of zona incerta [Citation44] and pedunculopontine nucleus [Citation45] DBS have been too rarely studied to permit confirmation of the initial impression of relative cognitive safety.
3. Predictors of and risk factors for cognitive decline after STN DBS
Correlates or predictors of post-DBS cognitive decline can be conceptualized as falling into two broad categories: patient characteristics and surgical and stimulation characteristics [Citation42,Citation46]. summarizes some important characteristics of studies examining correlates and predictors of cognitive decline after DBS, including the study sample size, target, length of post-surgical follow-up, whether the study is a RCT and includes a neuropsychological test battery (NP), and key findings.
Table 1. Properties of studies examining correlates and predictors of cognitive decline after deep brain stimulation.
Here the focus is on studies utilizing NP to document pre- to post-operative cognitive changes, and especially those studies reporting risk estimates or odds ratios. Numerous studies have used only cognitive screening or focused on verbal fluency or another single cognitive aspect (e.g. verbal fluency, memory). Such circumscribed outcome studies are mentioned here when they provide new insights or findings rarely documented by NP and needing replication.
3.1. Patient characteristics
3.1.1. Demographic characteristics
Age is the demographic characteristic most frequently evaluated as a predictor of DBS cognitive outcome. DBS has come to be used for increasingly older patients, and the predictive utility of age in earlier studies might have been blunted by narrow age range inclusion criteria. At least four studies using multiple NP measures and cognitive composites indicate older age to be a risk factor for cognitive decline after DBS.
In a prospective NP study of 123 of 156 patients participating in a RCT comparing bilateral STN DBS and BMT all patients were under age 75 years (and free of dementia per DRS > 130) [Citation56]. Patients were administered tests of global cognitive function, memory, working memory, attention, and executive functions. In comparison to BMT, the DBS group showed a significantly greater decline in the executive function composite score (comprising phonemic and semantic verbal fluency and the Stroop interference task) 6 months after surgery and this decline was associated with higher age, more severe axial motor symptoms, and higher baseline daily levodopa equivalence dosage (LEDD). No relationship was found between cognitive (executive function) decline and baseline DRS or executive function score, overall motor symptom severity, disease duration or LEDD reduction post-surgery ().
Table 2. Patient characteristics implicated in cognitive outcomes of deep brain stimulation for Parkinson’s disease and sample references*.
Another controlled study, albeit not RCT, also informed regarding the risk age might confer for cognitive deterioration [Citation83]. In this study 105 STN DBS patients (average age 58 years) underwent NP evaluation before and 12 months after surgery and 40 non-surgical PD patients (average age 64 years) served as controls. After surgery the STN DBS, relative to the control group, evidenced declines in global cognitive function (DRS), verbal memory, verbal fluency, complex attention/processing speed (Stroop) and visuospatial reasoning. Using a multivariate normative approach which compares patient to normative control test score profiles (rather than individual tests) [Citation101], the study found a cognitive decline in 38/105 (36%) of STN DBS patients. Predictors of this decline included the baseline composite scores of processing speed and attention, age, and baseline levodopa response (motor symptom severity off medication versus after levodopa challenge). Especially instructive is the simultaneous use of significant predictors to estimate risk of cognitive decline. For example, the probability of cognitive decline in a 65-year-old patient with 30% levodopa response and a composite attention score 1 standard deviation (SD) below the normative mean was about 60%, whereas the probability in a 55-year-old with the same levodopa response and attention score was about 50%.
A study based on the CSP-468 cohort comparing bilateral STN and GPi DBS (n = 164; mean age 61 years in both the STN and GPi groups) to BMT (n = 116; mean age 62 years), examined predictors of cognitive decline in 84 STN and 80 GPi DBS patients 6 months after surgery [Citation79]. Multidomain cognitive decline was defined as one exceeding the reliable change index (RCI) in at least one third of the NP measures in two or more domains. Using this criterion, 18/164 DBS patients (11%) experienced cognitive decline. Age and baseline DRS score were associated with cognitive decline, but when the two variables were considered simultaneously, only age remained significant. This study too examined differential risk of cognitive decline when simultaneously considering age (less than 70 vs. 70 and above), DRS score (<130 vs. ≥130), and occurrence of a serious adverse event (SAE, which was not necessarily surgery or stimulation related). Those persons experiencing SAEs and with DRS < 130, regardless of age, were at highest risk of cognitive decline (67%) but one must consider that some of the cells contain very small numbers of patients. Patients ≥70 years were at about threefold risk of cognitive decline (22.9% vs 7.8%) in comparison to younger patients. Similarly, patients with DRS < 130 were at about threefold risk (28.6% vs 9.3%) of cognitive decline compared to those with higher scores.
Multiple comparisons between those with and without cognitive decline failed to find significant differences between the two groups’ disease duration, disease severity, LEDD, gender, or education. The groups differed on several baseline NP measures: WAIS-III Arithmetic and Similarities (working memory), Trailmaking (processing speed), Brief Visuospatial Memory test – Revised delayed recall and Hopkins Verbal Learning Test – Revised recognition discrimination (memory).
Another randomized (but uncontrolled) comparison of bilateral STN (n = 56) and GPi DBS (n = 58) defined cognitive deterioration as a decline exceeding the RCI on at least three of 14 tests administered [Citation29]. At 12 months, comparable percentages of patients showed declines after GPi (29%) and STN DBS (39%). Declines were predicted by older age and higher baseline semantic verbal fluency scores, but not disease duration, LED or DRS. Advancing age has also been associated with post-operative delirium [Citation96] and lesser QoL gains [Citation1]. Several other studies have also found age to be related to cognitive decline after STN DBS [Citation76,Citation80], but they are either retrospective, have small samples, and/or are uncontrolled. By contrast, a RCT using a delayed stimulation activation control did not find age to be associated with decline in a composite score of executive function and memory measures after STN DBS [Citation85]. Similarly, meta-regression analyses have not identified age as a predictor of verbal fluency decline [Citation31,Citation37] or decline in other cognitive domains [Citation31], but these meta-analytic studies included lower-quality studies in the analyses.
Other demographic factors evaluated include education (which is often considered a proxy for cognitive reserve) and gender. Neither education [Citation79] nor gender [Citation51,Citation79] was identified as a predictor of cognitive decline, but higher education may be associated with better verbal fluency after DBS [Citation48] and women may experience greater depression improvement [Citation102]. These findings bear replication.
In summary, age seems to represent a risk for cognitive decline 6–12 months after surgery when such decline is measured by comprehensive NP, composites test scores or decline on multiple measures exceeding the RCI. Age was also associated with development of dementia within 3 years of bilateral STN DBS [Citation51]. Gender and education have only rarely been implicated in cognitive decline, but further study is warranted.
3.1.2. Disease characteristics
Disease duration, age at disease onset, pre-surgical LEDD and response to levodopa, post-operative LEDD, and motor phenotype and asymmetry have been investigated as predictors of cognitive decline after DBS. These studies have yielded conflicting findings but generally do not support these characteristics as reliable correlates or predictors of neuropsychologically verified cognitive decline after DBS. For completeness, some of the better studies are described below (see also for findings).
Higher-quality studies already discussed under demographic predictors failed to find associations between cognitive outcome and disease duration [Citation29,Citation56], motor symptom severity [Citation56,Citation79], and post-operative LEDD reduction [Citation56]. One study found an association between cognitive decline and baseline levodopa response [Citation83]. Whereas one study found an association between cognitive decline and higher pre-surgical LEDD [Citation56], others did not [Citation29,Citation79]. Another study [Citation68] also found cognitive decline to be associated with pre-surgical LEDD, but findings are difficult to interpret for methodological reasons.
Cognitive outcome appears to be favorable in persons with relatively short disease duration given reports of intervention in early stage PD. In a study of 30 patients (Hoehn and Yahr stage II and treated with dopaminergic medication for at least 6 months but less than 4 years) randomized to bilateral STN DBS or BMT, 2 persons with more marked cognitive changes in the DBS group had a stroke or infected hardware. Among the remaining patients, the DBS group, relative to BMT, showed greater declines in verbal fluency, processing speed, and aspects of attention and processing speed [Citation38]. These findings are similar to those generally reported for STN DBS in other patients, and the group differences diminished or were no longer significant at 2 years [Citation38] and 5 years [Citation103]. The EARLYSTIM trial of PD patients with early motor complications did not find differences in cognitive function between the STN DBS and BMT groups, but that study only used a cognitive screening instrument (DRS) [Citation104].
Meta-regression studies of potential disease characteristics have failed to associate disease severity, disease duration, or levodopa response with cognitive change [Citation31] or disease duration and LEDD reduction as predictors of verbal fluency decline [Citation37].
There exists limited support for the notion that motor phenotype is associated with NP-verified cognitive decline. Congruently, one study identified the postural instability and gait disturbance (PIGD) subtype of PD to have worse cognitive (attention and memory) outcome than tremor-dominant PD 12 months after bilateral STN DBS [Citation89]. Studies found that more severe axial motor symptoms were associated with executive function decline 6 months after surgery [Citation56] and worse MMSE outcome [Citation60]. A retrospective study reported poorer executive functions and different cognitive declines 1 and 5 years after STN DBS in persons with asymmetric motor symptoms. Whereas the authors reported poorer cognitive outcome in patients with left-predominant motor symptom patients at 12 months, there was a worse cognitive outcome in predominant right-sided motor symptom patients at 5 years [Citation87,Citation88].
