ABSTRACT
Objectives: Bazedoxifene was found to be effective and well tolerated for the treatment and prevention of osteoporosis in postmenopausal women. This post-marketing surveillance study (PMSS) examined the safety of bazedoxifene in postmenopausal Korean women with osteoporosis, in a real-world setting.
Methods: This PMSS was conducted from 2013 to 2017. A total of 3,423 subjects from 68 centers were enrolled and monitored for about 3 months (± 2 weeks). Bazedoxifene was prescribed at a dose of 20 mg/day. The safety of bazedoxifene was evaluated based on the number and nature of adverse events (AEs), serious AEs (SAEs), adverse drug reactions (ADRs) and serious ADRs (SADRs) in routine medical practice.
Results: The mean age of study subjects was 69.51 years. The incidence of AEs and ADRs was 6.11% and 3.86%, respectively, and significantly decreased with increasing age (p= 0.0007). AE and ADR rates with bazedoxifene treatment of 3 months or more were significantly lower than those of less than 3 months (AE, 3.64% vs 30.00%, p < 0.0001; ADR, 1.74% vs 24.38%, p < 0.0001).
Conclusion: In this study, bazedoxifene was well tolerated in the management of postmenopausal osteoporosis in Korean women, including those aged 70 years or more.
1. Introduction
Osteoporosis, the most common skeletal disorder, is characterized by decreased bone mass and bone strength as well as deterioration of the bone microarchitecture that collectively lead to an increased risk of fracture [Citation1,Citation2]. World Health Organization (WHO) defined osteoporosis as the condition in which bone mineral density (BMD) is more than 2.5 standard deviations below the young adult mean. Presently, osteoporosis affects more than 200 million people worldwide [Citation3,Citation4]. According to the 2010 Fifth Korean National Health and Nutrition Examination Survey data, 32.3% and 49.9% of female respondents suffer from osteoporosis and osteopenia, respectively. Especially, 37.7% of female respondents are at high-risk for osteoporotic fracture [Citation5].
Bazedoxifene acetate is a novel, chemically distinct selective estrogen receptor modulator (SERM) developed for the prevention and treatment of osteoporosis in postmenopausal women [Citation6,Citation7]. Pivotal studies conducted with bazedoxifene suggested that it is effective and well tolerated for the treatment and prevention of osteoporosis in postmenopausal women [Citation7–Citation9]. However, to our knowledge, no large-scale clinical study has been performed for bazedoxifene in the Asian population.
Bazedoxifene acetate was approved as a new drug on 16 November 2011 in Korea. For the approval of bazedoxifene in Korea by the Ministry of Food and Drug Safety (MFDS), safety information of bazedoxifene must be gathered from the routine medical practice setting during the re-examination period of 6 years from the approval date.
Therefore, the purpose of this post-marketing surveillance study (PMSS) is to identify the safety profile of bazedoxifene in postmenopausal Korean women with osteoporosis, in a real-world setting. Furthermore, we seek to identify factors that may have influence on the safety profile of bazedoxifene in these patients.
2. Patients and methods
2.1. Study population
Women for whom the treatment and prevention of postmenopausal osteoporosis were required were eligible for enrollment in this study. Subjects were excluded if they had hypersensitivity to the active substance or any of the excipients, had an active or past history of venous thromboembolic events including deep vein thrombosis, pulmonary embolism and retinal vein thrombosis, or unexplained uterine bleeding. In addition, women with signs or symptoms of endometrial cancer, rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption were excluded from this study.
In accordance with the ethical principles outlined in the International Conference on Harmonization Good Clinical Practice (ICH-GCP) guidelines, all subjects (or legally acceptable representatives) have been informed of all pertinent aspects of the study and signed a written informed consent before study enrollment. The informed consent form and study protocol (including any amendments) were submitted to the institutional review board (IRB) of each participating center for review and written approvals were obtained.
