?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objectives
To describe the cumulative incidences of adverse drug reactions (ADRs) associated with disease-modifying anti-rheumatic drugs (DMARDs) in rheumatoid arthritis (RA) patients from real-world data (RWD), using the DREAM-RA registry, and to compare these with incidence frequencies mentioned in the Summary of Product Characteristics (SmPC).
Methods
All ADRs in patients with recorded use of adalimumab, etanercept, hydroxychloroquine, leflunomide, oral and subcutaneous methotrexate, and sulfasalazine from a single center participating in the DREAM-RA registry (n = 1,098 patients) that were directly sent to the Netherlands Pharmacovigilance Center Lareb were assessed. Cumulative incidences were calculated, described and compared to the most recently revised SmPCs.
Results
In total, 14 ADRs (≥5 case reports) associated with the use of one of the included DMARDs were reported with a higher estimated cumulative incidence compared to the SmPC. For hydroxychloroquine and sulfasalazine, 5 ADRs (≥5 case reports) mentioned with an ‘unknown’ incidence in the SmPC were reported as ‘common’ in this study.
Conclusions
Although ADR data in the DREAM-RA registry were partly comparable with data in the SmPCs, RWD from this patient registry provided an added value to the currently available information on the incidences of ADRs associated with DMARDs in RA patients as described in SmPCs.
1. Introduction
Rheumatoid Arthritis (RA) is a chronic inflammatory disease, characterized by synovitis and joint destruction and may cause progressive disability and premature death [Citation1]. Disease modifying anti-rheumatic drugs (DMARDs), both conventional synthetic (csDMARDs) and biologicals (bDMARDs), have proven to be effective in reducing or reversing symptoms, preventing disability, and preventing progression of joint damage. DMARDs can be used as monotherapy or in combination therapies [Citation2]. With the broad spectrum of treatment options with DMARDs, toxicity may be a limiting factor [Citation1,Citation3].
The Summary of Product Characteristics (SmPC) is a summary of data from clinical trials, post-authorization marketing studies, and spontaneous reports, and provides information on the properties and officially approved conditions for use of a medicine [Citation4]. For each DMARD, the known adverse drug reactions (ADRs) are summarized in the SmPC, including the observed incidences of these ADRs. In turn, patient information leaflets (PIL) and decision aids are based on information summarized in the SmPC. Therefore, ideally, the information in the SmPC matches observations in real-world data (RWD). However, as these data were not specifically gathered in the context of the continuously changing daily clinical care of RA patients, and information on the frequency of events is probably based on preclinical trials and controlled circumstances, both the PIL and the SmPC may not be comprehensive or entirely representative.
The Netherlands Pharmacovigilance Center (Lareb) is responsible for collecting and analyzing reports of ADRs of medicines and vaccines. New information on ADRs should promptly be used to update sources like the SmPC. In order to detect discrepancies on (incidences of) ADRs between the SmPC and data from daily clinical practice, RWD from the growing number of available disease registries may be used. The online Dutch Rheumatoid Arthritis Monitoring (DREAM-RA) registry [Citation5] is an initiative from a network of Dutch hospitals to stimulate quality of care and clinical research, by sharing knowledge, experience and results. DREAM-RA contains multiple prospective, still ongoing, cohorts of RA patients treated in rheumatology departments across the Netherlands. The registry currently includes over 2,500 patients, leading to a large and representative high quality database of, among others, reported ADRs from real-world clinical practice.
Several studies have previously been conducted analyzing selections of ADRs for specific types of DMARDs in specific treatment cohorts in DREAM-RA [Citation6,Citation7,Citation8,Citation9]. However, the question remains whether the frequency of the real-world reported ADRs in RA patients in the DREAM-RA registry matches with data in the SmPC. Knowledge on the incidences of possible ADRs in real life as compared to the information in the SmPCs creates safer pharmacotherapy and may also contribute to shared decision making and subsequent treatment adherence, since this knowledge can be used to better inform patients on both the benefits and possible harms when using a specific drug. The aim of this study is to assess the cumulative incidences of ADRs in the DREAM-RA registry and compare these to those mentioned in the SmPC. We describe all real-world ADR incidences that are higher than those listed in the SmPCs, new drug-ADR associations that are not mentioned in the SmPCs, and observed cumulative incidences of ADRs with an unknown incidence in the SmPCs.
