ABSTRACT
Background
The cardiovascular and kidney safety of glucose-lowering drugs is a key concern in type 2 diabetes (T2D). We evaluated cardiorenal outcomes with glucose-lowering drugs in Asian patients, who comprise over half of T2D cases globally.
Research design and methods
A rapid evidence assessment was conducted for phase III or IV, double-blind, randomized clinical trials of glucose-lowering drugs reporting cardiovascular or kidney outcomes for Asian T2D patients (Embase, Medline, Cochrane Library databases: 1 January 2008–14 June 2020).
Results
Fifty-four publications reported exploratory data for Asians from 18 trials of dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and insulin analogs. SGLT2 inhibitors and several GLP-1 receptor agonists were associated with reduced cardiovascular risk in Asian T2D patients, while DPP-4 inhibitors exhibited cardiovascular safety. SGLT2 inhibitors also appeared to reduce renal risk; however, kidney outcomes were lacking for DPP-4 inhibitors other than linagliptin and GLP-1 receptor agonists in Asian patients. Insulin data were inconclusive as the only trial conducted used different types of insulin as both treatment and comparator.
Conclusions
Cardiorenal outcomes with glucose-lowering drugs in Asian T2D patients were similar to outcomes in the overall multinational cohorts of these trials. DPP-4 inhibitors appear to demonstrate cardiovascular safety in Asians, while SGLT2 inhibitors and some GLP-1 receptor agonists may reduce cardiorenal and cardiovascular risk, respectively.
1. Introduction
Type 2 diabetes (T2D) is characterized by chronic hyperglycemia, which is associated with both microvascular and macrovascular complications [Citation1]. Consequently, people with T2D are at increased risk for kidney disease and cardiovascular disease [Citation1], conditions that are closely interconnected pathophysiologically, with mechanisms and crosstalk involving metabolic, hemodynamic, neurohormonal, and inflammatory pathways [Citation2,Citation3]. Cardiovascular disease, including atherosclerotic conditions and heart failure, is the leading cause of premature mortality in people with T2D [Citation4] while chronic kidney disease affects up to 50% of T2D patients [Citation5].
The prevalence of diabetes has escalated dramatically over recent years to become a global pandemic affecting an estimated 463 million people in 2019, around 90% of whom suffer from T2D [Citation1]. Asia has not been spared this trend; in fact, South-East Asia (including India) and the Western Pacific region (including Japan and China) together account for over half of diabetes cases worldwide [Citation1].
Because of the interconnected cardio-renal-metabolic complications of T2D, it is critical that glucose-lowering drugs do not have off-target adverse effects on the cardiovascular system or kidneys, and ideally they would provide additional cardiorenal benefit. Based on cardiovascular safety signals for peroxisome proliferator-activated receptor agonists and sulfonylureas [Citation6–10], the United States Food and Drug Administration (FDA) mandated in 2008 that novel glucose-lowering drugs must demonstrate no excess risk for cardiovascular disease [Citation11]. In response, more than 15 large, multinational, cardiovascular outcomes trials (CVOTs) of glucose-lowering drugs have been conducted over the past 12 years, mostly involving the modern drug classes of dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors.
The pathophysiology of T2D differs in some respects between Asians and the non-Asian patients who comprised the majority of the CVOT cohorts, and there may also be differences in their cardiorenal risk [Citation5,Citation12,Citation13]. Compared with their white counterparts, Asians with T2D generally have lower body mass index but greater abdominal obesity. Asian patients also tend to have greater insulin resistance (especially South Asians), reduced pancreatic β-cell function in the setting of lower insulin sensitivity that often necessitates early insulin treatment (particularly East Asians), and higher postprandial glucose excursions (likely resulting from carbohydrate-rich diets) [Citation5,Citation12–14]. Recent research has revealed genetic factors in East Asian individuals that may contribute to T2D [Citation15]. Furthermore, East Asian patients with T2D have a greater risk of stroke and kidney complications than white patients [Citation12], while South Asian patients also have a higher risk of kidney complications [Citation5]. Interestingly, the incretin effect – i.e. postprandial amplification of insulin secretion by gastrointestinal peptide hormones – may not be blunted in East Asians with T2D as it is in white patients [Citation16]. This may explain apparent differences between Asians and non-Asians in glycemic response to incretin therapies (DPP-4 inhibitors and GLP-1 receptor agonists); namely, the seemingly greater improvements in glycemic control in Asians [Citation17–20]. Furthermore, some analyses have suggested that the glycemic response to SGLT2 inhibitors may also be greater in Asians than whites [Citation20], although others have found no differences in the pharmacokinetics or glycemic efficacy of SGLT2 inhibitors between Asians and non-Asians, including those with chronic kidney disease [Citation21,Citation22].