2.2. Study design
This non-interventional, multi-center, PMSS was conducted from 24 October 2013 to 31 May 2017 according to the routine medical practice of accredited physicians in primary, secondary, and tertiary care medical centers in Korea. A total of 70 investigators from 68 sites participated. Bazedoxifene was prescribed at a dose of 20 mg per day. Subjects were enrolled by continuous registration method and monitored for 3 months (± 2 weeks) if they had been continuing the treatment, or for 1 month if they had stopped taking the drug before 3 months (± 2 weeks) period (≥ 3 months was 90.7%). This monitoring duration was determined according to the pivotal study of bazedoxifene and as adverse events to SERM mostly occur within 1 month of treatment, the 3-month monitoring was deemed as sufficiently long period and was accepted by MFDS.
2.3. Drug information
Bazedoxifene is approved in Korea with indication for the treatment and prevention of osteoporosis in postmenopausal women. It has been shown in previous clinical studies to reduce the incidence of vertebral fractures [Citation8,Citation9] and should be taken once daily at 20 mg dose, irrespective of food intake [Citation10]. Patients with renal or hepatic impairment are advised to use the drug with caution, although dose adjustment for patients with mild to moderate renal impairment is not necessary. As with other SERMs, bazedoxifene is known to increase the risk of intravenous thrombosis, and the risk is suggested to be the highest within the first month of initiation of treatment [Citation11]. The most common adverse events reported in clinical studies are hot flushes and leg cramps. Other common adverse events include hypersensitivity reactions, dry mouth, drowsiness, urticaria, rash, pruritus, and elevated serum triglycerides and liver enzymes [Citation9].
2.4. Assessment of safety
The safety of bazedoxifene was evaluated based on the number and nature of adverse event (AE), serious adverse events (SAE), adverse drug reactions (ADR) and serious adverse drug reactions (SADR). The study subjects were treated in routine clinical practice at the discretion of the treating investigator according to the label approved by MFDS and performed with the Korean New Drug Re-Examination Guidelines. All safety parameters were collected if available or measured as part of routine clinical practice.
The investigators were required to assess and record the causal relationship of any AE with bazedoxifene. For all AEs, sufficient information should be obtained by the investigator to adequately determine the causality. Causality of AE to the study drug was allocated by the physician according to the following criteria: 1) certain; 2) probable/likely; 3) possible; 4) unlikely; 5) conditional/unclassified; and if the information is insufficient or conflicting and the causal relationship cannot be determined and cannot be supplemented or confirmed, was classified to 6) unassessible/unclassifiable. All AEs, except for ‘unlikely’, were considered as an AE whose causal relationship to the study drug cannot be excluded (hereinafter ‘ADR’).
We defined SAE and SADR as any untoward medical occurrence in a patient administered a medicinal product at any dose that: 1) results in death; 2) is life-threatening; 3) requires inpatient hospitalization or prolongation of hospitalization; 4) results in persistent or significant disability/incapacity (substantial disruption of the ability to conduct normal life functions); 5) results in congenital anomaly/birth defect; or 6) is an important medical event.
Severity of AEs and ADRs was evaluated and categorized as mild (not having caused any significant problem to the subject), moderate (caused a problem that does not interfere significantly with the subject’s usual daily activities or clinical status), or severe (caused a problem that interferes significantly with the subject’s usual daily activities or clinical status).
2.5. Statistical analysis
Descriptive analysis was performed on the data set which consists of AE cases, safety evaluable cases, discontinuation cases and the causalities collected during the study. Data were expressed as mean ± standard deviation (SD) for continuous variables and number (N) and percentage (%) for categorical variables.
Incidences of safety parameters (AE, ADR, SAE, SADR) were categorized according to the duration of administration (<3 months and ≥3 months), physical organ and disease and symptoms (System Organ Class, SOC). Also, differences in the incidence rates of AE and ADR by age groups (<50, 50–59, 60–69, 70–79, 80–89 and ≥90 years old) were evaluated. The p-values were calculated from Chi-squared test (X2-test) and Fisher`s exact test.
