ABSTRACT
Objectives: The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin is indicated for type 2 diabetes mellitus (T2DM). However, the long-term safety, effectiveness, and clinical relationship with cardiovascular events of vildagliptin have not been evaluated in Japan.
Methods: The authors conducted post-marketing surveillance (PMS) to evaluate the safety and effectiveness of vildagliptin in more than 3000 Japanese T2DM patients for up to 3 years. Main assessments included demographics, major adverse cardiovascular events (MACE), adverse events (AEs), adverse drug reactions (ADRs), and glycated hemoglobin (HbA1c).
Results: In this PMS, 3831 patients (775 sites) were registered in April 2010 − April 2012. The safety analysis population comprised 3769 patients; 2085 patients were aged ≥65 years, and 240, 411, and 114 had renal impairment, hepatic impairment, and heart failure, respectively. The median treatment duration was 2.7 years. The incidence of MACE was 6.04 cases/1000 person-years, mostly attributable to cerebrovascular events (4.27 cases/1000 person-years). The AE and ADR incidences were 26.0% and 5.3%, respectively. The incidence of hypoglycemia was 0.6%. No significant changes in body weight occurred and mean change in HbA1c from baseline at final assessment was −0.74 ± 1.41% (p < 0.0001).
Conclusions: In real-world clinical settings, vildagliptin was well tolerated, with similar profiles as previously reported.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disorder of glucose metabolism and is associated with obesity, hypertension, and dyslipidemia. It is also a major risk factor for cardiovascular disease (CVD) and mortality [Citation1–Citation3]. The current treatments for T2DM are aimed at tight glycemic control and the control of CVD risk factors, such as hypertension, dyslipidemia, smoking, and overweight/obesity.
The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control by preventing the degradation of glucagon-like polypeptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) and improves pancreatic β-cell and α-cell function [Citation4,Citation5]. Accordingly, DPP-4 inhibitors provide effective glycemic control with less risk of hypoglycemia and weight gain [Citation6]. Several multicenter randomized controlled trials have shown that vildagliptin treatment, as monotherapy or add-on therapy, is effective and well tolerated [Citation7–Citation11]. Despite the ample evidence supporting the efficacy and relatively few adverse events (AEs) of DPP-4 inhibitors, concerns have been raised about the increased risk of cardiovascular (CV) events (CV death, myocardial infarction [MI], or ischemic stroke) in patients with T2DM [Citation12,Citation13].
These concerns regarding CV events were initially evaluated in the SAVOR-TIMI 53 (saxagliptin) and EXAMINE (alogliptin) trials in 2008, and then more recently in the TECOS (sitagliptin) trial [Citation14–Citation16]. In the SAVOR-TIMI 53 study, it was estimated that the rate of a CV event with saxagliptin treatment was similar to placebo (hazard ratio [HR] 1.00, 95% confidence interval [CI] 0.89–1.12) [Citation14]. In the EXAMINE trial, alogliptin treatment was assessed in T2DM patients who had a high CV event risk [Citation15]. In this study, alogliptin was not associated with an increased risk of a CV event compared with placebo (HR 0.96, 95% CI upper boundary ≤1.16). Similarly, in the TECOS trial there was no significant difference in CV event endpoints between patients receiving placebo or the DPP-4 inhibitor sitagliptin (HR 0.98, 95% CI 0.88–1.09) [Citation16].
In a meta-analysis of vildagliptin treatment in non-Japanese patients, it was shown that there was no increase in the incidence of major CV events (risk ratio 0.82, 95% CI 0.61–1.11) [Citation17]. Murayama et al recently reported results of a 2-year post-marketing surveillance (PMS) study of vildagliptin in nearly 20,000 Japanese T2DM patients, in which the incidence of macrovascular disease (including MI and stroke) was 1.14% [Citation18]. However, the entire CV risk was not directly evaluated and the study had a limited follow-up period [Citation18]. Another PMS in a large Japanese T2DM population recently provided real-world efficacy and safety data of vildagliptin used concomitantly with oral antidiabetic drugs and insulin for a period of up to 52 weeks. However, CV outcomes were not specifically evaluated [Citation19]. Thus, further study is necessary to clarify the incidence of CV events in Japanese patients receiving long-term vildagliptin treatment. This is relevant because the incidence and nature of CV events in Japanese and Caucasian patients are different: the incidence of stroke is higher in Japanese patients while the incidence of MI is lower [Citation20]. Moreover, the long-term safety profile of vildagliptin in patients with different patient backgrounds, including renal impairment, hepatic impairment, and heart failure, has not been confirmed. Therefore, the present 3-year PMS was conducted to evaluate the real-world, long-term safety, focusing on the CV safety of vildagliptin in a large group of Japanese patients with diverse baseline characteristics.