2. Methods
2.1. Study design
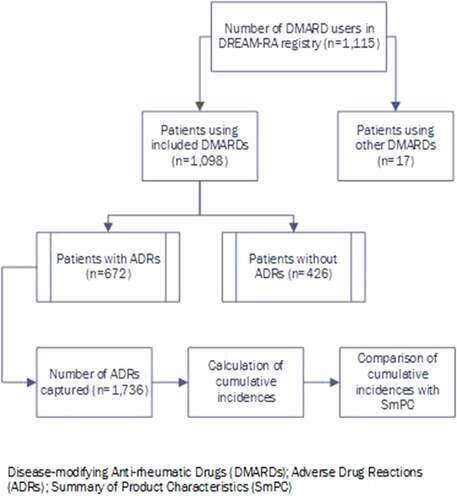
In this observational study, the cumulative incidences of reported ADRs from RWD collected in one large hospital (Medisch Spectrum Twente (MST)) which participated in the DREAM-RA registry from the very beginning are described. The data gathered in MST are representative for the average rheumatology center in the Netherlands. The online data collection system mijnreumacentrum.nl [Citation10] was used for registration of all data in DREAM-RA. RA patients were extracted from the system by selecting only those patients that were intensively monitored in the biologicals cohort or one of the two remission-induction cohorts of DREAM, defined as the DREAM-RA registry [Citation5]. All reports on ADRs from 1 January 2003 up to 29 February 2020 in patients from MST using DMARDs for RA in the DREAM-RA registry and subsequently directly sent to Lareb were assessed. The cumulative incidences associated with the use of the six DMARDs with the largest numbers of reported ADRs were included for analysis. These DMARDs were adalimumab, etanercept, hydroxychloroquine, leflunomide, oral methotrexate, subcutaneous (sc.) methotrexate, and sulfasalazine. These also represent the overall most commonly used csDMARDs and bDMARDs in RA in the Netherlands. The selection of patients for analysis is presented in . In total, 1,098 RA patients (>90% of RA patients from MST included in the DREAM-RA registry) used at least one of the selected DMARDs. For each DMARD, the cumulative incidences of the associated ADRs were calculated, described and compared with the most recently revised version of the SmPCs of this DMARD.
2.2. Data source
In DREAM-RA, data on the quality of current rheumatic care, encompassing both clinical aspects and patient-reported outcomes, have been systematically collected and reported since 2003. Starting with the monitoring of bDMARD users [Citation11], the registry has expanded from 2006 with cohorts of very early RA patients treated according to specific treat-to-target (T2T) remission induction strategies [Citation12,Citation13] and finally by including all RA patients treated in hospitals participating in DREAM-RA. Both healthcare professionals (HCPs) and patients can register an ADR in mijnreumacentrum.nl, an online patient portal. However, all patient reported ADRs are always verified by rheumatology HCPs before they are definitively reported in the system. Therefore, ADRs were never reported double. Since December 2015, all ADRs collected in the DREAM-RA registry are directly forwarded to Lareb. In addition, all ADRs prospectively collected between 2003 and 2015 were retrospectively forwarded to Lareb [Citation5]. Written consent was given by all patients before inclusion in the DREAM-RA cohorts. This informed consent also covers the use of their data by Lareb [Citation14].
In this study, for each DMARD only one SmPC was used as a reference, derived from the online database of the Dutch medicine evaluation board [Citation15]. Since all manufacturers compose their own unique SmPC for every individual drug product, in this study the most recently revised verison of the SmPC of each DMARD was chosen. Regarding biologicals, the innovator is leading. For methotrexate, the most recently revised versions of both oral methotrexate as sc. methotrexate were used. Therefore, a total of seven SmPCs were used in this study [Citation16,Citation17,Citation18,Citation19,Citation20,Citation21,Citation22].