All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided, and the statistical significance was determined as p-value < 0.05.
3. Results
A total of 3,430 subjects were enrolled in this study. Among them, 7 subjects were excluded (1 subject did not receive bazedoxifene, 1 subject was lost to follow up, 5 subjects had inclusion and/or exclusion criteria violations) and the remaining 3,423 subjects were included for safety analysis. The mean (± SD) age of the subjects was 69.51 (± 10.03) years and more than half of the subjects were aged 70 years or older ().
Table 1. Baseline characteristics of subjects in this study.
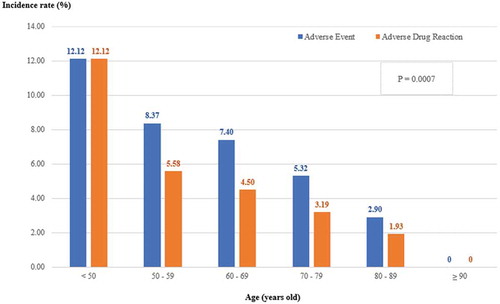
Overall AE and ADR rates were 6.11% and 3.86%, respectively. Serious AE and Serious ADR occurred in 0.50% and 0.09% of the total group (). AE and ADR rates in subjects who continued the treatment with bazedoxifene for a period of 3 months or longer were found to be significantly lower than those who were treated of the drug for less than 3 months () (AE, 3.64% vs 30.00%, p < 0.0001; ADR, 1.74% vs 24.38%, p < 0.0001). When compared between different age groups, incidence rates of AEs and ADRs decreased significantly with increasing age (p = 0.0007) ().
Table 2. Incidence of adverse events (AEs) and adverse drug reaction (ADR) by duration of administration.
shows the summary of AE, SAE, ADR, and SADR as classified by SOC. Overall, the incidence rate of AEs in this study was 6.11% (comprising 209 subjects and 261 events). Most of the AEs were graded as ‘mild’ (86.21%) and ‘moderate’ (11.49%) in severity. The incidence rate of SAEs was 0.50% (17 subjects and 19 events) ().
Table 3. A summary of adverse events (AE), serious AE (SAE), adverse drug reaction (ADR) and serious ADR (SARD) by system organ class (SOC).
Of the 261 AEs reported, bazedoxifene was discontinued permanently in 129 (49.43%) cases, temporarily discontinued in 11 (4.21%) cases, while no further action was taken in the remaining 119 (45.59%) cases, and not applicable in 2 (0.77%) cases. The mean duration of temporary drug discontinuation was 15.64 days, when 2 patients’ discontinuation dates, which were not precisely known, were counted at their minimum possible discontinuation duration. Fifty-three subjects reported on having pre-existing liver and biliary system disorder and 32 subjects reported on having renal disorder at enrollment. AEs were reported from six subjects (11.32%) with liver disorder which included arthralgia, dyspepsia, and hepatocellular damage. Three subjects (9.38%) with renal disorder reported on pharyngitis, nausea, asthenia, and laceration as AE. When compared to the other sub-populations without liver and renal disorders, the incidence rates of AE were not significantly different (p = 0.1359, p = 0.4421) (date not shown). Thirty-three (1.49%) subjects have been taking drugs that affect bone metabolism and 15 (0.68%) subjects have been taking estrogen, progesterone or their related agents. Among the subjects taking drugs that affect bone metabolism, six (18.18%) patients had reported on AE. Among the subjects taking estrogen, progesterone or their related agents, 1 (6.67%) patient reported on AE (data not shown).
During the study period, three confirmed cases of SADR were reported, which consist of fracture, cerebral infarct and one case of death. Overall, there were 4 reported cases of fracture in this study; two vertebral fractures and two non-vertebral (femoral neck, pelvis bone) fractures. However, only one case (pelvis bone fracture) was found to have a causal relationship with bazedoxifene (data not shown).
