ABSTRACT
Introduction
Since calcitonin gene-related peptide (CGRP) plays an important role in the pathophysiology of migraine via the activation of the trigeminovascular system, the newest prophylactic treatments directly block CGRP or its receptor. However, the safety of these novel antimigraine drugs is not yet sufficiently established.
Areas covered
Based on the blockade of CGRP or its receptor, this review considers: (i) the effects of the novel prophylactic antimigraine drugs (i.e. gepants and monoclonal antibodies) in clinical trials; and (ii) the potentially negative effects of blocking CGRP or its receptor in terms of safety.
Expert opinion
In the last decade, clinical trials have demonstrated the efficacy of new drugs for the preventive treatment of migraine which aim to (i) block CGRP or its receptor; (ii) increase tolerability as compared to the currently available prophylactics; and/or (iii) be more effective and safer than other treatments. However, these trials are limited to study the safety on the short term, and a cardiovascular risk with prolonged use cannot be excluded. Clearly, basic science experimental studies and long-term clinical trials (i.e. Phase IV) are required to delineate the safety of the newest prophylactic antimigraine drugs without causing unwanted side effects due to chronic CGRP (receptor) blockade.
1. Introduction
Migraine is a common neurovascular disorder characterized by recurrent moderate to severe headaches accompanied by nausea, vomiting, phonophobia, and/or photophobia, lasting from 4 to 72 hours [Citation1,Citation2]. Due to its high prevalence and high socioeconomic and personal impact worldwide, it has been considered as the third most prevalent disorder, the second-highest specific cause of disability, and the first in those people under 50 years of age [Citation2,Citation3].
The exact pathophysiological mechanisms underlying the onset of a migraine attack remain unclear. However, extensive research in the last three decades has demonstrated that calcitonin gene-related peptide (CGRP) plays an important role in the genesis of migraine via the activation of the trigeminovascular system [Citation4–7]. In this respect, as previously reported: (i) activation of the trigeminovascular system (which includes the meninges and intracranial blood vessels) results in cranial vasodilation mainly mediated by CGRP release [Citation6]; (ii) serum levels of CGRP are elevated during migraine attacks [Citation4]; and (iii) intravenous administration of CGRP produces vasodilation of the middle meningeal artery [Citation12] and triggers migraine-like headaches [Citation13,Citation14]. These findings, which further support the key role of CGRP in the pathophysiology of migraine, indicate that CGRP and its receptor may be therapeutic targets for developing new antimigraine drugs [Citation15].
While the triptans (which act as agonists at 5-HT1B/1D/1F receptors) are considered the gold standard for acute antimigraine treatment [Citation16,Citation17], some patients with a high frequency of migraine attacks or chronic migraine require prophylactic antimigraine treatment [Citation18,Citation19] in order to reduce the frequency, severity, and duration of attacks, as well as the associated disability [Citation18,Citation20,Citation21]. This prophylactic treatment, which encompasses a wide variety of drugs with mechanisms of action unrelated to blocking the CGRPergic system, includes β-blockers, antiepileptics, Ca2+ channel blockers, 5-HT receptor antagonists, angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists [Citation17,Citation20]. It is noteworthy that these drugs were not originally developed for the prophylactic treatment of migraine, but over time they proved useful in migraine management. Moreover, their specific mechanisms of action are not well understood and, in most cases, remain speculative [Citation17].
Since migraine is related to several comorbidities (e.g. cardiovascular, pain disorders, psychiatric, or neurological comorbidities) [Citation20], the use of these prophylactic drugs should be considered based on the comorbidity presented by the migraine patient. In addition, the possibility of recurrent migraine attacks may result when the use of the prophylactic medications is withdrawn.
Interestingly, novel prophylactic antimigraine treatments, which directly target CGRP or its receptor, have been explored in the last decade () [Citation15,Citation22]. These novel antimigraine treatments include: (i) small-molecule CGRP receptor antagonists (i.e. gepants); and (ii) monoclonal antibodies targeting either CGRP or its receptor (see ), which have now been approved by the Food and Drug Administration (FDA) and/or by the European Medicines Agency (EMA) for the preventive treatment of migraine in adults. Additionally, the Spiegelmers (CGRP-neutralizing mirror-image L-aptamers) have been proposed as a new candidate for the prophylactic antimigraine treatment [Citation23]. However, to our knowledge, no further studies have demonstrated their antimigraine effectiveness.
Figure 1. An overview of some antimigraine drugs that directly block CGRP or its receptor. Since CGRP plays an important role in the pathophysiology of migraine, in the last decades new antimigraine drugs have been developed for migraine treatment. These drugs include: (i) gepants, which act by directly blocking the CGRP receptor (i.e. rimegepant and atogepant); and (ii) monoclonal antibodies, which directly block CGRP (i.e. eptinezumab, fremanezumab and galcanezumab) or its receptor (i.e. erenumab). CLR, calcitonin receptor-like receptor; RAMP1, receptor activity-modifying protein 1
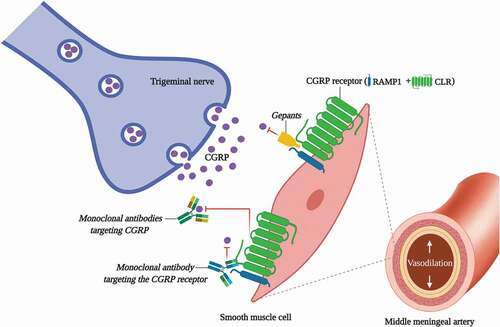
Table 1. The new antimigraine drugs targeting CGRP or its receptor
Despite the highly promising potential of the gepants and monoclonal antibodies for the prophylactic antimigraine treatment, the safety of these drugs and their mechanisms of action have not yet been explored in long-term trials [Citation15,Citation25–27]. Accordingly, this review attempts to provide an overview of the contributions of blocking CGRP or its receptor in terms of: (i) tolerability and safety, covering the efficacy of the new prophylactic antimigraine treatments (see ); and (ii) the possible negative side effects and/or risks produced by the long-term blockade of the CGRPergic system.
2. CGRP and migraine
In addition to serotonin, histamine, and nitric oxide [Citation28], CGRP plays an important role in the pathophysiology of migraine [Citation4–7]. CGRP is a 37-amino acid neuropeptide localized in the peripheral and central sensory nervous system, acting as a potent vasodilator as well as a neurotransmitter [Citation29,Citation30]. CGRP mainly mediates its biological effects through its interactions with the CGRP receptor. This canonical receptor is a complex of two subunits, namely: (i) a G-coupled protein receptor called calcitonin receptor-like receptor (CLR); and (ii) a receptor activity-modifying protein 1 (RAMP1) [Citation31] (see ). In humans, CGRP is present in two isoforms, α-CGRP and β-CGRP. Since α-CGRP is the principal isoform localized in the peripheral and central sensory nervous system [Citation32], its biological activity seems more important in the pathophysiology of migraine. However, in terms of side effects, we cannot exclude the β-CGRP isoform (which plays a role in the enteric transmission) [Citation33] since some CGRP-blocking drugs (i.e. monoclonal antibodies such as eptinezumab, fremanezumab, and galcanezumab) are not selective and can block both the α-CGRP and β-CGRP isoforms.
