ABSTRACT
Introduction: A radiation countermeasure that can be used prior to radiation exposure to protect the population from the harmful effects of radiation exposure remains a major unmet medical need and is recognized as an important area for research. Despite substantial advances in the research and development for finding nontoxic, safe, and effective prophylactic countermeasures for the acute radiation syndrome (ARS), no such agent has been approved by the United States Food and Drug Administration (FDA).
Area covered: Despite the progress made to improve the effectiveness of amifostine as a radioprotector for ARS, none of the strategies have resolved the issue of its toxicity/side effects. Thus, the FDA has approved amifostine for limited clinical indications, but not for non-clinical uses. This article reviews recent strategies and progress that have been made to move forward this potentially useful countermeasure for ARS.
Expert opinion: Although the recent investigations have been promising for fielding safe and effective radiation countermeasures, additional work is needed to improve and advance drug design and delivery strategies to get FDA approval for broadened, non-clinical use of amifostine during a radiological/nuclear scenario.
1. Introduction
Radiation exposure from radiological accidents or other types of radiological/nuclear incidents (intentional or otherwise) can lead to various types of radiation injuries; all of which will require good diagnosis and treatment. Acute radiation syndrome (ARS) is an acute illness caused by radiation exposure of the whole or partial body by a high dose of penetrating ionizing radiation in a short time. In this age of terrorism and possible use of weapons of mass destruction, there is a compelling need to develop and establish federal, state and local capacities to counter these threats [Citation1–Citation4]. Such ‘medical threat-countering’ strategies are referred to as radiation countermeasures. Such agents are divided into three classes: radioprotectors (prophylaxis), radiomitigators (to be used soon after exposure), and therapeutics [Citation5]. There are several radiation countermeasures under development that have been considered safe and efficacious to be used as radioprotective agents for radiological/nuclear events; however, they have not yet received FDA approval and need more studies [Citation6–Citation10]. All three currently FDA-approved radiation countermeasures (Neupogen/filgrastim/granulocyte colony-stimulating factor (G-CSF), Neulasta/pegfilgrastim/G-CSF, and Leukine/sargramostim/granulocyte-macrophage colony-stimulating factor (GM-CSF)) are defined as radiomitigators. These agents are approved specifically for a major ARS sub-syndrome, known as the hematopoietic sub-syndrome of ARS (H-ARS) [Citation11–Citation17]. It is important to note that at the present time no radioprotector for either H-ARS or for gastrointestinal-ARS (GI-ARS) has been approved by the FDA [Citation18].
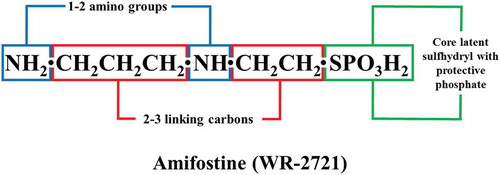
During the era of World War II, under the Manhattan Project, screening of more than 4,400 agents was carried out at the Walter Reed Army Research Institute of the US Army Drug Development Program to identify drugs to protect against injuries caused by ionizing radiation from nuclear/radiological detonation. This resulted in the selection of amifostine (WR-2721, WR implies Walter Reed, 2-[(3-aminopropyl)amino]ethanethiol dihydrogen phosphate) based on its excellent radioprotective efficacy and safety profile () [Citation19]. Since then, amifostine has been pursued as the radioprotective agent of choice by diverse institutions, investigators, and government/funding agencies over the last six decades [Citation20–Citation24]. Though the brief message of this endeavor has been mixed [Citation25–Citation28], initial studies in animal models showed that amifostine could protect treated animals from high doses of ionizing radiation. Unlike other organ specific radioprotectants, amifostine is a broad-spectrum systemic cytoprotective agent. Preclinical studies in different animal models tend to encourage the belief that amifostine can protect almost all normal tissues from insults arising from irradiation or from particular types of chemotherapeutic agents. It is a strong, systemically effective radioprotector when used at high doses. However, it is toxic when administered at high doses required for radioprotection (survival benefits). The drug is hypotensive, causing both upper and lower GI disturbances [Citation29,Citation30]. These qualities lead to adverse behavioral responses and decreased performance [Citation31–Citation35]. As a result, this promising agent has been considered not suitable for use as a radioprotector for special operation forces, high-risk individuals, or for the general civilian population [Citation36,Citation37]. However, investigators have tried through various approaches to limit its side effects while retaining its robust radioprotective characteristics [Citation38]: such efforts include, but not limited to threshold dosing regimens, modulating the drug delivery, and modifying the drug itself in order to retain its superior radioprotective activities.
Amifostine was referred to as Ethyol (trihydrate form of amifostine) after its purchase by MedImmune (Gaithersburg, MD, USA) in October 2001 from ALZA Corporation (Mountain View, CA, USA). In 2007, Astra Zeneca (Cambridge, UK) acquired MedImmune. Next, amifostine was acquired by the Clinigen Group (Staffordshire, UK) in August 2014. Clinigen and Cumberland Pharmaceuticals (Nashville, TN, USA) entered into an agreement for commercialization of amifostine in USA in May 2016. Generic version of amifostine is also marketed by Sun Pharmaceutical (Goregaon, Mumbai, India), Taj Pharmaceuticals (Goregaon West, Mumbai, India), Merro Pharmaceutical (Islamabad, Rawalpindi, Punjab, Pakistan), Luye Pharma (Shanghai, China), Mylan Pharmaceuticals (Canonsburg, PA, USA), and Kaifeng Mingren Pharmaceutical (Zhengzhou, Henan, China). It has obtained US FDA approval for two indications: (i) to reduce the accumulative renal toxicity associated with repeated administration of cisplatin in patients with ovarian cancer in 1996, (ii) to diminish the occurrence of xerostomia in patients undergoing post-operative radiotherapy for head and neck cancers (where the radiation port includes a considerable segment of the parotid glands, in 1999 ()) [Citation39,Citation40]. An enhanced uptake by the salivary gland and kidneys may be responsible for privileged protection of these tissues and organs. A recent study suggested that amifostine does not have a significant effect for preventing xerostomia and the benefits of amifostine should be weighed against its high cost and side effects [Citation41].
Table 1. Indications for which amifostine has received full FDA approval.
2. Preclinical development of amifostine using various animal models
The development of radioprotectors has been dominated by the investigation of sulfhydryl compounds, specifically aminothiols and phosphorothioates. Several mechanisms have been suggested for radioprotection by the use of sulfhydryl compounds, such as radiation-induced repair of DNA damage, free radical scavenging, genomic stabilization, modulation of cellular metabolism, modification of cell-cycle progression, augmentation of selective DNA repair enzymes, and scavenging of metals () [Citation42,Citation43]. The most meticulously studied, efficient, and valuable radioprotective thiols produced are WR-2721 (amifostine) and other phosphorothioates, such as WR-3689 – S-2-(3-methylaminopropylamino) ethylphosphorothioic acid and WR-151,327 – S-2-(3-methylaminopropylamino) propylphosphorothioic acid [Citation26,Citation27]. These phosphorylated compounds are prodrugs for the active free aminothiols, such as WR-1065 – [2-(3-aminopropylamino)ethanethiol] from WR-2721, WR-151326 – [3-(3-methylaminopropylamino)propanethiol] from WR-151327, and their disulfides generated in vivo. These free thiols can form disulfides, and WR-2721 can also be metabolized to cysteamine [Citation44,Citation45]. All of these WR-associated phosphorothioates exhibit effective, but distinct degrees of radioprotectiveness and drug toxicity/side effects when assessed using small animal rodent models [Citation46]. Furthermore, different routes of administration have been documented as well [Citation47]. The inadequately studied WR-159243 is well-tolerated agent that can be used orally soon before acute radiation exposure, while still attaining high levels of radioprotection (30 days survival, dose reduction factor (DRF) of ~1.3 and therapeutic index of ~7.5).
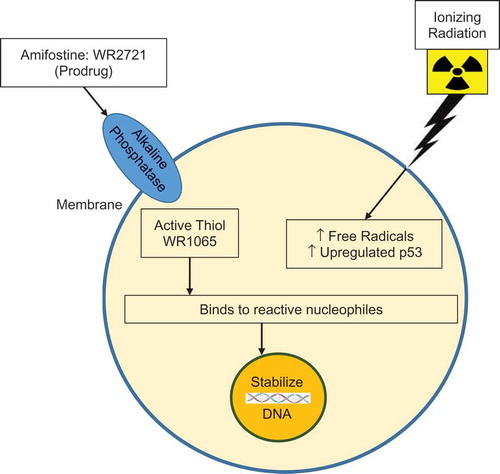
Figure 2. Simplified diagram for the mechanism of action of amifostine. Amifostine protects against radiation/chemotherapeutic agent-induced DNA damage. It has anti-mutagenic, anti-carcinogenic characteristics, affect redox-sensitive transcription factors, gene expression, chromatin stability, enzymatic activity, affects growth and cell cycle progression, and prevents the up-regulation of inflammatory pathways.
