ABSTRACT
Introduction: Epidural analgesia is a popular approach to postoperative and labor pain. Neurotoxicity and drug-specific systemic side effects can occur after epidural administration. As an increasing number of epidural drugs are studied and clinically applied, drug efficacy and safety evaluation are crucial.
Areas covered: In this narrative review, the authors provide a thorough overview on the safety of the most widely used epidural drugs, focusing on potential neurotoxicity, side effects, and complications in the adult, non-pregnant population. A combined text and MeSH heading search strategy was used to identify relevant publications.
Expert opinion: The search for the ideal epidural medication has resulted in a surplus of drug combinations with extensive heterogeneity amongst studies, while the value of investigating these is not always evident. Epidural drugs pose a potential threat of neurotoxicity and other side effects. Consequently, we should pursue safe epidural drug administration to patients and refrain from drugs with minimal proven benefit. Also, studies should compare epidural with systemic application. Because why use a drug epidurally, which can be safely used systemically? Future research should focus on providing solid evidence regarding efficacy of epidural analgesia compared to new and already existing modalities and optimizing presently used medicinal regimens.
1. Introduction
Epidural analgesia is a popular method for treating postoperative and labor pain, with over 3,7 million adult patients receiving epidural analgesia between 1998 and 2010 in the United States alone[Citation1]. However, some concerns exist regarding epidural safety and efficacy. Observed severe complications, albeit rare, include epidural hematoma or abscess and neurological injury. Epidurally administered drugs have a higher potential for inducing neurotoxicity as compared to systemically. Hence, administering drugs systemically is the preferred route when efficacy is comparable to epidural application. In addition, drug-specific systemic side effects like hemodynamic instability or intoxication can also occur after epidural administration. In this narrative review, we aim to give a thorough overview on the safety of the most widely used epidural drugs, focusing on potential neurotoxicity, known side effects and possible complications of specific drug groups.
2. Search strategy
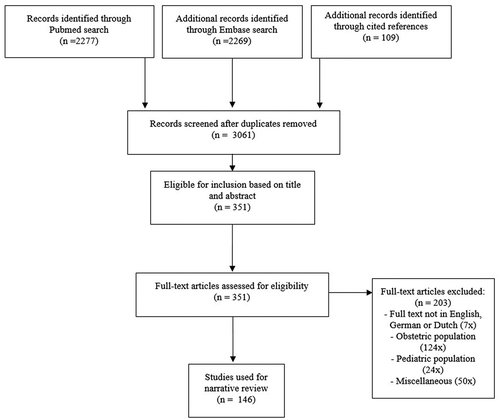
The search strategy is summarized in . We included studies on epidural drug safety for perioperative analgesia in the adult, non-pregnant population, excluding papers on epidural drug safety in chronic pain, pregnant and pediatric patients. In addition, we included studies regarding systemic versus epidural drug efficacy. Medical Subject Headings terms used were, among others, epidural analgesia, local anesthetics, glucocorticoids, steroids, dexamethasone, bupivacaine, mepivacaine, lidocaine, opioid analgesics, buprenorphine, morphine, fentanyl, sufentanil, adrenergic alpha-agonists, epinephrine, clonidine, dexmedetomidine, ketamine, drug-related side effects and adverse reactions, hypotension and synonyms. Titles and abstracts were screened and cross-referenced for possible inclusion by MvZ, while when in doubt discussing eligibility with other authors (WtH, JH, MFS). Selected articles were restricted to articles in English, German or Dutch. Full text articles were retrieved and reviewed for all studies that seemed relevant and were assessed for eligibility.
3. Local anesthetic
Local anesthetics (LA) are the main component in most epidural mixtures used in daily clinical practice. LA main site of action is a specific intracellular portion of voltage-gated sodium channels, which they block reversibly. The onset of action of LA is related to their pKa. A high pKa indicates a slow onset of action. Potency and duration of action of a LA, on the other hand, depend mostly on lipid solubility and protein-binding capacity: the more lipophilic the LA, the easier it diffuses across nerve membranes, increasing its potency. Increased protein-binding ensures LA are more tightly bound to the receptor sites and therefore dissociate more slowly [Citation2] (see ).
Table 1. Widely used local anesthetics.
3.1. Neurotoxicity
All LA have time- and dose-dependent neurotoxic properties [Citation6,Citation7] which correlate with their clinical potency [Citation8]. Neurotoxicity is mediated through a variety of different mechanisms leading to apoptosis and necrosis [Citation7]. Epidural neurotoxicity predominantly occurs after supra-clinical LA doses and therefore has little clinical impact in daily practice. Transient neurological symptoms (TNS) have been described after intrathecal LA administration, specifically lidocaine [Citation9]. However, only one case report described TNS after epidural administration of lidocaine, and this was after a high dose (≥600 mg) was administered over a short (20 min) period of time [Citation10].
3.2. Systemic toxicity
LA toxicity after systemic absorption of high doses of LA can produce central nervous system excitation with seizures, central nervous system inhibition, loss of airway reflexes, respiratory arrest, hemodynamic instability, and in extreme cases, coma or cardiovascular collapse. Case reports have shown possible benefit from the use of intravenous lipid emulsions in case of LA overdose [Citation11], however its effect on lidocaine central nervous system toxicity has been debated [Citation12].
In the sixties [Citation13] the antiarrhythmic properties of lidocaine were described, which has resulted in its intentional intravenous administration in case of ventricular arrhythmias. In recent years, more widespread use of systemically administered lidocaine has been promoted as a possible alternative to epidural analgesia. Even high intravenous doses (up to 300 mg) of systemic lidocaine rarely give rise to problems like conduction disturbances or ventricular arrhythmias [Citation14,Citation15].
At equipotent dosing, all LA have similar toxic properties, however systemic LA toxicity after epidural injection is rare [Citation16]. It has been suggested that ropivacaine has a superior safety profile compared to racemic bupivacaine. When given systemically to healthy volunteers, the tolerated maximum dosage of ropivacaine before start of neurotoxic and cardiovascular effects was twice as high as that of racemic bupivacaine [Citation17,Citation18]. Ropivacaine has a lower potency than both levo- and racemic bupivacaine and is approximately 10 times less lipophilic. At equipotent dose [Citation19], it is uncertain whether there is really clinically relevant lower toxicity. Several cases in which intended epidural doses of ropivacaine were accidentally administered either intrathecally or intravenously resulted in severe complications [Citation20,Citation21]. Thus, whether ropivacaine actually has a safer clinical profile is questionable.
Finally, all LA can cause hypotension when administered epidurally. Increased hypotension after epidural LA administration, either with or without adjuvant opioids, was reported in two recent systematic reviews [Citation22,Citation23]. In both studies the incidence of hypotension was lower when a lower dose of LA was combined with an opioid, nonetheless it was still markedly higher than in the control group.