Figure 2. Composition of the human calcitonin-family receptors and the relative affinity/potency of binding of CGRP, AM and AMY to canonical and non-canonical receptors. CGRP can exert its pathophysiological effects via the activation of its receptor (CGRP receptor) or via the activation of distinct CGRP-responsive receptors with moderate affinity/potency for AM receptors, and practically the same potency for the AMY receptors, as compared to their endogenous ligands. CGRP, calcitonin gene-related peptide; AM, adrenomedullin; AMY, amylin; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein. Human and *rodents values of apKi, bpEC50, and cpKd [Citation98,Citation100]
![Figure 2. Composition of the human calcitonin-family receptors and the relative affinity/potency of binding of CGRP, AM and AMY to canonical and non-canonical receptors. CGRP can exert its pathophysiological effects via the activation of its receptor (CGRP receptor) or via the activation of distinct CGRP-responsive receptors with moderate affinity/potency for AM receptors, and practically the same potency for the AMY receptors, as compared to their endogenous ligands. CGRP, calcitonin gene-related peptide; AM, adrenomedullin; AMY, amylin; CLR, calcitonin receptor-like receptor; CTR, calcitonin receptor; RAMP, receptor activity-modifying protein. Human and *rodents values of apKi, bpEC50, and cpKd [Citation98,Citation100]](/cms/asset/e0536de5-80b2-4d1c-a267-8bcd09658efc/ieds_a_1811229_f0002_oc.jpg)
In the last decades, different studies have shown the role of CGRP during a migraine attack. In this respect, CGRP is released from the trigeminal ganglion via the activation of the trigeminovascular system [Citation34]. As previously reviewed by Iyengar et al. [Citation35], the trigeminal ganglion sends central afferent projections to the trigeminal nucleus caudalis in the brain stem and also to the C2 segments of the cervical spinal cord (trigeminocervical complex); and peripheral projections through sensory nerves, which innervate the cranial vasculature and the dura mater. Then, the released neuropeptide can bind to its receptor localized in the human cranial arteries producing vasodilation [Citation36]. Furthermore, it has been reported that CGRP concentrations in serum [Citation4,Citation8–11] and saliva [Citation37] are elevated in migraine patients, and that these levels are normalized or reduced after treatment with triptans [Citation8,Citation13,Citation14]. Moreover, intravenous injections of CGRP can trigger moderate to severe migraine-like headaches in migraine-prone patients (but not in non-migraine patients), suggesting that migraine patients are unusually sensitive to CGRP [Citation13,Citation14]. This approach has led to the development of experimental animal models for the study of migraine, which include, among others: (i) electrical stimulation of the trigeminal ganglion; (ii) electrical/chemical stimulation of the meninges; (iii) administration of CGRP; (iv) genetic manipulation of CGRP [Citation38,Citation39]; and (v) in vivo (using infusions of headache-inducing substances) and in vitro (using isolated cranial and coronary arteries) human models [Citation40]. These models have been used in an attempt to clarify and understand the pathophysiological mechanisms of migraine, as well as to carry out preclinical studies that lead to the development of new antimigraine drugs.
Finally, the fact that there are selective drugs targeting CGRP (or its receptor) with antimigraine efficacy (as discussed below), supports the link between CGRP and migraine [Citation41].
3. Blockade of CGRP or its receptor as a treatment for migraine
Based on the background showing the role of CGRP in migraine, new antimigraine drugs targeting CGRP or its receptor have been developed in the last decades. These new antimigraine drugs are focused on reducing vasodilation and neurogenic inflammation, relieving pain, reducing the associated symptoms of migraine and avoiding the occurrence or intensity of migraine attacks. Moreover, they have been shown to be effective, well tolerated and safer than other drugs initially used in the preventive treatment of migraine.
As previously reviewed by de Vries et al. [Citation15], antimigraine drugs targeting CGRP or its receptor can currently be divided into: (i) drugs for the acute treatment of migraine (i.e. gepants, which block the CGRP receptor); and (ii) prophylactic antimigraine drugs (i.e. gepants and monoclonal antibodies against CGRP or its receptor) (see ).
In this respect, seven CGRP receptor blockers have been developed for migraine therapy. Four have been discontinued due to several disadvantages including: (i) poor oral bioavailability (i.e. olcegepant, previously known as BIBN4096BS) [Citation42]); and (ii) hepatotoxicity [i.e. telcagepant (MK-0974), MK-3207, and BI 44,370 TA] [Citation43–45]. In contrast, both ubrogepant (MK-1602) [Citation46] and rimegepant (BMS-927,711) [Citation47] have been approved by the FDA in 2019 and 2020, respectively, for acute antimigraine treatment. Interestingly, rimegepant is currently undergoing a clinical trial for approval as a prophylactic treatment (phase II/III trial, NCT0372368) [Citation48]. The seventh CGRP receptor antagonist, atogepant (MK-8031, AGN-241,689) is the first gepant specifically developed for the prophylaxis of episodic and chronic migraine [Citation49–52].
On the other hand, next to the gepants, the monoclonal antibodies against CGRP or its receptor represent a new hope for the treatment of migraine. Currently, there are four monoclonal antibodies that have been approved by the FDA and/or the EMA for the prophylactic treatment of migraine, namely: (i) erenumab; (ii) eptinezumab; (iii) fremanezumab; and (iv) galcanezumab (see ). These monoclonal antibodies against either the CGRP receptor (the former) or the CGRP receptor (the latter three) seem to be promising for the prophylaxis of migraine and have been shown to be safe in the short term [Citation15,Citation25,Citation26].
3.1. Prophylactic antimigraine treatment
As previously described, the goal of prophylactic antimigraine drugs is to reduce the frequency, duration and severity of migraine attacks and, as a result, to decrease the suffering and disability associated with migraine. Some of these drugs have been approved for use in migraine treatment since 2018 and, due to their mechanisms of action on the trigeminal system, have offered specificity and tolerability over the existing classical treatments (i.e. β-adrenoceptor antagonists, antiepileptics, Ca2+ channel blockers or 5-HT receptor antagonists). Therefore, CGRP receptor antagonists (i.e. rimegepant and atogepant) and monoclonal antibodies against CGRP (eptinezumab, fremanezumab and galcanezumab) or its receptor (erenumab) have been shown to be effective for migraine treatment and seem to have fewer or no side effects compared to ergots, triptans, or even the existing classical prophylactic treatments [Citation15,Citation26].
3.1.1. CGRP receptor antagonists: second-generation gepants
Gepants are small-molecule CGRP receptor antagonists developed in the last decades for the treatment migraine. These small molecules, which avoid the interaction between CGRP and its receptor (located in the trigeminal ganglion and the vascular smooth muscle cells; ), can be divided into: (i) first-generation gepants (developed for the acute treatment of migraine); and (ii) second-generation gepants, used for the acute (i.e. ubrogepant and rimegepant) and prophylactic [rimegepant (ongoing clinical trials) and atogepant] treatment of migraine.
3.1.1.1. Rimegepant
Rimegepant (BMS-927,711) is a small-molecule CGRP receptor antagonist developed by Biohaven Pharmaceutical Holding Company Ltd., and has recently been approved by the FDA (February 2020) for the acute treatment of migraine [Citation47].
In a Phase II clinical trial, 75, 150, and 300 mg rimegepant showed an excellent tolerability profile [Citation53] (similar to that placebo), with no serious side effects (see ). The results after 2 hours rimegepant 75, 150 and 300 mg showed that the percentage of pain-free patients (31.4%; 32.9%; and 29.7%, respectively) was significantly higher than that of the placebo group (15.3%). Additionally, a secondary endpoint showed that 27.9%; 25.9%; and 23.4% of patients treated with rimegepant 75, 150 and 300 mg, respectively, had complete migraine freedom (i.e. pain freedom, and no symptoms of phonophobia, photophobia, or nausea) at 2 hours post-dose [Citation53]. Based on the results obtained in this clinical trial, the dose of 75 mg was chosen for Phase III trials [Citation54] to test its efficacy, safety, and tolerability. In this study, the authors reported that rimegepant 75 mg was more effective in 21% of patients (who were pain free) compared with the placebo group (11%). The most common side effect in the rimegepant-treated patients were nausea (2–8%), vomiting (2–3%), dizziness (2–4%), and urinary tract infection (2–3%) [Citation53,Citation54]. Moreover, since rimegepant produced no cardiovascular side effects or hepatotoxicity in the population [Citation53–56], it was suggested that this gepant is safe for the treatment of migraine. Furthermore, the efficacy and safety of rimegepant as a prophylactic antimigraine treatment is being evaluated through a randomized, double-blind, placebo-controlled, phase II/III clinical trial (NCT0372368). This trial will evaluate the efficacy and safety of 75 mg rimegepant as compared with placebo in migraine patients, with a study completion date of 30 January 2021 [Citation48].
Table 2. Overview of side effects reported for the prophylactuc anti-migraine drugs
3.1.1.2. Atogepant
Atogepant (MK-8031 or AGN-241,689) is a CGRP receptor antagonist with a higher potency and longer half-life than ubrogepant (which is used for the acute treatment of migraine). For this reason, its use has been proposed for the prevention of episodic and chronic migraine (see ) [Citation49–52].