2. Patients and methods
2.1. Patients
This was an observational PMS conducted in accordance with the Japanese Good Post-marketing Study Practice guidelines. Patients were centrally registered from April 2010 to April 2012 and were observed for up to 36 months until March 2015 across 775 clinics and hospitals throughout Japan. Patients were included in the PMS if they were prescribed vildagliptin during the above registration period, in accordance with the approved label, for T2DM that was inadequately controlled by diet and exercise, or by diet and exercise combined with sulfonylurea treatment. Patients who had previously been prescribed vildagliptin were not included in this study.
2.2. Treatment
Vildagliptin was administered orally twice daily (in the morning and evening) at a dose of 50 mg. Depending on the patient’s condition, the regimen could be changed to once daily (in the morning) at a dose of 50 mg at the discretion of the investigators.
2.3. Survey
In brief, the following background variables were recorded: sex, age, treatment start date, reason for vildagliptin administration, duration of illness, medical complications, disease history, and body mass index (BMI). Patients with hepatic or renal impairment had their severity of dysfunction assessed and recorded. Concomitant drugs were also recorded, including the name of drug, dosage, route of dosing, and dosing period.
Adverse events (AEs) and adverse drug reactions (ADRs) that occurred during the surveillance and up to 1 month after were also recorded using the Japanese Medical Dictionary for Regulatory Activities (MedDRA-J) version 20.0. This included the date of onset, name of the AE, severity, outcome, date of outcome assessment, causal relationship to the drug, other factors responsible for the event, and patient’s history of ADRs. AEs coded as diabetes mellitus were defined as worsening or exacerbation of T2DM as well as the presence of elevated blood glucose levels. CV mortality (cardiac and cerebrovascular), cardiac arrest (resuscitated and non-resuscitated), acute coronary syndrome (ST-segment elevation MI [STEMI] and non-STEMI), cerebrovascular events (stroke [ischemic/hemorrhagic] and transient ischemic attack), and hospitalization due to unstable angina were defined as major adverse cardiovascular events (MACE). The clinical course outcomes included in the surveillance were: glycated hemoglobin (HbA1c), fasting blood glucose, urinary glucose, urinary protein, 12-lead electrocardiogram, blood pressure, heart rate, and body weight. Patients’ clinical data were evaluated every 3 months from the start of the survey, after 12 months, and then every 6 months thereafter until the end of the survey period (3 years). Laboratory variables included in the surveillance were liver enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), serum total bilirubin, serum creatinine, serum uric acid, urea nitrogen, creatine kinase, and lipids. These were measured during routine clinical management.
2.4. Statistical methods
The sample size calculation included 3000 cases to enable detection of ADRs that would occur at an incidence of 0.1% with a probability of 95%. The safety analysis population included all registered patients who received vildagliptin. The effectiveness analysis population included all patients in the safety analysis population who also had their glycemic control assessed.