4. Opioids
Epidural opioids have been used for over three decades, after receiving their United States Food and Drugs Administrations (FDA) approval in 1984, with the first studies ranging back as far as 1979. Most epidural mixtures currently used combine a lipophilic opioid (fentanyl, sufentanil) with a long-acting LA (levo- or racemic bupivacaine, ropivacaine) because of their presumed synergism and the avoidance of delayed respiratory depression. A meta-analysis [Citation22] suggested slightly better analgesic efficacy of epidural analgesia when compared with an intravenous pain regimen. A Cochrane systematic review supports this limited superior analgesic effect of epidural analgesia over systemic opioid administration in patients undergoing open aortic surgery, reporting a mean difference in VAS scores on movement on postoperative day 1 of −1.78 (95% CI −2.32 to −1.25) [Citation24]. The clinical relevance of this difference is however debatable. According to the requirements for a clinically superior pain regimen of two points NRS reduction or 30% relative pain reduction from baseline recommended by the International Association for the Study of Pain (IASP), this difference cannot be seen as clinically superior.
4.1. Neurotoxicity
Nearly every opioid has shown at least some degree of neurotoxicity, including hydromorphone, tramadol, oxycodone, fentanyl, sufentanil and buprenorphine [Citation25–Citation35]. For neuraxial morphine, this is less consistent [Citation26,Citation36]. Researchers studying neurotoxicity of the DepoFoam technology, a lipid-based depot used for extended release morphine, deemed it safe [Citation37] (see ). Epidural nalbuphine [Citation27] showed no neurotoxicity, but long-term clinical research regarding its safety is missing.
Table 2. Widely used epidural opioids.
It’s worth noting that very little neurotoxicity is reported in daily clinical practice, thus it can be assumed that the most frequently used epidural opioids (i.e. morphine, fentanyl, sufentanil) are safe when epidurally administered doses are in accordance with the current standard of care.
4.2. Systemic response and respiratory depression – hydrophilic opioids
Since the introduction of epidural opioids, it is well-defined that they all share, to some extent, the adverse effects as known from their systemic administration. These include, but are not limited to, hypotension, sedation, nausea, vomiting, pruritus, urinary retention and respiratory depression, whereby pruritus occurs more often after epidural opioid administration [Citation41].
Morphine is one of the most frequently used opioids in epidural anesthesia. It has long-lasting analgesic effects, especially in combination with LA. Epidural morphine can produce delayed respiratory depression and it’s an ongoing discussion whether the respiratory depression is a result of its active metabolite morphine-6-glucuronide or because of the rostral spread of morphine in the cerebrospinal fluid into the brainstem [Citation42]. It is worth noting that the pharmacokinetics of morphine seems to differ depending on the injection site (i.e. lumbar vs. thoracic catheter placement). This could result in a concentration gradient in the cerebrospinal fluid with concurrent alterations in the rostral spread, resulting in increased systemic side effects if similar doses of morphine are used in a thoracic relative to lumbar epidural [Citation43]. Late respiratory depression has been found to occur up to 24 h [Citation44] after an epidural dose. The incidence for delayed respiratory depression was found to be 0–2.8%[Citation45], however a clear definition of respiratory depression is missing in the literature [Citation45,Citation46]. Older studies used larger dosages of epidural morphine and excluding those would result in a lower incidence.
The recently introduced extended-release variant of morphine should guarantee sufficient analgesia for up to 48 h, whilst simultaneously decreasing total opioid consumption. The sterile suspension of multivesicular liposomes contains morphine sulfate and works as a depot, ensuring a controlled release. After FDA approval in 2004, a high incidence (>10%) of adverse events, including respiratory depression, has been reported, especially after doses >15 mg [Citation47–Citation52], leading to the current-advised dose of ≤15 mg. Interestingly, this decreased dose resulted in less adverse events, but consequently also reduced the duration of action [Citation53]. It has been debated whether it should be combined with LA because of potential physicochemical interactions which could alter the release of the morphine [Citation54], triggering adverse effects like respiratory depression [Citation55].
Hydromorphone is a hydrophilic opioid similar to morphine, but significantly more potent (for systemic action 1.5 mg hydromorphone equals 10 mg morphine). Its analgesic efficacy is comparable to epidural fentanyl, sufentanil [Citation56] and morphine [Citation57,Citation58]. Delayed respiratory depression up to 4,5 hours after epidural drug administration at even modest dosages is reported [Citation59] but seems to have a lower incidence than with morphine. Similar to morphine, an extended release preparation is available.
Many risk factors for delayed respiratory depression, like usage of hydrophilic opioids, advanced age, morbid obesity or obstructive sleep apnea, have been identified [Citation45]. Considering these risk factors, the lowest clinically effective dose and adequate monitoring are essential for safe epidural usage of hydrophilic opioids.
4.3. Systemic response and respiratory depression – lipophilic opioids
For lipophilic opioids, the distribution to, and clearance from, the spinal cord and epidural space are rapid as compared to hydrophilic opioids, decreasing the risk of delayed respiratory depression when given epidurally [Citation42]. Indeed, lower sedation levels and less respiratory depression are reported when comparing fentanyl to morphine [Citation42,Citation60]. Buprenorphine is a lipophilic long-lasting mixed agonist-antagonist. Its specific site of action is still unclear, but seems to be predominantly supraspinal of nature [Citation61]. The use of epidural buprenorphine showed no advantages over epidural morphine [Citation62]. Naloxone-resistant respiratory depression has been reported for epidural buprenorphine [Citation63], but the incidence appears to be low.
4.4. Clinical implication
We suggest avoiding all widely used opioids that are proven neurotoxic and have no proven benefit when administered epidurally, being hydromorphone [Citation64], buprenorphine [Citation62] and tramadol [Citation29,Citation30].
Individual patient risk stratification is advised when using morphine and especially extended release morphine considering late respiratory depression. Dosing needs to be done carefully as adjustments cannot be made after administering the drug [Citation51]. Because of its long duration of action, continuous monitoring for 24–48 h is recommended. Whether the risks of (extended-release) morphine outweigh its benefits remains to be seen.
Even though multiple studies have shown no added benefit for epidural sufentanil compared to its intravenous administration [Citation65,Citation66], it remains one of the most widely used adjuvants in epidural analgesia, presumably due to the LA sparing effect and thereby reducing the adverse effects of both drugs [Citation67]. The use of epidural fentanyl and sufentanil appears safe when standard precautions for the use of opioids are taken. Because their mechanism of action is predominantly systemic [Citation43], equianalgesic doses of intravenous lipophilic opioids are similar to epidural doses [Citation65,Citation66], compared to the much lower epidural doses of hydrophilic opioids necessary for equianalgesia.
In summary, none of the opioids reviewed show ideal epidural analgesic properties without any side effects. This does, however, not mean that there is no place for opiates in epidural anesthesia. The benefits of the synergistic LA/opioid mixture which result in less side effects are well established. Using the lowest effective dose and applying adequate monitoring should minimize complications.