In a preclinical study, WR-2721 demonstrated a DRF of 2.7 for H-ARS and 1.8 for GI-ARS, when administered intraperitoneally (ip) at a dose of 500 mg/kg to mice before irradiation () [Citation48–Citation51]. The drug dose used was relatively high in terms of adverse side-effects, as LD50 dose for the drug alone was 704 mg/kg [Citation48]. In subsequent studies, several compounds were evaluated for the protection of the hematopoietic system, the GI system, the integumentary system, and pulmonary tissue. It was observed that the most effective aminothiol had the basic features of a free sulfhydryl group separated by no more than three carbon atoms from a nitrogen functional group () [Citation27,Citation52]. In these reports, drugs were compared for dosing schedules, one-half of the maximum tolerated dose (MTD, the dose that is lethal to no more than 10% of mice tested). Administration of phosphorothioates offered a DRF of 1.7–2.3 for H-ARS and 1.2–1.6 for GI-ARS. Cysteamine, cystamine, and AET (2-aminoethylisothiouronium Br.HBr) demonstrated low therapeutic indexes compared to phosphorothioates. Earlier, a high DRF was attained at doses close to their MTD [Citation26]. Not all phosphorothioates are effective as radioprotective agent when administered orally to rodents; furthermore, the phosphorothioate, namely amifostine, proved not to be effective in nonhuman primates (NHPs) when dispensed orally () [Citation26]. When amifostine was administered intramuscularly (im) at a dose of 300 mg/kg in mice 15 min prior or up to 2 h before irradiation, its DRF was 2.7 [Citation53]. Phosphorothioates were useful against high-linear energy transfer (LET) exposures [Citation54] indicating a protective mechanism. This implicates hydrogen donation to damaged DNA rather than solely scavenging reactive oxygen species (ROS).
Table 2. Radioprotective efficacy of amifostine (WR-2721) in mice.
Table 3. Radioprotective efficacy of selected agents.
Table 4. Radioprotective efficacy of amifostine (WR-2721) in rodent, canine, and NHP.
A low level of radioprotection was observed when phosphorothioates were dispensed to mice at a low dose, such as 75 mg/kg [Citation26]. Although not fully authenticated, it is likely that the dose of amifostine suggested for use in head and neck cancer patients undergoing radiotherapy (200 mg/m2 intravenous (iv)) [Citation39] would be adequate to afford radioprotection, regardless of whether or not clinical support was used. There are several potentially radioprotective thiols, such as diethyldithiocarbamate (DDC), mercaptopropionylglycine (MPG), N-acetylcysteine (NAC), mesna, and cysteamine that have FDA approval for other indications and for which there is considerable clinical experience. Although these agents are less radioprotective for lethal doses of radiation, they may be far less toxic and well tolerated. Toxicity/side effect assessments of these agents indicate that NAC has the lowest toxicity, followed by amifostine, cysteamine, and DDC [Citation34]. Researchers continued synthesizing and assessing newer derivatives of thiols, such as thiazolidine, prodrugs of cysteine, cysteamine, and WR-1065, for finding an optimal radioprotective agent for ARS [Citation55].
The concept of dose conversion between various species of animal models and human is important for drug development [Citation56]. Interspecies allometric scaling for dose conversion from animal to human (dose per kg to dose for body surface area) takes into the consideration the differences in body surface area, which is associated with animal weight. Allometric scaling is an approach where the conversion of drug dose is based on normalization of dose/kg to body surface area. This approach considers differences of attributes of anatomical, biochemical, and physiological processes among species, as well as differences in pharmacokinetics. Different equations described in a recently published article can be used as a reference for ‘dose extrapolation’ among species [Citation56].
3. Mechanisms and toxicities of amifostine
Amifostine is an inactive phosphorylated aminothiol prodrug and it cannot protect until dephosphorylated to WR-1065, its active and free radical-scavenging sulfhydryl metabolite. It is dephosphorylated by alkaline phosphatase. Alkaline phosphatase is available in high concentrations in normal tissues and in relatively low concentrations in tumor/malignant cells. WR-1065 only provides protection to non-central nervous system (CNS) tissues, but not the CNS itself since it is not able to cross the blood–brain barrier. Once inside the cell, WR-1065 scavenges free radicals, allowing for the protection of intracellular constituents. Several studies demonstrate that there are additional mechanisms relative to radioprotective efficacy of amifostine [Citation6,Citation57,Citation58]. Oxidation of WR-1065 to its polyamine-like disulfide metabolite (WR-33,278) is followed by a quick consumption of oxygen, suggesting that cellular anoxia may be a mechanism for radioprotection. This was supported by an increase in the oxygen saturation of the venous blood following iv administration of amifostine in the absence of hemoglobin-bound-oxygen. This promotes a decreased oxygen consumption by normal tissue; a process related to radioprotection by amifostine [Citation59,Citation60]. The latter effect has been referred to as the ‘Walberg-effect’ [Citation61]. Furthermore, a high concentration of WR-33,278 condenses DNA and limits potential target sites for free-radical damage and for subsequent free radical scavenging [Citation62]. Such event leads to a decrease in the number of DNA double-strand breaks. The drug-induced mitigation of a radiation-induced inhibition of cellular proliferation may be important to accelerate the recovery of endothelial and mucosal cells. Amifostine may upregulate the expression of proteins responsible for DNA repair and inhibition of apoptosis through Bcl-2 and hypoxia-inducible factor-1α (HIF-1α) [Citation63–Citation65].
In sum, amifostine and its immediate, dephosphorylated metabolite WR1065, exert radioprotective efficacy through various molecular and cellular processes [Citation29]. Very often the efficacy of amifostine is attributed to free-radical scavenging, along with DNA protection and repair; all of which are coupled with the initial induction of cellular hypoxia [Citation29,Citation47,Citation55,Citation57–Citation60]. At the cellular level, amifostine has significant effects on growth and cell cycle progression. In brief, amifostine protects against the DNA damage induced by ionizing radiation and chemotherapeutic agent associated free radicals. It has anti-mutagenic and anti-carcinogenic characteristics [Citation29]. These latter endpoints for amifostine’s prophylactic efficacy have been demonstrated using small animal models [Citation29], while (to our knowledge) the drug’s mitigative, anti-mutagenic actions have only been reported using in vitro testing systems [Citation66,Citation67]. At the molecular level, it has been demonstrated to affect redox-sensitive transcription factors, gene expression, chromatin stability, and enzymatic activity. At the cellular level, it has important effects on growth and cell cycle progression. Amifostine prevents the up-regulation of inflammatory pathways related to cancer therapy toxicity [Citation23].
Tumor/malignant tissues are not well vascularized and demonstrate hypoxic microenvironments with low interstitial pH, and decreased alkaline phosphatase. Such microenvironments lead to low buildup of active drug within malignant tissues. Normal tissues may have as much as a 100 fold higher concentration of free thiol compared to malignant tissues [Citation68]. Nonetheless, in some reports using animal models, it has been shown that amifostine does indeed diffuse into malignant tissues, perhaps slowly as compared to normal tissues. Still, it raises the question about its potential to protect malignant tissue from radiotherapy and chemotherapy [Citation61,Citation69].
The usual toxic effects of amifostine are hypotension, emesis, somnolence, and sneezing in patients who received single or multiple doses [Citation46]. Other minor adverse effects are malaise, hypocalcemia, metallic taste, hiccups, chills, flushed feeling, idiosyncratic reactions, fever, and/or rash.
4. Strategies for use and regulatory approval of amifostine as a prophylaxis agent in limiting ARS and associated delayed pathological sequelae
Substantial effort has been made by several investigators to make amifostine more user-friendly by trying to reduce its toxicity and side effects by developing novel chemical analogs, drug formulations, and drug delivery systems. The overall goal of this endeavor is to maintain the efficacy and to extend the time window for effective drug administration. Specifically, such efforts have comprised of (i) the chemical synthesis of better tolerated analogs [Citation26,Citation27]; (ii) the radioprotective synergy by combining lower doses of amifostine with lower doses of another pharmacological agent with less or fewer side effects (such as α-tocopherol, γ-tocotrienol (GT3), selenomethionine); i.e., a poly-pharmacy approach [Citation70,Citation71]; (iii) minimize side effects of amifostine by additional use of supplemental pharmacological agent designed to minimize emetic effects of amifostine (or use of anti-emetics) [Citation6]; (iv) use of low doses of amifostine to protect against carcinogenesis and/or mutagenesis, while forgoing some of inherently toxic cytoprotective attributes of amifostine [Citation72,Citation73]; and (v) improve the approaches for ‘slow-release’ drug delivery [Citation74–Citation76]. Generally, these approaches have proven to be successful in lowering drug toxicity/side effects, but have not been successful in totally eliminating it. Considering current rigorous regulatory environment that controls drug use in humans, such an effort to reduce toxicity of amifostine to a minimum will be critical if amifostine, phosphonol or any other phosphorothioates are to be approved by the FDA or other international drug regulatory agencies (such as The European Medicines Agency, Pharmaceuticals and Medical Devices Agency of Japan) for use as a radioprotector. Some important and ongoing approaches for improving amifostine use are discussed below:
4.1. Chemically re-engineering better tolerated analogs of amifostine
Attempts to improve the radioprotective efficacy, improve the drug pharmacology, and reduce the side effects of amifostine by re-engineering a phosphorothioate analog have been pursued for the past several decades [Citation26,Citation27,Citation76]. The radioprotective domain of this class of drugs seems to be its hydrophilic chemical core, containing a sulfhydryl group, protected by a phosphate, and linked by two or three carbons to at least one or two amino groups ().