Persons with PD patients with glucocerebrosidase (GBA) gene mutations have higher risk of cognitive decline and dementia [Citation105] and perform more poorly on various neuropsychological tests than non-carriers [Citation106]. Cognitive deficit severity and frequency is less well characterized in alpha synuclein (SNCA) gene mutations and multiplications, but those with gene triplications may have a higher rate of dementia [Citation105]. Despite suggestion that GBA and SNCA carriers, although benefitting from DBS, may have poorer cognitive outcomes [Citation77,Citation100] these preliminary studies’ findings are deemed controversial. Two oft-cited studies [Citation72,Citation77] have small numbers of mutation carriers, and one of these studies found cognitive outcome differences after DBS for GBA mutation carriers with one cognitive screening test but not another. Further studies are thus needed, especially as regards whether cognitive outcome differs in mutation carriers treated with DBS or not.
3.1.3. Brain morphometry and neurophysiology
Brain morphometric measurements have been associated with both motor and non-motor outcome after DBS [Citation107]. Few studies, often with small samples, have examined baseline brain morphometric and neurophysiologic characteristics in DBS neuropsychological outcome and none are controlled studies. Findings discussed should thus be considered as implicating, but not confirming these characteristics as risks for poor cognitive outcome after DBS. The studies examining morphologic predictors of cognitive decline vary in follow-up duration and outcome measures, with studies variously using screening measures, composite neuropsychological scores, measures of specific cognitive functions, or clinical diagnoses (mild cognitive impairment [MCI] or dementia). One retrospective study based on comprehensive NP before and 6 months after bilateral STN DBS in 43 patients [Citation90] found only limited evidence that cortical atrophy or white matter lesion (WML) burden is related to cognitive declines, even though declines in attention, executive function, verbal fluency, memory, and visuospatial functions were observed. Greater WML volume before surgery was associated with visuospatial declines (after accounting for potential confounds), but not with declines in other cognitive domains. Presurgical forebrain parenchyma and hippocampal volumes were not associated with declines on individual cognitive tests, except for a single correlation between right hippocampal volume and change in word list recognition. Costentin and colleagues also found no association between proportion of WM fiber disconnection and verbal fluency decline 6 months after STN DBS [Citation55]. By contrast, a retrospective study using NP in only a subset of patients (17/40) with subjective cognitive complaints on average 21 months after STN DBS, found the composite cognitive score change to be associated with pre-surgical WML burden [Citation51]. In summary, better quality studies have not found a robust association between WML burden and DBS cognitive outcome.
Other morphometric studies are only mentioned briefly here because many used limited outcome measures and/or have not been replicated. One study found that persons with intermammillary distance (indexing third ventricle width and thus indirectly brain atrophy) exceeding 8 mm were at 60% risk of behavioral problems (e.g. confusion, protracted drowsiness, aggressiveness, paranoia) within 3 days of surgery [Citation65]. One study reported that nucleus basalis of Meynert (nbM) volume in 55 patients was related to cognitive changes indexed by either MMSE or DemTect 12 months after STN DBS [Citation61]. Another study of 42 STN DBS patients found that pre-operative left nucleus accumbens volume strongly predicted decline in the DRS Initiation/Perseveration score (a score heavily influenced by verbal fluency). Additionally, left lateral ventricle volume was associated with DRS Total and Initiation/Perseveration score changes [Citation78]. A retrospective study of 40 STN DBS patients who underwent memory testing an average of about 7 months before and 9 months after surgery found an association between word list learning change (using the BIRT Memory and Information Processing Battery) and pre-operative left thalamic and bilateral hippocampal volumes [Citation97]. Subcortical iron accumulation (indexed by MRI transverse relaxation rate or R2*) has also been proposed to be related to post-DBS cognitive outcome. In a retrospective study of 32 patients who underwent NP before and an abbreviated repeat assessment within 3 months of STN DBS, higher caudate R2* was associated with better executive function outcome, but higher putamen R2* was predictive of poorer attention outcome [Citation53]. This seemingly counterintuitive finding awaits replication and explanation.
A few studies have examined pre-operative neurophysiologic function (electroenecephalographic or EEG) correlates of cognitive outcome after STN DBS. One retrospective study of 30 patients found ‘Grand Total EEG’ score to predict cognitive decline occurring in 6 of the patients (defined by DemTect score in most patients) 4 to 12 months after surgery [Citation73]. By contrast, another study of 17 patients reported that increased theta power in pre-operative EEG predicted decline 1 year after surgery in neuropsychologically defined visuoperceptual and spatial function [Citation94]. Geraedts and colleagues [Citation62] found that an automated machine learning model developed from 16,674 features extracted from pre-surgical EEG predicted clinically diagnosed cognitive deterioration occurring in 17 among 42 patients with positive predicted value (PPV) of 91.4% and negative predictive value (NPV) of 85.0%. The evaluation of EEG as a predictor of cognitive decline after DBS will require a more extensive and systematic investigation before conclusions can be drawn.
3.1.4. Presurgical neuropsychological characteristics
Several studies reported relationships between pre-surgical NP characteristics and cognitive outcome, defined either by NP or clinical diagnoses such as MCI or dementia. Additional studies have reported on the association between pre-surgical cognition and DBS outcomes such as motor function [Citation99], post-operative confusion or delirium [Citation96] and length of hospitalization [Citation47].
Very few studies have examined the association between pre-surgical psychiatric diagnoses and DBS cognitive outcome. These studies suggest a link between pre-surgical apathy [Citation58,Citation76], depression [Citation70], anxiety [Citation82], hallucinations [Citation51,Citation69] and cognitive outcome. REM-sleep behavior disorder (RBD), despite sharing cognitive features with and being a risk factor for cognitive decline in PD, was found not to impact cognitive and QoL outcomes one year after DBS [Citation108]. Patients with RBD may, however, have poorer axial motor symptom outcome 3 years after DBS [Citation109]. By contrast, some studies fail to find an association between psychiatric symptoms or conditions and DBS cognitive outcome [Citation81]. Given the association of various psychiatric conditions with adverse outcomes in PD generally (e.g. cognitive impairment, disability, disease progression, poor life quality) [Citation110], high-quality DBS studies closely attending to the distinction between psychiatric symptoms and diagnoses are urgently needed.
Some studies have examined whether scores on specific NP tests are associated with cognitive decline. The CSP-468 study found that patients who experienced cognitive decline by 6 months after GPi or STN DBS scored significantly lower than those without decline on pre-surgical measures of verbal reasoning (Similarities), attention/working memory (Arithmetic, Trailmaking) and verbal and visual memory (HVLT-R and BVMT-R) [Citation79]. The predictive utility of these tests was not evaluated.
Several other studies associated poorer performance in verbal fluency, reasoning/executive functions, memory, attention/working memory, and visuoperceptual functions with post-DBS cognitive decline. In a retrospective study of 39 bilateral STN DBS patients, 18% experienced cognitive deterioration defined as a decline exceeding the RCI on at least 4 of 9 neuropsychological tests 6 months after surgery. In comparison to patients who did not experience decline, those declining had significantly lower pre-surgical verbal fluency and executive function (Wisconsin Card Sorting Test; WCST) scores [Citation76]. Pre-surgical verbal fluency (and verbal memory) also have been associated with motor outcome 12 months after DBS [Citation99] and the same factors and verbal reasoning have been linked to patient- and caregiver-reported multidomain cognitive change 4 months after DBS [Citation111]. Verbal reasoning (assessed with Similarities) and visuoperceptual and constructional task performance (Object Assembly) were also associated with visuospatial deficits one year after STN DBS (OR 10.2 and 9.5, respectively) [Citation98]. By contrast, as already noted, a large sample RCT of GPi and STN DBS (NSTAPS) found that higher verbal fluency scores at baseline were associated with poorer cognitive outcome (decline exceeding the RCI on at least 3 neuropsychological tests) 12 months after surgery (OR 1.06) [Citation29], a finding that might represent a regression to the mean phenomenon or difficulty detecting significant declines in verbal fluency among persons with already very low scores before surgery.
In the study by Smeding and colleagues [Citation83] baseline attention (a composite of Trailmaking B and the Stroop task scores) was predictive of cognitive decline (OR = 0.41). Others have reported lower baseline attention (composite of Letter Number Sequencing and Digit Span scores) to be associated with longer post-operative hospitalization and a trend toward post-operative confusion [Citation47]. However, a few studies have not found change in cognitive screening or composite cognitive scores to be associated with poorer pre-surgical attention/working memory or executive scores [Citation29,Citation85]. In summary, pre-operative scores on tests of attention/working memory, executive function, and, to a lesser extent, memory and rarely visuospatial function are associated with poorer cognitive outcomes.