5. Alpha-adrenergic receptor agonists
In recent years alpha-adrenergic receptor agonists have increasingly been used for epidural analgesia. Epinephrine as an adjuvant to LA is thought to decrease the clearance and distribution of LA from the epidural space. Intravenous clonidine and dexmedetomidine are known for their analgesic properties and, when administered epidurally, are thought to have an analgesic and LA sparing effect. Clonidine can be administered systemically, epidurally or intrathecally, because alpha2-adrenoceptors are localized throughout the central nervous system. Recent studies propose a predominantly spinal site of action suggesting neuraxial administration to be preferable over systemic clonidine for its analgesic effect [Citation68,Citation69]. When clonidine is administered epidurally, lower doses of LA and opioids can be used whilst still maintaining sufficient analgesia [Citation70–Citation72]. This dose-sparing effect could limit possible complications and adverse effects associated with the use of epidural LA and/or opioids. In contrast, oral clonidine similarly decreased epidural morphine dosages without any clonidine specific side effects [Citation73]. This gives rise to the hypothesis that at least one extra pathway, apart from the spinal site of action, for clonidine’s analgesic properties exist. Oral administration of clonidine may reduce the required doses of epidurally applied drugs and medication-associated side effects to a similar extent as epidural clonidine.
5.1. Neurotoxicity
Safety concerns were initially issued regarding possible spinal cord ischemia after epidural epinephrine administration, however since then, studies in both animals and humans have debunked this [Citation74]. Neurotoxicity or spinal cord ischemia in epidural use of epinephrine seems to be highly unlikely. Ambiguity remains regarding the safety of epinephrine in patients with an already compromised spinal circulation, where epidural epinephrine may perhaps aggravate already present LA-induced neurotoxic injury [Citation74] (see ).
Table 3. Epidural alpha-adrenergic receptor agonists.
Clonidine has been thoroughly studied in both animals and humans as an epidural adjuvant. Neurotoxicity seems to be low and epidural administration safe [Citation76,Citation77]. There is no consensus regarding the epidural neurotoxicity of dexmedetomidine and it is therefore currently not approved by the FDA [Citation78].
5.2. Side effects
Common side effects of alpha-adrenergic receptor agonists include sedation, hypotension, and bradycardia. This resulted in discussions regarding its safety and the risk–benefit ratio for epidural administration. The adverse events reported for clonidine are dose dependent [Citation79]. Similar to morphine, the systemic side effects increase when clonidine is given via thoracic epidural catheter, perhaps as a reflection of rostral spread [Citation68]. Dexmedetomidine shares most of clonidine’s dose-dependent adverse effects [Citation80], but displays a significant higher incidence of bradycardia and clearly increases sedation scores [Citation81]. Pooled data did, however, not show any statistically significant increase in hypotension. Similar to clonidine less systemic opioids were needed.
5.3. Clinical implications
A recent systematic review concluded that the possible impact of adding epinephrine to epidural local anesthetics remains uncertain due to insufficient evidence. In addition, the side effects of clonidine and dexmedetomidine should prompt caution before using them as adjuvants in epidural analgesia, because even a low epidural dose of approximately 50ug/h of clonidine can cause considerable hypotension [Citation68,Citation71,Citation82] care should be taken to assess and maintain hemodynamic stability in patients receiving clonidine.
Finally, more data from large comprehensive trials assessing long-term safety and efficacy of dexmedetomidine used as an adjuvant in epidural analgesia are necessary before recommending dexmedetomidine for neuraxial use.
6. Other frequently used epidural adjuvants
6.1. Dexamethasone
In 2014 the FDA issued a warning concerning the use of epidural steroids after a series of serious neurological problems including loss of vision, stroke, paralysis, and death [Citation83,Citation84]. Dexamethasone is a water-soluble steroid known for its analgesic, anti-inflammatory and antiemetic properties [Citation85–Citation87]. It is thought that water-soluble steroids like dexamethasone are safer than the more lipid soluble steroids like triamcinolone when given epidurally [Citation88].
Multiple studies have emerged where dexamethasone has been used as an adjuvant to LA in epidural mixtures. The rationale for using dexamethasone epidurally was an assumed analgesic effect that was at least similar to other adjuvants, but with less side effects than other adjuvants like opioids or alpha-adrenergic receptor agonists [Citation89]. To date, however, no studies assessed dexamethasone as the sole adjuvant to LA and comparing it to other LA/adjuvant mixtures.
Some researchers have deemed epidural dexamethasone safe. Nevertheless, safety issues still surround the use of epidural corticosteroids in general, and dexamethasone in particular [Citation90,Citation91] (see ). For example, high (≥15 mg) doses of epidural dexamethasone were associated with transient adrenal suppression [Citation92].
Table 4. Other epidural medication.
Very little is known about neurotoxicity of dexamethasone. The potential for neurotoxicity was not actively sought in the majority of the studies performed [Citation96]. Water-soluble steroids have been implicated in seizures when given intrathecally [Citation97–Citation99] and higher doses of intrathecal dexamethasone were associated with increased inflammation of the subarachnoid space in rats [Citation100]. In vitro, neurotoxicity was suggested when combining dexamethasone with ropivacaine [Citation101].
6.2. Ketamine
Ketamine is a selective, non-competitive NMDA-receptor antagonist with known analgesic and in particular anti-hyperalgesic action. Evidence suggests that ketamine has neurotoxic properties when administered intrathecally [Citation102,Citation103]. Epidural ketamine should also be used with caution, especially when ketamine with preservatives is used. Particularly the well-known neurotoxic preservative benzethonium could worsen ketamine’s own neurotoxicity [Citation104]. The added benefits of epidural ketamine over systemic ketamine are still debated [Citation105,Citation106], even though a small potentiating effect was seen when epidural ketamine was given in combination with morphine [Citation107]. Systemic adverse reactions include psychotomimetic side effects and a mild sympathomimetic action, whereby the latter only occurs at plasma levels of >243 ± 54 ng/mL [Citation93]. Considering the above, epidural ketamine should be reserved for very specific individual cases, for example, in palliative care. Only a few human studies assessed neurological complications [Citation108] associated with the neuraxial use of ketamine and more data on its safety should be gathered.
Interestingly, ketamine had been advocated as an adjuvant for pediatric caudal anesthesia [Citation109,Citation110] based on a meta-analysis of 13 randomized controlled trials demonstrating its effectiveness [Citation111]. However, doses used in those studies make a predominantly systemic analgesic effect likely. Thus, there is no evidence suggesting superiority of epidural over systemic application. There is considerable experimental data demonstrating neurotoxicity yet only one clinical study looked at permanent neurological damage. Therefore, neuraxial use of ketamine cannot be advocated.
6.3. Magnesium
Magnesium is a NMDA-receptor antagonist, similar to ketamine. The main reason for adding magnesium to an epidural drug-mixture is to reduce side effects of epidural LA and/or opioids, whilst assuring the same level of analgesia [Citation112]. A high dose of magnesium intravenously (resulting in plasma levels >5 mmol/L) can produce flushing and hypotension that is not seen when applied via the epidural route, presumably due to a dose-dependent effect. Very high doses of magnesium resulting in plasma levels exceeding 6mmol/L could lead to hypermagnesemia, a potentially lethal condition.