4. Discussion
This real-world study on the safety of bazedoxifene demonstrated that bazedoxifene is well tolerated in postmenopausal Korean women with osteoporosis, including those aged 70 years or older. To our knowledge, this is the first large-scale study of over 3,000 subjects, which evaluated the safety of bazedoxifene in postmenopausal women with osteoporosis and the resulting safety profile of bazedoxifene was consistent with previous studies done in Asian people. Moreover, in our study, incidence rates of AE and ADR decreased with increasing age. So, we suggest that this study presents useful and meaningful information on safety issue, especially in elderly patients.
The overall safety profile of bazedoxifene in this study with Korean patients was favorable and the results were consistent with the findings from the earlier published studies, even though the mean age of study subjects was 5 ~ 11 years older than those of previous studies (). In a 2-year, pivotal bazedoxifene Phase III, multicenter, double-blind, randomized, placebo- and raloxifene-controlled clinical study performed in 101 sites in Canada, Europe, and the United States with 1,583 postmenopausal women, there was no difference in the incidences of AE, SAE and withdrawal due to AEs between all groups, although the incidences of hot flushes were higher in the treatment arms [Citation8]. Similar outcomes were observed in a phase Ⅱ trial of 429 postmenopausal Japanese women with osteoporosis, reporting favorable safety and tolerability profile of bazedoxifene [Citation12].
Table 4. Comparison of adverse event (AE) of special interests with other studies testing Bazedoxifene 20 mg.
The well-being of elderly people is of growing concern, due to an increase in the proportion of this age group worldwide. It is important to assess the safety of drugs in elderly patients, as this population can demonstrate greater comorbidity and frailty than younger adults. When compared to raloxifene which showed about 10% of overall ADR rate in a PMS study conducted in Japan [Citation15], overall AE and ADR rates of bazedoxifene-treated patients of our study were lower. Moreover, in contrast to the above-mentioned raloxifene PMS study in which there was no significant difference in ADR rates between groups of women younger than 75 years and women aged 75 years and older, incidence rates of AE and ADR decreased with increasing age in our study subjects (p = 0.0007). Although SERM has been recommended to be used in very early stage of postmenopause or age around sixties [Citation14], this study has shown that bazedoxifene can be safely used in elderly patients as well as relatively young postmenopausal women. The exact rationale for this greater tolerability of bazedoxifene with increasing age is however not readily known. A possible explanation for this finding is that elderly patients have less active immune system due to aging-related immunosenescence and immune remodeling and may be less likely to have an overt reaction. The likelihood of spontaneous reporting of AEs or ADRs may be lower in the elderly population due to communication problems including hearing and speech deficits.
Additionally, it appeared that majority of subjects continued bazedoxifene for a period of 3 months or longer, with AE and ADR rates for this group significantly lower than those who stayed on treatment for less than 3 months. It would be expected that those who withdrew the drug have a lower frequency of serious AE or ADR than the patients who stayed on bazedoxifene for more than 3 months probably due to more exposure duration. However, as adverse events to SERM mostly occur during early phase of treatment that is, within 1 month, the patients who continued treatment may not have experienced AE once they’ve gone through the 1-month period without an event. This seems to suggest that the main reason for early discontinuation of treatment is the occurrence of AE or ADR. Given the much larger number of subjects continuing treatment for 3 months or more (3,103 subjects versus 320 subjects), it can be suggested that bazedoxifene has favorable safety profile and positive tolerability. However, a longer observation period would be needed to clarify this. The permanent discontinuation rate for bazedoxifene in our study was rather high (more than 49%) compared to previous clinical trials (around 30%) [Citation8,Citation9]. The fact that our study was a non-interventional, observational study could partially explain this result. However, further studies are needed for better understanding.
There were study subjects who have been taking concomitant drugs during the study period which might affect bone homeostasis. However, only 6 cases of AE were reported from the subjects taking drugs that affect bone metabolism (33 patients, 1.49%), and only 1 case of AE was reported from the subjects taking estrogen, progesterone or related agents (15 patients, 0.68%). Although the percentage of subjects taking these medicines was quite low and it seems not to have a major impact on the overall safety profile results, concomitant administration of these drugs could still have influenced on the results of this study.