The statistical tests used were Fisher’s exact test for nominal variables and the Mann–Whitney U-test for ordinal variables. However, in cases where ordinal variables were summarized in a 2 × 2 contingency table, Fisher’s test was used. Quantitative data on HbA1c, fasting blood glucose, AST, ALT, sitting blood pressure (systolic, diastolic), heart rate, body weight, and waist circumference are presented as the mean, standard deviation (SD), and the change from baseline. SAS® version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
3. Results
3.1. Patient disposition and baseline characteristics
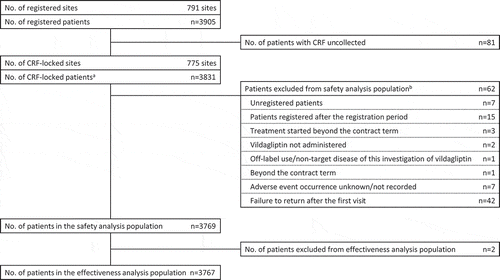
A total of 3905 patients were registered, and 3831 patients had locked case report forms. Of these, a further 62 patients were excluded from the safety analysis population, for reasons such as ‘failure to return after the first visit’, ‘registered after the registration period’, and ‘unregistered patient’. Therefore, the safety analysis population included a total of 3769 patients. As the primary effectiveness evaluation was missing or not recorded for two patients, these were therefore excluded from the effectiveness analysis population, which included a total of 3767 patients ().
Figure 1. Patient disposition.
aNo. of CRF-locked patients includes seven patients whose CRFs were collected despite being unregistered or ineligible for registration.bSome patients were excluded for more than one reason. CRF, case report form.
The baseline patient treatment characteristics are shown in . There were 2085 (55.3%) elderly patients (aged ≥65 years), 240 (6.4%) patients with renal impairment, 411 (10.9%) patients with hepatic impairment, and 114 (3.0%) patients with heart failure. There were 2274 (60.3%) patients with hypertension and 2198 (58.3%) patients with dyslipidemia. The mean (SD) and median durations of the observation period were 2.0 (1.11) and 2.7 years, respectively, with 43.0% of patients observed for more than 3.0 years.
Table 1. Baseline patient characteristics (safety and effectiveness analysis populations).
3.2. Safety outcomes
In this PMS, the incidence of MACE was 48 cases (6.04 cases per 1000 person-years) with the majority caused by cerebrovascular events (34 cases), followed by acute coronary syndrome (8 cases), and CV mortality (6 cases) (). There was no cardiac arrest or hospitalization due to unstable angina.
Table 2. Incidence of MACE (safety analysis populationa).
The incidence of AEs was 26.0% (979/3769 patients) and AEs occurring in ≥1% of patients are shown in . The most common AEs that occurred in more than 50 patients were hypertension (1.7%, 64 patients), blood glucose increased (1.7%, 63 patients), diabetes mellitus (i.e., worsening of T2DM) (1.6%; 60 patients), blood triglycerides increased (1.6%, 60 patients), diabetes mellitus inadequate control (1.6%, 59 patients), and hepatic function abnormal (1.5%, 55 patients).
Table 3. Most common adverse events (occurring in ≥1%, safety analysis population).
The incidence of serious AEs (SAEs) was 6.0% (225/3769 patients). The most common SAEs were cerebral infarction (21 patients) followed by pneumonia (16 patients) and angina pectoris (10 patients). There were 47 deaths; however, all of the fatal AEs were judged to be not related to vildagliptin except for a case of aplastic anemia in one patient, in whom the relationship with vildagliptin was judged to be unknown. The incidence of specific AEs related to DPP-4 inhibitor administration included 24 cases (0.6%) of hypoglycemia, two cases (0.05%) of acute pancreatitis, five cases (0.1%) of pancreatic carcinoma, and one case (0.03%) of metastatic pancreatic carcinoma. In terms of body weight changes, there were no significant changes from the start of treatment until the final assessment.
The overall incidence of ADRs was 5.3%, and 246 ADRs were reported in 200 patients. The most common ADRs included 21 cases of hypoglycemia (0.56%), 16 cases of constipation (0.42%), 13 cases of hepatic function abnormal (0.34%), and 12 cases of diabetes mellitus inadequate control (0.32%). There were 29 serious ADRs, the most common of which were two cases each of hypoglycemia, diabetes mellitus inadequate control, and hepatic function abnormal.