Because of these potential interethnic differences, it is important to explore whether glucose-lowering drugs have cardiovascular and kidney safety in Asian patients. Consequently, we aimed to identify phase III or IV randomized clinical trials conducted after the FDA 2008 guidance that assessed the effects of glucose-lowering drugs on cardiovascular or renal outcomes in Asians with T2D, and narratively synthesize data by drug class.
2. Patients and methods
2.1. Rapid evidence assessment
2.1.1. Eligibility criteria
The Embase, Medline, and Cochrane Library databases were searched for manuscripts published between 1 January 2008 and 14 June 2020 on phase III or IV, double-blind, randomized clinical trials of glucose-lowering drugs in Asian T2D patients with either inactive (placebo) or active comparators that reported cardiovascular or kidney outcomes.
Trials evaluating T2D patients aged ≥18 years were eligible for inclusion if they met one of the following criteria: they were conducted in an Asian country or countries (author-defined) but did not report ethnicity/race of the population; conducted in any country or countries and all of the trial population was described by the authors as Asian by ethnicity/race; or conducted in any country or countries and reported relevant outcomes for Asian subpopulations (region or ethnicity/race).
To meet eligibility criteria, the trials also had to investigate the effects of treatment for at least one year with an oral or injectable glucose-lowering drug from one of the following classes added to usual care: alpha-glucosidase inhibitors, DPP-4 inhibitors, GLP-1 receptor agonists, insulin, meglitinides (glinides), biguanides (e.g. metformin), SGLT2 inhibitors, sulfonylureas, or thiazolidinediones (glitazones). Trials of glucose-lowering drugs added to other specific glucose-lowering drugs, i.e. investigating specific combination therapies, were not considered for this analysis.
Trials also had to report at least one cardiovascular or kidney outcome, or all-cause mortality. Cardiovascular outcomes were defined as the following: the composite of 3-point major adverse cardiovascular events (3P-MACE: cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke), 4-point major adverse cardiovascular events (4P-MACE: hospitalization for unstable angina or any of the 3P-MACE endpoints), myocardial infarction or stroke (any first or recurrent fatal or non-fatal events), unstable angina, cardiovascular death, and hospitalization for heart failure. Kidney outcomes were defined as the following: doubling of serum creatinine; sustained decline (≥30%/40%/50%) in estimated glomerular filtration rate (eGFR); death due to kidney disease; end-stage kidney disease; urine albumin-to-creatinine ratio (UACR); progression to macroalbuminuria (UACR >300 mg/g); renal replacement therapy; and composite kidney outcomes that included one or more eligible individual kidney outcome. Composite kidney outcomes were ineligible if they included an individual non-kidney/non-cardiovascular outcome.
Subgroups of Asian patients for which outcomes were reported must have included at least 100 patients. Post-hoc analyses of relevant trials were eligible if they provided data for eligible Asian populations. Trials must have been published in peer-reviewed journals between 2008 (i.e. after the 2008 FDA guidance) and 2020 in the English language. Unpublished data and data from conference abstracts were not eligible.
2.1.2. Screening and data extraction
The initial results of the search were pre-screened, where clearly irrelevant results were removed by a single reviewer, followed by single-reviewer screening of titles and abstracts of the remaining records with 20% also screened by a second reviewer (double screening), and then single-reviewer screening of full-text records with 20% double screening.