An important issue is whether pre-surgical MCI is a risk for further cognitive decline or with de novo MCI after surgery. Many studies examining MCI before and after DBS are uncontrolled and differ in whether and how they used the Movement Disorder Society (MDS) criteria for MCI [Citation112]. There is a noteworthy prevalence of MCI among PD patients eventually undergoing surgery: figures presented include 47% [Citation93], 60% [Citation47], 63% [Citation68], 65% [Citation51], and 76% [Citation64]. There seems to be an increase in MCI after DBS (one study not using the MDS criteria) found that MCI prevalence increased from 47% to 63% by 9 months after STN DBS [Citation93]. This increase in MCI is difficult to interpret in the absence of an unoperated control group. The increase is, nonetheless, noteworthy if one considers NP test score changes and MCI evolution in unoperated persons with PD. Specifically, changes exceeding reliable change indices over 12 months on common NP tests are usually rare [Citation113] and groupwise changes over 12 months in a PD non-surgical control group are typically not significant [Citation114]. Furthermore, development of MCI in persons with PD and initially normal cognition has variously been reported to occur in about 10% in one year [Citation115], less than 8% over 18 months [Citation116], and 10% over 2 years [Citation117], that is, considerably less than the reported increase in MCI of 16% over 9 months after DBS. Despite the increase in MCI, there is generally consensus that dementia prevalence and incidence after DBS are comparable to those in the general PD population [Citation118].
Studies differ in regard to whether there is an association between pre-operative MCI and subsequent dementia. One study (limited by the inconsistency of NP) failed to find an association between pre-surgical cognitive scores or MCI and dementia within 3 years of surgery [Citation51], while another study found a trend association between MCI and dementia [Citation64]. This latter study is difficult to interpret because it was reported that of 37 patients available for long-term follow-up (3.6–10.5 years), 76% had MCI before surgery and post-operatively 19% had no cognitive impairment while 41% had dementia or MCI. It is unclear what cognitive status the remaining 40% of patients might have had and why the MCI prevalence is lower after than before surgery. One study of 103/135 patients who had pre-operative NP and were followed for 1–7 years (mean 42 months) did find an association between pre-surgical MCI and subsequent dementia [Citation68]. Given that MMSE score decline was similar in the MCI and non-MCI groups, it is probable that development of dementia reflects the natural history of the disease and the heightened risk of dementia among persons with MCI in PD in general [Citation119,Citation120]. This conclusion is also supported by the observations that dementia incidence within 3 years of DBS was comparable to that in unoperated PD patients [Citation121], and that while MCI operates experienced a more precocious development of dementia (median 6 years after surgery) than did non-MCI operates (median 11 years), dementia was not observed early in the course after DBS. Furthermore, dementia incidence and prevalence over 10 years after DBS are comparable to those in the general PD population [Citation118].
A retrospective study reported that 40 patients with moderate cognitive impairment experienced motor symptom benefit 1 year after thalamic, pallidal, or subthalamic DBS to a similar extent as a cognitively unimpaired group [Citation122]. Adverse event rates were similar in the two groups. Unfortunately, detailed cognitive outcome data were not presented.
In summary, while some consider MCI to be a relative contraindication to DBS [Citation123], others do not and yet other centers may consider whether the MCI pattern is more typical of PD or Alzheimer’s disease in recommending surgery.
4. Surgical characteristics
The seemingly most investigated DBS surgical characteristic impacting cognition (usually verbal fluency) is electrode passage through the caudate nucleus. Other factors investigated included electrode cortical entry point, the number of microelectrode tracks, anesthesia type (usually awake vs asleep surgery), and the size and location of the electrical stimulation field derived from modeling or active contact location in the target.
Witt and colleagues [Citation92] found that the patients who declined on the DRS and backward digit span (working memory) tended to have a more medial electrode trajectory passing through the caudate. By contrast, a study of 46 patients (23 of whom had electrode trajectories through the caudate) who experienced declines in verbal fluency and on Trailmaking B (cognitive set shifting and psychomotor speed) did not find caudate penetration to be associated with cognitive decline 6–18 months after surgery [Citation84]. Another study found no relationship between 12 months post-DBS change in verbal fluency, executive function tests, and DRS and caudate penetration [Citation124]. Whereas electrode entry in the left superior frontal gyrus was associated with verbal fluency decline in another study of 56 patients before and 9 months after STN DBS, caudate penetration was not [Citation49]. One possible explanation for divergent findings is that caudate penetration may impact cognition on early in the post-operative course [Citation66] ().
Table 3. Surgical and stimulation characteristics implicated in cognitive outcomes of deep brain stimulation for Parkinson’s disease and sample references*.
An anterior left entry/electrode trajectory was associated with a variety of gross mental changes soon after surgery [Citation65] and decline in verbal fluency [Citation71], while another study found that electrode position along the inferior/superior axis of the left STN was associated with verbal fluency decrements [Citation67]. Trajectories associated with declines on specific neuropsychological tests seem quite variable, with some functions more impacted by posterior frontal trajectories while others are impacted more by superior or lateral trajectories in one hemisphere or the other [Citation95], but the study reporting these findings had a small sample size and has not been replicated.
The association between the number of microelectrode recording (MER) tracks and cognitive decline has also only been inconsistently reported. Whereas simultaneous insertion of multiple electrodes compared to use of one or fewest possible tracks was associated with verbal fluency reduction within 12 months of surgery [Citation50], other studies have not found an association [Citation75,Citation92].
Type of anesthesia (local vs. general anesthesia or ‘awake’ vs. ‘asleep’ surgery) and its effect on cognition was investigated in a randomized trial (GALAXY) of 110 PD patients undergoing bilateral STN DBS with MER (although macroelectrode stimulation was performed only in the ‘awake’ group). No differences were found between the two groups’ composite cognitive scores 6 months after DBS [Citation126], but subtle differences were observed on specific cognitive tests [Citation127]. Both groups had higher scores after surgery on delayed recall of a word list but the gain was much smaller in the awake group and this group also had a greater decline on the Stroop task. The asleep group had a lesser improvement on a story recall task. Overall, however, cognitive outcome similarities far outweighed any differences. This echoes the findings of earlier studies reporting minimal cognitive changes [Citation128] or better verbal fluency in the asleep group [Citation129].
5. Stimulation characteristics
Studies examining the role of active contact location and cognitive decline have yielded rather variable findings. A study of 46 STN DBS patients found that stimulation in the left medial STN was related to semantic verbal fluency declines, while posterior stimulation produced phonemic verbal fluency declines [Citation57]. Yet another study found superior left STN stimulation to produce the largest verbal fluency decrements [Citation67], and two studies found anterior-ventral stimulation to be associated with cognitive or fluency declines [Citation86,Citation125]. One possibility is that both contact location and volume of tissue stimulated need to be considered simultaneously. For example, Mikos et al. [Citation125] observed that whereas location of active contact within STN was not related to verbal fluency, phonemic verbal fluency declines were associated with activation of a larger volume of tissue in the ventral region. Indeed, preliminary findings based on 10 patients suggest that one also might need to consider connectivity of various STN regions when attempting to minimize cognitive decline with DBS. Specifically, it was reported that stimulation sites producing cognitive decline (per DRS) are more connected to caudate, hippocampus, anterior cingulate, and cognitive regions of the cerebellum [Citation130]. Stimulation of GPi, perhaps due to the larger size of the structure, may be more forgiving, and studies have not found an association between different GPi stimulation sites and verbal fluency declines [Citation24,Citation131]. In summary, there is a paucity of large sample, controlled studies that examined contact location and its impact on cognition and findings are inconsistent or remain to be replicated. The finding that anterior and ventral STN stimulation leads to cognitive or verbal fluency declines has some support. By contrast, studies have failed to find an association between active contact location in GPi and cognitive deterioration.
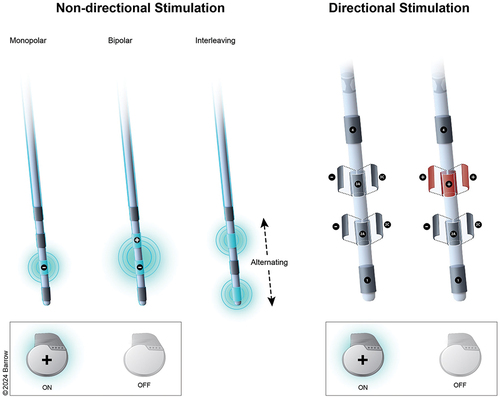
Few studies have examined the NP impact of stimulation parameters (e.g. amplitude, frequency, pulse width) or electrode configurations (the most common of which are illustrated in ). The limited number of studies available has significant methodological shortcomings and do not allow one to draw firm conclusions. One study found that higher total energy delivered via the left STN electrode was associated with verbal fluency declines [Citation54] while another related higher right STN DBS voltage to memory decline [Citation57]. It is possible that the increase in stimulation values is a proxy for sub-optimally placed electrodes. Small sample studies suggest that amplitude, pulse width, and stimulation frequency may each have different effects on different cognitive functions, even simultaneously affecting some functions positively but others negatively [Citation59,Citation82] but such findings await replication in large samples. The observation that acute stimulation (on-off) comparisons not finding stimulation effects on verbal fluency suggests a possible surgical or microlesion effect [Citation24,Citation132], but such comparisons may be less instructive when done after clinical stimulation optimization. Indeed, one RCT using a delayed stimulation activation control found some shared cognitive declines between the immediate and delayed stimulation groups, but some declines unique to the stimulation group, suggesting that both surgical and stimulation effects operate in cognitive declines [Citation85].