The domain responsible for side effects is attributed to the length and terminal nature of the aminoalkyl group and its hydrophobicity. The pharmacology of phosphorothioate species seems to be an amalgamation of both these functional groups, the aminoalkyl and sulfhydryl [Citation26,Citation27]. The chemical modifications within the two functional domains producing greater hydrophilicity, along with increased coverage of sulfur group, resulted in improved therapeutic indices [Citation26]. It is vital to note, that such improvement did not generate ideal pharmacologic profiles. The time window for radioprotection and the poor bioavailability remained important concerns.
Phosphonol (WR-3689) is close to amifostine in its radioprotective efficacy (polyamine like affinities and targeted action on DNA) but suffers from side effects comparable to amifostine although to somewhat lesser extent. Its bioavailability is marginally higher than amifostine when administered orally. In brief, phosphonol may be considered an improved drug compared with amifostine due to is higher protective effects [Citation77,Citation78].
A new and quite promising radioprotective aminothiol analog, PrC-210, has been developed and evaluated in preclinical animal models. PrC-210 is a five carbon, single thiol alkylamine (aminothiol derived organosulphur compound). Important considerations in the designing this aminothiol were (i) a small molecular size for efficient transmembrane diffusion, (ii) positive charged amines on an alkyl backbone for strong ionic interaction with DNA, and (iii) a perpendicular, alkyl side-chain with a terminal thiol projecting away from the DNA backbone to enable scavenging of ROS around DNA [Citation79]. PrC-210, when administered prior to irradiation (prophylaxix) via ip injection, provided 100% protection in ICR mice against total-body irradiation which was 100% lethal to untreated control mice (8.63 Gy). In this study, PrC-210 dose was 252 mg/kg and it was administered 30 min prior to irradiation, similar to the amifostine. Furthermore, PrC-210 was effective when administered anywhere between 7.5 and 60 min before irradiation to mice [Citation80]. When administered topically (370 mM in ethanol:propylene glycol:water solution) to Sprague-Dawley female rats prior to cutaneous irradiation (17.3 Gy to skin), it provided 100% prevention against grade 2–3 radiation-induced dermatitis. The DRF for PrC-210 was 1.6 in prophylaxed, acutely irradiated (i.e., at a dose of 252 mg/kg ip 30 min prior to 8.75 Gy 137Cs γ-irradiation) ICR female mice [Citation79]. Furthermore, PrC-210 also protected mice and rats against total-body irradiation, 100% survival in rat and mouse models against 100% lethal dose of 137Cs γ-radiation. This drug appeared to lack retching and emesis-producing properties when tested in a ferret model [Citation73]. In addition, there was no evidence of drug-induced hypotension in arterial cannulated rats treated with PrC-210 [Citation81]. The comparison of PrC-210 and amifostine side effects was striking because PrC-210 was free of all side effects investigated.
A variety of N-(aminoalkanoyl)-S-acylcysteamine and N,N’-is(aminoalkanoyl)cystamine salt derivatives have been synthesized and such agents have been tested for their toxicity/side effects and radioprotective efficacy using murine model, and also compared with amifostine and S-acetylcysteamine hydrochloride. One of the most attractive compounds of this series was N-glycyl-S-acetylcysteamine trifluoroacetate (16, I 102) [Citation82]. Side effects and radioprotective efficacy were investigated in murine model and found to be promising when compared to amifostine and S-acetylcysteamine hydrochloride. The structure–activity relationships of these agents have been well studied.
Several analogs of amifostine (such as DRDE-07: (S-2(2-aminoethylamino) ethyl phenyl sulfide), DRDE-30: (S-2(2-aminoethyl amino) ethyl propyl sulfide), and DRDE-35: (S-2(2-aminoethyl amino) ethyl butyl sulfide)) have been synthesized by Defense Research and Development Establishment, Gwalior, India (Government of India, Ministry of Defense Institution), and tested for their radioprotective efficacy using C57BL/6 mice model of total-body irradiation. These agents were reported to be promising as radioprotectors when administered 30 min before irradiation [Citation83]. Several parameters such as animal survival, body weight, hematological parameters, GI histology (structural integrity, vilus height, regenerating crypts and mitotic figures), and colony forming unit – spleen (CFU-S) were examined. These analogs significantly improved all above listed parameters in a dose dependent manner. All three analogs were effective orally as well as through ip route of administration. Earlier, these agents were reported to be effective against Sulfur Mustard [Citation84,Citation85]. Recently, DRDE-30 has also demonstrated to attenuate bleomycin-induced pulmonary fibrosis in murine model [Citation86].
Overall, only a small number of rationally designed analogs of amifostine have been synthesized and investigated for side effects/toxicity, pharmacology, and radioprotective efficacy using various preclinical animal models. Further effort is warranted and might prove fruitful from a radiation countermeasure development perspective.
4.2. Nanoparticle preparation, PEGylation and molecular conjugates designed to extend amifostine (or it’s active metabolite, WR 1065) circulating half-life
Although amifostine is an efficacious and potentially useful radiation countermeasure, its limitations due to short systemic circulation time and fast clearance from the tissues hamper its sustained bioactivity. With the development of nanotechnology in the area of drug development, the nanoparticles have proved to be powerful drug delivery systems for improving the biological activity of the drug [Citation87]. Two oral formulations of amifostine have been prepared through nanotechnology with median particle sizes of 257 and 240 nm. After oral administration to mice, tissue distribution study demonstrated presence of its active metabolite, WR-1065, in various organs with some degree of selectivity in bone marrow, jejunum, and kidneys [Citation76]. Radioprotective efficacy evaluation orally demonstrated enhanced mouse survival, hematopoietic cell survival, and surviving crypt cell counts in animals irradiated with 137Cs γ-radiation source [Citation88]. The nanoparticles of WR1065 prepared by spray drying using polylactide-co-glycolide (PLGA) resulted in significant radioprotection (mice survival, bone marrow recovery, and intestinal injury mitigation) when administered orally 1 h before irradiation at a dose of 500 mg/kg [Citation89]. These nanoparticles were of 206 nm in diameter and having 21.7% w/w WR1065. There are reports for dual targeting functionality and dual drug delivery using nanoparticle technology [Citation90].
PEGylation, covalently conjugating drug with polyethylene glycol (PEG), has demonstrated enormous potential in improving drug safety, pharmacokinetics, and pharmacodynamics. Since PEG is FDA approved for clinical use, PEGylation is used for the development of drugs [Citation91]. PEGylated amifostine was prepared by its conjugation with the 4-arm PEG (5,000 Da). The large PEG-amifostine conjugated molecules could be internalized into cells and translocated to acidic organelles and demonstrate prolonged hydrolysis time. Results demonstrated sustained transformation of amifostine to WR1065 with higher efficiency in the elimination of ROS, prevention of cisplatin-induced nephrotoxicity, and impeded the interaction between free sulfhydryls and functional biomolecules providing improved safety profile [Citation92].
4.3. Use of low doses of amifostine for cytoprotection
It is of great interest to know whether low doses of amifostine can be used to radioprotect humans at the risk to ionizing radiation exposures. Preclinical studies using rodent model have demonstrated that low, nontoxic doses of amifostine can protect hematopoietic progenitors within blood-forming tissues of irradiated mice. Although the radioprotection may not be enough to provide an overall survival benefit, it might however be enough to partially protect vital tissues. It is reasonable to assume that fractional protection by a pre-exposure drug can be successfully leveraged by the use of another agent used post-exposure [Citation6,Citation37,Citation93].
As mentioned above, it has been demonstrated in a murine model that radioprotective doses for amifostine to protect hematopoietic organs of mice appear to be between 25 and 50 mg/kg. The mature and lineage-restricted progenitors are more responsive to the low doses of amifostine than the more primitive, multipotential progenitors [Citation73]. Amifostine had a marginal effect on the bipotential and multipotential progenitors of bone marrow under a steady-state. Prophylactic doses of >50 mg/kg of amifostine resulted in moderate regeneration of multipotential progenitors and the significant regeneration of bipotential progenitors in irradiated mice. There was no regeneration of more primitive progenitors. Amifostine dose of 25 mg/kg failed to stimulate radioprotective effects on any of the progenitor subtypes. In brief, radioprotective doses for amifostine appear to lie between 25 and 50 mg/kg. Furthermore, the protection afforded by low doses of amifostine can be enhanced by the combined administration of other nontoxic agents [Citation70,Citation71,Citation94].