After screening, the remaining records were grouped according to trial and data were extracted by a single researcher into a bespoke template. A second researcher checked all of the extracted data against the original publications, with disparities rechecked against the original publication, and corrections made to the extracted data in the template as required.
2.1.3. Risk of bias assessment
As part of the data extraction process, a single researcher assessed the trials for bias using the single technology appraisal instrument for randomized clinical trials from the National Institute for Health and Care Excellence in the UK. A second researcher reviewed the assessment for agreement, and any discrepancies were discussed between researchers or by consulting a third researcher.
2.2. Additional searches
Data from the systematic search were supplemented with additional trials meeting the eligibility criteria (section 2.1.1) published after the search, where identified between 15 June 2020 and 15 October 2020.
2.3. Narrative synthesis of data
Key outcomes data were synthesized narratively with consideration given to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [Citation23].
3. Results
3.1. Trial selection and characteristics
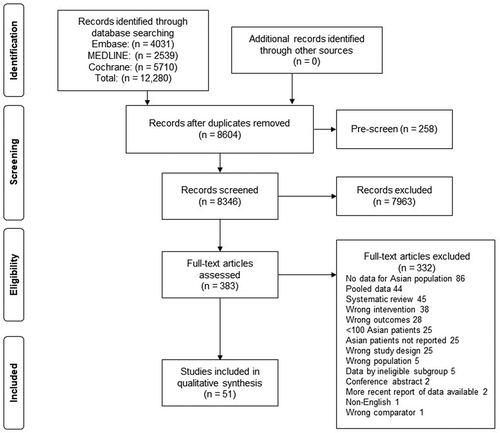
Database searches were conducted on 14 June 2020, retrieving 12,280 records initially, which were reduced after screening to 51 publications (). Three additional publications published after this date was subsequently retrieved. These publications were derived from 18 individual trials that reported cardiovascular or kidney outcomes for Asian patients with T2D ().
Table 1. Cardiovascular outcomes trials of glucose-lowering drugs with Asian participants
Figure 1. PRISMA flow diagram of literature search and selection process. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses
Five trials investigated four DPP-4 inhibitors: the CARMELINA [Citation24–27] and CAROLINA [Citation28–30] trials of linagliptin; the SAVOR-TIMI 53 trial of saxagliptin [Citation31–33], the EXAMINE trial of alogliptin [Citation34–37], and the TECOS trial of sitagliptin [Citation38–41]. Five trials investigated the following four SGLT2 inhibitors: empagliflozin (EMPA-REG OUTCOME [Citation42–47]), canagliflozin (CANVAS Program [Citation48–51] and CREDENCE [Citation52–54]), dapagliflozin (DECLARE-TIMI 58 [Citation55–58]), and ertugliflozin (VERTIS CV) [Citation59,Citation60]. Seven trials investigated GLP-1 receptor agonists. Lixisenatide, liraglutide, once-weekly exenatide, albiglutide, and dulaglutide were evaluated in the ELIXA [Citation61,Citation62], LEADER [Citation63,Citation64], EXSCEL [Citation65–67], HARMONY Outcomes [Citation68,Citation69], and REWIND [Citation70–72] trials, respectively. Injectable and oral formulations of semaglutide were investigated in the SUSTAIN-6 [Citation73] and PIONEER-6 [Citation74,Citation75] trials, respectively. The only study to evaluate insulin was the DEVOTE trial of degludec, an ultralong-acting basal analog of human insulin [Citation76,Citation77].
All of these trials were designed and powered to primarily investigate cardiovascular outcomes with the glucose-lowering drug of interest versus the comparator, except CREDENCE, which was designed to evaluate kidney outcomes but did include cardiovascular outcomes as secondary endpoints [Citation54]. Nearly all trials used placebo as a comparator, except the CAROLINA trial of linagliptin, which used the sulfonylurea glimepiride [Citation29], and the DEVOTE trial of insulin degludec, in which the comparator was insulin glargine U100 [Citation77]. The primary endpoint in the large majority of trials was 3P-MACE; however, the primary endpoint in ELIXA and TECOS was the similar composite outcome of 4P-MACE [Citation38,Citation61]. CREDENCE, in contrast, used as primary outcome a cardiorenal composite endpoint: namely, end-stage kidney disease (dialysis, transplantation, or sustained eGFR of <15 ml per minute per 1.73 m2), doubling of serum creatinine level, or death from kidney or cardiovascular causes [Citation53].