A Phase IIb/III randomized, double-blind, placebo controlled in parallel-group study clinical trial evaluated the efficacy, safety, and tolerability of atogepant at different daily (10, 30, or 60 mg) or twice daily (30 or 60 mg) oral doses or placebo during 12 weeks. All active treatment groups showed a significant reduction in their mean monthly migraine/probable migraine headache days (−4.00 for 10 mg; −3.76 for 30 mg; −3.55 for 60 mg; and −4.23 or 4.14 for the twice daily 30 or 60 mg, respectively) compared to placebo (−2.85 days). Moreover, atogepant was well tolerated with not significant and/or serious side effects (placebo = 19.35%; atogepant 10, 30 and 60 mg daily = 18.28%, 25.68%, and 27.96%, respectively; or atogepant 30 and 60 mg twice daily = 24.42% and 28.57%, respectively) and ≥5% patients presented the most common side effects, namely: nausea (5–11%), fatigue (1–10%), constipation (2–6%), nasopharyngitis (1–7%) and urinary tract infection, with not sings of hepatoxicity [Citation49,Citation50]. Currently, a Phase III clinical trial (NCT03855137) is in progress, with a study completion date of 31 August 2021 [Citation52].
In addition, data obtained from in vitro studies using human intracranial and coronary arteries showed that atogepant is more effective and potent in antagonizing CGRP-induced vasodilation in human meningeal arteries as compared with human coronary arteries, suggesting that atogepant would have a safety benefit when considering cardiovascular side effects [Citation57]. Obviously, long-term clinical trials are necessary to fully verify the safety of atogepant.
3.1.2. Monoclonal antibodies
The development of monoclonal antibodies targeting CGRP or its receptor represents a strategy complementary to gepants for migraine treatment. Since their plasma half-life is much longer than that of the gepants, their use has been proposed and recommended by current European Headache Federation guidelines as prophylactic drugs to prevent episodic and chronic migraine attacks [Citation58]. Nevertheless, their specific site and/or mechanisms of action in the trigeminal system are still unknown. Interestingly, as previously reviewed by Edvinsson et al. [Citation59], the therapeutic effect of monoclonal antibodies can be completely peripheral due to its low permeability into the blood-brain barrier), and it is likely to affect targets within the trigeminovascular system [Citation59]. Currently, there are four different monoclonal antibodies (see above and ): (i) one fully human monoclonal antibody (i.e. erenumab) that directly blocks the CGRP receptor, preventing the binding of CGRP to its canonical receptor; and (ii) three human monoclonal antibodies that directly block CGRP (i.e. eptinezumab, fremanezumab, and galcanezumab) (see ). Therefore, the monoclonal antibodies targeting the CGRP pathway symbolize a novel and specific therapeutic approach in the prevention of migraine.
3.1.2.1. Erenumab
Erenumab (AMG-334) is the only fully human monoclonal antibody that directly blocks the canonical CGRP receptor (see ) [Citation60], and the first to be approved by the FDA (May 2018) and by the EMA (July 2018) for the prophylactic treatment of migraine (see ) [Citation61,Citation62].
Data from two Phase II clinical trials have been presented. First, in a 12-week multicenter, randomized, double-blind, placebo-controlled monthly dose of 7, 21 and 70 mg erenumab were tested. Only the 70 mg dose reduced the number of monthly headache days to −3.4 days when compared with the placebo group (−1.1 days) with a difference of −1.1 days (P = 0.021) [Citation63]. In the second clinical trial, doses of 70 and 140 mg produced a significant reduction in the number of monthly headache days, both doses −6.6 days versus placebo −4.2 (P < 0.0001) post-dose [Citation64]. Furthermore, there are data from two Phase III clinical trials, namely, the ARISE and STRIVE studies. In the ARISE study (NCT02483585), 557 patients were randomized to monthly subcutaneous injections of 70 mg erenumab or placebo. The results showed a reduction in the number of monthly headache days at the third moth post-dose. In this trial, erenumab-treated patients (70 mg) showed a change of −2.9 days in monthly migraine days compared with −1.8 days for placebo-treated patients (difference of −1.1 days, P = 0.001); achieving a ≥ 50% reduction in monthly migraine days by 39.7% for erenumab 70 mg and 29.5% for the placebo group (P = 0.01) [Citation65]. Similar results were obtained from the STRIVE study (NCT02456740), in which a total of 955 patients (monitored during 6 months) were randomized and divided into (i) 317 patients for testing erenumab 70 mg; (ii) 319 for testing erenumab 140 mg; and (iii) 319 for the placebo group. Erenumab produced a reduction in monthly migraine days by 3.2 days in the 70 mg erenumab group and 3.7 days in the 140 mg erenumab group, as compared with 1.8 days in the placebo group (P < 0.001). In addition, a ≥ 50% reduction in the monthly migraine days was achieved by 43.3% and 50% after erenumab 70 and 140 mg, respectively, as compared with 26.6% in the placebo group (P < 0.001) [Citation66].
On the other hand, erenumab has also been evaluated in the long term in episodic [Citation67] and chronic [Citation68] migraine patients. In this respect, during the open-label treatment phase clinical trial for episodic migraine (NCT01952574), patients received monthly erenumab 70 mg. After 2 years, the initial dose was increased to 140 mg in order to evaluate the long-term safety and tolerability of the higher dose. The side effects patient incidence rate was 132.0 per 100 patient-years (142.0 after erenumab 70 mg and 128.1 after erenumab 140 mg). Moreover, the most frequent side effects in at least ≥4.0 per 100 patient-years were (expressed in incidence rate): viral upper respiratory tract infection (12.9), upper respiratory tract infection (7.2), sinusitis (4.6), influenza (4.2) and back pain (4.2); these effects did not increase relative to placebo based on the results obtained from the placebo-controlled clinical trials [Citation67]. Regarding the chronic migraine patients, an open-label extension study (NCT02174861) evaluated the effect of monthly erenumab 70 mg following erenumab 140 mg (which was increased between weeks 4 or 28) to evaluate the long-term efficacy and safety during 52 weeks [Citation68]. The results showed an efficacy with a reduction in the monthly migraine days of 9.4 and 8.8 days at weeks 40 and 52, respectively. In addition, a ≥ 50%, ≥75% and ≥100% reductions in the monthly migraine days was achieved at week 52 by 59.0%, 33.2% and 8.39% of patients, respectively. In terms of safety, the total incidence rate in erenumab-treated patients was 126.3 per 100 patient-years, and 132.0 and 148.5 per 100 patient-years pots-dose erenumab 70 mg and 140 mg, respectively. The most common side effects (expressed as incidence rate per 100 patient-year) were: viral upper respiratory tract infection (16.4), upper respiratory tract infection (7.2), sinusitis (7.1) and arthralgia (4.2), which did not increase when compared with the double-bind treatment phase [Citation68].
Moreover, an in vitro study by Rubio-Beltrán et al. [Citation69] showed that erenumab is capable of inhibiting CGRP-induced vasodilatory responses in human-isolated middle meningeal, internal mammary and coronary arteries. This monoclonal antibody showed no direct contractile or relaxant effects, as well as specificity at inhibiting the vasodilator responses to CGRP without interactions with other vasoactive compounds [Citation69]. These findings seem favorable in terms of vascular safety. However, as discussed below, some other issues must be considered to adequately explore the cardiovascular safety of erenumab, including the interaction of CGRP with non-canonical receptors [Citation31,Citation70].
In all clinical trials, erenumab showed to be well tolerated, effective and safe. The most common side effects (without showing differences with the placebo group) (see ) were injection-site pain (0.3–6%), fatigue (2–4%), nasopharyngitis (2–11%), upper respiratory tract infection (3–7%) and nausea (2–3%) [Citation63–66]. Indeed, erenumab has proven to be a safe monoclonal antibody for the prevention of migraine (in short term) since its administration does not produce cardiovascular side effects or hepatotoxicity (due to its route of administration). In addition, this monoclonal antibody for the CGRP receptor resulted 5000-fold more selective for the canonical receptor, as compared with other human calcitonin family receptors [i.e. adrenomedullin (AM), calcitonin (CT) or amylin (AMY) receptors] [Citation60]. However, we cannot categorically rule out whether CGRP receptor blockade will be selective in the long term or whether there will be involvement of non-canonical receptors. If this were the case, blocking the CGRP receptor with erenumab would leave the possibility open for CGRP to bind to the amylin AMY1 receptors, keeping in mind that CGRP can mediate its effects through CGRP receptors or distinct CGRP-responsive receptors [Citation31]. In fact, it has been shown that the AMY1 receptor: (i) could potentially function as an additional CGRP receptor in coronary arteries [Citation70]; and (ii) is located in the trigeminal system [Citation71], which could suggest an important role in migraine. Nevertheless, further studies are necessary to elucidate the safety of erenumab and its exact mechanism(s) of action (in the long term), as well as the role of AMY1 receptors in migraine.