2.3. Data processing
All ADRs were coded by trained pharmacovigilance assessors of Lareb according to Medical Dictionary for Regulatory Activities (MedDRA) codes, version 20.1 [Citation23]. MedDRA is a medical terminology that has a structured hierarchy and provides options for retrieving data by specific or broad groupings, according to the level of specificity required. In this study, preferred terms (PTs) were used to describe ADRs [Citation4]. A PT is the second-last level of the MedDRA terminology and is, in this study, a distinct descriptor for a symptom. Related PTs are grouped into High Level Terms (HLT) based upon characteristics such as anatomy, physiology, etiology or function. HLTs are in turn subordinated to High Level Group Terms (HGLTs), and finally HGLTs are grouped into system organ classes (SOCs). The SOC is the highest level of the hierarchy and provides the broadest concept for data retrieval. Each PT has a primary SOC [Citation24]. All ADR reports were extracted from databases of Lareb using queries in Microsoft SQL Server Management Studio 17. The following were queried: reference ID, reporting number, date of birth, gender, DMARDs (adalimumab, etanercept, hydroxychloroquine, leflunomide, methotrexate and sulfasalazine), route of administration, description of ADR, and ADR as both PT and SOC. For methotrexate, ADRs were analyzed separately for either oral or sc. administration. If the route of administration was unknown, it was assumed the route of administration was oral, as rheumatologists are used to explicitly mention sc. administration.
In each SmPC, the incidences of ADRs are based on one or more data sources (i.e. clinical trial or post-authorization safety study), determining the frequency category [Citation4]. The known ADRs can be found in the section ‘undesirable effects’ (4.8) of the SmPC as PT, per SOC. The frequency is described as ‘very common’ (≥10%), ‘common’ (≥1% to <10%), ‘uncommon’ (≥0.1% to <1%), ‘rare’ (≥0.01% to <0.1%), ‘very rare’ (<0.01%) or unknown.
2.4. Data analysis
Statistics were performed using IBM SPSS Statistics (version 22). Baseline characteristics of all included patients were expressed in means with standard deviations (SD) or frequency with percentage, where appropriate. Cumulative incidences for ADRs included in this study were calculated by using the following equation:
In case more than one drug was the suspect drug for a specific ADR, all suspect drug-ADR associations were reported in the registry. Thereby, the ADR was included in the numerator of the fraction for calculation of the cumulative incidences of all suspect drugs.
Whenever a patient experienced the same drug-ADR association for the second time, this was considered a new case report. No intended rechallenges were included in this study. Subsequently, all cumulative incidences were compared to the corresponding SmPC [Citation16–22]. For this, the reported ADR as PT had to match with the ADR in the corresponding SmPC exactly. However, in case a PT was not mentioned in the SmPC exactly, but was clinically captured by another PT, this was also described. First, all ADRs associated with a higher cumulative incidence in DREAM-RA compared to the incidence in the corresponding SmPC were described. Second, all ADR-drug associations which were not described in the corresponding SmPC, were also checked against all other SmPCs of the concerned DMARD. Only in cases where the ADR-drug association was not found in any of these SmPCs, the ADR was reported as a new ADR-drug association. Third, all ADRs that are mentioned with a frequency unknown in the SmPC, but with an estimated cumulative incidence in DREAM-RA, were described.
2.5. Data presentation
First, only those ADRs with a higher cumulative incidence reported in DREAM-RA (with ≥5 case reports) compared to the cumulative incidence mentioned in the SmPC were tabulated and ranked according to observed incidence. Next, new drug-ADR associations observed in the DREAM-RA registry with ≥5 case reports were described. Finally, ADRs that are mentioned in the SmPC with an unknown frequency but with ≥5 case reports in DREAM-RA, were described. In addition, all ADRs stratified by DMARD were described in detail in the appendix, including cumulative incidences.
3. Results
A total of 1,098 RA patients included in the DREAM-RA registry with a total follow-up period of 7,137 years (mean 2,816 days (±1,430)) used one of the studied DMARDs, from which a total of 672 (61.2%) unique patients experienced at least one ADR. They experienced a total of 1,736 ADRs, from which 142 ADRs (8.2%) were serious ADRs.
3.1. Patient characteristics
ADRs were reported by 58.1% of leflunomide users, 41.7% of oral methotrexate users, 38.7% of sulfasalazine users, 30.3% of adalimumab users, 32.0% of etanercept users, 24.8% of sc. methotrexate users, and 21.0% of hydroxychloroquine users. Patient characteristics can be found in . Patients were on average 66.5 (±13.8) years old and the majority were female (65.1%). Patients who reported ADRs were on average 61.4 (±13.6) years old, and more often female (68.8%).