Despite the advancement in electrode capability of affording directional stimulation and suggestion that adverse event thresholds are increased by directional stimulation [Citation133], a small (n = 31), randomized cross-over study of ring and directional STN and GPi DBS did not disclose a cognitive advantage from directional stimulation [Citation134].
6. Conclusions
DBS for PD, absent SAE associated with surgery, is relatively safe cognitively. Mild cognitive changes, according to RCT using comprehensive neuropsychological evaluations occur in 10–35% of patients [Citation29,Citation30] and cognitive decline attenuates quality of life gains after DBS [Citation30]. Verbal fluency and response inhibition declines are the most common cognitive changes, but these declines are only rarely associated with QoL dissatisfaction [Citation85]. More pronounced cognitive changes may occur with serious surgical AEs [Citation38,Citation79]. A plethora of risk factors and predictors have been implicated in post-DBS cognitive declines, albeit generally inconsistently and sometimes in a very limited number of studies. The limited number of meta-regression analyses available have failed to identify reliable predictors or risks, but this failure may reflect the inclusion in meta-analyses of many small sample, uncontrolled studies of lower quality. Precise risk estimates associated with risk factors still cannot be confidently offered, but higher-quality RCTs suggest that age and pre-surgical cognitive characteristics may be among the more robust predictors of post-DBS cognitive decline. Pallidal and subthalamic stimulation have more similar than dissimilar cognitive outcomes, with minor differences favoring pallidal stimulation. While there appears to be an increase in MCI soon after surgery, dementia after DBS appears to be a manifestation of the natural disease evolution and occurring very rarely within a year of surgery.
7. Expert opinion
Consideration of pre-surgical and surgical predictors of cognitive decline after DBS is important to optimal patient selection and enhancing the safety of DBS and thus patient QoL and satisfaction. While some dismiss cognitive declines after DBS as perhaps ‘inconsequential’ relative to motor improvement [Citation135], others have shown the importance of cognitive outcome to the patient. Geraedts and colleagues [Citation62] found that whereas 100% of patients with stable cognition would again choose to have surgery, only two-thirds of those with cognitive declines would do so.
Several challenges face studies seeking to identify predictors of post-DBS cognitive decline. One of the biggest challenges is that persons with dementia or concerning cognitive impairment are excluded from studies. The resulting narrow range of pre-surgical NP test scores limits the magnitude of correlations between test scores and cognitive outcome. Furthermore, for ethical reasons it is not possible to carry out DBS RCTs with persons with marked cognitive compromise. Consequently, the utility of pre-surgical NP tests in predicting more serious cognitive declines after DBS is often assumed and inferred from isolated cases [Citation136] rather than empirically verified. Similar restrictions in the range of other pre-surgical patient characteristics of those selected for DBS (e.g. age, levodopa responsiveness, cognitive screening values, disease duration and severity), coupled with uniformity of surgical approach and programming algorithms at a specific center, hamper the ability of single center studies to identify predictors of cognitive decline. Data analyses and reliable predictor identification are also complicated by the relatively low incidence of clinically meaningful cognitive decline. Particular challenges in identifying active contact locations and stimulation parameters in clinic during device programming visits also exist. Neuropsychological evaluation is time and cost intensive and typically impractical during programming visits. Additionally, while changes in motor symptoms as a function of stimulation parameters reveal themselves quickly in clinic [Citation137], cognitive changes may not declare themselves until the patient is outside the clinic environment and engaged in more cognitively demanding tasks. A programming algorithm for managing speech changes after DBS has been proposed [Citation138], and development and validation of an algorithm specifically for managing the common verbal fluency problems post-DBS would be helpful. The promise of brief, automated cognitive assessment with small portable devices yielding valid and reliable results remains to be fulfilled.
Another challenge is the question of the generalizability of NP findings in early RCTs to current clinical practice. Clinical practice is less likely to employ the stringent study inclusion/exclusion criteria of RCTs, especially those relating to device approval. Furthermore, once devices are approved, patients may be more reluctant to participate in onerous RCTs making it difficult to obtain confirmatory findings. Generalizability of findings is also called into question by limited access of diverse populations to DBS [Citation139,Citation140]. The remarkable technical advances in DBS, including in pulse generators, electrodes that permit current steering and sensing and remote programming capability [Citation141], neuroimaging and surgical workflow (increased surgeon experience, use of robots, alternative methods of anesthesia) all leave open for new RCTs the question whether these advances are associated with different cognitive outcomes.
Because many predictors have been implicated in DBS cognitive outcome, likely only very large sample studies will be able to adequately address the utility of simultaneously using the strongest predictors of cognitive decline after DBS. It is worth bearing in mind that risk ratios and odds ratios are not synonymous, and only similar in value when odds ratios are small [Citation142]. Future studies should provide both odds and risk ratios to allow more precise information to be given to the patient and treatment team about expected risk of cognitive decline (and this can be contrasted to risk of cognitive decline in the absence of DBS). Given difficulty recruiting large samples, one avenue forward is to employ study center consortia. Development of data reporting guidelines, routine provision of predictive values, sensitivity, specificity, and odds or risk ratios would be beneficial in determining meaningful and reliable risk estimates. Future studies will need to include diverse populations and reduce barriers to assessment, something that may be facilitated by telehealth evaluations [Citation143–145], and capitalize on artificial intelligence advances. Indeed, machine learning models are beginning to be used to identify predictors of DBS cognitive outcomes [Citation62]. Because cognitive decline and dementia in PD can be predicted accurately by using a combination of biomarkers, cognitive measures, and patient and disease characteristics [Citation146], future DBS RCTs would do well to explore such predictor combinations (including common gene mutations such as in GBA) and to determine whether there exists an interaction between a predictor combination of cognitive decline or dementia with DBS. Such studies would address whether marker combinations predict a more rapid cognitive decline in DBS patients than might be expected during the natural course of PD.
Finally, I reiterate a point made previously [Citation42,Citation46]. Cognitive screening instruments have a place in patient selection for DBS and avoid potentially lengthy and costly NP evaluations especially in those with clear and considerable cognitive impairment. The screening measures, however, do not suffice to accurately characterize (or reliably predict) cognitive outcome or to identify the specific and most appropriate rehabilitation strategies that are occasionally needed after DBS.
Article highlights
Mild cognitive declines, according to randomized, controlled clinical trials (RCT) using comprehensive neuropsychological evaluations, occur in 10-35% of patients undergoing DBS for Parkinson’s disease.
The declines most often involve verbal fluency, working memory, information processing speed and executive functions and can attenuate quality of life gains after DBS.
A host of patient, surgical, and stimulation characteristics have been inconsistently identified as correlates or predictors of cognitive decline.
Age and pre-surgical cognitive characteristics are the most robust predictors of post-DBS cognitive decline in the absence of surgical adverse events such as stroke or infection.
It is unclear if cognitive decline incidence estimates yielded by early RCT and many uncontrolled, small sample observational studies are modified by recent technical advances in DBS (e.g. directional stimulation), neuroimaging, and surgical workflow.