3.1.2.2. Eptinezumab
Eptinezumab (ALD-403) is the first intravenously administered humanized monoclonal antibody that directly blocks CGRP. It was developed by Alder BioPharmaceuticals Inc. and H. Lundbeck A/S, and has recently been approved by FDA (February 2020) for the preventive treatment for migraine (see ) [Citation72].
In a first Phase II, randomized, double-bind, placebo-controlled clinical trial, intravenous eptinezumab (1000 mg) was tested for 24 weeks and showed a significant reduction in monthly migraine days in patients with episodic migraine as compared with placebo. In this respect, the mean change in migraine days between baseline and weeks 1–4; 5–8; and 9–12 in eptinezumab (1000 mg) and the placebo group was −5.6 and −3.9 days (difference of −1.7 days); −5.6 and −4.6 days (difference of −1.0 days); and −5.6 and −4.6 days (difference of −1.0 days), respectively. Furthermore, 33% of eptinezumab-treated patients experienced a ≥ 75% reduction in monthly migraine days over 12 weeks compared with 9% in the placebo group, as well as 51% of eptinezumab-treated patients showed ≥50% reduction in monthly migraine days compared to 33% in the placebo group [Citation73]. Additionally, in a Phase III clinical trial, eptinezumab 30, 100 and 300 mg (PROMISE-I) or eptinezumab 100 and 300 mg (PROMISE-II) showed to be effective in reducing monthly migraine days in episodic migraine (PROMISE-I) by reducing the frequency of migraine days during weeks 1–2 from baseline to −4.0 days (30 mg); −3.9 days (100 mg); and −4.36 days (300 mg) when compared to the placebo group (−3.2 days), with a difference of −0.82; −0.69; and −1.11 days, respectively (P < 0.0.01) [Citation74]; and by reducing the monthly migraine days in chronic migraine patients treated with eptinezumab (100 and 300 mg) by −7.7 and −8.2 days, respectively, versus the placebo group (−5.6 days) with a difference of −2.0 and −2.6 days (P < 0.0001) (PROMISE-II) [Citation75].
The most frequent side effects reported in the eptinezumab-treated group were (see ): upper respiratory tract infection (4–11%), urinary tract infection (1–3%), fatigue (2–4%), nausea and vomiting (1–4%); which were similar in the placebo group (5–7%; 1–5%; 1–4%; 1–4%; respectively, [Citation72–75], with no signs of hepatotoxicity or cardiovascular side effects. Therefore, the results from clinical trials showed the efficacy, tolerability, and short-term safety of eptinezumab for the preventive treatment of migraine.
3.1.2.3. Fremanezumab
Fremanezumab (TEV-48,125) is another humanized monoclonal antibody that directly blocks the α-CGRP and β-CGRP isoforms [Citation76], avoiding the binding of CGRP to its receptor (). This monoclonal antibody is the second one to be approved by the FDA (September 2018), and the third one to be approved by the EMA (March 2019) for use in the prevention of episodic and chronic migraine (see ) [Citation77,Citation78].
For episodic migraine, results from a Phase IIb clinical trial showed that subcutaneous doses of 225 and 675 mg (given once every 28 days for 12 weeks) reduced the number of monthly headache days by 2.81 days in the 225 mg fremanezumab group and 2.64 days in the 675 mg fremanezumab group compared to the placebo group (P < 0.001). In this respect, the percentage of patients treated with fremanezumab 225 or 675 mg with at least 50% of reductions in migraine days was 53% and 59%, respectively, compared with the placebo group, 28% (P < 0.001) [Citation79]. Similar results were obtained from a Phase III clinical trial [Citation80]. A total of 875 patients were randomized to monthly 225 mg injections or quarterly 675 mg injections of fremanezumab. The high dose (675 mg) produced a decrease in the number of monthly migraine days by 1.3 days compared to placebo, while the low dose (225 mg) reduced the number of monthly headache days by 1.5 days. Moreover, at least 50% of reduction in migraine days was observed at week 12 in 47% of patients treated with fremanezumab 225 mg; and in 44.4% of the patients treated with fremanezumab 675 mg (38%); compared with 27.9% in the placebo group (P < 0.001 when comparing both fremanezumab-treated groups with the placebo-treated group) [Citation80].
Regarding chronic migraine, a Phase IIb clinical trial showed that subcutaneous administration of fremanezumab 675 mg (in the first treatment cycle) plus 225 mg (in the second and third treatment cycles) produced a reduction in the number of monthly headache hours with 22.74 hours compared to placebo. Additionally, fremanezumab 900 mg (in all three treatment cycles) produced a decrease of 30.41 hours in the number of monthly headache hours compared to placebo [Citation81]. Moreover, in a Phase III clinical trial, patients with chronic migraine received a quarterly subcutaneous injection of fremanezumab 675 mg at baseline (and placebo at weeks 4 and 8) or a single dose of fremanezumab 675 mg followed by two doses of 225 mg at weeks 4 and 8. Both groups showed a reduction in the number of monthly headache days with 1.8 days (quarterly 675 mg fremanezumab) and 2.1 days (monthly 675 mg plus 225 mg fremanezumab) compared to placebo. Additionally, a reduction of at least 50% in the average number of headache days per month was observed in 38% in the fremanezumab-quarterly group; in 41% in the fremanezumab-monthly group; and 18% in the placebo group (P < 0.001 when comparing both fremanezumab-treated groups with placebo group) [Citation82].
In all cases, fremanezumab 225 and 675 mg showed to be safe, well tolerated and effective for the prevention of episodic and chronic migraine in the short term. The most common side effects (see ) were pain at the injection site, upper respiratory infection, nasopharyngitis, urinary tract infection, fatigue, and nausea, without cardiovascular side effects or hepatotoxicity [Citation76,Citation79–85]. On this basis, it has been suggested that fremanezumab seems a promising option (in terms of safety) as a preventive treatment for migraine, even in the long term [Citation85].
3.1.2.4. Galcanezumab
Galcanezumab (LY-2,951,742) is a humanized monoclonal antibody that directly blocks both the α-CGRP and β-CGRP isoforms, preventing their binding to the CGRP receptor () [Citation86]. This monoclonal antibody received FDA approval in September 2018 [Citation86] and EMA approval in November 2018 [Citation87] for the prophylaxis of migraine in adults (see ). More recently, Eli Lilly and Company applied for an extension of indication of galcanezumab to add the prevention of attacks throughout a cluster period in adults with episodic cluster headache. However, EMA recommended the refusal of a change to the marketing authorization for this drug [Citation88].
Results from a Phase IIb clinical trial for episodic migraine showed that i.v. administration of galcanezumab (120 mg given monthly during 3 months) produced a significant reduction in the monthly headache days as compared with placebo by −4.8 versus −3.7 days, respectively (compared to the baseline values of 6.6 and 6.7 days, respectively). Moreover, in a secondary endpoint, the galcanezumab-treated patients (120 mg) reported 50% and 100% reduction in the number of monthly headache days during the third study period [Citation89]. Additionally, in two Phase III clinical trials (EVOLVE-1 and EVOLVE-2), monthly injections of galcanezumab 120 and 240 mg (during 6 months) produced a reduction in the monthly migraine headache days as compared with placebo, without showing differences between both doses [Citation90,Citation91]. In this respect, during the EVOLVE-1 trial, the treatment with galcanezumab 120 and 240 mg produced a significant reduction in the monthly migraine headache days by −4.7 and −4.6 days, respectively, compared with placebo (−2.8 days), with a difference of −1.9 days for galcanezumab 120 mg and −1.8 days for galcanezumab 240 mg (P < 0.001 versus placebo [Citation90]. Similar results were obtained during the EVOLVE-2 trial, where the patients showed a reduction in the monthly migraine headache days by −4.3 and −4.2 days post-dose of galcanezumab 120 and 240 mg, respectively, compared with the placebo group (−2.3 days) [Citation91]. Regarding chronic migraine, results from Phase II clinical trials showed that both doses of galcanezumab (120 and 240 mg, given subcutaneously) during 9 months [Citation92] or 12 months [Citation93] are effective in reducing the number of monthly migraine headache days by −4.8 and −4.6 days (when compared with placebo −2.7 days, P < 0.001) [Citation92]; and by −5.6 and −6.5 days post-dose of galcanezumab 120 and 240 mg, respectively [Citation93]. Likewise, this monoclonal antibody has been shown to be well tolerated since the incidence rate of side effects was low (see ) both in patients with episodic (51–65% for galcanezumab 120 mg; 67–71% for galcanezumab 240 mg; when compared with the 50–63% in the placebo group) [Citation89–91] and chronic migraine (58% for galcanezumab 120 mg; 57% for galcanezumab 240 mg; compared with the placebo group, 50%) [Citation92].