Table 1. Patient characteristics of DREAM-RA patients using one or more of the DMARDs of interest (n = 1,098)
3.2. Higher cumulative incidence of ADRs in DREAM-RA than mentioned in the SmPC
For a complete overview of all ADRs and corresponding cumulative incidences reported in DREAM-RA, see Appendix 1. In total, 14 ADRs (with ≥5 case reports) were associated with a higher cumulative incidence in the DREAM-RA data compared to the cumulative incidence in the corresponding SmPC. All other ADRs captured in DREAM-RA were mentioned with an equal or lower cumulative incidence compared to the SmPC, or had not been mentioned at all in the SmPC. With respect to cumulative incidences of ADRs that were comparable with the SmPC, those of oral methotrexate use were the most comparable. In contrast, reported ADRs associated with the use of hydroxychloroquine were the least comparable with mentioned ADRs in the SmPC. Overall, cumulative incidences of ADRs in the SOC skin and subcutaneous tissue disorders were the most comparable to the incidences mentioned in the SmPC. In addition, observed cumulative incidences of ADRs in the SOC gastrointestinal disorders were often higher compared to the incidences mentioned in the SmPC. An example is nausea associated with oral methotrexate use, with an estimated incidence of 15.8% in the DREAM-RA registry. In the SmPC, this was mentioned with an incidence ‘common’ (≥1% to <10%). Other interesting results are opportunistic infections and serious infections with the use of etanercept, which have higher estimated cumulative incidences in the DREAM-RA registry compared to the SmPC (1.9% vs ≥0.01% to <0.1% and 2.3% vs ≥0.1% to <1%, respectively). All ADRs with a higher cumulative incidence compared to the SmPC are summarized in .
Table 2. ADRs with a higher cumulative incidence compared to the SmPC
3.3. New drug-ADR associations
There were no new drug-ADR associations reported in the DREAM-RA data with 5 or more case reports, which had not been mentioned in one of the concerned SmPCs. Although some ADRs as PT were not explicitly mentioned in the SmPCs, these were captured by other PTs described in the SmPC. An example is abdominal discomfort, which was observed with the use of all DMARDs except sulfasalazine, and was captured in all respective SmPCs by ‘abdominal pain’ and/or ‘abdominal problems’. This is in line with the SmPC guidelines, which states that when effects are mentioned under different terms but represent the same phenomenon, they should be grouped as a single ADR [Citation4].
3.4. ADRs with an incidence ‘unknown’ in the SmPC
Dizziness, pruritus, blurred vision and headache associated with the use of hydroxychloroquine were mentioned with an incidence ‘unknown’ in the SmPC. However, cumulative incidences of respectively 2.6%, 1.6%, 1.5% and 1.0% were found in DREAM-RA (). This suggests that the estimated cumulative incidences of dizziness, pruritus, blurred vision and headache are in fact ‘common’ (≥1% to <10). The same was observed for mucositis (including mouth ulceration and stomatitis) with the use of sulfasalazine, with an incidence in DREAM-RA of 1.0%, suggesting that the incidence is ‘common’ (≥1% to <10%).
Table 3. ADRs with an unknown incidence in the SmPC
4. Discussion
A higher cumulative incidence was found for 14 ADRs associated with the use of etanercept, hydroxychloroquine, leflunomide, oral and sc. methotrexate, and sulfasalazine in RWD compared to the SmPCs. In addition, dizziness, pruritus, blurred vision and headache with the use of hydroxychloroquine, and mucositis associated with the use of sulfasalazine, which were all mentioned with an incidence ‘unknown’ in the SmPC, were observed with an estimated cumulative incidence corresponding with ‘common’ (≥1% to <10%) in this RWD study. All other ADRs observed in DREAM-RA had a cumulative incidence within the range of or lower than mentioned in the SmPC. All ADRs that were not mentioned in the SmPC, but reported in the DREAM-RA registry, had <5 case reports.