Declaration of interest
The author has received royalties from Oxford University Press as well as an Associate Editor stipend from the Journal of the International Neuropsychological Society. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Dafsari HS, Reker P, Stalinski L, et al. Quality of life outcome after subthalamic stimulation in parkinson’s disease depends on age. Mov Disord. 2018 Jan;33(1):99–107. doi: 10.1002/mds.27222
- Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for parkinson’s disease. N Engl J Med. [2006 Aug 31];355(9):896–908. doi: 10.1056/NEJMoa060281
- Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012 Feb;11(2):140–149. doi: 10.1016/S1474-4422(11)70308-8
- Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in parkinson’s disease (intrepid): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020 Jun;19(6):491–501. doi: 10.1016/S1474-4422(20)30108-3
- Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced parkinson disease: a randomized controlled trial. JAMA. [2009 Jan 7];301(1):63–73. doi: 10.1001/jama.2008.929
- Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced parkinson’s disease (pd surg trial): a randomised, open-label trial. Lancet Neurol. 2010 Jun;9(6):581–591. doi: 10.1016/S1474-4422(10)70093-4
- Martinez-Martin P, Deuschl G, Tonder L, et al. Interpretation of health-related quality of life outcomes in parkinson’s disease from the earlystim study. PLOS ONE. 2020;15(8):e0237498. doi: 10.1371/journal.pone.0237498
- Lachenmayer ML, Murset M, Antih N, et al. Subthalamic and pallidal deep brain stimulation for parkinson’s disease-meta-analysis of outcomes. NPJ Parkinsons Dis. [2021 Sep 6];7(1):77. doi: 10.1038/s41531-021-00223-5
- Bratsos S, Karponis D, Saleh SN. Efficacy and safety of deep brain stimulation in the treatment of parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Cureus. [2018 Oct 22];10(10):e3474. doi: 10.7759/cureus.3474
- Jahanshahi M, Leimbach F, Rawji V. Short and long-term cognitive effects of subthalamic deep brain stimulation in parkinson’s disease and identification of relevant factors. J Parkinsons Dis. 2022;12(7):2191–2209. doi: 10.3233/JPD-223446
- Racki V, Hero M, Rozmaric G, et al. Cognitive impact of deep brain stimulation in parkinson’s disease patients: A systematic review. Front Hum Neurosci. 2022;16:867055. doi: 10.3389/fnhum.2022.867055
- Bucur M, Papagno C. Deep brain stimulation in parkinson disease: A meta-analysis of the long-term neuropsychological outcomes. Neuropsychol rev. 2023 Jun;33(2):307–346. doi: 10.1007/s11065-022-09540-9
- Geraedts VJ, Feleus S, Marinus J, et al. What predicts quality of life after subthalamic deep brain stimulation in parkinson’s disease? A systematic review. Eur J Neurol. 2020 Mar;27(3):419–428. doi: 10.1111/ene.14147
- Cartmill T, Skvarc D, Bittar R, et al. Deep brain stimulation of the subthalamic nucleus in parkinson’s disease: a meta-analysis of mood effects. Neuropsychol rev. 2021 Sep;31(3):385–401. doi: 10.1007/s11065-020-09467-z
- Lo Buono V, Luca Trombetta M, Palmeri R, et al. Subthalamic nucleus deep brain stimulation and impulsivity in parkinson’s disease: A descriptive review. Acta Neurol Belg. 2021 Aug;121(4):837–847. doi: 10.1007/s13760-021-01684-4
- Wilt JA, Merner AR, Zeigler J, et al. Does personality change follow deep brain stimulation in parkinson’s disease patients? Front Psychol. 2021;12:643277. doi: 10.3389/fpsyg.2021.643277
- Lang AE, Houeto JL, Krack P, et al. Deep brain stimulation: Preoperative issues. Mov Disord. 2006 Jun;21(Suppl 14):S171–96. doi: 10.1002/mds.20955
- Caparros-Lefebvre D, Blond S, Pécheux N, et al. Evaluation neuropsychologique avant et après stimulation thalamique chez 9 parkinsoniens. Rev Neurol (Paris). 1992;148(2):117–122.
- Tröster AI, Fields JA, Wilkinson SB, et al. Neuropsychological functioning before and after unilateral thalamic stimulating electrode implantation in parkinson’s disease [electronic manuscript]. Neurosurg Focus. 1997;2(3):Article 9 (pp. 1–6. doi: 10.3171/foc.1997.2.3.12
- Woods SP, Fields JA, Lyons KE, et al. Neuropsychological and quality of life changes following unilateral thalamic deep brain stimulation in parkinson’s disease: A 12-month follow-up. Acta Neurochir (Wien). 2001;143(12):1273–1278. doi: 10.1007/s007010100024
- Merello M, Nouzeilles MI, Kuzis G, et al. Unilateral radiofrequency lesion versus electrostimulation of posteroventral pallidum: a prospective randomized comparison. Mov Disord. 1999;14(1):50–56. doi: 10.1002/1531-8257(199901)14:1<50:AID-MDS1010>3.0.CO;2-6
- Tröster AI, Fields JA, Wilkinson SB, et al. Unilateral pallidal stimulation for parkinson’s disease: Neurobehavioral functioning before and 3 months after electrode implantation. Neurology. 1997;49(4):1078–1083. doi: 10.1212/WNL.49.4.1078
- Vingerhoets G, van der Linden C, Lannoo E, et al. Cognitive outcome after unilateral pallidal stimulation in parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;66(3):297–304. doi: 10.1136/jnnp.66.3.297
- Okun MS, Fernandez HH, Wu SS, et al. Cognition and mood in parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The compare trial. Ann Neurol. 2009 May;65(5):586–595. doi: 10.1002/ana.21596
- Fields JA, Tröster AI, Wilkinson SB, et al. Cognitive outcome following staged bilateral pallidal stimulation for the treatment of parkinson’s disease. Clin Neurol Neurosur. 1999;101(3):182–188. doi: 10.1016/S0303-8467(99)00044-X
- Rothlind JC, Cockshott RW, Starr PA, et al. Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for parkinson’s disease. J Int Neuropsychol Soc. 2007 Jan;13(1):68–79. doi: 10.1017/S1355617707070105
- Ardouin C, Pillon B, Peiffer E, et al. Bilateral subthalamic or pallidal stimulation for parkinson’s disease affects neither memory nor executive functions: a consecutive series of 62 patients. Ann Neurol. 1999;46(2):217–223. doi: 10.1002/1531-8249(199908)46:2<217:AID-ANA11>3.0.CO;2-Z
- Pillon B, Ardouin C, Damier P, et al. Neuropsychological changes between “off” and “on” stn or gpi stimulation in parkinson’s disease. Neurology. 2000;55(3):411–418. doi: 10.1212/WNL.55.3.411
- Odekerken VJ, Boel JA, Geurtsen GJ, et al. Neuropsychological outcome after deep brain stimulation for parkinson disease. Neurology. 2015 Mar 31;84(13):1355–1361. doi: 10.1212/WNL.0000000000001419
- Rothlind JC, York MK, Carlson K, et al. Neuropsychological changes following deep brain stimulation surgery for parkinson’s disease: comparisons of treatment at pallidal and subthalamic targets versus best medical therapy. J Neurol Neurosurg Psychiatry. 2015 Jun;86(6):622–629. doi: 10.1136/jnnp-2014-308119
- Combs HL, Folley BS, Berry DT, et al. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in parkinson’s disease: A meta-analysis. Neuropsychol rev. 2015 Dec;25(4):439–454. doi: 10.1007/s11065-015-9302-0
- Bonenfant J, Drapier S, Houvenaghel JF, et al. Pallidal stimulation in parkinson’s patients with contraindications to subthalamic target: a 3 years follow-up. Parkinsonism Relat Disord. 2017 Jan;34:20–25. doi: 10.1016/j.parkreldis.2016.10.007
- Rughani A, Schwalb JM, Sidiropoulos C, et al. Congress of neurological surgeons systematic review and evidence-based guideline on subthalamic nucleus and globus pallidus internus deep brain stimulation for the treatment of patients with parkinson’s disease: Executive summary. Neurosurgery. [2018 Jun 1];82(6):753–756. doi: 10.1093/neuros/nyy037
- Elgebaly A, Elfil M, Attia A, et al. Neuropsychological performance changes following subthalamic versus pallidal deep brain stimulation in parkinson’s disease: a systematic review and metaanalysis. CNS Spectr. 2017 Feb;23(1):10–23. doi: 10.1017/S1092852917000062
- Wang JW, Zhang YQ, Zhang XH, et al. Cognitive and psychiatric effects of stn versus gpi deep brain stimulation in parkinson’s disease: a meta-analysis of randomized controlled trials. PLOS ONE. 2016;11(6):e0156721. doi: 10.1371/journal.pone.0156721
- Tröster AI, McTaggart AB, Heber IA. Neuropsychological issues in deep brain stimulation of neurological and psychiatric disorders. In: Tarsy D, Vitek J, and Starr P, editors. Deep brain stimulation in neurological and psychiatric disorders. (NY): Springer; 2008. p. 399–452.
- Parsons TD, Rogers SA, Braaten AJ, et al. Cognitive sequelae of subthalamic nucleus deep brain stimulation in parkinson’s disease: A meta-analysis. Lancet Neurol. 2006 Jul;5(7):578–588. doi: 10.1016/S1474-4422(06)70475-6
- Tramontana MG, Molinari AL, Konrad PE, et al. Neuropsychological effects of deep brain stimulation in subjects with early stage parkinson’s disease in a randomized clinical trial. J Parkinsons Dis. 2015;5(1):151–163. doi: 10.3233/JPD-140448
- Cobb JL, Wolf PA, Au R, et al. The effect of education on the incidence of dementia and alzheimer’s disease in the framingham study. Neurology. 1995;45(9):1707–1712. doi: 10.1212/WNL.45.9.1707
- Martinez-Martinez AM, Aguilar OM, Acevedo-Triana CA. Meta-analysis of the relationship between deep brain stimulation in patients with parkinson’s disease and performance in evaluation tests for executive brain functions. Parkinsons Dis. 2017;2017:9641392. doi: 10.1155/2017/9641392
- Xie Y, Meng X, Xiao J, et al. Cognitive changes following bilateral deep brain stimulation of subthalamic nucleus in parkinson’s disease: A meta-analysis. Biomed Res Int. 2016;2016:3596415. doi: 10.1155/2016/3596415
- Tröster AI. Some clinically useful information that neuropsychology provides patients, carepartners, neurologists, and neurosurgeons about deep brain stimulation for parkinson’s disease. Arch Clin Neuropsychol. [2017 Nov 1];32(7):810–828. doi: 10.1093/arclin/acx090
- Meader N, King K, Llewellyn A, et al. A checklist designed to aid consistency and reproducibility of grade assessments: Development and pilot validation. Syst Rev. [2014 Jul 24];3(1):82. doi: 10.1186/2046-4053-3-82
- Philipson J, Blomstedt P, Fredricks A, et al. Short- and long-term cognitive effects of deep brain stimulation in the caudal zona incerta versus best medical treatment in patients with parkinson’s disease. J Neurosurg. [2020 Feb 7];134(2):357–365. doi: 10.3171/2019.12.JNS192654
- Ricciardi L, Sarchioto M, Morgante F. Role of pedunculopontine nucleus in sleep-wake cycle and cognition in humans: A systematic review of dbs studies. Neurobiol Dis. 2019 Aug;128:53–58. doi: 10.1016/j.nbd.2019.01.022
- Tröster AI, Ponce FA, Moguel-Cobos G. Deep-brain stimulation for parkinson’s disease: Current perspectives on patient selection with an emphasis on neuropsychology. JPRLS. 2018 Nov 17;8(null):33–48. doi: 10.2147/JPRLS.S125332
- Abboud H, Floden D, Thompson NR, et al. Impact of mild cognitive impairment on outcome following deep brain stimulation surgery for parkinson’s disease. Parkinsonism Relat Disord. 2015 Mar;21(3):249–253. doi: 10.1016/j.parkreldis.2014.12.018
- Alhourani A, Wylie SA, Summers JE, et al. Developing predictor models of postoperative verbal fluency after deep brain stimulation using preoperative neuropsychological assessment. Neurosurgery. [2022 Aug 1];91(2):256–262. doi: 10.1227/neu.0000000000001964
- Askari A, Greif TR, Lam J, et al. Decline of verbal fluency with lateral superior frontal gyrus penetration in subthalamic nucleus deep brain stimulation for parkinson disease. J Neurosurg. 2022Jan 28;137:729–734.