4.4. Toxicity mitigation with anti-emetic agents
The upper and lower GI tract disturbances associated with the use of high doses of amifostine can be reduced with the use of 5-HT3-receptor antagonist-based antiemetic drugs, such as granisetron or ondansetron [Citation38,Citation95]. Canines treated with increasing doses of amifostine (12.5–100 mg/kg) demonstrated a dose-dependent emesis. The use of granisetron at a dose of 80 mg/kg shortly before amifostine administration diminished the emesis and also the percentage of emetic animals [Citation6]. A few canines remained sensitive to amifostine irrespective of the relatively low dose used.
4.5. Poly-pharmacy approach – combination of lower doses of amifostine and another radiation countermeasure
There are several reports for the poly-pharmacy approach in the area of radiation countermeasures [Citation96–Citation98]. The combination of three cytokines/growth factors, PEGylated G-CSF, PEGylated GM-CSF, and PEGylated interleukin-11 (IL-11) has been shown to enhance radiomitigation in the murine model demonstrating enhanced recovery in complete blood counts [Citation99,Citation100]. This study supports and extends earlier findings of the beneficial effects of combined cytokine therapy that serves to enhance hematopoietic recovery of vital trilineal bone marrow compartments of acutely irradiated mice [Citation101]. Another example of the utility of the ‘poly-pharmacy’ approach to mitigate ARS includes the combination of angiotensin-converting enzyme (ACE) inhibitor, Captopril, and EUK-207, selenomethionine complexes in limiting the extent of radiation-induced lung damage in Sprague-Dawley rats. This rodent model has been shown to be a well-established animal model for radiation-induced lung damage [Citation96,Citation102]. It is important to note that captopril is effective, well tolerated, and has widespread clinical use.
Based on existing information with other radiation countermeasures, some of which are mentioned above, it is a logical step to investigate whether amifostine could enhance the radioprotective efficacy of another radiation countermeasure that acts through a different pathway, or if the efficacy of amifostine can be improved by another unrelated agent. Such poly-pharmacy approaches need to be tried with amifostine as its adverse, toxic side-effects clearly limited its non-clinical use as a sole radioprotective agent [Citation71,Citation103,Citation104]. The advantageous therapeutic usefulness of amifostine is well known and the combined therapy with amifostine with other agents for the potential prevention and treatment of individuals at high risk of developing ARS is becoming an increasingly important strategy.
4.5.1. Combination of amifostine with growth factor/cytokine
The combination of amifostine and G-CSF has been attempted successfully for mitigating ARS in murine model where amifostine was used as a radioprotector and G-CSF as a radiomitigator [Citation105]. The study was conducted to determine whether amifostine can be used to protect hematopoietic stem cells against irradiation, and G-CSF can be used to stimulate the amifostine-protected cells to proliferate and reconstitute the hematopoietic system [Citation105,Citation106]. C3H/HeN mice received treatment of 200 mg/kg amifostine ip 30 min before 60Co γ-irradiation and 125 µg/kg G-CSF sc from day 1–16 post-irradiation. The LD50/30 values for mice administered saline, G-CSF, amifostine, and amifostine plus G-CSF were 7.85 Gy, 8.30 Gy, 11.30 Gy, and 12.85 Gy, respectively. Based on above LD50/30 values, it is obvious that the DRF of the combined treatment of amifostine and G-CSF was higher than the DRFs for either amifostine or G-CSF treatment. Accelerated recovery of bone marrow and splenic multipotential stem cells (CFU-s), granulocyte-macrophage progenitor cells, and peripheral blood cells, was detected in mice treated with amifostine and G-CSF. Furthermore, the G-CSF administered post-irradiation accelerated hematopoietic reconstitution with amifostine-protected stem and progenitor cells and leading to an unambiguous survival-enhancing effect of the pre-irradiation administered amifostine [Citation106]. Such combination treatment approach using amifostine and cytokine has also been discussed in relation to oncology patients with chemotherapy and radiotherapy [Citation107,Citation108].
4.5.2. Combination with vitamin E
Vitamin E is a less effective radioprotector compared to amifostine but it has a wider window of protection against radiation injury, as well as it is less toxic. Amifostine or phosphorothioate WR-3689 (S-2([3-methylaminopropyl]amino)ethylphosphorothioic acid) have been combined with α-tocopherol (a component of vitamin E), and also selenomethionine with positive, survival promoting outcomes [Citation70,Citation109,Citation110]. A prophylactically administered combination of amifostine and vitamin E in acutely irradiated rats has been shown to be beneficial, in terms of protecting and maintaining organ-specific (liver) functions as well [Citation111].
GT3 is currently under advanced development as a radiation countermeasure [Citation112]. Optimal radioprotective doses of GT3 for acutely, potentially lethally irradiated mice have been estimated to be 200 mg/kg [Citation113]. However, the radioprotective efficacy of much lower doses of GT3 (50 mg/kg) can be enhanced significantly by complementary prophylaxis with low, but nontoxic doses of amifostine (30 and 50 mg/kg) [Citation71]. This study using one fourth of the optimal dose of GT3 for murine H-ARS and a very low dose of amifostine suggests that both agents can be used at lower doses which may be free of any side effects of either agent and can still achieve optimal radioprotection.
4.5.3. Combination with metformin
Metformin (1,1-dimethylbiguanide hydrochloride, a biguanide derivate) is the most prescribed agent for the treatment of type 2 diabetes. It has been reported to have radiomitigative potential for ARS [Citation114,Citation115]. When administered 24 h after irradiation as a single dose, metformin significantly potentiated the radioprotective efficacy of amifostine and other sulfhydryl agents in several cell types [Citation114]. It has been proposed that metformin reduces endogenous ROS, cell-cycle progression of radiation-injured cells and increases time for repair. Metformin administered 24 h post-irradiation and in combination with sulfhydryl agents had comparable radioprotective efficacy to that observed for amifostine administered 30 min prior to irradiation. As such, metformin might well be considered as a potentially useful radiation countermeasure for combination with other radioprotective agents.
4.5.4. Combined effects of amifostine and antioxidative salts
The radioprotective efficacy of the metal-containing compounds has been shown to be relatively low, but they have been found to enhance the radioprotective effects of amifostine and other thiols, while reducing toxicity of thiols [Citation27,Citation110]. Several theories (relative to selenium-based enhancements) have been suggested: these include but not limited to: (a) dampening of alkaline phosphatase activity in bone marrow and (b) the stimulation of glutathione peroxidase activity [Citation116]. These observations have resulted in clinical efforts to combine selenium (Se) and amifostine to attenuate side effects of radiotherapy and chemotherapy of malignancies [Citation103,Citation117]. Preclinical studies, using rodent models, have been performed in order to better understand the nature of the adjuvant effect of selenium on radioprotection. For example, the effect of Se pretreatment on the toxicity and radioprotective efficacy of amifostine was studied in male CD2F1 mice [Citation110]. Administration of 1.6 mg/kg Se 24 h before ip injection of amifostine (800–1,200 mg/kg) decreased the toxicity of amifostine. Se alone (1.6 mg/kg) administered 24 h prior to 60Co γ-irradiation increased the survival with a DRF of 1.1. When Se was injected 24 h before ip administration of amifostine (200–600 mg/kg 30 min before irradiation), a synergistic, radioprotective effect was observed. The DRF with 400 mg/kg of amifostine and Se was 2.6, a surprisingly high value as compared to a DRF of 2.2 without Se pretreatment.
4.5.5. Combination of amifostine with other agents
Amifostine has been tested in combination with prostaglandin E2 (PGE2) or its synthetic analog, misoprostol. The DRF values of amifostine alone, PGE2 alone, or PGE2 plus amifostine have been reported to be 1.9, 1.45, and 2.15, respectively [Citation118]. Both PGE2 and misoprostol used singly have been found to be effective in mitigating GI-ARS [Citation119]. The protective role of amifostine in mitigating GI-ARS by the prostaglandin synthesis inhibitor indomethacin has substantiated a positive role of amifostine on the prostaglandin metabolism [Citation120]. Interestingly, prostaglandins have been shown to have a hematopoietic effect as well. The prostaglandins have been reported to enhance erythroid and multilineage progenitors, homing of hematopoietic stem cells, and hematopoietic stem cell survival [Citation121,Citation122].
β-glucan, a polyglucose administered post-irradiation, has been shown to enhance the radioprotective efficacy of amifostine administered before irradiation. The DRF values for mice survival were shown to be 1.37, 1.08, and 1.52 for amifostine, β-glucan, and amifostine plus β-glucan, respectively [Citation123]. The combination of amifostine (200 mg/kg, ip, 30 min prior to irradiation), Se (0.8 mg/kg, ip, 20 h before irradiation), and β-glucan (75 mg/kg iv, 20 h before irradiation) to C3H/HeN mice has been reported as a successful poly-pharmacy approach [Citation124]. The additive effect of amifostine administered pre-irradiation and glucan administered post-irradiation has also been reported by another group [Citation125].