One notable underestimation of ADRs in the SmPC concerned nausea associated with oral methotrexate use. Previous studies indicate that nausea is one of the most frequently occurring ADRs in oral methotrexate use in inflammatory arthritis [Citation25,Citation26], whereas Dhir et al. [2012, Citation27] found that the estimated frequency of nausea in oral methotrexate users in RA routine practice patients is 21.8%. This is in line with our findings in RWD with an estimated incidence of 15.8%, implying that nausea in oral methotrexate use is ‘very common’ (≥10%). Another underestimation of frequency of ADRs in the SmPC of etanercept concerned opportunistic infections and serious infections. A systematic review of Minozzi et al. [2016, Citation28] showed comparable outcomes, with an incidence of 2.6% (‘common’) for serious infections and 0.3% (‘uncommon’) for opportunistic infections. Improved knowledge on associations between genetic polymorphisms and ADRs may allow us to better understand which patients to treat or not to treat with etanercept, reducing the risk of ADRs [Citation29]. For hydroxychloroquine, nausea and diarrhea were mentioned with an incidence ‘uncommon’ in the SmPC, but in our study an estimated incidence of ‘common’ was observed (3.1% and 2.1%, respectively). Literature supports these findings by reporting both ADRs as frequently occurring with the use of hydroxychloroquine [Citation30], with an incidence of 10.0% reported by the Australian Rheumatology Association [Citation31].
There were several ADRs observed in our RWD study with an incidence ‘unknown’ in the SmPC. This was mostly the case for ADRs related to the use of hydroxychloroquine and sulfasalazine. An example is blurred vision (n = 11, 1.5%) associated with the use of hydroxychloroquine. Although no incidences are known, blurred vision is an ADR which is commonly described in literature [Citation26,Citation32,Citation33]. Pruritus associated with the use of hydroxychloroquine was also mentioned with an incidence ‘unknown’ in the SmPC, but in our study an incidence of 1.6% (n = 12) was observed. A systematic literature review of Sharma et al. [Citation34] supports this finding by describing pruritus as one of the most common dermatologic reactions reported with the use of hydroxychloroquine. The review included 94 articles encompassing 689 dermatologic ADRs. A total of 62 cases of pruritus were reported, with incidences of pruritus in RA patients varying from 0.8% (‘uncommon’) to 25.0% (‘very common’). Mucositis (including mouth ulceration and stomatitis) associated with the use of sulfasalazine is mentioned with an incidence ‘unknown’ in the SmPC, but is a well-known ADR in literature [Citation35]. A retrospective study of Okubo et al. [2002, Citation35] reported an estimated incidence of 5.9% (n = 9), implying the incidence is ‘common’. The current study adds to this knowledge that an incidence corresponding to ‘common’ (n = 5, 1.0%) was found.
With regard to the study population, the female/male ratio in RA patients in this study is about 1.9. This ratio is in line with literature, suggesting the female/male ratio in RA patients above the age of 60 is 2.0 [Citation36], which represents the study population in our study. When looking at the patients who experienced ADRs, the ratio in our study increases to 2.6 (72.1% females compared to 27.9% males). This suggests that female RA patients either experience more ADRs or tend to report ADRs more often to HCPs during their visits to outpatient clinics. These findings are in line with previous reports in literature [Citation37,Citation38], and was also confirmed by Holm et al. [2017, Citation39], who concluded that the frequency of reported ADRs by HCPs was higher for women than for men.
A limitation of this study could be that patients from only one hospital (MST) were included, introducing the possibility of selection bias. However, as this hospital includes and intensively monitors all their RA patients seen in daily practice in the DREAM-RA registry, this center provided the most complete data on a broad range of RA patients. Given that MST is a large top-clinical hospital, the study population is likely to be representative for the overall Dutch population of RA patients in the Netherlands. Another limitation of real-world observational data is that it remains unclear whether each HCP reports every ADR all the time, in particular those with a known incidence of ‘common’ or ‘very common’. HCPs are likely to assume that such ADRs are already well known and might therefore refrain from reporting them. Additionaly, as an ADR might not always have a clinical impact, HCPs may not always register every ADR in the online patient portal mijnreumacentrum.nl. As a result, the cumulative incidences of ADRs reported in this study are likely to be an underestimation of the actual experienced ADRs. However, as this is already a good approximation and we should take into account the registration burden of HCP, the calculated cumulative incidences can be seen as a lower limit of true occurrence.