4. New antimigraine treatments?
In an attempt to find new alternatives for migraine treatment, more recent studies have analyzed the direct effects of small RNA sequences on CGRP. A first approach to block the effect of CGRP is the use of specific CGRP-neutralizing L-aptamers or Spiegelmers. Indeed, next to gepants or monoclonal antibodies, Spiegelmers seem to be candidates for the treatment/prevention of migraine [Citation23]. Spiegelmers are single-stranded mirror-image RNA oligonucleotides, which are capable of specifically binding to CGRP and of inhibiting its function [Citation94].
In this respect, two Spiegelmers have been shown to bind selectively to CGRP, namely, NOX-C89 and NOX-L41. Indeed, NOX-C89 was shown to reduce CGRP release from the cranial dura mater caused by antidromic activation of meningeal afferents, and produced a dose-dependent inhibition of the electrically evoked increases in meningeal blood flow [Citation94]. Moreover, NOX-L41 can inhibit both CGRP-induced cAMP formation and neurogenic plasma protein extravasation in the rat dura mater [Citation23]. Based on these findings, the use of these Spiegelmers was proposed as an alternative for the treatment of migraine. However, since NOX-C89 preferentially binds to mouse/rat CGRP than human CGRP and it is cross-reactive to AMY, it was considered insufficient for further clinical development [Citation94]. In contrast, no compelling study has yet reported the effectiveness of NOX-L41 in migraine, but its potential use has been suggested for migraine prevention [Citation23].
On the other hand, a second approach would be the possibility of genetically manipulating the gene expression of CGRP or its receptor. Since the use of RNA interference (RNAi) and small interfering RNA (siRNA) have been proposed for the treatment of some neurological disorders, we can speculate that post-transcriptional silencing of CGRP could be used as a therapeutic tool for migraine attacks. However, in view that CGRP exerts protective functions, the complete silencing of gene may or may not be effective in the prevention of migraine, keeping in mind that it could alter the vascular function or increase the possible harmful cardiovascular consequences. Therefore, we hypothesize that if the silencing of gene expression is an alternative for the treatment of migraine, it should be considered at what time and/or how long should the genetic silencing be applied, as well as if it is necessary to apply a complete or partial genetic silencing and/or intermittent or continuous. This leads us to pose the question: will genetic engineering be safe to treat migraine? This still remains a crucial issue that needs to be further analyzed before RNAi or siRNA are ready for clinical use.
5. Side effects associated with CGRP (receptor) blockade
An important concern related to the long-term use of CGRP blockers for the prophylactic treatment of migraine (specifically using monoclonal antibodies) is that CGRP acts as an potent vasodilator, maintaining an important role in the homeostasis of the gastrointestinal [Citation95–97] and cardiovascular [Citation98] systems under pathological conditions. Therefore, long-term blockade of CGRP or its receptor could cause a loss of the protective effect of CGRP.
In this respect, since the monoclonal antibodies eptinezumab, fremanezumab and galcanezumab are nonselective for directly blocking CGRP and can bind to the α-CGRP and β-CGRP isoforms [Citation73,Citation76,Citation86], a long-term CGRP blockade could cause gastrointestinal system-related side effects (e.g. mucosal damage, ulcers, constipation, and/or diarrhea). Evidently, its long-term effects should be investigated to rule out these possible side effects and to verify the safety of these monoclonal antibodies.
Regarding the cardiovascular side effects, this issue seems to be relevant since migraine patients may have an increased cardiovascular risk [Citation98]. While data obtained from several clinical trials have shown that the four monoclonal antibodies do not produce cardiovascular adverse effects in the short term [Citation63–66,Citation73–76,Citation79–84,Citation91–93], some fatal cardio – and cerebrovascular events such as atherosclerosis, right thalamic infarction or transient attack of ischemia occurred during the evaluation of erenumab or eptinezumab [Citation15], even during the long-term clinical trials [Citation67]. It is noteworthy that most of these clinical trials were limited to the evaluation time of the treatment (no more than 2 years). In addition, although most short-term clinical trials to assess cardiovascular safety of monoclonal antibodies include patients without cardiovascular complications, data showing that erenumab 140 mg shows vascular safety in patients with cardiovascular disorders (i.e. angina and coronary artery disease) after exercise treadmill suggesting that inhibition of the canonical CGRP receptor with erenumab does not worsen myocardial ischemia [Citation99]. Certainly, this study was designed with the purpose of demonstrating the safety of erenumab including patients with cardiovascular disorders. However, this study has important limitations that cast doubt on its validity, including that: (i) 78% of the study population were male considering that migraine is a predominantly female disorder; therefore, it could be assumed that blocking the effects of CGRP receptor with erenumab may have different effects in female patients than in male patients; (ii) although the study included patients with cardiovascular diseases (i.e. stable angina, a mainly macrovascular disease), patients with microvascular diseases better represent the population at cardiovascular risk; and (iii) there is no evidence that intravenous infusion with erenumab 140 mg already induces CGRP receptor blockade after 30 min [Citation100]. On this basis, we consider that additional studies are still needed to assess the cardiovascular safety of monoclonal antibodies.
On the other hand, as previously reviewed by MaassenVanDenBrink et al. [Citation98], women might be at a higher cardiovascular risk during CGRP (receptor) blockade than men, specifically after menopause or during pregnancy. Therefore, in an attempt to clarify the cardiovascular safety from long-term use of monoclonal antibodies and considering the pharmacokinetic differences over gepants (e.g. longer half-lives), further studies should be conducted in patients with cardiovascular diseases and in pregnant (or preeclamptic) women.
Since this experimental strategy would obviously raise ethical concerns, an approach using relevant animal models seems most appropriate. In this respect, several studies have demonstrated the effects resulting from CGRP or its receptor by using gepants and/or monoclonal antibodies in animal models. One such example is a mouse study, where a harmful effect of CGRP receptor blockade by olcegepant (1 mg/kg) or rimegepant (10 mg/kg) on cerebral ischemic outcome was described. Both gepants worsened ischemic stroke in mice with familial hemiplegic migraine type 1 via collateral dysfunction [Citation101]. In addition, it has been reported that CGRP-induced diarrhea in C57BL/6 J mice (transgenic nestin/hRAMP1 mice) is blocked by anti-CGRP antibodies [Citation102]. Finally, α-CGRP knockout mice model showed to have increased hypertension and aortic hypertrophy, suggesting that upregulation of CGRP plays a protective role in the vascular system [Citation103]. Notwithstanding the use of gepants and/or monoclonal antibodies to block the CGRPergic system has shown no vascular or gastrointestinal side effects in short-term clinical trials, the long-term effects in specific patient group still need to be explored.
6. Conclusion
Based on the advances in basic and clinical research, CGRP has emerged as one of the main targets for the treatment of migraine. In this respect, during the last decades, the development of drugs for the acute or prophylactic treatment of migraine has been based on the direct blockade of the CGRP pathway, including either CGRP or the CGRP receptor. Regarding the new prophylactic antimigraine drugs, results from clinical trials have shown the gepants or monoclonal antibodies to be safe in the short term, and even in the long term in the case of erenumab. These findings could suggest that direct blockade of CGRP or its receptor represents a novel option for the prevention of this disorder, particularly in patients who: (i) fail to respond to other treatments: or (ii) have contraindications to existing treatments (i.e. triptans). In addition, some basic research studies have shown the ‘vascular safety’ of some of these prophylactic antimigraine drugs. However, we think that chronic or long-term blockade of the CGRPergic pathway (which has not been explored yet) for the prevention of migraine attacks could cause unwanted (mainly cardiovascular) side effects. Therefore, basic science experimental studies and Phase IV clinical trials are necessary to further confirm the long-term safety of the new prophylactic antimigraine drugs.