Broncho-Vaxom, a bacterial lysate, has been investigated in combination with amifostine and shown to potentiate its efficacy as radioprotector as well as radiomitigator when amifostine was administered as a radioprotector [Citation126,Citation127]. When both the agents were administered prior to irradiation, the DRF values in mouse survival study have been reported as 1.92, 1.17, and 2.07 for amifostine, Broncho-Vaxom, and amifostine plus Broncho-Vaxom, respectively [Citation126].
The polysaccharide from Sipunculus nudus (a species of unsegmented marine worms) administered post-irradiation along with IL-11 and G-CSF, has been shown to enhance the radioprotective efficacy of amifostine administered as a radioprotector in irradiated mice [Citation128]. Amifostine administered prior to irradiation and peptidoglycan (a polymer from the bacterial cell wall consisting of sugars and amino acids) administered post-irradiation have enhanced the survival of irradiated mice [Citation129].
5. Recent developments with amifostine
Regardless of the lack of FDA approval to use amifostine non-clinically as a radioprotector for ARS in humans associated with nuclear/radiological incidents, we strongly believe this agent, with its several encouraging attributes, merits additional consideration in order to get FDA approval for additional generalized use during radiation exposure emergencies. However, few laboratories have continued to investigate different aspects of its radioprotective efficacies, as well as pursuing work molecular targets, pharmacology, ways to limit its toxicity, and developing modern strategies for drug delivery and extending drug action. Notwithstanding these investigative deficits, a recent investigation demonstrated differential gene expression in lymphohematopoietic tissues of mice administered 100 mg/kg (minimally toxic doses) and exposed to sublethal doses of 60Co γ-radiation. Irradiation elicited both early and late-arising gene responses that were altered by amifostine prophylaxes, with such treatments leading to wide-spread dampening of irradiation-related gene response [Citation130]. A select group of proto-oncogenes reacted in much the same manner as did entire group of arrayed genes. Disparities in gene expression caused by sublethal dose of radiation were observed and amifostine prophylaxis considerably changed gene expression. This investigation suggests that amifostine may have additional useful attributes which we are not aware of. At present, we are conducting metabolomics and lipidomic studies using blood and various tissue samples collected from amifostine-treated and irradiated/unirradiated animals. In these studies, comparisons are being made between animals treated with survival-optimal doses of amifostine but associated with toxic side effects, and animals treated with sub-optimal doses of amifostine that are devoid of any side effects. The goal of such investigations is to identify the downstream metabolic products in animals treated with different doses of amifostine to identify biomarkers which may help to identify the dose of amifostine that is devoid of any side effects. Our initial observations are encouraging and incoming data suggest that the extent to which amifostine can correct radiation-induced perturbations of given metabolic pathways are better with lower dose 50 mg/kg compared to higher dose of 200 mg/kg (unpublished observations). In brief, there is a need for further investigations to optimize the drug dose, administration route, and treatment schedule to reduce the undesired side effects to broaden its clinical application.
6. Conclusion
The pursuit of an ideal radioprotective agent to be used under various radiological/nuclear scenarios has continued for more than 60 years. However to date, we do not have any FDA-approved radioprotective agents that can be administered to individuals at high risk to injurious radiation exposures resulting from radiological/nuclear accidents or from intentional terrorism. There are several agents that have demonstrated potential, but these agents need to be thoroughly investigated and further developed for FDA approval following Animal Rule.
The production of ROS and reactive nitrogen species (RNS) largely underpin cell and tissue injuries induced by ionizing radiation. Efficient defensive mechanisms have evolved within cells in order to cope with such oxidative stresses generated by radiation exposure. As a result of an understanding of such critical radiobiological processes, earlier pharmacologic work concentrated on sulfhydryl drugs and other antioxidants to counter ionizing radiation-induced harmful effects. A primary goal of such investigations was to recognize radioprotective sulfhydryl drugs that generated higher DRF values compared to agents with marginal radioprotective efficacy and low toxicity. Initially, amifostine (WR 2721) was recognized as an encouraging radioprotector among the vast collection of potential radiation-countering pharmacologic agents. It was demonstrated that the drug successfully quenched DNA damage by scavenging free radicals induced by irradiation. However, as in the case of amifostine and several other aminothiols and phosphorothioates, toxicity limits its effectiveness. As the radioprotective efficacy of amifostine increases with higher doses of the drug, so does its detrimental, toxic side-effects. Amifostine has several side effects, such as upper and lower GI disturbances as well as hypotension, making it incompatible for its use as a prophylactic drug administered to population during emergencies. Further R&D investigations for its use outside clinical settings are required to exploit its positive attributes while minimizing its toxic side effects. By either modifying drug dose, reworking its structure, altering its route of administration, or blending it with other potent radioprotective agents at lower doses, amifostine may retain its optimal efficacy and minimize the side effects. This agent might also be tested and used in combination with other FDA-approved drugs for other indications following the poly-pharmacy approach since there is a good possibility that additive or synergistic effects can be achieved [Citation42].
Due to the scientific community’s continued interest and the increased funding in the US post 9/11 for terrorism-associated radiation countermeasure research, significant progress has been and continues to be made to identify, develop, and to receive FDA-approval for countermeasures that can protect population at large from acute radiation injuries. Even with all of its positive characteristics, clinical use of amifostine remains limited. It is difficult to discard amifostine as a viable radioprotector for human use only because of side effects that are quite manageable. Amifostine might well be dispensed safely, with significant benefit, to populations under risk to the radiation injury that are not tasked to execute emergency duties. In brief, amifostine is an effective, promising, and systemically active drug that provide not only cytoprotection to various vital tissues, but also to promote survival in fatally exposed individuals. Despite the reasonable advancements in the usefulness of amifostine as a radioprotector for ARS, none of the strategies described to date completely remove amifostine’s toxic side effects. Although the work-effort on amifostine and other aminothiols has been assuring for developing a safe and effective radioprotector, additional work is definitely required to advance the current drug design and delivery approaches.
7. Expert opinion
Amifostine is a promising, well-studied, effective radioprotector (an agent administered prior to radiation exposure) for ARS. However, to date, no radioprotector for ARS has received FDA approval for non-clinical use in humans. There are several reasons for such failure in fielding of radioprotectors compared to radiomitigators. The use of radioprotectors presents a higher level of risk. They would require greater scrutiny by FDA since such countermeasures would be dispensed to healthy individuals under threat of radiation exposure. This situation is quite different than for the radiomitigators that would be dispensed to actually exposed persons. Furthermore, all three radiomitigators approved by the FDA for radiological/nuclear exposure contingencies and for the unwanted radiation exposure-associated H-ARS, were already approved for other clinical indications [Citation11–Citation17] and were in clinic for several decades. These agents were approved following the FDA Animal Rule established for such purpose.
Despite the recent progress in optimizing amifostine’s radioprotective use, none of the approaches taken to date (for using amifostine as a radioprotector for H-ARS) have reduced side effects to sufficiently low levels that would satisfy the regulatory requirements of the FDA. Although the preclinical work with amifostine and related aminothiols have indeed been promising in terms of improving drug efficacy and safety profiles, still further work is needed for better drug design and delivery strategies. We consider that a realistic, practical ‘game-plan’ to get amifostine approved for H-ARS would be to use a ‘poly-pharmacy approach’ where more than one agent, particularly with different modes of action, are combined at lower doses to attain the synergistic or additive outcomes. Under such situation, low doses of few agents without any side effects can be combined. Several poly-pharmacy approaches with amifostine have been tried over last 25 years, with some reported accomplishments using preclinical, murine animal models. One such approach has been to combine low doses of amifostine with lower doses of GT3, a component of vitamin E. GT3 is under advanced development following the FDA Animal Rule and provided consistent efficacy in irradiated murine and NHP models. Recent studies using combined treatment with these two agents demonstrated promising results in the murine model of H-ARS using total-body 60Co γ–radiation [Citation71]. This approach of combined treatment was based on earlier reports of combining amifostine with another component of vitamin E, α-tocopherol, and few other thiols [Citation70].
In addition to the side effects, other constraints of amifostine are route of administration (i.e., iv route/sc) and the narrow time-window (~30 min prior to radiation exposure) for optimal efficacy [Citation131]. The utility of amifostine as a radiation countermeasure is unlikely to be completely appreciated if the mode of administration is limited to iv dispensation. Efforts have been made to develop noninvasive options of delivery (e.g. transdermal patches, pulmonary inhalers and microspheres for oral sustained-release) [Citation132]. Such endeavors need to be continued for ultimate success. Furthermore, the window of amifostine administration needs to be improved to make amifostine effective during a radiological or nuclear situation. Though significant effort has been made to increase the treatment window of amifostine, such attempts need to be continued until we get complete success [Citation26,Citation42,Citation133].
