Several new drugs are under development for the treatment of multiple myeloma (MM) and the introduction of immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) has prolonged both progression-free survival (PFS) and overall survival (OS). However, patients may become resistant to these drugs and new strategies are needed to improve disease control and outcome. Here, we provide an overview of new monoclonal antibodies (MoAbs) used for the treatment of MM, in particular anti-CD38 and anti-CS1 MoAbs.
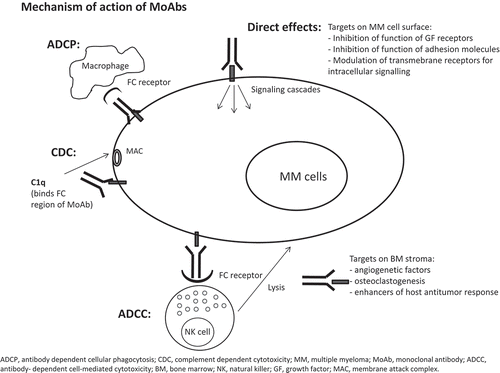
MoAbs may interact with myeloma cells through different mechanisms of action and targets: MoAbs can elicit antibody-dependent cell-mediated cytotoxicity (ADCC), activate complement-dependent cytotoxicity (CDC), activate antibody-dependent cellular phagocytosis (ADCP), and/or directly induce tumor cell apoptosis. Alternatively, they may target bone marrow microenvironment, resulting in neutralization of growth factors, inhibition of angiogenesis, modulation of mediators of bone disease, or enhancement of the host antitumor immune response () [Citation1].
Figure 1. Monoclonal antibodies can induce tumor cell death through different mechanisms: directly on MM cells via modulation of activity of the targeted antigens or via Fc-dependent effector mechanisms (ADCC, CDC and ADCP). The ADCC process activates the NK effector cells and induces cytotoxicity by the release of perforin and granzymes from effector cells and thanks to FasL and TNF-related apoptosis-inducing ligand (TRAIL) process. CDC results by the lineage of the antibody Fc domains with C1q protein and subsequent formation of the membrane attack complex (MAC), that forms holes in the MM cells. ADCP is due to phagocytosis of tumor cells by macrophages.
CD38 is a multifunctional cell surface glycoprotein highly expressed on myeloma cells, at low levels on normal lymphoid and myeloid cells. CD38-targeting antibodies kill MM cells via CDC, ADCC, ADCP, direct induction of apoptosis, and modulation of CD38 ectoenzyme function [Citation1]. Currently, there are three anti-CD38 MoAbs under clinical development for MM patients: daratumumab, SAR650984, and MOR202 ().
Table 1. Anti-CD38 clinical trials and results.
In particular, daratumumab was first investigated as single agent. In a phase I study, daratumumab at 16 mg/kg showed to be safe and effective as compared with the 8 mg/kg dose, with an overall response rate (ORR) of 36% versus 10%, respectively. Daratumumab had an acceptable safety profile, with grade 1–2 infusion-related events (IREs) including mild and transient bronchospasm, headache, dyspnea, and fever. Most events occurred during the first infusion, and no patient discontinued treatment because of IREs [Citation2]. Subsequently, a phase II study assessed daratumumab at 16 mg/kg in 106 relapsed/refractory MM (RRMM) patients: the ORR was 29%, the median PFS was 3.7 months, and only 5% of grade 3 IREs were observed [Citation3]. Of note, these patients were heavily pretreated, almost all of them did not respond to therapy with IMiDs or PIs, they were refractory to alkylators or had relapsed after autologous stem transplantation. Therefore, these results are quite impressive, and responses and survival outcomes are better than expected [Citation11].
Daratumumab was then tested in combination with other drugs, such as with the association lenalidomide-dexamethasone (RD). Also in this case, the 16 mg/kg dose proved to be superior to lower doses of daratumumab in terms of efficacy (ORR: 81%), with a favorable safety profile (grade 3 IREs occurred in only 6% of patients, and only one patient discontinued treatment due to grade 3 laryngeal edema). Neutropenia was the most frequently reported grade 3 or 4 treatment-related adverse event (75%) [Citation4]. Overall, this trial demonstrated significant activity in the context of refractory myeloma and paved the way for the recent Food and Drug Administration approval of daratumumab for the treatment of myeloma. Other trials are testing daratumumab with bortezomib-based strategies in newly diagnosed multiple myeloma (NDMM) and RRMM patients, and preliminary results are promising [Citation5]. Daratumumab plus pomalidomide and dexamethasone led to an ORR of 71% in a heavily pretreated population resistant to previous lenalidomide therapy, and responses deepened over time. Moreover, the addition of daratumumab to pomalidomide and dexamethasone was associated with only a moderate toxicity: most of the events occurred on the first day of the first cycle, and they included mainly chills (13%), cough (13%), and dyspnea (11%) [Citation6]. It is important to be aware of daratumumab interference with laboratory test results, particularly with serum protein electrophoresis and immunofixation assays. This complicates the evaluation of complete responses, causing unexpected false-positive results. Moreover, daratumumab interferes with routine pre-transfusion laboratory tests, because CD38 is also present on the surface of red blood cells, possibly leading to false-positive results of the indirect antiglobulin tests (IATs) [Citation1]. New laboratory tests are, therefore, needed and are under evaluation to overcome these ‘new adverse events’. An ongoing trial is also investigating the role of daratumumab in asymptomatic myeloma and results are awaited (clinical trilas.gov: NCT02316106).