- Bjerknes S, Toft M, Brandt R, et al. Subthalamic nucleus stimulation in parkinson’s disease: 5-year extension study of a randomized trial. Mov Disord Clin Pract. 2022 Jan;9(1):48–59. doi: 10.1002/mdc3.13348
- Blume J, Lange M, Rothenfusser E, et al. The impact of white matter lesions on the cognitive outcome of subthalamic nucleus deep brain stimulation in parkinson’s disease. Clin Neurol Neurosur. [2017 May 31];159:87–92. doi: 10.1016/j.clineuro.2017.05.023
- Bot M, van den Munckhof P, Schmand BA, et al. Electrode penetration of the caudate nucleus in deep brain stimulation surgery for parkinson’s disease. Stereotact Funct Neurosurg. 2018;96(4):223–230. doi: 10.1159/000489944
- Brown G, Du G, Farace E, et al. Subcortical iron accumulation pattern may predict neuropsychological outcomes after subthalamic nucleus deep brain stimulation: a pilot study. J Parkinsons Dis. 2022;12(3):851–863. doi: 10.3233/JPD-212833
- Clement G, Wirth T, Haumesser L, et al. Language and verbal fluency outcome after bilateral subthalamic nucleus deep brain stimulation in parkinson’s disease. Parkinsonism Relat Disord. 2022 Dec;105:15–18. doi: 10.1016/j.parkreldis.2022.10.023
- Costentin G, Derrey S, Gerardin E, et al. White matter tracts lesions and decline of verbal fluency after deep brain stimulation in parkinson’s disease. Hum Brain Mapp. [2019 Jun 15];40(9):2561–2570. doi: 10.1002/hbm.24544
- Daniels C, Krack P, Volkmann J, et al. Risk factors for executive dysfunction after subthalamic nucleus stimulation in parkinson’s disease. Mov Disord. 2010;25(11):1583–1589. doi: 10.1002/mds.23078
- Floden DP, Matias CM, Wathen CA, et al. Contact location and neuropsychological outcomes in subthalamic deep brain stimulation. Neurosurgery. [2017 Oct 18];83(4):666–674. doi: 10.1093/neuros/nyx475
- Foley JA, Foltynie T, Zrinzo L, et al. Apathy and reduced speed of processing underlie decline in verbal fluency following dbs. Behav Neurol. 2017;2017:1–10. doi: 10.1155/2017/7348101
- Francel P, Ryder K, Wetmore J, et al. Deep brain stimulation for parkinson’s disease: Association between stimulation parameters and cognitive performance. Stereotact Funct Neurosurg. 2004;82(4):191–193. doi: 10.1159/000082208
- Fukaya C, Watanabe M, Kobayashi K, et al. Predictive factors for long-term outcome of subthalamic nucleus deep brain stimulation for parkinson’s disease. Neurol Med Chir (Tokyo). [2017 Apr 15];57(4):166–171. doi: 10.2176/nmc.oa.2016-0114
- Kubler D, Wellmann SK, Kaminski J, et al. Nucleus basalis of meynert predicts cognition after deep brain stimulation in parkinson’s disease. Parkinsonism Relat Disord. 2022 Jan;94:89–95. doi: 10.1016/j.parkreldis.2021.12.002
- Geraedts VJ, Koch M, Kuiper R, et al. Preoperative electroencephalography-based machine learning predicts cognitive deterioration after subthalamic deep brain stimulation. Mov Disord. 2021 Oct;36(10):2324–2334. doi: 10.1002/mds.28661
- Greif TR, Askari A, Cook Maher A, et al. Anterior lead location predicts verbal fluency decline following stn-dbs in parkinson’s disease. Parkinsonism Relat Disord. 2021 Nov;92:36–40. doi: 10.1016/j.parkreldis.2021.10.012
- Gruber D, Calmbach L, Kuhn AA, et al. Longterm outcome of cognition, affective state, and quality of life following subthalamic deep brain stimulation in parkinson’s disease. J Neural Transm (Vienna). 2019 Mar;126(3):309–318. doi: 10.1007/s00702-019-01972-7
- Hrabovsky D, Balaz M, Rab M, et al. Factors responsible for early postoperative mental alterations after bilateral implantation of subthalamic electrodes. Br J Neurosurg. 2017 Apr;31(2):212–216. doi: 10.1080/02688697.2016.1226256
- Isler C, Albi A, Schaper FL, et al. Neuropsychological outcome in subthalamic nucleus stimulation surgeries with electrodes passing through the caudate nucleus. Stereotact Funct Neurosurg. 2016;94(6):413–420. doi: 10.1159/000453278
- John KD, Wylie SA, Dawant BM, et al. Deep brain stimulation effects on verbal fluency dissociated by target and active contact location. Ann Clin Transl Neurol. 2021 Mar;8(3):613–622. doi: 10.1002/acn3.51304
- Kim HJ, Jeon BS, Paek SH, et al. Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in parkinson’s disease. J Neurol. 2014 Jun;261(6):1090–1096. doi: 10.1007/s00415-014-7321-z
- Kratter IH, Karp JF, Chang YF, et al. Association of preoperative visual hallucinations with cognitive decline after deep brain stimulation for parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2021 Spring;33(2):144–151. doi: 10.1176/appi.neuropsych.20040077
- Kratter IH, Jorge A, Feyder MT, et al. Depression history modulates effects of subthalamic nucleus topography on neuropsychological outcomes of deep brain stimulation for parkinson’s disease. Transl Psychiatry. [2022 May 27];12(1):213. doi: 10.1038/s41398-022-01978-y
- Le Goff F, Derrey S, Lefaucheur R, et al. Decline in verbal fluency after subthalamic nucleus deep brain stimulation in parkinson’s disease: A microlesion effect of the electrode trajectory? J Parkinsons Dis. [2015 Jan 1];5(1):95–104. doi: 10.3233/JPD-140443
- Mangone G, Bekadar S, Cormier-Dequaire F, et al. Early cognitive decline after bilateral subthalamic deep brain stimulation in parkinson’s disease patients with gba mutations. Parkinsonism Relat Disord. 2020 Jul;76:56–62. doi: 10.1016/j.parkreldis.2020.04.002
- Markser A, Maier F, Lewis CJ, et al. Deep brain stimulation and cognitive decline in parkinson’s disease: The predictive value of electroencephalography. J Neurol. 2015 Oct;262(10):2275–2284. doi: 10.1007/s00415-015-7839-8
- Merola A, Rizzi L, Artusi CA, et al. Subthalamic deep brain stimulation: clinical and neuropsychological outcomes in mild cognitive impaired parkinsonian patients. J Neurol. 2014 Sep;261(9):1745–1751. doi: 10.1007/s00415-014-7414-8
- Mulders AEP, Temel Y, Tonge M, et al. The association between surgical characteristics and cognitive decline following deep brain stimulation of the subthalamic nucleus in parkinson’s disease. Clin Neurol Neurosur. 2021 Jan;200:106341. doi: 10.1016/j.clineuro.2020.106341
- Nimura T, Nagamatsu KI, Ando T, et al. An investigation into the effects and prognostic factors of cognitive decline following subthalamic nucleus stimulation in patients with parkinson’s disease. J Clin Neurosci. 2017 Oct;44:164–168. doi: 10.1016/j.jocn.2017.06.018
- Pal G, Mangone G, Hill EJ, et al. Parkinson disease and subthalamic nucleus deep brain stimulation: Cognitive effects in gba mutation carriers. Ann Neurol. 2022 Mar;91(3):424–435. doi: 10.1002/ana.26302
- Planche V, Munsch F, Pereira B, et al. Anatomical predictors of cognitive decline after subthalamic stimulation in parkinson’s disease. Brain Struct Funct. 2018 Sep;223(7):3063–3072. doi: 10.1007/s00429-018-1677-2
- Rothlind JC, York MK, Luo P, et al. Predictors of multi-domain cognitive decline following dbs for treatment of parkinson’s disease. Parkinsonism Relat Disord. 2022 Feb;95:23–27. doi: 10.1016/j.parkreldis.2021.12.011
- Saint-Cyr JA, Trépanier LL, Kumar R, et al. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in parkinson’s disease. Brain. 2000;123(10):2091–2108. doi: 10.1093/brain/123.10.2091
- Sarno M, Gaztanaga W, Banerjee N, et al. Revisiting eligibility for deep brain stimulation: Do preoperative mood symptoms predict outcomes in parkinson’s disease patients? Parkinsonism Relat Disord. 2019 Jun;63:131–136. doi: 10.1016/j.parkreldis.2019.02.019