The number and proportion of ADRs when stratified by patient characteristics are shown in Supplementary Table 1. The incidence of ADRs in elderly patients (aged ≥65 years) was 6.04% (126/2085 patients). Patients with renal impairment had an ADR incidence of 6.67% (16/240 patients), and those with hepatic impairment had an ADR incidence of 6.08% (25/411 patients). The safety analysis population included 114 patients with prior heart failure complications. By New York Heart Association class, the incidence of ADRs was 8.22% (6/73 patients) in the class I group, 12.90% (4/31 patients) in the class II group, 0.00% in the class III group (0/6 patients) and 0.00% in the class IV group (0/2 patients).
The incidences of ADRs were significantly associated with elderly age (p = 0.0281) and disease duration (p = 0.0081). However, after adjusting for the other characteristics, a statistically significant difference in the incidence of ADRs was found only for disease duration.
3.3. Effectiveness outcomes
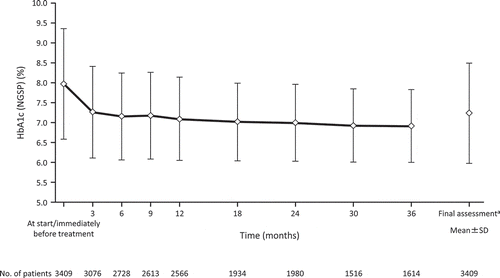
The mean changes in HbA1c (%) from baseline until the final assessment are shown in . The change in HbA1c [mean (SD)] from baseline to the final assessment was −0.74% (1.41) (p < 0.0001). At the final assessment, the mean (SD) HbA1c had decreased from 7.97% (1.39) to 7.23% (1.26). Additionally, the mean (SD) change in fasting blood glucose from baseline to the final assessment was −26.7 mg/dL (69.5) (p < 0.0001) (Supplementary Figure 1).
Figure 2. Time course of HbA1c levels.
aFinal assessment refers to the last measurement closest (but not later than 30 days) to the last administration of the drug.HbA1c, glycated hemoglobin; NGSP, National Glycohemoglobin Standardization Program; SD, standard deviation
The mean changes in HbA1c (%) from baseline by patient characteristics are shown in Supplementary Table 2. The mean (SD) change in HbA1c in elderly patients was −0.66% (1.26). The mean (SD) changes in HbA1c in patients with renal impairment, hepatic impairment, and heart failure were −0.56% (1.31), −0.68% (1.53), and −0.58% (1.51), respectively.
4. Discussion
This PMS registered more than 3000 Japanese T2DM patients across 775 clinics and hospitals and included an appropriate number of elderly patients, as well as those with renal impairment, hepatic impairment, and heart failure. Because patients with diverse baseline characteristics and medical backgrounds were included, it was possible to investigate vildagliptin use in a broad variety of T2DM patients. Although mean HbA1c was higher among patients in the present study in comparison to the 54,503 T2DM patients registered in a nationwide database as of 2010 (7.96% vs. 7.20%), mean age and BMI were similar [Citation21].
The incidence of MACE in this study was a composite of 6.04 cases per 1000 person-years. In comparison to other DPP-4 inhibitor clinical trials, vildagliptin-treated patients in this study experienced fewer CV events according to the reported Kaplan–Meier estimates for saxagliptin, alogliptin, and sitagliptin [Citation14–Citation16]. This may be supported by the observed low incidence of hypoglycemia and blood glucose fluctuations in vildagliptin-treated patients, as hypoglycemia is believed to be associated with increased CVD risk [Citation22,Citation23]. Vildagliptin has previously been shown not to inhibit the action of GIP, which is required to increase blood glucose levels during hypoglycemia [Citation24], and data show that vildagliptin can prevent blood glucose fluctuations [Citation25–Citation27]. These data support the suggestion that vildagliptin does not increase the risk of CV events. This is further confirmed by the findings of a 2-year PMS analyzing the micro- and macrovascular complications in Japanese T2DM patients treated with vildagliptin, which concluded that vildagliptin did not increase or worsen diabetic micro- or macrovascular complications. After 2 years, the incidence of macrovascular complications, including MI and stroke, was low at 1.14% [Citation18]. Moreover, it was reported that background patient characteristics associated with macrovascular disease were age and history of macrovascular disease [Citation18]. In the Japan Diabetes Complications Study (JDCS), the incidences of coronary heart disease and stroke were reported to be 9.59 and 7.45 cases per 1000 person-years [Citation28]. In the JDDM20 cohort study, it was shown that Japanese T2DM patients without a history of CV disease in the primary care setting had incidences of coronary heart disease, ischemic stroke, and peripheral artery disease of 4.4, 3.1, and 0.7 cases per 1000 person-years, respectively [Citation29]. The corresponding incidences found in this PMS were not higher than those obtained from these other studies/registries. In addition, two meta-analyses assessing the cerebrovascular, CV, and heart failure safety profile of vildagliptin concluded vildagliptin is not associated with an increased risk of MACE, CV events, or heart failure relative to its comparators, even in patients at increased risk of cerebrovascular and CV events [Citation17,Citation30].