In controlled clinical studies, bazedoxifene was shown to have a favorable breast and endometrial safety profile and majority of adverse reactions occurring during those clinical trials were mild to moderate in severity and did not lead to discontinuation of therapy. The most frequent drug-related adverse reactions in double-blind, randomized studies were hot flushes and muscle spasms (includes leg cramps). These results were reproduced in our study, in which no new safety concerns were revealed.
Regarding the hot flushes, the incidence was found to be lower in this PMSS as compared to the other bazedoxifene studies. Hot flushes are generally known to be more severe in the first 2 years of menopause and tend to wane over time. Given the age makeup of this study where the majority of subjects were relatively older than the age of onset of menopause, the incidence of hot flushes may have been lower than expected.
Most of the ADR reported in this study were described as mild in nature and tolerable, with three confirmed cases of SADR which consist of fracture, cerebral infarct and one case of death. The occurrence of fracture in this study population was anticipated, given that the target patients had postmenopausal osteoporosis and the average age of the subjects was almost 70 years. In this study, fracture was diagnosed by study investigators by individually checking for radiologic evidence. There were 4 reported cases of fracture in this study, including two vertebral fractures and two non-vertebral fractures, but only in one case, the causal relationship with bazedoxifene could not be excluded. This patient had pelvis bone fracture, which was assessed by the investigator as conditional/un-classifiable and whether to accompany trauma was unknown. However, there seems to be the possibility of steroid-associated pelvic insufficiency fracture, as the patient had been taking prednisolone 5 mg for 4 years. In the case of the subject who developed cerebral infarct, the event was assessed by the investigator as having possible causality. This subject incidentally had a pre-existing medical condition of Behcet disease and was on concomitant treatments with a statin and an anti-platelet agent, making her a high-risk subject. In the case where death occurred, the subject was an 83-year-old patient with known lung cancer. This SAE was assessed as un-assessible/un-classifiable by the investigator, that is, a causal relation with bazedoxifene cannot be excluded here.
It was of note that no cases of deep vein thrombosis (DVT) were reported in this study of over 3,000 patients with a significant elderly cohort of whom we expect higher risk. The lack of DVT finding was similarly observed in an Asian study reported by Xu et al., which observed the patients for a longer period of 6 months, albeit with a much smaller cohort [Citation7]. From this and a low DVT rate of 0.1% reported by Takeuchi et al. with raloxifene, we may infer an inherent lower rate of DVT in the Asian population [Citation15]. In this study, retinal vein occlusion was not reported, but a case of retinal hemorrhage was. As this patient was suffering from diabetes, hypertension, and hyperlipidemia, the investigator concluded that the underlying hypertension contributed to the retinal hemorrhage and assessed it as unlikely to have a causal relationship with bazedoxifene. There were two cases of ischemic stroke. The first case was a patient with atrial fibrillation and was diagnosed of MCA infarction while taking bazedoxifene. In this case, the investigator confirmed the causal relationship with bazedoxifene to be unlikely. In the other case of ischemic stroke, causal relationship with bazedoxifene was identified as possible. There were no cases of myocardial infarction or pulmonary embolism reported in this study. However, as being an observational study, we investigated for symptomatic arterial/venous thromboembolism cases without using screening tools, such as computed tomography.
There are several limitations in this study that need to be acknowledged. First, the incidence rate of AE appeared to be somewhat lower than that observed in controlled clinical studies. This is likely to have attributed from the real-world setting of the current study and the rate could have been underestimated due to relatively short observation period. However, most AEs and ADRs are known to occur in the early period of treatment. Second, the results of the present study derive from an older population (with an average of 69.5 years and more than half of the subjects being 70 years old) compared to other international studies. Nevertheless, this study is meaningful as it provides important information on the safety of bazedoxifene in routine clinical practice for Asian post-menopausal osteoporosis patients. Third, the present study is a non-interventional observational study, thus laboratory data were collected at the physicians’ discretion. The results from these laboratory tests may not be sufficient to be published, however they may still contribute to our understanding of bazedoxifene, especially in the elderly cohort.