Regardless of the current lack of FDA approval to use amifostine non-clinically as a radioprotector for H-ARS in humans associated with nuclear/radiological incidents, we strongly believe that this agent, with its many positive, radioprotective attributes, merits additional consideration in order to secure full regulatory approval for generalized use during radiation exposure emergencies. However, few laboratories have continued to investigate different aspects of its radioprotective efficacies, including investigations of molecular targets, pharmacology, ways to limit its toxicity, and developing modern strategies for drug delivery and extending drug action. To achieve this goal, a recent investigation show differential gene expression in lymphohematopoietic tissues of mice administered 100 mg/kg (minimally toxic doses) and exposed to sublethal doses of 60Co γ-radiation. Irradiation introduced both early and late-arising gene responses that were altered by amifostine prophylactic use and such treatment led to wide-spread dampening of irradiation-related gene response [Citation130]. A group of proto-oncogenes reacted to the entire group of arrayed genes. Disparities in gene expression caused by sublethal dose of radiation were observed and amifostine prophylaxis considerably changed gene expression. This investigation suggests that amifostine may have additional useful attributes which we are not aware of. At present, we are conducting metabolomics and lipidomic studies using blood and various tissue samples collected from amifostine-treated and irradiated/unirradiated animals. In these studies, comparisons are being made between animals treated with the optimal dose of amifostine, where a significant survival benefit was observed but may be associated with side effects, and animals treated with the sub-optimal dose of amifostine which may be devoid of any side effects. The goal of such investigations is to identify the downstream metabolic products in animals treated with different doses of amifostine to identify biomarkers which may help to identify the dose of amifostine that is devoid of any side effects. Our initial observations are encouraging and incoming data suggest that pathway corrections are better with lower dose 50 mg/kg compared to higher dose of 200 mg/kg (unpublished observations). In brief, there is a need for further investigations to simplify the drug dose, administration route, and treatment schedule to reduce the undesired side effects to broaden its clinical application.
Article highlights
Amifostine is a promising radioprotector but it has not received FDA approval for the indication of ARS due to its side effects at doses required for radioprotection against ARS, and other clinically relevant limitations.
It has received FDA approval for limited indications; to reduce the renal toxicity as a result of cisplatin treatment of individuals with ovarian cancer and to reduce the xerostomia in patients undergoing radiotherapy treatment for head and neck cancer.
The radioprotective doses for amifostine to protect hematopoietic tissues of mice are significantly lower; lie between 25 and 50 mg/kg, compared to its dose used in survival studies.
One important approach is poly-pharmacy with the use of small doses of two or more radiation countermeasures with different modes of action to improve the treatment outcome.
Several approaches are being tried to get amifostine or its improved analogs approved by FDA for ARS.
Amifostine modulates several genes in irradiated mice and appears to reverse the radiation-induced changes on some genes.
This box summarizes key points contained in the article.
Author contributions
VK Singh and TM Seed drafted the manuscript, reviewed critically for intellectual content, and approved the final version to be published. Both authors agree to be accountable for all aspects of the work.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Armed Forces Radiobiology Research Institute, the Uniformed Services University of the Health Sciences, or the Department of Defense. Mention of specific therapeutic agents does not constitute endorsement by the U.S. Department of Defense, and trade names are used only for the purpose of clarification. We apologize to those having contributed substantially to the topics discussed herein that we were unable to cite because of space constraints.
Additional information
Funding
References
- Ferguson CD, Potter WC, Sands A, et al. The four faces of nuclear terrorism. Monterey, CA: Center for Nonproliferation Studies, Monterey Institute of International Studies Nuclear Threat Initiative; 2004.
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123.
- Coleman CN, Blakely WF, Fike JR, et al. Molecular and cellular biology of moderate-dose (1-10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17- 18,2001. Radiat Res. 2003;159:812–834.
- Benjamin GC, McGeary M, McCutchen SR. Assessing medical preparedness to respond to a terrorist nuclear event: workshop report. Washington, DC: The National Academies Press; 2009.
- Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3- 4, 2003. Radiat Res. 2004;162:711–728.
- Seed TM. Radiation protectants: current status and future prospects. Health Phys. 2005;89:531–545.
- Singh VK, Garcia M, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part II. Countermeasures for limited indications, internalized radionuclides, emesis, late effects, and agents demonstrating efficacy in large animals with or without FDA IND status. Int J Radiat Biol. 2017;93:870–884.
- Singh VK, Hanlon BK, Santiago PT, et al. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part III. Countermeasures under early stages of development along with ‘standard of care’ medicinal and procedures not requiring regulatory approval for use. Int J Radiat Biol. 2017;93:885–906.
- Singh VK, Newman VL, Romaine PL, et al. Radiation countermeasure agents: an update (2011–2014). Expert Opin Ther Pat. 2014;24:1229–1255.
- Yahyapour R, Shabeeb D, Cheki M, et al. Radiation protection and mitigation by natural antioxidants and flavonoids: implications to radiotherapy and radiation disasters. Curr Mol Pharmacol. 2018;11:285–304.
- Singh VK, Santiago PT, MacVittie TJ. Opportunities and challenges with animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2018;13:987–992.
- U.S. Food and Drug Administration. FDA approves Neupogen for treatment of patients with radiation-induced myelosuppression following a radiological/nuclear incident. 2015. [cited 2016 July 6]. Available from: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/ucm443245.htm
- National Institute of Allergic and Infectious Diseases. Pegfilgrastim approved for treating acute radiation syndrome. 2015; [cited 2016 Aug 18]. Available from: https://www.niaid.nih.gov/topics/radnuc/Pages/pegfilgrastim.aspx.
- U.S. Food and drug Administration. FDA approves Leukine for acute radiation syndrome. 2018. [cited 2018 Apr 01]. Available from: https://www.fda.gov/downloads/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/UCM603226.pdf.
- Hankey KG, Farese AM, Blaauw EC, et al. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat Res. 2015;183:643–655.
- Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc). 2015;51:537–548.
- Singh VK, Seed TM. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today (Barc). 2018;54:679–693.
- Singh VK, Seed TM. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int J Radiat Biol. 2017;93:851–869.
- Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Semin Radiat Oncol. 2003;13:62–72.
- Capizzi RL. The preclinical basis for broad-spectrum selective cytoprotection of normal tissues from cytotoxic therapies by amifostine. Semin Oncol. 1999;26:3–21.
- Capizzi RL, Oster W. Chemoprotective and radioprotective effects of amifostine: an update of clinical trials. Int J Hematol. 2000;72:425–435.
- Capizzi RL. Clinical status and optimal use of amifostine. Oncology (Williston Park). 1999;13: 47–59. discussion 63, 67.
- Nicolatou-Galitis O, Sarri T, Bowen J, et al. Systematic review of amifostine for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21:357–364.
- Gu J, Zhu S, Li X, et al. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One. 2014;9:e95968.
- Piper JR, Stringfellow CR Jr., Elliott RD, et al. S-2-(omega-aminoalkylamino)ethyl dihydrogen phosphorothioates and related compounds as potential antiradiation agents. J Med Chem. 1969;12:236–243.
- Davidson DE, Grenan MM, Sweeney TR. Biological characteristics of some improved radioprotectors. In: Brady LW, editor. Radiation sensitizers, their use in the clinical management of cancer. New York, Masson: Masson Publishing; 1980. p. 309–320.
- Brown DQ, Graham WJ 3rd, MacKenzie LJ, et al. Can WR-2721 be improved upon? Pharmacol Ther. 1988;39:157–168.
- Giambrarresi LI, Walker RI. Prospects of radioprotection. In: Office of the Surgeon General DotA, United States of America, editor. Medical consequences of nuclear warfare. Falls Church, VA: TMM Publications. 1989. p. 245–273.
- Grdina DJ, Kataoka Y, Murley JS. Amifostine: mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact. 2000;16:237–279.
- Ryan SV, Carrithers SL, Parkinson SJ, et al. Hypotensive mechanisms of amifostine. J Clin Pharmacol. 1996;36:365–373.
- Turrisi AT, Glover DJ, Hurwitz S, et al. Final report of the phase I trial of single-dose WR-2721 [S-2-(3-aminopropylamino)ethylphosphorothioic acid]. Cancer Treat Rep 1986;70:1389–1393
- Landauer MR, Davis HD, Dominitz JA, et al. Dose and time relationships of the radioprotector WR-2721 on locomotor activity in mice. Pharmacol Biochem Behav. 1987;27:573–576.
- Landauer MR, Davis HD, Dominitz JA, et al. Long-term effects of radioprotector WR-2721 on locomotor activity and body weight of mice following exposure to ionizing radiation. Toxicology. 1988;49:315–323.
- Landauer MR, Davis HD, Dominitz JA, et al. Comparative behavioral toxicity of four sulfhydryl radioprotective compounds in mice: WR-2721, cysteamine, diethyldithiocarbamate, and N-acetylcysteine. Pharmacol Ther. 1988;39:97–100.
- Dorr RT. Radioprotectants: pharmacology and clinical applications of amifostine. Semin Radiat Oncol. 1998;8:10–13.