The 3-year follow-up RUBY PMS evaluated 10,696 Japanese patients with T2DM treated with teneligliptin [Citation31], and reported similar incidence of ADRs (3.85%) and ADRs in patients with renal impairment (3.24%–7.14% in grade 1–5) as in the present PMS (5.3% and 6.67%, respectively). The most frequent ADRs reported with teneligliptin in the RUBY PMS were gastrointestinal disorders, including constipation (0.68%), hypoglycemia (0.36%), and hepatic-impairment-related ADRs (0.44%), which were similar to the ADR rates observed in the present PMS (hypoglycemia [0.56%], constipation [0.42%], and hepatic function abnormal [0.34%]). In the RUBY PMS, the incidence of CV events (broadly defined as MI and central nervous system hemorrhage and cerebrovascular condition) reported as AEs was 0.33 cases per 100 patient-years (85 events/10,696 patients [0.79%]) and that of CV events reported as ADRs was 0.07 per 100 patient-years (18 events/10,696 patients [0.17%]) [Citation31]. A recent PMS evaluated 2235 Japanese T2DM patients who were initiating linagliptin monotherapy and who were followed-up for up to 3 years [Citation32]. The reported ADR rate was higher at 10.7% than in the present PMS, with the most frequent ADRs being worsening diabetes (1.6%), constipation (0.9%), diabetes mellitus inadequate control (0.6%) and hypertension (0.6%). CV events were classed as serious ADRs, of which the most common was cerebral infarction (0.2%) followed by MI, pancreatic carcinoma, death, sudden death, and fall (each, 0.1%). The incidence of ADRs was higher in patients aged ≥65 years (12.0%) than in those aged <65 years (8.9%) [Citation32]. Altogether, these PMS data from other DDP-4 inhibitors are consistent with those previously reported and further validate the safety profile of DDP-4 inhibitors, and vildagliptin in particular.
The results of this PMS showed that renal impairment, hepatic impairment, and heart failure (complications of T2DM) were not associated with an increased incidence of ADRs. Age and disease duration were significantly associated with a higher incidence of ADRs, but after adjusting for other characteristics, disease duration was the only significant patient characteristic. Age was a significant factor in a previous long-term PMS of T2DM Japanese treated with vildagliptin [Citation18], while the incidence rates of ADRs were not significantly different in elderly patients (>65 years) or non-elderly patients (≥65 years) in a 52-week PMS of vildagliptin [Citation19]. Because a higher number of AEs and ADRs are generally attributed to longer-term treatment, a degree of caution is still required when prescribing vildagliptin to patients with these background characteristics.
There is extensive published evidence demonstrating the effectiveness of vildagliptin treatment in T2DM patients, as either monotherapy or add-on therapy [Citation7–Citation11]. However, it is important to consider that there are ethnic differences in DPP-4 inhibitor effectiveness between non-Asian [Citation14–Citation16] and Asian populations, as reported in the subanalysis of the JDCS [Citation28] and the JDDM20 cohort study [Citation29]. The glucose-lowering effect of DPP-4 inhibitors is more pronounced in Asian populations than in Caucasians, primarily due to differences in BMI and the pathophysiological features of T2DM [Citation33]. This was recently highlighted in a post hoc analysis of the TECOS trial, which showed that DPP-4 inhibitor treatment is more effective in patients with Asian ethnicity [Citation34]. When comparing the time courses of HbA1c and fasting blood glucose reported from vildagliptin pre-approval data, reduced HbA1c (range of 0.64% to 1.00%) was observed in studies of various lengths and settings [Citation8,Citation9,Citation35–Citation38], while fasting blood glucose was reduced by 27.1mg/dL in a phase II trial [Citation11]. No noticeable differences were observed between these pre-approval data and the present PMS data. Therefore, the present PMS data further confirm the amply demonstrated real-world effectiveness of vildagliptin.