Similar to ketamine, the main concern regarding neuraxial magnesium pertains to its neurotoxic potential [Citation113,Citation114]. Case reports in the obstetric population describe patients suffering from disorientation [Citation115] or burning pain [Citation116] following supra-clinical doses of magnesium accidentally given neuraxially.
6.4. Midazolam
Midazolam is a benzodiazepine acting on GABAA receptors thereby facilitating chloride influx into the cell resulting in neural inhibition. Midazolam showed some analgesic action when epidurally administered as an adjuvant to racemic bupivacaine [Citation117,Citation118]. Side effects for epidural midazolam are mainly sedation and hypotension, however, when using the lowest clinically effective dose of midazolam, those adverse events should be rare.
Neurotoxicity is a considerable concern and neurologic damage has been reported in animals when midazolam was given neuraxially [Citation119,Citation120]. Apoptosis induction is known to be mediated via the same mechanism as described for LA [Citation121]. However, the quality of some animal studies reporting on neurotoxicity of midazolam has been questioned [Citation122]. Therefore, no conclusive statement on the safety of neuraxial midazolam can be given. Worth mentioning, midazolam aggravated the neurotoxic properties of lidocaine [Citation123]. Because all LA are neurotoxic [Citation7], midazolam added to any mixture containing an LA could potentiate neurotoxicity.
6.5. Neostigmine
Neostigmine inhibits the enzyme acetylcholinesterase, thus interfering with the breakdown of acetylcholine in the synaptic cleft. Epidural neostigmine has shown, at best, doubtful efficacy. Some studies reported a dose-independent analgesic effect when combined with lidocaine [Citation124]. Adding neostigmine to epidural morphine results in a longer time to first analgesic rescue medication, albeit, without a reduction in the total opioid consumption [Citation125].
The main clinically relevant side effect for epidural neostigmine was significant hypotension [Citation126], while nausea, vomiting, and sedation were also reported [Citation127]. Epidural administration of neostigmine appears to be safe after extensive neurotoxicity research [Citation123,Citation128–Citation130].
6.6. Clinical implications
When searching for a clinically useful epidural adjuvant, safety of the drug should have been proven and the epidural administration should have a clear beneficial effect. With this in mind, all adjuvants mentioned in the latter part of our review do not meet the aforementioned criteria. Epidural dexamethasone, ketamine, and midazolam are possibly neurotoxic and the analgesic efficacy of neostigmine or dexamethasone has not been well established. Potentially neurotoxic drugs should not be given epidurally, especially when systemic administration seems to work equally well.
Other epidurally used drugs like prilocaine, calcitonin or haloperidol fall beyond the scope of this review, due to a lack of appropriate studies.
7. Conclusion
As presented above, the optimal mixture for epidural analgesia does not exist. Consequently, individual patient characteristics, safety of the chosen medication (see ), and a risk–benefit analysis should be leading in deciding which epidural medications to choose. Specific hospital safety measures should be in place before the application of epidural drugs in order to handle possible adverse events.
Table 5. Recommended safe doses* for commonly used epidural drugs in the adult non-obstetric population.
When deciding on epidural medication, patient safety is most important. Whenever medication is dispensed close to the central nervous system it poses a potential risk for neurotoxicity or other adverse effects. If questions regarding drug safety are still unanswered or high-quality long-term studies have not unequivocally shown the safety of an epidurally administered drug, they should not be used in routine clinical practice.
Where most local anesthetics and some opioids clearly have their advantages when used in an epidural mixture, most adjuvant drugs described in this review have no or minor benefits when administered epidurally compared to alternative routes (mainly systemic administration). Especially drugs without any proven benefit when applied epidurally compared to their systemic administration should be avoided. Therefore, adjuvants beyond morphine, sufentanil, fentanyl, and clonidine cannot be advocated unequivocally.
Several relevant factors other than epidural drug safety, such as doses and side effects of systemic drugs, or the impact of a chosen analgesic regimen on patient outcome, fall outside the scope of this narrative review. However, in recent years many papers worth reading have been published addressing these issues [Citation23,Citation24,Citation43,Citation144,Citation145].
8. Expert opinion
The search for new epidural medication seems never-ending and an abundance of research is dedicated to finding the optimal combination of drugs. This has resulted in an enormous amount of heterogeneous studies, which all study different mixtures in very specific groups of patients and procedures. Conclusive evidence regarding safety and efficacy is therefore difficult to interpret. There is a paucity of large randomized, multicenter, placebo-controlled studies for epidural drug usage. In particular, studies where the epidural adjuvant is compared to systemic administration are warranted. Furthermore, there are no large-scale prospective observational studies regarding the safety of frequently used epidural drugs.
In recent years regulatory oversight of clinical trials has greatly approved. It has become common practice to use multiple well-characterized animal models studying supra-clinical doses and concentrations of perineural drugs before administering them in humans. However, in rare cases, some epidural medications are still used experimentally without ensuring safety in humans. It sometimes seems minor advantages were weighed against perceivable, yet probably rare, deleterious neurologic outcomes.
Even if medication has thoroughly been tested, and no neurotoxicity has been shown in both animal and human studies, it still should be questioned whether it is necessary to administer these drugs epidurally. If a drug shows no added benefit when administered epidurally compared to intravenously, or when epidural efficacy is questioned, systemic administration should be preferred.
Our literature search focused on safety and efficacy of epidural drugs in non-pregnant adults. However, in vulnerable patient groups like pregnant women or children even more caution should be taken. The particularities of transplacental transport, teratogenic properties of drugs and increased neurotoxicity in a developing central nervous system should ensure even more precautions to be taken to avoid any possible harm.
Where in some fields, like obstetrics, the role of epidural analgesia is still widely accepted as the gold standard with regards to analgesic efficacy, the role of postsurgical epidural analgesia has somewhat diminished in recent years. Cumulative evidence supports the notion that there is no added benefit of epidural analgesia over a systemically administered pain regimen or peripheral/local nerve blocks (e.g. pre-peritoneal wound catheter [Citation146]) for analgesic purposes for a growing number of perioperative indications.
Future research should focus on the risk–benefit analysis of epidural analgesia compared to new modalities and on optimizing presently used regimens rather than on fast-tracking the search for new epidural drugs. This is, in particular, true whenever basic safety issues have not been addressed properly.
This review has focused on the safety of epidural drugs. It is however important to keep in mind that other factors should be taken into account when using epidural analgesia. For instance, primary and secondary epidural failure rates are relatively high and minimally invasive surgical techniques, opiate sparing regimens and peripheral regional anesthesia, ranging from paravertebral blocks to pre-peritoneal or pre-pleural wound catheters, offer numerous options and alternatives to new experimental epidural drugs.
Article highlights
Research regarding new epidural drugs and drug combinations is ever increasing.
Clinically safe epidural usage of drugs should be pursued, reducing possible neurotoxicity and other side effects. In vitro and in vivo data can prescreen new drugs and drug combinations before administering them to patients, but only large-scale observational prospective studies looking for short- and long-term neurological change can demonstrate clinical safety.