7. Expert opinion
Migraine is a complex neurovascular disorder that requires specific drugs for its acute or prophylactic treatment. However, many patients use nonspecific drugs to alleviate migraine-related pain or symptoms (i.e. nonsteroidal anti-inflammatory drugs, analgesics, or a combination of both). In an attempt to improve the quality of life of migraine patients, new drugs have been developed in order to reduce the side effects produced by the classical antimigraine drugs (e.g. ergots and triptans). In this respect, the association of CGRP with the pathophysiology of migraine has led to the development of specific drugs that directly block CGRP or its receptor (see ). These drugs have been shown to be safe in the short-term prophylactic antimigraine treatment. However, their mechanism(s) of action and their long-term effects with reference to safety have not yet been explored. Consequently, there are still some pending issues (described right below) to ensure the safety of these novel prophylactic antimigraine drugs.
Firstly, CGRPergic blockers are not highly ‘selective’. This means that some prophylactic antimigraine drugs can block both the α-CGRP and β-CGRP isoforms (i.e. eptinezumab, fremanezumab, and galcanezumab). Since β-CGRP is mainly present in the gastrointestinal system and its function is to regulate the enteric tone (i.e. by maintaining the mucosal integrity and gastrointestinal motility [Citation95–97]), it is reasonable to question whether it would be safe to block CGRP at long term in the gastrointestinal system. In fact, long-term CGRP blockade could cause damage on the gastrointestinal mucosa, contribute to inflammatory bowel disease, or alter gastrointestinal motility (which could exacerbate episodes of diarrhea or constipation). Therefore, it would be prudent to consider the appropriate treatment of migraine in patients with gastrointestinal disorders (including ulcers).
Secondly, CGRP plays an important role in maintaining cardiovascular homeostasis, by acting as a potent vasodilator. Moreover, migraine is associated with increased risk of cardio – and cerebrovascular events [Citation98]. Within this framework, CGRP seems to be relevant in some vascular diseases by preventing hypertension [Citation103], preeclampsia [Citation104], cerebral ischemia [Citation105], as well as myocardial infarction and heart failure after cardiac ischemia [Citation106]. From this perspective, the prophylactic treatment with drugs that block CGRP or its receptor in patients with migraine turns out to be beneficial to prevent it. Notwithstanding, would the blockade of the CGRPergic system be beneficial in the long term?; or would this blockade trigger unwanted (cerebro)vascular events? Certainly, clinical trials have evaluated the safety of approved drugs for the prevention of migraine, reporting that patients have no cardiovascular side effects. Nonetheless, these trials were conducted in the short term (lasting less than 2 years) and excluded higher-risk patients. Hence, future clinical trials should include patients with preexisting cardiovascular disorders.
Thirdly, as shown in and described above, CGRP can also bind to CGRP-responsive receptors, namely, the AM and AMY receptors [Citation31]. Since these receptors can be found in the trigeminovascular system [Citation59], they may well play a role in the pathophysiology of migraine. However, to the best of our knowledge, there is no compelling evidence of their significance. In this respect, it is noteworthy that CGRP displays: (i) a high‐affinity/potency for the human AMY1, AMY2, and AMY3 receptors (human pEC50 values of 8.7–10.7; 6.2–9.7; and 7.6–9.7, respectively); and (ii) a moderate affinity for the human AM1 and AM2 receptors (human pKd or pKi values of 6.0 and 6.5–6.8, respectively) [Citation107]. Accordingly, CGRP receptor blockade by the use of gepants or monoclonal antibodies (i.e. erenumab), particularly in the long term, would also allow CGRP to bind to the AMY or AM receptors (see ). Hence, since the AMY1 receptor is considered as a second physiological CGRP receptor [Citation71], the possibility that this receptor may play a role during migraine attacks cannot be categorically excluded. In addition, the direct blockade of CGRP by using monoclonal antibodies (i.e. eptinezumab, fremanezumab, or galcanezumab) would allow the AM or AMY ligands to bind to the CGRP receptor. If this were indeed possible: what effects could trigger the binding of these vasodilators (less potent than CGRP) to the CGRP receptor? In fact, even the less potent effects produced by AM or AMY could explain a lower risk of (cardio)vascular disorders such as brain injury, hypertension, and ischemic heart disease [Citation85]. However, despite the fact that CGRP shows a moderate affinity for both the AM1 and AM2 receptors (pKd and pKi values of 6.0 and 6.5–6.8, respectively) (see ), the role of AM in migraine seems less likely since intravenous administration of this neuropeptide fails to cause migraine (unlike intravenous administration of CGRP) [Citation108]. On the other hand, AMY has moderate potency to bind at the CGRP receptor (pEC50 value of 6.63) [Citation109] (see ) and exerts biological actions similar to those of CGRP. Therefore, we can question whether AMY plays an important role in migraine triggering or in migraine pathophysiology. Hence, further studies are needed in order to determine: (i) the potential role and the possible mechanism(s) of action of the non-canonical CGRP receptors and their ligands in migraine; and (ii) whether blocking CGRP is more effective than blocking its receptor, or vice versa.
Fourthly, migraine is associated with comorbid illnesses besides cardiovascular diseases, especially painful disorders which include visceral pain, myofascial pain syndromes, or fibromyalgia [Citation20,Citation110,Citation111]. Indeed, in most short-term clinical trials, the patient selection criteria were strict, excluding patients with medical comorbidities, which can be a limitation to evaluate the tolerability of monoclonal antibodies against the CGRPergic system. Definitively, consideration should be given to whether the CGRPergic system blockade can be beneficial in treating pain-related comorbidities such as visceral pain or fibromyalgia, keeping in mind that in most cases the treatment of migraine and comorbidities may require two different medications [Citation20]. Therefore, more long-term studies are needed to determine whether the monoclonal antibodies can help alleviate various pain-related syndromes, and even to determine their effects in migraine patients with a variety of comorbidities. On the other hand, despite the results obtained from clinical trials are promising; certainly there are still several limitations including the assessment of anti-migraine drugs in the real-life condition. Recently, data from a real-life study showed a higher efficacy and safety of erenumab than those of the clinical trial in chronic migraine patients with previous preventive failures and with medication overuse [Citation112,Citation113]. Moreover, the real-world population could be more susceptible to the side effects produced by drugs to treat migraine due to their comorbidities [Citation113]. Therefore, future studies should focus on evaluating the effect of anti-migraine drugs on real-life populations in order to compare the data obtained from short-term clinical trials, and considering the most frequent comorbidities and the treatment duration.
Finally, the human clinical trials analyzing the monoclonal antibodies for migraine prevention have been carried out in the short term (except for erenumab, which has been evaluated in the long term [Citation67,Citation68]. Nevertheless, it is still unknown whether or not long-term blockade of CGRP or its receptor may generate few (especially cardiovascular) side effects; this is indeed a reasonable concern in view that the blockade of CGRP-induced vasodilator effects could represent potential cardiovascular risks. Furthermore, another interesting issue that cannot be excluded is the immunological safety of monoclonal antibodies; in this regard, undoubtedly, there may be a potential for the production of autoantibodies, despite the fact that these monoclonal antibodies are humanized antibodies. Therefore, basic science experimental studies and long-term studies (i.e. Phase IV) in patients with cardiovascular comorbidities (e.g. obesity, diabetes mellitus, etc.) are required to delineate the safety of the new prophylactic antimigraine drugs.
Article highlights
Migraine is a highly disabling, complex, and multifactorial neurological disorder that involves activation of the trigeminovascular system, resulting in the release of CGRP from the sensory nerves.
Although there is a wide variety of prophylactic antimigraine drugs that act via different mechanisms of action (e.g. β-blockers, antiepileptics, Ca2+ channel blockers, 5-HT receptor antagonists, etc.), CGRP (receptor) blockade is critical for the novel prophylactic treatments of migraine.
For prophylactic antimigraine treatment based on CGRP (receptor) blockade, the gepants (rimegepant and atogepant) and the monoclonal antibodies (erenumab, eptinezumab, fremanezumab, and galcanezumab) are effective and safe in the short term.
Regarding the novel antimigraine treatments that involve CGRP (receptor) blockade, basic science experimental studies and long-term clinical trials and/or registries (Phase IV) are required to confirm their safety and effectiveness and to delineate their potential cardiovascular side effects.