5. Conclusion
In this PMSS for safety of bazedoxifene in postmenopausal Korean women with osteoporosis, bazedoxifene showed a low rate of AEs, ADRs, and SAEs and was well tolerated. Furthermore, bazedoxifene established its safety in the age group of 70 years and over. DVT was not reported in this study, consistent with the results of several previous studies in the Asian population, suggesting that the use of bazedoxifene is relatively safe compared to the western population.
Although limited by the study set-up which was primarily designed to conform with regulatory requirements and the relatively short observation period, this study generates usable and practical information and could result in the improvement of medical care of patients with postmenopausal osteoporosis in a real-world setting.
Authors’ contributions
Conception and design of the study were provided by YS Chung, JW Kim and JY Lee. Analysis and interpretation of the data, drafting and critical revising of the paper and final approval of the version to be published were provided by YS Chung, JW Kim, JY Lee, HE Park and SH Kim. Medical writing support (including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables, and figures, collating author comments, copy-editing, fact-checking, and referencing) was provided by Honorem. We thank Honorem for support with statistical analyses. All authors agree to be accountable for all aspects of the work.
Declaration of interest
YS Chung, JW Kim and JY Lee have received research grants/honorarium for this study from Pfizer. HE Park and SH Kim are full-time employees of Pfizer Pharmaceuticals Korea Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
All investigators participated in this study are listed as below table. Study sites were written at the time of study in alphabetical order.
Additional information
Funding
References
- Cole Z, Dennison E, Cooper C. Update on the treatment of post-menopausal osteoporosis. Br Med Bull. 2008;86:129–143.
- Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther. 2008;12(3):157–170.
- Jang HD, Hong JY, Han K, et al. Relationship between bone mineral density and alcohol intake: a nationwide health survey analysis of postmenopausal women. PLoS One. 2017 Jun 29;12(6):e0180132.
- Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res. 2004 Aug;425:126–134.
- Kim JW, Jeon Y-J, Baek D-H, et al. Percentage of the population at high risk of osteoporotic fracture in South Korea: analysis of the 2010 fifth Korean national health and nutrition examination survey data. Osteoporos Int. 2014 Apr;25(4):1313–1319.
- Komm BS, Lyttle CR. Developing a SERM: Stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann N Y Acad Sci. 2001 Dec;949:317–326.
- Xu L, Tsai KS, Kim GS, et al. Efficacy and safety of bazedoxifene in postmenopausal Asian women. Osteoporos Int. 2011 Feb;22(2):559–565.
- Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008 Apr;23(4):525–535.
- Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res. 2008 Dec;23(12):1923–1934.
- Ministry of Food and Drug Safety, Korea. Bezadoxifene Information. [cited 2019 Feb 24]. Available from: http://drug.mfds.go.kr/html/bxsSearchDrugProduct.jsp?item_Seq=201110095
- Lippuner K, Buchard PA, De Geyter C, et al. Recommendations for raloxifene use in daily clinical practice in the Swiss setting. Eur Spine J. 2012 Dec;21(12):2407–2417.
- Itabashi A, Yoh K, Chines AA, et al. Effects of bazedoxifene on bone mineral density, bone turnover, and safety in postmenopausal Japanese women with osteoporosis. J Bone Miner Res. 2011 Mar;26(3):519–529.
- de Villiers TJ, Chines AA, Palacios S, et al. Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int. 2011 Feb;22(2):567–576.
- Ki-Chan A. Selective estrogen receptor modulators. Asian Spine J. 2016 Aug;10(4):787–791.
- Takeuchi Y, Hamaya E, Taketsuna M, et al. Safety of 3-year raloxifene treatment in Japanese postmenopausal women aged 75 years or older with osteoporosis: a postmarketing surveillance study. Menopause. 2015 Oct;22(10):1134–1137.