Promising results were also seen with the anti-CD38 SAR650984, which showed a strong proapoptotic activity, as well as CDC, ADCC, and ADCP functions. Moreover, SAR650984 induces lysosomal-mediated cell death [Citation12]. Interestingly, this agent demonstrated an efficacy and safety profile similar to daratumumab; however, studies are still ongoing and no definitive conclusions can be drawn [Citation8,Citation9].
The human immunoglobuline G1 (IgG1) anti-CD38 MOR202 is another MoAb currently under investigation. It showed effective ADCC, ADCP, and high activity in preclinical models of MM. In the RRMM setting, MOR202 at 16 mg/kg was safe and effective, and the most important grade 3 adverse events were lymphopenia (18%) leucopenia (11%), and hypertension (9%) [Citation10].
CS1 signaling lymphocyte activation molecule number 7 (SLAM7) is a cell membrane glycoprotein highly expressed on myeloma cells, and, at lower levels, on normal plasma cells, natural killer (NK) cells, and T cells. It is involved in MM cell adhesion to bone marrow stromal cells (BMSCs); inhibition of apoptosis by regulating pro- and anti-apoptotic pathways, lowering phosphorylation of ERK1, AKT, and STAT; and regulation of NK cell cytolytic activity [Citation1].
Elotuzumab, the humanized anti-CS1 MoAb, was first tested in preclinical studies and then in clinical trials. As single agent in RRMM patients, no objective responses were observed () [Citation13], and a potential role in combination with other MM agents was hypothesized. Recently, elotuzumab in combination with bortezomib-dexamethasone (VD; ELO-VD) induced an ORR of 66% and a median PFS approximately 3 months longer than the one reported with VD alone [Citation14]. No additional clinically significant adverse events occurred with ELO-VD versus VD. Grade 1–2 IRE rate was low (5% ELO-VD) and mitigated with premedication. A more pronounced effect was evident when elotuzumab was combined with lenalidomide-dexamethasone (ELO-RD) [Citation15]. In fact, the ORR was higher in the ELO-RD arm versus RD alone (79% vs 66%) and the 2-year PFS was 41% versus 27%, with a 30% reduction in the risk of progression or death. Lymphocytopenia was observed in elotuzumab-treated patients, which may reflect alterations in lymphocyte trafficking. Infections were higher in the elotuzumab group (81% vs 74%), with an equalization after adjustment of drug exposure, particularly in terms of nasopharyngitis and upper tract respiratory infections.
Table 2. Clinical trials and results of elotuzumab-based therapies.
Ongoing trials are evaluating the role of elotuzumab in high-risk NDMM patients, asymptomatic MM patients, and as maintenance after autologous stem cell transplantation (clinicaltrial.gov NCT01668719; NCT02279394; NCT02420860).
Interleukin-6 (IL-6) is another important target for myeloma immunotherapy. It is involved in the survival and proliferation of MM cells, with a key role in the early stages of the disease. Siltuximab, an anti-IL-6 MoAb, showed to be a promising agent when combined with dexamethasone, melphalan, and bortezomib in preclinical studies. Unfortunately, this agent did not improve OS and PFS, unless in combination with bortezomib-melphalan-prednisone (VMP) in NDMM [Citation16]. Ongoing studies are evaluating the role of early treatment with IL-6 in smoldering myeloma (clinical trial.gov NCT01484275).
The immune checkpoint blockade therapy is another area of great interest. The programmed death-1 (PD-1) pathway was found to play a role in the regulation of T-cell activation and in apoptotic pathways of effector/memory T lymphocytes. The binding with PD-L1 (expressed on tumor cell surface) downregulates T-cell proliferation, representing an escape for the tumor cell [Citation16]. PD1-PD1L blockade is a very promising treatment for hematological malignancies. In particular, pembrolizumab and nivolumab are under evaluation in myeloma. In a recent study, pembrolizumab was combined with lenalidomide and low-dose dexamethasone for heavily pretreated RRMM patients. The maximum tolerated dose was defined as pembrolizumab 200 mg fixed dose in combination with lenalidomide 25 mg. This combination led to an ORR of 76% and was also well tolerated: most frequent treatment-related toxicities were hematological events, hyperglycemia, and muscle spasms. When dose-limiting toxicities were observed (neutropenia, pneumonia, and tumor lysis syndrome in three patients), they all recovered without treatment discontinuation [Citation17]. Similarly, promising results were seen with pembrolizumab plus pomalidomide and dexamethasone: toxicities were manageable and were mostly hematological events, fatigue, and dyspnea, with no IREs [Citation18].