Declaration of interest
A Maassen van den Brink has received grants and/or fees from Amgen/Novartis, Lilly, and Teva. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership, or options, expert testimony, grants, or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have received personal fees or honoraria for lectures and/or advisory boards from Pharm Allergan, BIAL, Desitin, Eli Lilly, Hormosan, Novartis and TEVA. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Olesen J, Steiner TJ. The international c1assification of headache disorders, 2nd edn (ICDH-II). J Neurol Neurosurg Psychiatry. 2004;75:808–811.
- Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38:1–211.
- GBD2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of dise study 2016. Lancet Neurol. 2018;954:976.
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196.
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44.
- Villalón CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–323.
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl 1):S44–S53.
- Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–53.
- Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attack therapies. Brain. 1994;117:427–434.
- Edvinsson L, Goadsby PJ. Neuropeptides in migraine and cluster headache. Cephalalgia. 1994;14:320–327.
- Fanciullacci M, Alessandri M, Figini M, et al. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerine-induced cluster headache attack. Pain. 1995;60:119–123.
- Asghar MS, Hansen AE, Kapijimpanga T, et al. Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology. 2010;75:1520–1526.
- Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61.
- Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–1186.
- de Vries T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther. 2020 [published online 2020 Mar 12]. doi:https://doi.org/10.1016/j.pharmthera.2020.107528.
- Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–658.
- Goadsby PJ, Sprenger T. Current practice and future directions in the prevention and acute management of migraine. Neurol. 2010;9:285–298.
- Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55(Suppl 2):103–122.
- Weatherall MW. The diagnosis and treatment of chronic migraine. Ther Adv Chronic Dis. 2015;6:115–123.
- Silberstein SD. Preventive migraine treatment. Neurol Clin. 2009;27:429–443.
- Sun-Edelstein C, Rapoport AM. Update on the pharmacological treatment of chronic migraine. Curr Pain Headache Rep. 2016;20:6.
- Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet. 2019;394:1765–1774.
- Hoehlig K, Johnson KW, Pryazhnikov E, et al. A novel CGRP-neutralizing Spiegelmer attenuates neurogenic plasma protein extravasation. Br J Pharmacol. 2015;172:3086–3098.
- Amgen Inc. Erenumab (Aimovig™): US prescribing information. [ cited 2020 Apr 11]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
- Deen M, Correnti E, Kamm K, et al. Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain. 2017;18:96.
- González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, et al. Side effects associated with current and prospective antimigraine pharmacotherapies. Expert Opin Drug Metab Toxicol. 2018;14:25–41.
- Favoni V, Giani L, Al-Hassany L, et al. CGRP and migraine from a cardiovascular point of view: what do we expect from blocking CGRP? J Headache Pain. 2019;20:27.
- Gupta S, Nahas SJ, Peterlin BL. Chemical mediators of migraine: preclinical and clinical observations. Headache. 2011;51:1029–1045.
- Rosenfeld MG, Mermod JJ, Amara SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–135.
- Brain SD, Williams TJ, Tippins JR, et al. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56.
- Hay DL, Garelja ML, Poyner DR, et al. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR review 25. Br J Pharmacol. 2018;175:3–17.
- Russell FA, King R, Smillie SJ, et al. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94:1099–1142.
- Mulderry PK, Ghatei MA, Bishop AE, et al. Distribution and chromatographic characterisation of CGRP-like immunoreactivity in the brain and gut of the rat. Regul Pept. 1985;12:133–143.
- Hoffmann J, Wecker S, Neeb L, et al. Primary trigeminal afferents are the main source for stimulus-induced CGRP release into jugular vein blood and CSF. Cephalalgia. 2012;32:659–667.
- Iyengar S, Johnson KW, Ossipow MH, et al. CGRP and the trigeminal system in migraine. Headache. 2019;59:659–681.
- Edvinsson L, Ekman R, Jansen I, et al. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blook Flow Metab. 1987;7:720–728.
- Cady RK, Vause CV, Ho TW, et al. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–1266.
- Russo AF, Kuburas A, Kaiser EA, et al. A potential preclinical migraine model: CGRP-sensitized mice. Mol Cell Pharmacol. 2009;1:264–270.
- Wattiez AS, Wang M, Russo AF. CGRP in animal models of migraine. Handb Exp Pharmacol. 2019;255:85–107.
- Arulmani U, Gupta S, MaassenVanDenBrink A, et al. Experimental migraine models and their relevance in migraine therapy. Cephalalgia. 2006;26:642–659.
- Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies – successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–350.
- Iovino M, Feifel U, Yong CL, et al. Safety, tolerability and pharmacokinetics of BIBN 4096 BS, the first selective small molecule calcitonin gene-related peptide receptor antagonist, following single intravenous administration in healthy volunteers. Cephalalgia. 2004;24:645–656.
- Ho TW, Connor KM, Xhang Y, et al. Randomized controlled trials of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–966.
- Hewitt DJ, Aurora SK, Dodick DW, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–722.
- Diener HC, Barbanti P, Dahhlof C, et al. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia. 2011;3:573–584.
- Allergan plc. Allergan receives U.S. FDA approval for UBRELVY™ for the acute treatment of migraine with or without aura in adults. [ cited 2020 Apr 24]. Available from: https://www.prnewswire.com/news-releases/allergan-receives-us-fda-approval-for-ubrelvy-for-the-acute-treatment-of-migraine-with-or-without-aura-in-adults-300979082.html
- Biohaven Pharmaceutical Company Holding Ltd. Biohaven’s NURTEC™ ODT (rimegepant) receives FDA approval for the acute treatment of migraine in adults. [ cited 2020 Apr 6]. Available from: https://www.prnewswire.com/news-releases/biohavens-nurtec-odt-rimegepant-receives-fdaapproval-for-the-acute-treatment-of-migraine-in-adults-301013021.html
- NCT0372368. Efficacy and safety trial of rimegepant for migraine prevention in adults. [ cited 2020 Apr 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT03732638
- NCT02848326. Efficacy, safety, and tolerability of multiple dosing regimens of oral atogepant (AGN-241689) in episodic migraine prevention. [ cited 2020 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT02848326
- Allergan plc. Allergan’s oral CGRP receptor antagonist atogepant demonstrates robust efficacy and safety in episodic migraine prevention in a phase </Collab>2b/3 clinical trial. [ cited 2020 10 Apr]. Available from: https://www.prnewswire.com/news-releases/allergans-oral-cgrp-receptor-antagonist-atogepantdemonstrates-robust-efficacy-and-safety-in-episodic-migraine-prevention-in-a-phase-2b3-clinical-trial-300663770.html
- Martelletti P, Cipolla F, Capi M, et al. Atogepant. calcitonin gene-related peptide (CGRP) receptor antagonist, preventive treatment of migraine. Drugs Future. 2020 [ published online 2020 May]. DOI:https://doi.org/10.1358/dof.2020.45.5.3123467.
- NCT03855137. Efficacy, safety, and tolerability of atogepant for the prevention of chronic migraine. [ cited 2020 Apr 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT03855137
- Marcus R, Goadsby PJ, Dodick D, et al. BMS-927711 for the acute treatment of migraine: A double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34:114–125.
- Croop R, Goadsby PJ, Stock DA, et al. Efcacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394:737–745.
- Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142–149.
- Biohaven Pharmaceutical Company Holding Ltd. Biohaven delivers positive phase 3 results with rimegepant zydis® orally dissolving tablet (ODT): rapid and lasting benefit for the acute treatment of migraine. [ cited 2020 Apr 6]. Available from: https://www.prnewswire.com/news-releases/biohaven-delivers-positivephase-3-results-with-rimegepant-zydis-orally-dissolving-tablet-odt-rapid-and-lasting-benefit-forthe-acute-treatment-of-migraine-300758762.html
- Rubio-Beltran E, Chan KY, Danser AJ, et al. Characterisation of the calcitonin gene-related peptide receptor antagonists ubrogepant and atogepant in human isolated coronary, cerebral and middle meningeal arteries. Cephalalgia. 2020;40:357–366.
- Sacco S, Bendtsen L, Ashina M, et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine preventionPractice Guideline. . J Headache Pain. 2019;20:6.
- Edvinsson JCA, Viganò A, Alekseeva A, et al. The fifth cranial nerve in headaches. J Headache Pain. 2020;00:65.
- Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther. 2016;356:223–231.
- Novartis. Novartis and Amgen announce FDA approval of Aimovig(TM) (erenumab), a novel treatment developed specifically for migraine prevention. [ cited 2020 Apr 11] Available from: https://www.novartis.com/news/media-releases/novartis-and-amgen-announce-fda-approval-aimovigtm-erenumab-novel-treatment-developed-specifically-migraine-prevention
- Novartis. Novartis receives European licence for Aimovig®?(erenumab), the first treatment designed specifically for the prevention of migraine. [ cited 2020 Apr 11]. Available from: https://www.novartis.co.uk/news/media-releases/novartis-receives-european-licence-aimovigrverenumab-first-treatment-designed
- Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:382–390.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425–434.
- Dodick DW, Ashina M, Brandes JL, et al. ARISE: A phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026–1037.
- Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of Erenumab for episodic migraine. N Engl J Med. 2017;377:2123–2132.
- Ashina M, Goadsby PL, Reuter U, et al. Long-term safety and tolerability of erenumab: three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;39:1455–1464.
- Tepper SJ, Ashina M, Reuter U, et al. Long-term safety and efficacy of erenumab in patients with chronic migraine: results from a 52-week, open-label extension study. Cephalalgia. 2020;40:543–553.
- Rubio-Beltrán E, Labastida-Ramírez A, Haanes KA, et al. Characterisation of vasodilatory responses in presence of the CGRP receptor antibody erenumab in human isolated arteries. Cephalalgia. 2019;39:1735–1744.
- Haanes K, Chan KY, MaassenVanDenBrink A. Comment on “A second trigeminal CGRP receptor: function and expression of the AMY1 receptor”. Ann Clin Transl Neurol. 2016;3:307–308.
- Walker CS, Eftekhari S, Bower RL, et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann Clin Transl Neurol. 2015;2:595–608.
- Lundbeck H, A S H. Lundbeck A/S. FDA approves Lundbeck’s Vyepti™ (eptinezumab-jjmr) – the first and only intravenous preventive treatment for migraine.[ cited 2020 Apr 10]. Available from: https://investor.lundbeck.com/news-releases/newsrelease-details/fda-approves-lundbecks-vyeptitm-eptinezumab-jjmr-first-and-only/
- Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13:1100–1107.
- Ashina M, Saper J, Schaeffler BA, et al. Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020. published online 2020 Feb 19. DOI:https://doi.org/10.1177/0333102420905132.
- Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine PROMISE 2. Neurology. 2020;94:e1365–e1377.
- Bigal ME, Escandon R, Bronson M, et al. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: results of the phase 1 program. Cephalalgia. 2014;34:483–492.
- U.S. Food and Drug Administration. Drug approval package: ajovy (fremanezumab-vfrm). Teva Pharmaceuticals USA, Inc; [ cited 2020 Apr 11]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761089Orig1s000TOC.cfm
- Teva Pharmaceutical Industries Ltd. Teva’s AJOVY® receives EU approval offering patients the first and only anti-CGRP treatment with both quarterly and monthly dosing for the prophylaxis of migraine in adults.[ cited 2020 Apr 14] Available from: https://www.tevapharm.com/news-and-media/latest-news/tevas-ajovy-receives-eu-approval-offering-patients-the-first-and-only-anti-cgrp-treatment-with-both-qua/
- Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14:1081–1090.
- Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;318:1999–2008.
- Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14:1091–1100.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113–2122.
- Cohen JM, Dodick DW, Yang R, et al. Fremanezumab as add-on treatment for patients treated with other migraine preventive medicines. Headache. 2017;57:1375–1384.
- Bigal ME, Walter S, Bronson M, et al. Cardiovascular and hemodynamic parameters in women following prolonged CGRP inhibition using LBR-101, a monoclonal antibody against CGRP. Cephalalgia. 2014;34:968–976.
- Lionetto L, Curto M, Cisale GY, et al. Fremanezumab for the preventive treatment of migraine in adults. Expert Rev Clin Pharmacol. 2019;12:741–748.
- Eli Lilly and Company. Lilly’s Emgality™ (galcanezumab-gnlm) receives U.S. FDA approval for the preventive treatment of migraine in adults. [ cited 2020 Apr 20] Available from: https://investor.lilly.com/news-releases/news-release-details/lillys-emgalitytm-galcanezumab-gnlm-receives-us-fda-approval
- European Medicines Agency. Emgality (galcanezumab). [ cited 2020 Apr 20]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/emgality
- European Medicines Agency. Emgality (galcanezumab). [ cited 2020 Apr 28]. Available from: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/emgality
- Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75:187–193.
- Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75:1080–1088.
- Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38:1442–1454.
- Detke HC, Goadsby PJ, Wang S, et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. 2018;91:e2211–e2221.
- Camporeale A, Kudrow D, Sides R, et al. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018;18:188.
- Denekas T, Tröltzsch M, Vater A, et al. Inhibition of stimulated meningeal blood flow by a calcitonin gene-related peptide binding mirror-image RNA oligonucleotide. Br J Pharmacol. 2006;148:536–543.
- Peskar BM, Wong HC, Walsh JH, et al. A monoclonal antibody to calcitonin gene-related peptide abolishes capsaicin-induced gastroprotection. Eur J Pharmacol. 1993;250:201–203.
- Reinshagen M, Flämig G, Ernst S, et al. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J Pharmacol Exp Ther. 1998;286:657–661.
- Bartho L, Koczan G, Maggi CA. Studies on the mechanism of the contractile action of rat calcitonin gene-related peptide and of capsaicin on the guinea-pig ileum: effect of hCGRP (8-37) and CGRP tachyphylaxis. Neuropeptides. 1993;25:325–329.
- MaassenVanDenBrink A, Meijer J, Villalón CM, et al. Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci. 2016;37:779–788.
- Depre C, Antalik L, Starling A, et al. A randomized, double-blind, placebo-controlled study to evaluate the effect of erenumab on exercise time during a treadmill test in patients with stable angina. Headache. 2018;58:715–723.
- MaassenVanDenBrink A, Rubio-Beltrán E, Duncker D, et al. Is CGRP receptor blockade cardiovascularly safe? Appropriate studies are needed. Headache. 2018;58:1257–1258.
- Mulder IA, Li M, de Vries T, et al. Anti-migraine CGRP receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. 2020. published online 2020 June 25. DOI:https://doi.org/10.1002/ana.25831.
- Kaiser EA, Rea BJ, Kubiras A, et al. Anti-CGRP antibodies block CGRP-induced diarrhea in mice. Neuropeptides. 2017;64:95–99.
- Smillie SJ, King R, Kodji X, et al. An ongoing role of a-calcitonin gene-related peptide as part of a protective network against hypertension, vascular hypertrophy, and oxidative stress. Hypertension. 2014;63:1056–1062.
- Yallampalli C, Wimalawansa SJ. Calcitonin gene-related peptide (CGRP) is a mediator of vascular adaptations during hypertension in pregnancy. Trends Endocrinol Metab. 1998;9:113–117.
- Schebesch KM, Herbst A, Bele S, et al. Calcitonin-gene related peptide and cerebral vasospasm. J Clin Neurosci. 2013;20:584–586.
- Homma S, Kimura T, Sakai S, et al. Calcitonin gene-related peptide protects the myocardium from ischemia induced by endothelin-1: intravital microscopic observation and (31)P-MR spectroscopic studies. Life Sci. 2014;118:248–254.
- IUPHAR/BPS Guide to PHARMACOLOGY. Calcitonin receptor. [ cited 2020 May 18]. Available from: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=11
- Petersen KA, Birk S, Kitamura K, et al. Effect of adrenomedullin on the cerebral circulation: relevance to primary headache disorders. Cephalalgia. 2009;29:23–30.
- Bailey R, Hay DL. Pharmacology of the human CGRP1 receptor in cos 7 cells. Peptides. 2006;27:1367–1375.
- Giamberardino MA, Affaitati G, Martelletti P, et al. Impact of migraine on fibromyalgia symptoms. J Headache Pain. 2016;17:28.
- Giamberardino MA, Costantini R. Challenging chronic migraine: targeting the CGRP receptor. Lancet Neurol. 2017;16:410–411.
- Ornello R, Casalea A, Frattale I, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21:32.
- Lambru G, Hill B, Murphy M, et al. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21:61.