If there is no added benefit of administering drugs epidurally as compared to systemically, the systemic application should be preferred.
The incidence of serious complications seems to be low, but the consequences can be devastating.
Future research should focus on providing solid evidence regarding efficacy of epidural analgesia compared to new and existing modalities and optimizing established regimens.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Mrs. Faridi S. van Etten-Jamaludin, Clinical Librarian from the Medical Library of the Amsterdam UMC, location AMC for her assistance with building our search strategy.
Additional information
Funding
References
- Rosero EB, Joshi GP. Nationwide incidence of serious complications of epidural analgesia in the United States. Acta Anaesthesiol Scand. 2016 Jul;60(6):810–820.
- Shah J, Votta-Velis EG, Borgeat A. New local anesthetics. Best Pract Res Clin Anaesth. 2018 Jun;32(2):179–185.
- Groban L, Dolinski SY. Differences in cardiac toxicity among ropivacaine, levobupivacaine, bupivacaine, and lidocaine. Tech Reg Anesthesia Pain Manage. 2001;5:48–55.
- Finucane BT, Ban C.H. Tsui. (Eds.). Complications of regional anesthesia principles of safe practice in local and regional anesthesia. Cham, Switzerland: Springer. Softcover reprint of the original 3rd ed 2017 edition 2017:44.
- Casati A, Putzu M. Bupivacaine, levobupivacaine and ropivacaine: are they clinically different? Best Pract Res Clin Anaesth. 2005;19(2):247–268.
- Verlinde M, Hollmann MW, Stevens MF, et al. Local anesthetic-induced neurotoxicity. Int J Mol Sci. 2016;17(3):339.
- Werdehausen R, Fazeli S, Braun S, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009 Nov;103(5):711–718.
- Sakura S, Bollen AW, Ciriales R, et al. Local anesthetic neurotoxicity does not result from blockade of voltage-gated sodium channels. Anesth Analg. 1995 Aug;81(2):338–346.
- Zaric D, Christiansen C, Pace NL, et al. Transient neurologic symptoms after spinal anesthesia with lidocaine versus other local anesthetics: a systematic review of randomized, controlled trials. Anesth Analg. 2005 Jun;100(6):1811–1816.
- Wong CA, Benzon H, Kim C. Bilateral radicular pain after epidural lidocaine. Reg Anesthesia. 1996 Nov-Dec;21(6):600–601.
- Lam SH, Majlesi N, Vilke GM. Use of intravenous fat emulsion in the emergency department for the critically ill poisoned patient. J Emerg Med. 2016 Aug;51(2):203–214.
- Heinonen JA, Litonius E, Salmi T, et al. Intravenous lipid emulsion given to volunteers does not affect symptoms of lidocaine brain toxicity. Basic Clin Pharmacol Toxicol. 2015;116(4):378–383.
- Harrison DC, Sprouse JH, Morrow AG. The antiarrhythmic properties of lidocaine and procaine amide: clinical and physiologic studies of their cardiovascular effects in man. Circulation. 1963;28(4):486–491.
- Weibel S, Jokinen J, Pace NL, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth. 2016;116(6):770–783.
- Wolfe JW, Butterworth JF. Local anesthetic systemic toxicity: update on mechanisms and treatment. Curr Opin Anaesthesiol. 2011;24(5):561–566.
- Brown DL, Ransom DM, Hall JA, et al. Regional anesthesia and local anesthetic-induced systemic toxicity: seizure frequency and accompanying cardiovascular changes. Anesth Analg. 1995;81(2):321–328.
- Knudsen K, Beckman Suurkula M, Blomberg S, et al. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997 May;78(5):507–514.
- Scott DB, Lee A, Fagan D, et al. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg. 1989 Nov;69(5):563–569.
- Christie LE, Picard J, Weinberg GL. Local anaesthetic systemic toxicity. BJA Educ. 2015;15(3):136–142.
- Abouleish EI, Elias M, Nelson C. Ropivacaine-induced seizure after extradural anaesthesia. Br J Anaesth. 1998 Jun;80(6):843–844.
- Jiang X, Huang W, Lin X. Ropivacaine-induced cardiac arrest and paraplegia after epidural anesthesia. Minerva Anestesiol. 2012 Nov;78(11):1309–1310.
- Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia - a meta-analysis. Jama. 2003 Nov 12;290(18):2455–2463.
- Pöpping DM, Elia N, Van Aken HK, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259(6):1056–1067.
- Guay J, Kopp S. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. 2016 Jan;5(1):Cd005059.
- Wright AW, Nocente ML, Smith MT. Hydromorphone-3-glucuronide: biochemical synthesis and preliminary pharmacological evaluation. Life Sci. 1998;63(5):401–411.
- Kokki M, Pesonen M, Vehviläinen P, et al. Cytotoxicity of oxycodone and morphine in human neuroblastoma and mouse motoneuronal cells: a comparative approach. Drugs R D. 2016;16(2):155–163.
- Rawal N, Nuutinen L, Raj PP, et al. Behavioral and histopathologic effects following intrathecal administration of butorphanol, sufentanil, and nalbuphine in sheep. Anesthesiology. 1991 Dec;75(6):1025–1034.
- Kugawa F, Arae K, Ueno A, et al. Buprenorphine hydrochloride induces apoptosis in NG108-15 nerve cells. Eur J Pharmacol. 1998 Apr 17;347(1):105–112.
- Lagard C, Chevillard L, Malissin I, et al. Mechanisms of tramadol-related neurotoxicity in the rat: does diazepam/tramadol combination play a worsening role in overdose? Toxicol Appl Pharmacol. 2016 Nov 1;310:108–119.
- Raffa RB, Friderichs E, Reimann W, et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992 Jan;260(1):275–285.
- Atici S, Cinel L, Cinel I, et al. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004 Aug;114(8):1001–1011.
- Pestean C, Taulescu M, Ober C, et al. The effect of chronic toxicity of pethidine on the spinal cord: an experimental model in rabbits. Rom J Morphol Embryol. 2013;54(3):617–622.
- Abut YC, Turkmen AZ, Midi A, et al. Neurotoxic effects of levobupivacaine and fentanyl on rat spinal cord. Braz J Anesth (English Edition). 2015 Jan;65(1):27–33.
- Perez-Alvarez S, Cuenca-Lopez MD, de Mera RM, et al. Methadone induces necrotic-like cell death in SH-SY5Y cells by an impairment of mitochondrial ATP synthesis. Biochim Biophys Acta. 2010 Nov;1802(11):1036–1047.
- Kofke WA, Garman RH, Stiller RL, et al. Opioid neurotoxicity: fentanyl dose-response effects in rats. Anesth Analg. 1996 Dec;83(6):1298–1306.
- Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. I. Mu and delta receptor profiles in the primate. J Pharmacol Exp Ther. 1983 Aug;226(2):303–316.
- Angst MS, Drover DR. Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet. 2006;45(12):1153–1176.