In conclusion, the most common adverse events for all MoAbs were IREs, which were easily manageable with adequate premedication including glucocorticoids, antihistamines, and acetaminophen.
1. Expert opinion
Immunotherapy is a crucial strategy for cancer patients, because it is based on agents aimed at engaging or augmenting the immune system to target cancer cells. The treatment armamentarium against myeloma is continuously evolving thanks to the availability of novel agents and MoAbs. In particular, anti-CD38 therapy in myeloma patients seems to be the equivalent to rituximab in lymphoma patients. Daratumumab appears to be effective in RRMM patients and we expect great results also in the NDMM setting, maintenance therapy, and high-risk asymptomatic MM patients. In addition, the safety profile of this agent is very appealing. However, a better understanding of interference in laboratory test results is critical to optimize its use. Elotuzumab and pembrolizumab seem to be very effective and many other MoAbs and small molecules are being investigated in early phase trials: filanesib (kinesin spindle protein inhibitor), ABT-199 (selective BCL2-inhibitor), lirilumab (anti-KIR inhibitor), dinaciclib (cyclin-dependent kinase inhibitor), and bone disease selective agents such as denosumab (anti-RANKL inhibitor). Although further investigation is needed, preliminary results are very encouraging since MoAbs do not add significant toxicities to classical anti-MM agents. Moreover, vigilance on potential cross-reactions that could occur with regimens including double MoAbs is fundamental to avoid excessive toxicity. We are increasingly moving toward personalized medicine and, in the future, we may be able to identify a subset of patients who express specific biomarkers and who will benefit more from therapy with specific antibodies.
Declaration of interest
S Oliva has received honoraria from Takeda and Celgene. A Palumbo has received honoraria, consultancy fees and Research funding from Amgen, Novartis, Bristo Meyers Squibb, Genmab, Celgene, Janssen-Cilag, Takeda, Sanofi and Merk. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- van de Donk NWCJ, Moreau P, Plesner T, et al. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood. 2016;127(6):681–695.
- Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–1219.
- Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560.
- Plesner T, Arkenau H-T, Gimsing P, et al. Daratumumab in combination with lenalidomide and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: updated results of a phase 1/2 study (GEN503). ASH. 2015;126(23):abstract 507.
- Mateos MV, Moreau P, Comenzo R, et al. An open-label, multicenter, phase 1b study of daratumumab in combination with pomalidomide-dexamethasone and with the backbone regimens in patients with multiple myeloma. EHA. 2015;100525:abstract P275.
- Chari A, Lonial S, Suvannasankha A, et al. Open-label, multicenter, phase 1b study of daratumumab in combination with pomalidomide and dexamethasone in patients with at least 2 lines of prior therapy and relapsed or relapsed and refractory multiple myeloma. ASH. 2015;126(23):abstract 508.
- Martin TG, Hsu K, Charpentier E, et al. A phase Ib dose escalation trial of SAR650984 (Anti-CD-38 mAb) in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma. ASCO. 2014;32(5s):abstract 8512.
- Martin T, Richter J, Vij R, et al. A dose finding phase II trial of isatuximab (SAR650984, Anti-CD38 mAb) as a single agent in relapsed/refractory multiple myeloma. ASH. 2015;126(23):abstract 509.
- Martin TG, Baz R, Benson DM, et al. A phase Ib dose escalation trial of SAR650984 (anti-CD38 mAb) in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma. ASH. 2014;124:abstract 83.
- Raab Marc S, Chatterjee M, Goldschmidt H, et al. Phase I/IIa study of the human anti-CD38 antibody MOR202 (MOR03087) in relapsed or refractory multiple myeloma. ASH. 2015;126(23):abstract 3035.
- Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128.
- Jiang H, Acharya C, An G, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399–408.
- Zonder JA, Mohrbacher AF, Singhal S, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120(3):552–559.
- Jakubowiak A, Offidani M, Pégourie B, et al. Randomized phase 2 study of elotuzumab plus bortezomib/dexamethasone (Bd) versus Bd for relapsed/refractory multiple myeloma. Blood. 2016;127(23):2833–2840.
- Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631.
- Magarotto V, Salvini M, Bonello F, et al. Strategy for the treatment of multiple myeloma utilizing monoclonal antibodies: a new era begins. Leuk Lymphoma. 2016;57(3):537–556.
- San Miguel J, Mateos M-V, Shah JJ, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma (RRMM): keynote-023. ASH. 2015;126(23):abstract 505.
- Badros AZ, Kocoglu MH, Ma N, et al. A phase II study of anti PD-1 antibody pembrolizumab, pomalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM). ASH. 2015;126(23):abstract 506.
- Jakubowiak AJ, Benson DM, Bensinger W, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol. 2012;30(16):1960–1965.
- Richardson PG, Jagannath S, Moreau P, et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015;2(12):e516–e527.