- Schoenberg MR, Mash KM, Bharucha KJ, et al. Deep brain stimulation parameters associated with neuropsychological changes in subthalamic nucleus stimulation for refractory parkinson’s disease. Stereotact Funct Neurosurg. 2008;86(6):337–344. doi: 10.1159/000163554
- Smeding HM, Speelman JD, Huizenga HM, et al. Predictors of cognitive and psychosocial outcome after stn dbs in parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011 Jul;82(7):754–760. doi: 10.1136/jnnp.2007.140012
- Tesio V, Rizzi L, Jiang T, et al. Deep brain stimulation of the subthalamic nucleus in parkinson’s disease: Relationship between the electrode trajectory and cognitive decline. Parkinsonism Relat Disord. 2019 Apr;61:45–49. doi: 10.1016/j.parkreldis.2018.12.005
- Tröster AI, Jankovic J, Tagliati M, et al. Neuropsychological outcomes from constant current deep brain stimulation for parkinson’s disease. Mov Disord. 2017 Mar;32(3):433–440. doi: 10.1002/mds.26827
- Tsai ST, Lin SH, Lin SZ, et al. Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in parkinson’s disease. Neurosurgery. 2007 Nov;61(5):E1024–9; discussion E1029-30.doi: 10.1227/01.neu.0000303198.95296.6f
- Voruz P, Pierce J, Ahrweiller K, et al. Motor symptom asymmetry predicts non-motor outcome and quality of life following stn dbs in parkinson’s disease. Sci Rep. [2022 Feb 22];12(1):3007. doi: 10.1038/s41598-022-07026-5
- Voruz P, Haegelen C, Assal F, et al. Motor symptom asymmetry predicts cognitive and neuropsychiatric profile following deep brain stimulation of the subthalamic nucleus in parkinson’s disease: A 5-year longitudinal study. Arch Clin Neuropsychol. [2023 Aug 24];38(6):904–912. doi: 10.1093/arclin/acad013
- Wang Z, You Z. Impacts of motor phenotype on cognitive function in patients with parkinson’s disease 1 year after subthalamic-nucleus deep brain stimulation. Geriatr Gerontol Int. 2023 Feb;23(2):85–90. doi: 10.1111/ggi.14524
- Weinkle LJ, Hoyt B, Thompson JA, et al. Association of mri measurements with cognitive outcomes after stn-dbs in parkinson’s disease. Mov Disord Clin Pract. 2018 Jul;5(4):417–426. doi: 10.1002/mdc3.12643
- Witt K, Daniels C, Krack P, et al. Negative impact of borderline global cognitive scores on quality of life after subthalamic nucleus stimulation in parkinson’s disease. J Neurol Sci. [2011 Nov 15];310(1–2):261–266. doi: 10.1016/j.jns.2011.06.028
- Witt K, Granert O, Daniels C, et al. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in parkinson’s disease: Results from a randomized trial. Brain. 2013 Jul;136(Pt 7):2109–2119. doi: 10.1093/brain/awt151
- Yaguez L, Costello A, Moriarty J, et al. Cognitive predictors of cognitive change following bilateral subthalamic nucleus deep brain stimulation in parkinson’s disease. J Clin Neurosci. 2014 Mar;21(3):445–450. doi: 10.1016/j.jocn.2013.06.005
- Yakufujiang M, Higuchi Y, Aoyagi K, et al. Predictive potential of preoperative electroencephalogram for neuropsychological change following subthalamic nucleus deep brain stimulation in parkinson’s disease. Acta Neurochir (Wien). 2019 Oct;161(10):2049–2058. doi: 10.1007/s00701-019-03991-5
- York MK, Wilde EA, Simpson R, et al. Relationship between neuropsychological outcome and dbs surgical trajectory and electrode location. J Neurol Sci. [2009 Dec 15];287(1–2):159–171. doi: 10.1016/j.jns.2009.08.003
- Zhou Y, Fan T, Ma Y, et al. Association between baseline cognitive score and postoperative delirium in parkinson’s disease patients following deep brain stimulation surgery. Parkinsons Dis. 2022;2022:1–8. doi: 10.1155/2022/9755129
- Geevarghese R, Lumsden DE, Costello A, et al. Verbal memory decline following dbs for parkinson’s disease: Structural volumetric mri relationships. PLoS One. 2016;11(8):e0160583. doi: 10.1371/journal.pone.0160583
- Yakufujiang M, Higuchi Y, Aoyagi K, et al. Predicting neurocognitive change after bilateral deep brain stimulation of subthalamic nucleus for parkinson’s disease. World Neurosurg. 2021 Mar;147:e428–e436. doi: 10.1016/j.wneu.2020.12.081
- Tang V, Zhu XL, Lau C, et al. Pre-operative cognitive burden as predictor of motor outcome following bilateral subthalamic nucleus deep brain stimulation in parkinson’s disease. Neurol Sci. 2022 Dec;43(12):6803–6811. doi: 10.1007/s10072-022-06370-8
- Asimakidou E, Xiromerisiou G, Sidiropoulos C. Motor and non-motor outcomes of deep brain stimulation across the genetic panorama of parkinson’s disease: A multi-scale meta-analysis. Mov Disord Clin Pract. [2024 Feb 6];11(5):465–477. doi: 10.1002/mdc3.13994
- Agelink van Rentergem JA, de Vent NR, Schmand BA, et al. Multivariate normative comparisons for neuropsychological assessment by a multilevel factor structure or multiple imputation approach. Psychol Assess. 2018 Apr;30(4):436–449. doi: 10.1037/pas0000489
- Hu T, Xie H, Diao Y, et al. Effects of subthalamic nucleus deep brain stimulation on depression in patients with parkinson’s disease. J Clin Med. [2022 Oct 1];11(19):5844. doi: 10.3390/jcm11195844
- Hacker ML, Tramontana MG, Pazira K, et al. Long-term neuropsychological outcomes of deep brain stimulation in early-stage parkinson’s disease. Parkinsonism Relat Disord. 2023 Aug;113:105479. doi: 10.1016/j.parkreldis.2023.105479
- Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for parkinson’s disease with early motor complications. N Engl J Med. [2013 Feb 14];368(7):610–622. doi: 10.1056/NEJMoa1205158
- Planas-Ballve A, Vilas D, Pisani A. Cognitive impairment in genetic parkinson’s disease. Parkinsons Dis. 2021;2021:1–11. doi: 10.1155/2021/8610285
- Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive performance of gba mutation carriers with early-onset pd: The core-pd study. Neurology. [2012 May 1];78(18):1434–1440. doi: 10.1212/WNL.0b013e318253d54b
- Wang F, Lai Y, Pan Y, et al. A systematic review of brain morphometry related to deep brain stimulation outcome in parkinson’s disease. NPJ Parkinsons Dis. [2022 Oct 13];8(1):130. doi: 10.1038/s41531-022-00403-x
- Besse-Pinot E, Pereira B, Durif F, et al. Preoperative rem sleep behavior disorder and subthalamic nucleus deep brain stimulation outcome in parkinson disease 1 year after surgery. Neurology. 2006 [2021 Nov 16];97(20):e1994–e. doi: 10.1212/WNL.0000000000012862
- Zibetti M, Rizzi L, Colloca L, et al. Probable REM sleep behaviour disorder and stn-dbs outcome in parkinson’s disease. Parkinsonism Relat Disord. 2010 May;16(4):265–269. doi: 10.1016/j.parkreldis.2010.01.001
- Burchill E, Watson CJ, Fanshawe JB, et al. The impact of psychiatric comorbidity on parkinson’s disease outcomes: A systematic review and meta-analysis. Lancet Reg Health Eur. 2024 Apr;39:100870. doi: 10.1016/j.lanepe.2024.100870
- Mills KA, Donohue K, Swaminathan A, et al. Neuropsychological predictors of patient-reported cognitive decline after deep brain stimulation in parkinson’s disease. J Clin Exp Neuropsychol. 2019 Apr;41(3):219–228. doi: 10.1080/13803395.2018.1526889
- Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in parkinson’s disease: Movement disorder society task force guidelines [Review]. Mov Disord. 2012 Mar;27(3):349–356. doi: 10.1002/mds.24893
- Tröster AI, Woods SP, Morgan EE. Assessing cognitive change in parkinson’s disease: Development of practice effect-corrected reliable change indices. Arch Clin Neuropsychol. 2007 Aug;22(6):711–718. doi: 10.1016/j.acn.2007.05.004