- Youssef N, Orlov D, Alie T, et al. What epidural opioid results in the best analgesia outcomes and fewest side effects after surgery? Meta-Anal Randomized Controlled Trials. 2014;119(4):965–977.
- Liu SS, Bieltz M, Wukovits B, et al. Prospective survey of patient-controlled epidural analgesia with bupivacaine and hydromorphone in 3736 postoperative orthopedic patients. Reg Anesth Pain Med. 2010;35(4):351–354.
- Donadoni R, Rolly G, Noorduin H, et al. Epidural sufentanil for postoperative pain relief. Anaesthesia. 1985 Jul;40(7):634–638.
- Jannuzzi RG. Nalbuphine for treatment of opioid-induced pruritus: a systematic review of literature. Clin J Pain. 2016;32(1):87–93.
- Congedo E, Sgreccia M, De Cosmo G. New drugs for epidural analgesia. Curr Drug Targets. 2009;10(8):696–706.
- Bernards CM, Shen DD, Sterling ES, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 1): differences among opioids. Anesthesiology. 2003;99:455–465.
- McCaughey W, Graham JL. The respiratory depression of epidural morphine. Time course and effect of posture. Anaesthesia. 1982 Oct;37(10):990–995.
- Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71(14):1807–1819.
- Shapiro A, Zohar E, Zaslansky R, et al. The frequency and timing of respiratory depression in 1524 postoperative patients treated with systemic or neuraxial morphine. J Clin Anaesth. 2005;17(7):537–542.
- Gambling D, Hughes T, Martin G, et al. A comparison of Depodur, a novel, single-dose extended-release epidural morphine, with standard epidural morphine for pain relief after lower abdominal surgery. Anesth Analg. 2005 Apr;100(4):1065–1074.
- Viscusi ER. Emerging techniques in the management of acute pain: epidural analgesia. Anesth Analg. 2005 Nov;101(5 Suppl):S23–9.
- Sumida S, Lesley MR, Hanna MN, et al. Meta-analysis of the effect of extended-release epidural morphine versus intravenous patient-controlled analgesia on respiratory depression. J Opioid Manag. 2009 Sep-Oct;5(5):301–305.
- Vaughan C. Development and implementation of a process to ensure safe use of morphine sulfate extended-release liposome injection. Am J Health-System Pharm. 2008 Mar 1;65(5):458–461.
- Hartrick CT, Hartrick KA. Extended-release epidural morphine (DepoDur): review and safety analysis. Expert Rev Neurother. 2008;8(11):1641–1648.
- Hartrick CT, Martin G, Kantor G, et al. Evaluation of a single-dose, extended-release epidural morphine formulation for pain after knee arthroplasty. J Bone Joint Surg Am Vol. 2006 Feb;88(2):273–281.
- Alam M, Hartrick CT. Extended-release epidural morphine (DepoDur): an old drug with a new profile. Pain Pract. 2005 Dec;5(4):349–353.
- Drugs.com. DepoDur Information from Drugs.com [Internet]. 2007 [ medically reviewed 2019 Jan 1; cited 2019 Jan]. Available from: https://wwwdrugscom/pro/depodurhtml
- Gambling DR, Hughes TL, Manvelian GZ. Extended-release epidural morphine (DepoDur) following epidural bupivacaine in patients undergoing lower abdominal surgery: a randomized controlled pharmacokinetic study. Reg Anesth Pain Med. 2009;34(4):316–325.
- Parker EO, Brookshire GL, Bartel SJ, et al. Effects of epinephrine on epidural fentanyl, sufentanil and hydromorphone for postoperative pain. Anesthesiology. 1985;63(3A):A235.
- Chaplan SR, Duncan SR, Brodsky JB, et al. Morphine and hydromorphone epidural analgesia. A prospective, randomized comparison. Anesthesiology. 1992 Dec;77(6):1090–1094.
- Drakeford MK, Pettine KA, Brookshire L, et al. Spinal narcotics for postoperative analgesia in total joint arthroplasty. A prospective study. J Bone Joint Surg Am Vol. 1991 Mar;73(3):424–428.
- Wust HJ, Bromage PR. Delayed respiratory arrest after epidural hydromorphone. Anaesthesia. 1987 Apr;42(4):404–406.
- Bujedo BM, Santos SG, Azpiazu AU. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag. 2012;8(3):177–192.
- Inagaki Y, Mashimo T, Yoshiya I. Mode and site of analgesic action of epidural buprenorphine in humans. Anesth Analg. 1996 Sep;83(3):530–536.
- Wolff J, Carl P, Crawford ME. Epidural buprenorphine for postoperative analgesia. A controlled comparison with epidural morphine. Anaesthesia. 1986 Jan;41(1):76–79.
- Knape JT. Early respiratory depression resistant to naloxone following epidural buprenorphine. Anesthesiology. 1986 Mar;64(3):382–384.
- Liu S, Carpenter RL, Mulroy MF, et al. Intravenous versus epidural administration of hydromorphone. Effects on analgesia and recovery after radical retropubic prostatectomy. Anesthesiology. 1995 Mar;82(3):682–688.
- Geller E, Chrubasik J, Graf R, et al. A randomized double-blind comparison of epidural sufentanil versus intravenous sufentanil or epidural fentanyl analgesia after major abdominal surgery. Anesth Analg. 1993 Jun;76(6):1243–1250.
- Swenson JD, Hullander RM, Bready RJ, et al. A comparison of patient controlled epidural analgesia with sufentanil by the lumbar versus thoracic route after thoracotomy. Anesth Analg. 1994 Feb;78(2):215–218.
- Hubler M, Litz RJ, Sengebusch KH, et al. A comparison of five solutions of local anaesthetics and/or sufentanil for continuous, postoperative epidural analgesia after major urological surgery. Eur J Anaesthesiol. 2001 Jul;18(7):450–457.
- Eisenach JC, De Kock M, Klimscha W. alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). Anesthesiology. 1996 Sep;85(3):655–674.
- Bernard J-M. Comparison of intravenous and epidural clonidine for postoperative patient-controlled analgesia. Anesth Analg. 1995;81(4):706–712.
- Rockemann MG, Seeling W, Brinkmann A, et al. Analgesic and hemodynamic effects of epidural clonidine, clonidine/ morphine,and morphine after pancreatic surgery – a double-blind study. Anesth Analg. 1995 May;80(5):869–874.
- Milligan KR, Convery PN, Weir P, et al. The efficacy and safety of epidural infusions of levobupivacaine with and without clonidine for postoperative pain relief in patients undergoing total hip replacement. Anesth Analg. 2000 Aug;91(2):393–397.
- Farmery AD, Wilson-MacDonald J. The analgesic effect of epidural clonidine after spinal surgery: a randomized placebo-controlled trial. Anesth Analg. 2009;108(2):631–634.
- Goyagi T, Tanaka M, Nishikawa T. Oral clonidine premedication enhances postoperative analgesia by epidural morphine. Anesth Analg. 1999 Dec;89(6):1487–1491.