This study has some limitations. The main limitations were the absence of a control group for comparison, absence of data on treatment regimens and details of which patients received specific treatment regimens, and the lack of generalizability outside the Japanese population. Furthermore, in real-world clinical settings, it cannot be ruled out that the safety profile and observed improvements in effectiveness could be attributable to the natural course of the disease or other unknown factors.
5. Conclusions
The present PMS results showed that the observed risk of major adverse CV events was acceptable. The safety profile and improvements in glycemic control observed in this PMS were consistent with those reported in pre-approval clinical trials; no differences in incidences of AEs were observed, and there were no differences in AE incidence according to patient background characteristics. The effectiveness of vildagliptin for the long-term treatment of patients with T2DM in Japan was confirmed.
Author contributions
Y Shinfuku was involved in the study design and study concept, T Sasajima was involved in the statistical analyses, and Y Ishida was involved in drafting the manuscript. All authors were involved in data interpretation, discussion of results, critical revision of the manuscript, and final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Declaration of Interest
Y Ishida, H Murayama, Y Shinfuku, T Taniguchi, T Sasajima and N Oyama report research funding and employment by Novartis Pharma K. K. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have been the principal investigator on two vildagliptin and vildagliptin/metformin studies in Belgium, which were sponsored by Novartis. These were contracted with a company in which they hold equity (Matrix45). By company policy, associates of Matrix45 cannot hold equity in sponsor and client companies, perform services independently, or receive compensation independently from sponsor and client organizations. Matrix45 provides services to biopharmaceutical companies on a non-exclusivity basis. All other peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
EQUA-PMS-CV_Suppl_fig.pdf
Download PDF (110.5 KB)EQUA-PMS-CV_Supp_tables_for_resubmission_clean.docx
Download MS Word (31.4 KB)Acknowledgments
The authors thank James Graham, PhD, of Edanz Medical Writing for providing medical writing support, which was funded by Novartis Pharma K.K., Japan through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Supplementary material
The supplementary data for this article can be accessed here.
Additional information
Funding
References
- Fox C, Coady S, Sorlie P, et al. The Framingham Heart Study. Increasing cardiovascular disease burden to diabetes mellitus. Circulation. 2007;115:1544–1550.
- Gerstein H, Capes S. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002;2:165–174.
- Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, et al. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5:444–470.
- Ahren B, Landin-Olsson M, Jansson P, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084.
- Utzschneider K, Tong J, Montgomery B, et al. The dipeptidyl peptidase-4 inhibitor vildagliptin improves beta-cell function and insulin sensitivity in subjects with impaired fasting glucose. Diabetes Care. 2008;31:108–113.
- Halimi S, Schweizer A, Minic B, et al. Combination treatment in the management of type 2 diabetes: focus on vildagliptin and metformin as a single tablet. Vasc Health Risk Manag. 2008;4:481–492.
- Bosi E, Camisasca R, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895.
- Garber A, Schweize A, Baron M, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–174.
- Garber A, Foley J, Banerji M, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–1056.
- Fonseca V, Baron M, Shao Q, et al. Sustained efficacy and reduced hypoglycemia during one year of treatment with vildagliptin added to insulin in patients with type 2 diabetes mellitus. Horm Metab Res. 2008;40:427–430.
- Kikuchi M, Abe N, Kato M, et al. Vildagliptin dose-dependently improves glycemic control in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:233–240.
- Nissen S, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471.
- Udell J, Cavender M, Bhatt D, et al. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3:356–366.