- Weiss JF. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect. 1997;105(Suppl 6):1473–1478.
- Seed TM, Fry SA, Neta R, et al. Prevention and treatments: summary statement. Mil Med. 2002;167:87–93.
- Roberts JT, Priestman TJ. A review of ondansetron in the management of radiotherapy-induced emesis. Oncology. 1993;50:173–179.
- Cumberland Pharmaceuticals Inc. ETHYOL- amifostine injection, powder, lyophilized, for solution. 2017. [cited 2018 Oct 28]. Available from: file:///C:/Users/vsingh/Downloads/20170606_f11c0753-ef0a-4e1b-83dd-8543649df9a5.pdf.
- Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345.
- Ferraiolo DM, Veitz-Keenan A. Insufficient evidence for interventions to prevent dry mouth and salivary gland dysfunction post head and neck radiotherapy. Evid Based Dent. 2018;19:30–31.
- Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol. 2009;85:539–573.
- Murray D, McBride WH. Radioprotective agents. In: Kirk-Othmer, editor. Kirk-Othmer encyclopedia of chemical technology. New York: John Wiley & Sons, Inc; 1996. p. 63–1006.
- Ziegler DM, Duffel MW, Pulsen LL. Studies on the nature and regulation of the cellular thiol: disulphide potential. In: Elliott K, Whelan J, eds. Ciba Foundation Symposium on Sulphur in Biology - Novartis Foundation Symposia; Amsterdam: Excerpta Medica; 1980. p. 191–204
- Weiss JF, Kumar KS. Antioxidant mechanisms in radiation injury and radioprotection. In: Chow CK, editor. Cellular antioxidant defense mechanisms. Boca Raton, FL: CRC Press; 1988. p. 163–189.
- Spencer CM, Goa KL. Amifostine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential as a radioprotector and cytotoxic chemoprotector. Drugs. 1995;50:1001–1031.
- Giambarresi LI, Walker RI. Prospects for radioprotection. In: Zajtchuk R, Jenkins DP, Bellamy RF, editors. Textbook of military medicine, part II, medical consequences of nuclear warfare. Falls Church, VA: TMM Publications, Office of the Surgeon General; 1996. p. 276–303.
- Yuhas JM, Storer JB. Chemoprotection against three modes of radiation death in the mouse. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;15:233–237.
- Sigdestad CP, Connor AM, Scott RM. The effect of S-2-(3-aminopropylamino)ethylphosphorothioic acid (WR-2721) on intestinal crypt survival. I. 4 MeV x-rays. Radiat Res. 1975;62:267–275.
- Rasey JS, Nelson NJ, Mahler P, et al. Radioprotection of normal tissues against gamma rays and cyclotron neutrons with WR-2721: LD50 studies and 35S-WR-2721 biodistribution. Radiat Res. 1984;97:598–607.
- Brown DQ, Pittock JW 3rd, Rubinstein JS. Early results of the screening program for radioprotectors. Int J Radiat Oncol Biol Phys. 1982;8:565–570.
- Doherty DG, Burnett WT Jr., Shapira R. Chemical protection against ionizing radiation. II. Mercaptoalkylamines and related compounds with protective activity. Radiat Res. 1957;7:13–21.
- Kuna P. Duration and degree of radioprotection of WR-2721 in mice following its intraperitoneal, intramuscular and subcutaneous administration. Radiobiol Radiother (Berl). 1983;24:357–364.
- Sigdestad CP, Grdina DJ, Connor AM, et al. A comparison of radioprotection from three neutron sources and 60Co by WR-2721 and WR-151327. Radiat Res. 1986;106:224–233.
- Wilmore BH, Cassidy PB, Warters RL, et al. Thiazolidine prodrugs as protective agents against gamma-radiation-induced toxicity and mutagenesis in V79 cells. J Med Chem. 2001;44:2661–2666.
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31.
- Santini V. Amifostine: chemotherapeutic and radiotherapeutic protective effects. Expert Opin Pharmacother. 2001;2:479–489.
- Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747.
- Purdie JW, Inhaber ER, Schneider H, et al. Interaction of cultured mammalian cells with WR-2721 and its thiol, WR-1065: implications for mechanisms of radioprotection. Int J Radiat Biol Relat Stud Phys Chem Med. 1983;43:517–527.
- Glover D, Negendank W, Delivoria-Papadopoulos M, et al. Alterations in oxygen transport following WR-2721. Int J Radiat Oncol Biol Phys. 1984;10:1565–1568.
- Koukourakis MI, Giatromanolaki A, Zois CE, et al. Normal tissue radioprotection by amifostine via Warburg-type effects. Sci Rep. 2016;6:30986.
- Savoye C, Swenberg C, Hugot S, et al. Thiol WR-1065 and disulphide WR-33278, two metabolites of the drug ethyol (WR-2721), protect DNA against fast neutron-induced strand breakage. Int J Radiat Biol. 1997;71:193–202.
- Rubin DB, Drab EA, Kang HJ, et al. WR-1065 and radioprotection of vascular endothelial cells. I. Cell proliferation, DNA synthesis and damage. Radiat Res. 1996;145:210–216.
- Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490.
- Shimizu S, Eguchi Y, Kamiike W, et al. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 1996;56:2161–2166.
- Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63(Suppl 2):2–10.
- Grdina DJ, Murley JS, Kataoka Y, et al. Radioprotectors: current status and new directions. Radiat Res. 2005;163:704–705.
- Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524.
- Koukourakis MI. Amifostine in clinical oncology: current use and future applications. Anticancer Drugs. 2002;13:181–209.
- Srinivasan V, Weiss JF, Kumar KS. Radioprotection by combination of WR-151327, vitamin E and selenomethionine. 40th Annual Meeting of the Radiation Research Society; Salt Lake City UT: Radiation Research Society; 1992
- Singh VK, Fatanmi OO, Wise SY, et al. The potentiation of the radioprotective efficacy of two medical countermeasures, gamma-tocotrienol and amifostine, by a combination prophylactic modality. Radiat Prot Dosimetry. 2016;172:302–310.
- Grdina DJ, Murley JS, Kataoka Y, et al. Relationships between cytoprotection and mutation prevention by WR-1065. Mil Med. 2002;167:51–53.
- Seed TM, Inal CE, Singh VK. Radioprotection of hematopoietic progenitors by low dose amifostine prophylaxis. Int J Radiat Biol. 2014;90:594–604.
- Fatome M, Courteille F, Laval JD, et al. Radioprotective activity of ethylcellulose microspheres containing WR 2721, after oral administration. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:21–29.
- Srinivasan V, Pendergrass JA Jr., Kumar KS, et al. Radioprotection, pharmacokinetic and behavioural studies in mouse implanted with biodegradable drug (amifostine) pellets. Int J Radiat Biol. 2002;78:535–543.
- Pamujula S, Graves RA, Freeman T, et al. Oral delivery of spray dried PLGA/amifostine nanoparticles. J Pharm Pharmacol. 2004;56:1119–1125.
- Steel-Goodwin L, Egan JE, Kendrick JM, et al. Comparative intestinal and testes toxicity of four aminothiols in irradiated and nonirradiated mice. Ann Clin Lab Sci. 1993;23:439–447.
- Weiss JF, Kumar KS, Srinivasan V, et al. Comparison of toxicity and radioprotective efficacy of WR-2721 and WR-3689 in mice. Proc Am Assoc Can Res. 1990;31:433.
- Peebles DD, Soref CM, Copp RR, et al. ROS-scavenger and radioprotective efficacy of the new PrC-210 aminothiol. Radiat Res. 2012;178:57–68.
- Copp RR, Peebles DD, Soref CM, et al. Radioprotective efficacy and toxicity of a new family of aminothiol analogs. Int J Radiat Biol. 2013;89:485–492.
- Soref CM, Hacker TA, Fahl WE. A new orally active, aminothiol radioprotector-free of nausea and hypotension side effects at its highest radioprotective doses. Int J Radiat Oncol Biol Phys. 2012;82:e701–7.
- Oiry J, Pue JY, Imbach JL, et al. Synthesis and radioprotective activity of new cysteamine and cystamine derivatives. J Med Chem. 1986;29:2217–2225.
- Soni R, Arora A, Singh S, et al. Radio-modifying effects of amifostine analogues in whole body gamma irradiated mice. International Conference on Radiation Biology: Frontiers in Radiobiology: Immunomodulation, Countermeasures & Therapeutics; Nov 11-13; New Delhi, India; 2014.
- Gautam A, Gupta A, Lomash V, et al. Prophylactic efficacy of combination of DRDE-07 and its analogues with amifostine against sulphur mustard induced systemic toxicity. Indian J Exp Biol. 2010;48:752–761.
- Kulkarni AS, Vijayaraghavan R, Anshoo G, et al. Evaluation of analogues of DRDE-07 as prophylactic agents against the lethality and toxicity of sulfur mustard administered through percutaneous route. J Appl Toxicol. 2006;26:115–125.