- Neal JM. Effects of epinephrine in local anesthetics on the central and peripheral nervous systems: neurotoxicity and neural blood flow. Reg Anesth Pain Med. 2003 Mar-Apr;28(2):124–134.
- Hetta DF, Fares KM, Abedalmohsen AM, et al. Epidural dexmedetomidine infusion for perioperative analgesia in patients undergoing abdominal cancer surgery: randomized trial. J Pain Res. 2018;11:2675–2685.
- Tamsen A, Gordh T. Clonidine is not neurotoxic. Lancet. 1984 Oct 13;2(8407):876.
- Yaksh TL, Rathbun M, Jage J, et al. Pharmacology and toxicology of chronically infused epidural clonidine.HCl in dogs. Fundam Appl Toxicol. 1994 Oct;23(3):319–335.
- Konakci S, Adanir T, Yilmaz G, et al. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25(5):403–409.
- De Kock M, Wiederkher P, Laghmiche A, et al. Epidural clonidine used as the sole analgesic agent during and after abdominal surgery. A dose-response study. Anesthesiology. 1997 Feb;86(2):285–292.
- Bajwa SJS, Bajwa SK, Kaur J, et al. Dexmedetomidine and clonidine in epidural anaesthesia: a comparative evaluation. Indian J Anaesth. 2011;55(2):116–121.
- Zhang X, Wang D, Shi M, et al. Efficacy and safety of dexmedetomidine as an adjuvant in epidural analgesia and anesthesia: a systematic review and meta-analysis of randomized controlled trials. Clin Drug Investig. 2017;37(4):343–354.
- Dobrydnjov I, Axelsson K, Gupta A, et al. Improved analgesia with clonidine when added to local anesthetic during combined spinal-epidural anesthesia for hip arthroplasty: a double-blind, randomized and placebo-controlled study. Acta Anaesthesiol Scand. 2005 Apr;49(4):538–545.
- FDA Drug Safety Communication I. FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. [cited 2019 Jan 25]. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM394286.pdf
- Dietrich TJ, Sutter R, Froehlich JM, et al. Particulate versus non-particulate steroids for lumbar transforaminal or interlaminar epidural steroid injections: an update. Skeletal Radiol. 2015 Feb 01;44(2):149–155.
- Fan Z, Ma J, Kuang M, et al. The efficacy of dexamethasone reducing postoperative pain and emesis after total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2018;52:149–155.
- Waldron NH, Jones CA, Gan TJ, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013 Feb;110(2):191–200.
- Allen TK, Jones CA, Habib AS. Dexamethasone for the prophylaxis of postoperative nausea and vomiting associated with neuraxial morphine administration: a systematic review and meta-analysis. Anesth Analg. 2012 Apr;114(4):813–822.
- Derby R, Lee SH, Date ES, et al. Size and aggregation of corticosteroids used for epidural injections. Pain Med. 2008 Mar;9(2):227–234.
- Khafagy HF, Refaat AI, El-Sabae HH, et al. Efficacy of epidural dexamethasone versus fentanyl on postoperative analgesia. J Anesth. 2010 Aug 01;24(4):531–536.
- Naghipour B, Aghamohamadi D, Azarfarin R, et al. Dexamethasone added to bupivacaine prolongs duration of epidural analgesia. Middle East J Anaesthesiol. 2013 Feb;22(1):53–57.
- Kim D, Brown J. Efficacy and safety of lumbar epidural dexamethasone versus methylprednisolone in the treatment of lumbar radiculopathy: a comparison of soluble versus particulate steroids. Clin J Pain. 2011;27(6):518–522.
- Maillefert JF, Aho S, Huguenin MC, et al. Systemic effects of epidural dexamethasone injections. Revue Du Rhumatisme (English Ed). 1995 Jun;62(6):429–432.
- Olofsen E, Sigtermans M, Noppers I, et al. The dose-dependent effect of S(+)-ketamine on cardiac output in healthy volunteers and complex regional pain syndrome type 1 chronic pain patients. Anesth Analg. 2012 Sep;115(3):536–546.
- Subramaniam B, Subramaniam K, Pawar DK, et al. Preoperative epidural ketamine in combination with morphine does not have a clinically relevant intra- and postoperative opioid-sparing effect. Anesth Analg. 2001;93(5):1321–1326.
- Daabiss MA, Kandil A. Evaluation of the effect of magnesium vs. midazolam as adjunct to epidural bupivacaine in patients undergoing total knee replacement. Br J Med Pract. 2013 Jan 1;6(2):a610.
- Jebaraj B, Khanna P, Baidya DK, et al. Efficacy of epidural local anesthetic and dexamethasone in providing postoperative analgesia: ameta-analysis. Saudi J Anaesth. 2016 Jul-Sep;10(3):322–327.
- Abram SE. Treatment of lumbosacral radiculopathy with epidural steroids. Anesthesiology. 1999;91(6):1937.
- Ildirim I, Furcolow ML, Vandiviere HM. A possible explanation of posttreatment convulsions associated with intrathecal corticosteroids. Neurology. 1970 Jun;20(6):622–625.
- Oppelt WW, Rall DP. Production of convulsions in the dog with intrathecal corticosteroids. Neurology. 1961 Oct;11:925–927.
- Kroin JS, Schaefer RB, Penn RD. Chronic intrathecal administration of dexamethasone sodium phosphate: pharmacokinetics and neurotoxicity in an animal model. Neurosurgery. 2000 Jan;46(1):178–182. discussion 82-3.
- Williams BA, Hough KA, Tsui BY, et al. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011 May-Jun;36(3):225–230.
- Vranken JH, Troost D, Wegener JT, et al. Neuropathological findings after continuous intrathecal administration of S(+)-ketamine for the management of neuropathic cancer pain. Pain. 2005 Sep;117(1–2):231–235.
- Vranken JH, Troost D, de Haan P, et al. Severe toxic damage to the rabbit spinal cord after intrathecal administration of preservative-free S(+)-ketamine. Anesthesiology. 2006 Oct;105(4):813–818.
- Yip KW, Mao X, Au PY, et al. Benzethonium chloride: a novel anticancer agent identified by using a cell-based small-molecule screen. Clin Cancer Res off J Am Assoc Cancer Res. 2006 Sep 15;12(18):5557–5569.
- Tena B, Gomar C, Rios J. Perioperative epidural or intravenous ketamine does not improve the effectiveness of thoracic epidural analgesia for acute and chronic pain after thoracotomy. Clin J Pain. 2014 Jun;30(6):490–500.
- Xie H, Wang X, Liu G, et al. Analgesic effects and pharmacokinetics of a low dose of ketamine preoperatively administered epidurally or intravenously. Clin J Pain. 2003 Sep-Oct;19(5):317–322.
- Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004 Aug;99(2):482–495. table of contents.
- Albrecht E, Kirkham KR, Liu SS, et al. The analgesic efficacy and safety of neuraxial magnesium sulphate: a quantitative review. Anaesthesia. 2013 Feb;68(2):190–202.