One strength of this study lies in the use of real-world daily clinical practice data from a large registry, including over 1,000 RA patients using one of the six DMARDs of interest over a mean follow-up period of almost eight years per patient. Data was collected as part of routine daily clinical practice, reducing selection bias. Consequently, this contributes to a high external validity, as the study population can be considered representative for the total population of RA patients treated in the Netherlands. Moreover, ADRs included in this study could be reported by both HCPs and patients, but were always confirmed by HCPs. Since these HCPs know their chronically ill patients very well and for a long time, including information on their comorbidities, co-medication and patient characteristics, only strong and verified drug-ADR associations were registered.
With regard to implications in practice, dizziness, pruritus, blurred vision and headache associated with the use of hydroxychloroquine and mucositis associated with the use of sulfasalazine were registered with an incidence ‘unknown’ in the SmPC, whereas in the current study estimated cumulative incidences of ‘common’ (≥1% to <10%) were observed. In reality, however, these cumulative incidences are probably even higher. According to the SmPC guidelines, SmPC incidences could be updated according to the findings in this study. The same is recommended for the ADRs with a higher estimated cumulative incidence in the current study compared to the SmPC.
5. Conclusion
This study shows that for 14 ADRs the incidences in the SmPCs were underestimated, and for five ADRs mentioned with an incidence ‘unknown’ in the SmPC, a cumulative incidence corresponding with ‘common’ was observed. This leads to the conlusion that ADRs captured in patient registries can be seen as a potentially important additional source of information on the estimate of incidences of ADRs in daily clinical practice and should preferably be integrated with the information in the SmPCs. In further research, real-world cumulative incidences of ADRs in other disease registries could be assessed by applying the methods of this study. Findings of these studies will contribute to bridging the gap between information available in SmPCs and the real-world clinical setting.
Author contributions
All authors were involved in the conception and design of the study. EL Giraud and NT Jessurun collected, researched, analyzed and interpreted the data. All authors critical reviewed, revised the paper for intellectual content, provided detailed feedback, read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Declaration of interest
A van Tubergen reports unrestricted research grants received from Pfizer, Abbvie, UCB, Janssen-Cilag, Celgene, Novartis and Biogen for the development and implementation of SpA-Net, a registry for spondyloarthritis in the Netherlands; consulting fee from Novartis, all outside the submitted work. HE Vonkeman reports grants received from AbbVie, and personal fees received from AbbVie, Amgen, AstraZeneca, BMS, Celgene, Celltrion, Gilead, GSK, Janssen-Cilag, Novartis, Pfizer, Roche and Safoni-Genzyme, all outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics approval
N/A.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_Material.docx
Download MS Word (81.6 KB)Data availability statement
The datasets used and/or analyzed during the current study are not publicly available due to legal restrictions related to data privacy protection. However, the data are available on reasonable request after authorization of the DREAM board. Researchers interested in data access may contact the DREAM board via http://www.dreamregistry.nl/en.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Guo Q, Wang Y, Xu D, et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
- Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
- Wilsdon TD, Hill CL. Managing the drug treatment of rheumatoid arthritis. Aust Prescr. 2017;40(2):51–58.
- Guideline A. On Summary Of Product Characteristics (SmPC). 2009. (Directorate-General ECEAI, editor). p. 29.
- DREAM-RA registry. 2020. [cited 2020 May 1]. Available from: https://www.dreamregistry.nl
- Kievit W, Fransen J, Adang EM, et al. Long-term effectiveness and safety of TNF-blocking agents in daily clinical practice: results from the Dutch Rheumatoid Arthritis Monitoring register. Rheumatology (Oxford). 2011;50(1):196–203.
- Ten Klooster PM, Versteeg LGA, Oude VMAH, et al. Radiographic progression can still occur in individual patients with low or moderate disease activity in the current treat-to-target paradigm: real-world data from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Arthritis Res Ther. 2019;21(1):237.
- van Dartel SA, Fransen J, Kievit W, et al. Predictors for the 5-year risk of serious infections in patients with rheumatoid arthritis treated with anti-tumour necrosis factor therapy: a cohort study in the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Rheumatology (Oxford). 2013;52(6):1052–1057.
- van Dartel SA, Fransen J, Kievit W, et al. Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis. 2013;72(6):895–900.