- Stebbins GT, Gabrieli JD, Shannon KM, et al. Impaired frontostriatal cognitive functioning following posteroventral pallidotomy in advanced parkinson’s disease. Brain Cogn. 2000;42(3):348–363. doi: 10.1006/brcg.1999.1109
- Pedersen KF, Larsen JP, Tysnes OB, et al. Natural course of mild cognitive impairment in parkinson disease: A 5-year population-based study. Neurology. [2017 Feb 21];88(8):767–774. doi: 10.1212/WNL.0000000000003634
- Lawson RA, Yarnall AJ, Duncan GW, et al. Stability of mild cognitive impairment in newly diagnosed parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017 Aug;88(8):648–652. doi: 10.1136/jnnp-2016-315099
- Santangelo G, Vitale C, Picillo M, et al. Mild cognitive impairment in newly diagnosed parkinson’s disease: A longitudinal prospective study. Parkinsonism Relat Disord. 2015 Oct;21(10):1219–1226. doi: 10.1016/j.parkreldis.2015.08.024
- Bove F, Fraix V, Cavallieri F, et al. Dementia and subthalamic deep brain stimulation in parkinson disease: A long-term overview. Neurology. [2020 Jul 28];95(4):e384–e392. doi: 10.1212/WNL.0000000000009822
- Hoogland J, Boel JA, de Bie RMA, et al. Mild cognitive impairment as a risk factor for parkinson’s disease dementia. Mov Disord. 2017 Jul;32(7):1056–1065. doi: 10.1002/mds.27002
- Hoogland J, Boel JA, de Bie RMA, et al. Risk of parkinson’s disease dementia related to level i mds pd-mci. Mov Disord. 2019 Mar;34(3):430–435. doi: 10.1002/mds.27617
- Aybek S, Gronchi-Perrin A, Berney A, et al. Long-term cognitive profile and incidence of dementia after stn-dbs in parkinson’s disease. Mov Disord. [2007 May 15];22(7):974–981. doi: 10.1002/mds.21478
- Block CK, Patel M, Risk BB, et al. Patients with cognitive impairment in parkinson’s disease benefit from deep brain stimulation: A case-control study. Mov Disord Clin Pract. 2023 Mar;10(3):382–391. doi: 10.1002/mdc3.13660
- Bari AA, Fasano A, Munhoz RP, et al. Improving outcomes of subthalamic nucleus deep brain stimulation in parkinson’s disease. Expert Rev Neurother. 2015 Oct;15(10):1151–1160. doi: 10.1586/14737175.2015.1081815
- Bot M, Schuurman PR, Odekerken VJJ, et al. Deep brain stimulation for parkinson’s disease: Defining the optimal location within the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2018 May;89(5):493–498. doi: 10.1136/jnnp-2017-316907
- Mikos A, Bowers D, Noecker AM, et al. Patient-specific analysis of the relationship between the volume of tissue activated during dbs and verbal fluency. Neuroimage. 2011 Jan;54(Suppl 1):S238–46. doi: 10.1016/j.neuroimage.2010.03.068
- Holewijn RA, Verbaan D, van den Munckhof PM, et al. General anesthesia vs local anesthesia in microelectrode recording-guided deep-brain stimulation for parkinson disease: The galaxy randomized clinical trial. JAMA Neurol. [2021 Oct 1];78(10):1212–1219. doi: 10.1001/jamaneurol.2021.2979
- Holewijn RA, Zoon TJC, Verbaan D, et al. Cognitive and psychiatric outcomes in the galaxy trial: Effect of anaesthesia in deep brain stimulation. J Neurol Neurosurg Psychiatry. [2024 Feb 14];95(3):214–221. doi: 10.1136/jnnp-2023-331791
- Sidiropoulos C, Rammo R, Merker B, et al. Intraoperative mri for deep brain stimulation lead placement in parkinson’s disease: 1 year motor and neuropsychological outcomes. J Neurol. 2016 Jun;263(6):1226–1231. doi: 10.1007/s00415-016-8125-0
- Brodsky MA, Anderson S, Murchison C, et al. Clinical outcomes of asleep vs awake deep brain stimulation for parkinson disease. Neurology. [2017 Oct 6];89(19):1944–1950. doi: 10.1212/WNL.0000000000004630
- Reich MM, Hsu J, Ferguson M, et al. A brain network for deep brain stimulation induced cognitive decline in parkinson’s disease. Brain. [2022 May 24];145(4):1410–1421. doi: 10.1093/brain/awac012
- Dietz J, Noecker AM, McIntyre CC, et al. Stimulation region within the globus pallidus does not affect verbal fluency performance. Brain Stimul. 2013 May;6(3):248–253. doi: 10.1016/j.brs.2012.05.011
- Leimbach F, Atkinson-Clement C, Wilkinson L, et al. Dissociable effects of subthalamic nucleus deep brain stimulation surgery and acute stimulation on verbal fluency in parkinson’s disease. Behav Brain Res. [2020 Jun 18];388:112621. doi: 10.1016/j.bbr.2020.112621
- Dembek TA, Reker P, Visser-Vandewalle V, et al. Directional dbs increases side-effect thresholds-a prospective, double-blind trial. Mov Disord. 2017 Oct;32(10):1380–1388. doi: 10.1002/mds.27093
- Del Bene VA, Martin RC, Brinkerhoff SA, et al. Differential cognitive effects of unilateral subthalamic nucleus deep brain stimulation for parkinson’s disease. Ann Neurol. 2024 Jun;95(6):1205–1219. doi: 10.1002/ana.26903
- Jain K, Ramesh R, Krishnan S, et al. Cognitive outcome following bilateral subthalamic nucleus deep brain stimulation for parkinson’s disease-a comparative observational study in indian patients. Acta Neurol Belg. 2022 Apr;122(2):447–456. doi: 10.1007/s13760-021-01778-z
- Hariz MI, Johansson F, Shamsgovara P, et al. Bilateral subthalamic nucleus stimulation in a parkinsonian patient with preoperative deficits in speech and cognition: persistent improvement in mobility but increased dependency: a case study. Mov Disord. 2000;15(1):136–139. doi: 10.1002/1531-8257(200001)15:1<136:AID-MDS1021>3.0.CO;2-5
- Hristova A, Lyons K, Tröster AI, et al. Effect and time course of deep brain stimulation of the globus pallidus and subthalamus on motor features of parkinson’s disease. Clin Neuropharmacol. 2000;23(4):208–211. doi: 10.1097/00002826-200007000-00007
- Swinnen B, Lotfalla V, Scholten MN, et al. Programming algorithm for the management of speech impairment in subthalamic nucleus deep brain stimulation for parkinson’s disease. Technol Neural Interface. 2024 Apr;27(3):528–537. doi: 10.1016/j.neurom.2023.05.002
- Deshpande N, Hadi M, Ali R. Healthcare disparities in deep brain stimulation access and utilization: a systematic review. J Neurosurg. 2023 Oct;6:1–11.
- Frassica M, Kern DS, Afshari M, et al. Racial disparities in access to dbs: results of a real-world U.S. claims data analysis. Front Neurol. 2023;14:1233684. doi: 10.3389/fneur.2023.1233684
- Merola A, Singh J, Reeves K, et al. New frontiers for deep brain stimulation: directionality, sensing technologies, remote programming, robotic stereotactic assistance, asleep procedures, and connectomics. Front Neurol. 2021;12:694747. doi: 10.3389/fneur.2021.694747
- Ranganathan P, Aggarwal R, Pramesh CS. Common pitfalls in statistical analysis: odds versus risk. Perspect Clin Res. 2015 Oct;6(4):222–224. doi: 10.4103/2229-3485.167092
- Palmese CA, Wyman-Chick KA, Racine C, et al. Assessment of deep brain stimulation candidacy during the COVID-19 pandemic: Lessons learned and future directions for neuropsychologists. Clin Neuropsychol. 2022 Jan;36(1):72–84. doi: 10.1080/13854046.2021.1929496
- Jitkritsadakul O, Rajalingam R, Toenjes C, et al. Tele-health for patients with deep brain stimulation: the experience of the ontario telemedicine network. Mov Disord. 2018 Mar;33(3):491–492. doi: 10.1002/mds.27230
- York MK, Farace E, Pollak L, et al. The global pandemic has permanently changed the state of practice for pre-dbs neuropsychological evaluations. Parkinsonism Relat Disord. 2021 May;86:135–138. doi: 10.1016/j.parkreldis.2021.04.029
- Backstrom D, Granasen G, Mo SJ, et al. Prediction and early biomarkers of cognitive decline in parkinson disease and atypical parkinsonism: A population-based study. Brain Com. 2022;4(2):fcac040. doi: 10.1093/braincomms/fcac040