- Arora A, Bhuria V, Hazari PP, et al. Amifostine analog, DRDE-30, attenuates bleomycin-induced pulmonary fibrosis in mice. Front Pharmacol. 2018;9:394.
- Xie J, Wang C, Zhao F, et al. Application of multifunctional nanomaterials in radioprotection of healthy tissues. Adv Healthc Mater. 2018;7:e1800421.
- Pamujula S, Kishore V, Rider B, et al. Radioprotection in mice following oral delivery of amifostine nanoparticles. Int J Radiat Biol. 2005;81:251–257.
- Pamujula S, Kishore V, Rider B, et al. Radioprotection in mice following oral administration of WR-1065/PLGA nanoparticles. Int J Radiat Biol. 2008;84:900–908.
- Li WM, Chiang CS, Huang WC, et al. Amifostine-conjugated pH-sensitive calcium phosphate-covered magnetic-amphiphilic gelatin nanoparticles for controlled intracellular dual drug release for dual-targeting in HER-2-overexpressing breast cancer. J Control Release. 2015;220:107–118.
- Kolate A, Baradia D, Patil S, et al. PEG - a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81.
- Yang X, Ding Y, Ji T, et al. Improvement of the in vitro safety profile and cytoprotective efficacy of amifostine against chemotherapy by PEGylation strategy. Biochem Pharmacol. 2016;108:11–21.
- Patchen ML, MacVittie TJ. Granulocyte colony-stimulating factor and amifostine (Ethyol) synergize to enhance hemopoietic reconstitution and increase survival in irradiated animals. Semin Oncol. 1994;21:26–32.
- Patchen ML, MacVittie TJ, Solberg BD, et al. Radioprotection by polysaccharides alone and in combination with aminothiols. Adv Space Res. 1992;12:233–248.
- Bardet E, Martin L, Calais G, et al. Preliminary data of the GORTEC 2000-02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Semin Oncol. 2002;29:57–60.
- Mahmood J, Jelveh S, Zaidi A, et al. Targeting the Renin-angiotensin system combined with an antioxidant is highly effective in mitigating radiation-induced lung damage. Int J Radiat Oncol Biol Phys. 2014;89:722–728.
- Hirouchi T, Ito K, Nakano M, et al. Mitigative effects of a combination of multiple pharmaceutical drugs on the survival of mice exposed to lethal ionizing radiation. Curr Pharm Biotechnol. 2015;17:190–199.
- Fish BL, Gao F, Narayanan J, et al. Combined hydration and antibiotics with lisinopril to mitigate acute and delayed high-dose radiation injuries to multiple organs. Health Phys. 2016;111:410–419.
- Plett PA, Chua HL, Sampson CH, et al. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2014;106:7–20.
- Cox G. Improving survival in a mouse H-ARS model using combinations of hematopoietic growth factors and an ACE inhibitor. A poly-pharmacy approach to mitigate acute radiation syndrome (ARS). Rockville, MD: National Institute of Allergy and Infectious Diseases, National Institutes of Health.
- Seed TM, Inal CE, Deen JE. Assessment of a combined G-CSF plus IL-11 cytokine treatment for radiation-induced hematopoietic injury. Proceeding of the 48th Annual Meeting of the Radiation Research Society; San Juan, Puerto Rico; 2001. p. 161
- Medhora M. 2018. Enhanced mitigation of radiation-induced injuries by combining angiotensin-converting enzyme (ACE) inhibitors with other countermeasures. A poly-pharmacy approach to mitigate acute radiation syndrome (ARS). Rockville, MD: National Institute of Allergy and Infectious Diseases, National Institutes of Health.
- Hofer M, Hoferova Z, Depes D, et al. Combining pharmacological countermeasures to attenuate the acute radiation syndrome -A concise review. In: Molecules. 2017;22.
- Hofer M, Falk M, Komurkova D, et al. Two new faces of amifostine: protector from DNA damage in normal cells and inhibitor of DNA repair in cancer cells. J Med Chem. 2016;59:3003–3017.
- Patchen ML. Amifostine plus granulocyte colony-stimulating factor therapy enhances recovery from supralethal radiation exposures: preclinical experience in animals models. Eur J Cancer. 1995;31A:S17–21.
- Patchen ML, MacVittie TJ, Souza LM. Post-irradiation treatment with granulocyte colony-stimulating factor and preirradiation WR-2721 administration synergize to enhance hemopoietic reconstitution and increase survival. Int J Radiat Oncol Biol Phys. 1992;22:773–779.
- Neumeister P, Jaeger G, Eibl M, et al. Amifostine in combination with erythropoietin and G-CSF promotes multilineage hematopoiesis in patients with myelodysplastic syndrome. Leuk Lymphoma. 2001;40:345–349.
- Winczura P, Jassem J. Combined treatment with cytoprotective agents and radiotherapy. Cancer Treat Rev. 2010;36:268–275.
- Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–845.
- Weiss JF, Hoover RL, Kumar KS. Selenium pretreatment enhances the radioprotective effect and reduces the lethal toxicity of WR-2721. Free Radic Res Commun. 1987;3:33–38.
- Kaplan B, Orhan O, Yazici C, et al. Radioprotective effects of amifostine (WR 2721) and vitamin E on whole-body-irradiated rat liver. Turk Klin Tip Bilim Derg. 2009;29:1055–1062.
- Singh VK, Hauer-Jensen M. Gamma-tocotrienol as a promising countermeasure for acute radiation syndrome: current status. Int J Mol Sci. 2016;17:e663.
- Kulkarni S, Singh PK, Ghosh SP, et al. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62:278–285.
- Miller RC, Murley JS, Grdina DJ. Metformin exhibits radiation countermeasures efficacy when used alone or in combination with sulfhydryl containing drugs. Radiat Res. 2014;181:464–470.
- Xu G, Wu H, Zhang J, et al. Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic Biol Med. 2015;87:15–25.
- Kumar KS, Vaishnav YN, Weiss JF. Radioprotection by antioxidant enzymes and enzyme mimetics. Pharmacol Ther. 1988;39:301–309.
- Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44:1173–1183.
- Hanson WR. Radiation protection of murine intestine by WR-2721, 16,16-dimethyl prostaglandin E2, and the combination of both agents. Radiat Res. 1987;111:361–373.
- Hanson WR, Houseman KA, Collins PW. Radiation protection in vivo by prostaglandins and related compounds of the arachidonic acid cascade. Pharmacol Ther. 1988;39:347–356.
- Mota JM, Soares PM, Menezes AA, et al. Amifostine (Wr-2721) prevents indomethacin-induced gastric damage in rats: role of non-protein sulfhydryl groups and leukocyte adherence. Dig Dis Sci. 2007;52:119–125.
- Lu L, Pelus LM, Broxmeyer HE. Modulation of the expression of HLA-DR (Ia) antigens and the proliferation of human erythroid (BFU-E) and multipotential (CFU-GEMM) progenitor cells by prostaglandin E. Exp Hematol. 1984;12:741–748.
- Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455.
- Patchen ML, MacVittie TJ, Jackson WE. Postirradiation glucan administration enhances the radioprotective effects of WR-2721. Radiat Res. 1989;117:59–69.
- Patchen ML, MacVittie TJ, Weiss JF. Combined modality radioprotection: the use of glucan and selenium with WR-2721. Int J Radiat Oncol Biol Phys. 1990;18:1069–1075.
- Pillai TG, Uma Devi P. Mushroom beta glucan: potential candidate for post irradiation protection. Mutat Res. 2013;751:109–115.
- Fedorocko P, Brezani P, Mackova NO. Radioprotective effects of WR-2721, Broncho-Vaxom and their combinations: survival, myelopoietic restoration and induction of colony-stimulating activity in mice. Int J Immunopharmacol. 1994;16:177–184.
- Mackova NO, Fedorocko P. Combined radioprotective effect of Broncho-Vaxom and WR-2721 on hemopoiesis and circulating blood cells. Neoplasma. 1995;42:25–30.
- Jiang S, Shen X, Liu Y, et al. Radioprotective effects of Sipunculus nudus L. polysaccharide combined with WR-2721, rhIL-11 and rhG-CSF on radiation-injured mice. J Radiat Res. 2015;56:515–522.
- Liu W, Chen Q, Wu S, et al. Radioprotector WR-2721 and mitigating peptidoglycan synergistically promote mouse survival through the amelioration of intestinal and bone marrow damage. J Radiat Res. 2015;56:278–286.
- Seed T, Singh VK, Hanlon BK. Early and late changes in radiation‑induced gene expression arrays following radioprotection with amifostine. J Radiat Cancer Res. 2019;10:44–57.
- Koukourakis MI, Simopoulos C, Minopoulos G, et al. Amifostine before chemotherapy: improved tolerance profile of the subcutaneous over the intravenous route. Clin Cancer Res. 2003;9:3288–3293.
- Praetorius NP, Mandal TK. Alternate delivery route for amifostine as a radio-/chemo-protecting agent. J Pharm Pharmacol. 2008;60:809–815.
- Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20.