- mijnreumacentrum: transparency in healthcare. 2020. [cited 2020 May 1]. Available from: https://www.mijnreumacentrum.nl/reuma-en-behandeling
- Kievit W, Fransen J, Oerlemans AJ, et al. The efficacy of anti-TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Ann Rheum Dis. 2007;66(11):1473–1478.
- Vermeer M, Kuper HH, Bernelot Moens HJ, et al. Adherence to a treat-to-target strategy in early rheumatoid arthritis: results of the DREAM remission induction cohort. Arthritis Res Ther. 2012;14(6):R254.
- Steunebrink LM, Vonkeman HE, Ten Klooster PM, et al. Recently diagnosed rheumatoid arthritis patients benefit from a treat-to-target strategy: results from the DREAM registry. Clin Rheumatol. 2016;35(3):609–615.
- van de Laar CJ, Oude Voshaar MAH, Vonkeman HE. Cost-effectiveness of different treat-to-target strategies in rheumatoid arthritis: results from the DREAM registry. BMC Rheumatol. 2019;3:16.
- CBG. Geneesmiddeleninformatiebank College ter Beoordeling van Geneesmiddelen. 2020. [cited 2020 May 1]. Available from: https://www.geneesmiddeleninformatiebank.nl/nl/
- AbbVie. Samenvatting van de productkenmerken Humira oplossing voor injectie in een voorgevulde spuit. 2008.
- Accord. Samenvatting van de productkenmerken Methotrexaat Accord 20 mg. 2020. (tabletten).
- Accord. Samenvatting van de productkenmerken Injexate oplossing voor injectie in voorgevulde spuit. 2019.
- Centrafarm. Samenvatting van de productkenmerken Leflunomide CF. 2017. (filmomhulde tabletten).
- Mylan. Samenvatting van de productkenmerken Sulfasalazine Mylan. 2019. (maagsapresistente tabletten).
- Pfizer. Samenvatting van de productkenmerken Enbrel 25 mg poeder voor oplossing voor injectie. 2010.
- Sanofi-aventis. Samenvatting van de productkenmerken Plaquenil. 2020. (filmomhulde tabletten).
- Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109–117.
- ICH. MedDRA maintenance and support services organization. 2010. (Introductory Guide to MedDRA Version 13.1).
- Patil P, Parker RA, Rawcliffe C, et al. Methotrexate-induced nausea and vomiting in adolescent and young adult patients. Clin Rheumatol. 2014;33(3):403–407.
- Ma SN, Zaman Huri H, Yahya F. Drug-related problems in patients with rheumatoid arthritis. Ther Clin Risk Manag. 2019;15:505–524.
- Dhir V, Aggarwal A. Methotrexate-related minor adverse effects in rheumatoid arthritis: more than a nuisance. J Clin Rheumatol. 2012;18(1):44–46.
- Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15(sup1):11–34.
- Murdaca G, Spanò F, Contatore M, et al. Pharmacogenetics of etanercept: role of TNF-α gene polymorphisms in improving its efficacy. Expert Opin Drug Metab Toxicol. 2014;10:12.
- Detert J, Klaus P, Listing J, et al. Hydroxychloroquine in patients with inflammatory and erosive osteoarthritis of the hands (OA TREAT): study protocol for a randomized controlled trial. Trials. 2014;15:412.
- Patient information on hydroxychloroquine. Australian Rheumatology Association; 2016.
- Browning DJ. Hydroxychloroquine and chloroquine retinopathy: screening for drug toxicity. Am J Ophthalmol. 2002;133(5):649–656.
- Grierson DJ. Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheum Dis. 1997;56(3):188–190.
- Sharma AN, Mesinkovska NA, Paravar T. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020;83(2):563–578.
- Okubo S, Nakatani K, Nishiya K. Gastrointestinal symptoms associated with enteric-coated sulfasalazine (Azulfidine EN tablets). Mod Rheumatol. 2002;12(3):226–229.
- Kvien TK, Uhlig T, Odegard S, et al. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069:212–222.
- Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999–1004.
- Watson S, Caster O, Rochon PA, et al. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17:100188.
- Holm L, Ekman E, Jorsater Blomgren K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf. 2017;26(3):335–343.