- Scirica B, Bhatt D, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326.
- White W, Cannon C, Heller S, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335.
- Green J, Bethel M, Armstrong P, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242.
- McInnes G, Evans M, Del Prato S, et al. Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17 000 patients. Diabetes Obes Metab. 2015;17:1085–1092.
- Murayama H, Toda M, Tsumiyama I, et al. Relationship of patient background with macro- and microvascular complications: a 2-year post-marketing surveillance of vildagliptin in nearly 20,000 Japanese diabetic patients. Expert Opin Pharmacother. 2019;20:1037–1047.
- Hayashi T, Murayama H, Shinfuku Y, et al. Safety and efficacy of vildagliptin: 52-week post-marketing surveillance of Japanese patients with type 2 diabetes in combination with other oral antidiabetics and insulin. Expert Opin Pharmacother. 2019;5:1–10.
- Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: A selected review. Circulation. 2008;118:2702–2709.
- Japan Diabetes Clinical Data Management Study Group [Internet]. C2009–2018. Japan Diabetes Clinical Data Management Study Group. [ cited 2018 Jul 23]. Available from: http://jddm.jp/data/index-2016.html
- Snell-Bergeon JK, Wadwa RP. Hypoglycemia, Diabetes, and Cardiovascular Disease. Diabetes Technol Ther. 2012;14(Suppl1):S-51–S-58.
- Hanefeld M. Hypoglycemia and cardiovascular risk: is there a major link? Diabetes Care. 2016;39(Suppl 2):S205–S209.
- Farngren J, Persson M, Schweizer A, et al. Glucagon dynamics during hypoglycaemia and food-re-challenge following treatment with vildagliptin in insulin-treated patients with type 2 diabetes. Diabetes Obes Metab. 2014;16:812–818.
- Sakamoto M, Nishimura R, Irako T, et al. Comparison of vildagliptin twice daily vs. sitagliptin once daily using continuous glucose monitoring (CGM): crossover pilot study (J-VICTORIA study). Cardiovasc Diabetol. 2012;11:92.
- Guerci B, Monnier L, Serusclat P, et al. Continuous glucose profiles with vildagliptin versus sitagliptin in add-on to metformin: results from the randomized Optima study. Diabetes Metab. 2012;38:359–366.
- Rizzo M, Barbieri M, Marfella R, et al. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35:2076–2082.
- Sone H, Tanaka S, Tanaka S, et al. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab. 2011;96:3448–3456.
- Yokoyama H, Matsushima M, Kawai K, et al. Low incidence of cardiovascular events in Japanese patients with Type 2 diabetes in primary care settings: a prospective cohort study (JDDM 20). Diabet Med. 2011;28:1221–1228.
- Schweizer A, Dejager S, Foley JE, et al. Assessing the cardio-cerebrovascular safety of vildagliptin: meta-analysis of adjudicated events from a large Phase III type 2 diabetes population. Diabetes Obes Metab. 2010;12:485–494.
- Kadowaki T, Haneda M, Ito H, et al. Long-term, real-world safety and efficacy of teneligliptin: a post-marketing surveillance of more than 10,000 patients with type 2 diabetes in Japan. Adv Ther. 2019 Dec 23. DOI:10.1007/s12325-019-01189-w
- Yamamoto F, Unno Y, Okamura T, et al. Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes mellitus: a 3-year post-marketing surveillance study. Diabetes Ther. 2019 Nov 11. DOI:10.1007/s13300-019-00723-x
- Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708.
- Davis TME, Mulder H, Lokhnygina Y, et al. Effect of race on the glycaemic response to sitagliptin: insights from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab. 2018;20:1427–1434.
- Pi-Sunyer F, Schweizer A, Mills D, et al. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diab Res Clin Pract. 2007;76:132–138.
- Dejager S, Razac S, Foley J, et al. Vildagliptin in drug-naive patients with type 2 diabetes: a 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007;39:218–223.
- Rosenstock J, Baron M, Dejager S, et al. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diab Care. 2007;30:217–223.
- Fonseca V, Schweizer A, Albrecht D, et al. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155.