- Lundblad M, Lonnqvist PA. Adjunct analgesic drugs to local anaesthetics for neuroaxial blocks in children. Curr Opin Anaesthesiol. 2016 Oct;29(5):626–631.
- Marhofer P, Krenn CG, Plochl W, et al. S(+)-ketamine for caudal block in paediatric anaesthesia. Br J Anaesth. 2000 Mar;84(3):341–345.
- Schnabel A, Poepping DM, Kranke P, et al. Efficacy and adverse effects of ketamine as an additive for paediatric caudal anaesthesia: a quantitative systematic review of randomized controlled trials. Br J Anaesth. 2011 Oct;107(4):601–611.
- Kroin JS, McCarthy RJ, Von Roenn N, et al. Magnesium sulfate potentiates morphine antinociception at the spinal level. Anesth Analg. 2000 Apr;90(4):913–917.
- Ozdogan L, Sastim H, Ornek D, et al. Neurotoxic effects of intrathecal magnesium sulphate. Braz J Anesthesiol (Elsevier). 2013 Jan-Feb;63(1):139–143.
- Saeki H, Matsumoto M, Kaneko S, et al. Is intrathecal magnesium sulfate safe and protective against ischemic spinal cord injury in rabbits? Anesth Analg. 2004 Dec;99(6):1805–1812. table of contents.
- Goodman EJ, Haas AJ, Kantor GS. Inadvertent administration of magnesium sulfate through the epidural catheter: report and analysis of a drug error. Int J Obstet Anesth. 2006 Jan;15(1):63–67.
- Dror A, Henriksen E. Accidental epidural magnesium sulfate injection. Anesth Analg. 1987 Oct;66(10):1020–1021.
- Nishiyama T, Matsukawa T, Hanaoka K. Continuous epidural administration of midazolam and bupivacaine for postoperative analgesia. Acta Anaesthesiol Scand. 1999 May;43(5):568–572.
- Nishiyama T, Matsukawa T, Hanaoka K. Effects of adding midazolam on the postoperative epidural analgesia with two different doses of bupivacaine. J Clin Anesth. 2002 Mar;14(2):92–97.
- Bozkurt P, Tunali Y, Kaya G, et al. Histological changes following epidural injection of midazolam in the neonatal rabbit. Paediatr Anaesth. 1997;7(5):385–389.
- Ugur B, Basaloglu K, Yurtseven T, et al. Neurotoxicity with single dose intrathecal midazolam administration. Eur J Anaesthesiol. 2005 Dec;22(12):907–912.
- Stevens MF, Werdehausen R, Gaza N, et al. Midazolam activates the intrinsic pathway of apoptosis independent of benzodiazepine and death receptor signaling. Reg Anesth Pain Med. 2011 Jul-Aug;36(4):343–349.
- Yaksh TL, Allen JW. Preclinical insights into the implementation of intrathecal midazolam: a cautionary tale. Anesth Analg. 2004 Jun;98(6):1509–1511.
- Werdehausen R, Braun S, Hermanns H, et al. The influence of adjuvants used in regional anesthesia on lidocaine-induced neurotoxicity in vitro. Reg Anesth Pain Med. 2011 Sep-Oct;36(5):436–443.
- Lauretti GR, de Oliveira R, Reis MP, et al. Study of three different doses of epidural neostigmine coadministered with lidocaine for postoperative analgesia. Anesthesiology. 1999 Jun;90(6):1534–1538.
- Omais M, Lauretti GR, Paccola CA. Epidural morphine and neostigmine for postoperative analgesia after orthopedic surgery. Anesth Analg. 2002 Dec;95(6):1698–1701. table of contents.
- Lauretti GR. The evolution of spinal/epidural neostigmine in clinical application: thoughts after two decades. Saudi J Anaesth. 2015 Jan;9(1):71–81.
- Hood DD, Eisenach JC, Tuttle R. Phase I safety assessment of intrathecal neostigmine methylsulfate in humans. Anesthesiology. 1995 Feb;82(2):331–343.
- Hood DD, Eisenach JC, Tong C, et al. Cardiorespiratory and spinal cord blood flow effects of intrathecal neostigmine methylsulfate, clonidine, and their combination in sheep. Anesthesiology. 1995;82(2):428–435.
- Yaksh TL, Grafe MR, Malkmus S, et al. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology. 1995 Feb;82(2):412–427.
- Gurun MS, Leinbach R, Moore L, et al. Studies on the safety of glucose and paraben-containing neostigmine for intrathecal administration. Anesth Analg. 1997 Aug;85(2):317–323.
- farmacotherapeutischkompas.nl. Lidocaine Information from farmacotherapeutischkompas.nl [Internet]. [ cited 2019 Mar]. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/l/lidocaine__parenteraal_
- medicines.org.uk. Bupivacaine Information from medicines.org.uk [Internet]. [ cited 2019 Mar]. Available from: https://www.medicines.org.uk/emc/product/6591/smpc
- farmacotherapeutischkompas.nl. Bupivacaine Information from farmacotherapeutischkompas.nl [Internet]. [ cited 2019 Mar]. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/b/bupivacaine
- farmacotherapeutischkompas.nl. Levobupivacaine Information from farmacotherapeutischkompas.nl [Internet]. [ cited 2019 Mar]. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/l/levobupivacaine
- medicines.org.uk. Levobupivacaine Information from medicines.org.uk [Internet]. [ cited 2019 Mar]. Available from: https://www.medicines.org.uk/emc/product/329/smpc
- medicines.org.uk. Ropivacaine Information from medicines.org.uk [Internet]. [ cited 2019 Mar]. Available from: https://www.medicines.org.uk/emc/product/9686/smpc
- farmacotherapeutischkompas.nl. Ropivacaine Information from farmacotherapeutischkompas.nl [Internet]. [ cited 2019 Mar]. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/r/ropivacaine
- medicines.org.uk. Lidocaine Information from medicines.org.uk [Internet]. [ cited 2019 Mar]. Available from: https://www.medicines.org.uk/emc/product/6277/smpc
- Smith H. Current Therapy in Pain. 1st ed. Philadelphia: Saunders; 2008:79.
- Mosby. Mosby’s drug reference for health professions. 6th ed. Amsterdam: Elsevier - Health Sciences Division; 2017:1097.
- Mosby. Mosby’s drug reference for health professions. 6th ed. Amsterdam: Elsevier - Health Sciences Division; 2017:643.
- Mosby. Mosby’s drug reference for health professions – e-book. 4th ed. Amsterdam: Elsevier - Health Sciences Division; 2013:782.
- Drugs.com. Clonidine Information from Drugs.com [Internet]. 2007 [medically reviewed 2019 Jan 4; cited 2019 Mar]. Available from: https://www.drugs.com/dosage/clonidine.html
- Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001 Jul;87(1):62–72.
- Bernards CM. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (Part 2): effect of epinephrine. Anesthesiology. 2003;99:466–475.
- Mungroop TH, Veelo DP, Busch OR, et al. Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): a randomised controlled, open-label, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016 Oct;1(2):105–113.