No data on cardiovascular or kidney outcomes in Asian T2D patients were found for other compounds from these drug classes marketed in Asia, including DPP-4 inhibitors (evogliptin, gemigliptin, teneligliptin) and SGLT2 inhibitors (ipragliflozin, luseogliflozin, tofogliflozin), or for compounds from other glucose-lowering drug classes (biguanides including metformin; sulfonylureas other than glimepiride (in CAROLINA); alpha-glucosidase inhibitors; thiazolidinediones/glitazones; glinides/meglitinides).
3.2. Risk of bias
All trials were multicenter, randomized clinical trials conducted to a high standard. Therefore, risk was predominantly low for randomization, allocation concealment and blinding criteria. Most publications explicitly reported how the random sequence was generated and which parties were blinded. Manuscripts also predominantly reported central treatment allocation (e.g. interactive voice or web response systems). These were considered sufficient to indicate that treatment allocation could not be predicted.
The publications for the EXAMINE trial of the DPP-4 inhibitor alogliptin [Citation34,Citation35] and the ELIXA trial of the GLP-1 receptor agonist lixisenatide [Citation61,Citation62] did not explicitly report their randomization methods but these are likely to have been robust given that the trials were designed in accordance with FDA guidance.
The publications for the following trials reported that analyses of cardiovascular outcomes by race and/or geographical region were prespecified: TECOS (sitagliptin), CARMELINA and CAROLINA (both linagliptin), EMPA-REG OUTCOME (empagliflozin), ELIXA (lixisenatide), SUSTAIN-6 (semaglutide injection), EXSCEL (exenatide once weekly), HARMONY Outcomes (albiglutide), and REWIND (dulaglutide). It is likely that subgroup analyses of race and/or region were also prespecified in many other trials, although the corresponding publications did not explicitly state this. Kidney outcomes in subgroups were prespecified in CARMELINA, CANVAS, CREDENCE and DECLARE-TIMI 58, and analyzed post hoc in EMPA-REG OUTCOME.
For many trials, data for Asian patients for baseline characteristics, imbalances, or drop-outs were not reported separate to those for the overall trial populations. However, as all trials were well conducted, it is reasonable to assume that randomization resulted in balanced distribution of key prognostic factors and effect modifiers across arms in these trials, including within the Asian subgroups. In this regard, the CARMELINA and CAROLINA trials of the DPP-4 inhibitor linagliptin [Citation27,Citation30], the TECOS trial of the DPP-4 inhibitor sitagliptin [Citation78], and the EMPA-REG-OUTCOME trial of the SGLT2 inhibitor empagliflozin [Citation43,Citation45,Citation46] were rated low risk because they all reported baseline characteristics for Asian subpopulations, which were similar across treatment arms.
3.3. Cardiovascular outcomes in Asian patients
In general, most trials reported cardiovascular outcomes for Asian patients defined by race or region as part of wider subgroup analyses of treatment effect across different races and regions; these analyses were mostly limited to the primary endpoint and reported in the primary publication. However, the CARMELINA and CAROLINA trials of the DPP-4 inhibitor linagliptin and the EMPA-REG OUTCOME trial of the SGLT2 inhibitor empagliflozin reported detailed analyses of Asian outcomes in separate publications [Citation27,Citation30,Citation45,Citation46].
3.3.1. Cardiovascular outcomes with DPP-4 inhibitors in Asian patients
Four placebo-controlled trials reported cardiovascular outcomes for DPP-4 inhibitors (). In the SAVOR-TIMI 53 trial, saxagliptin demonstrated non-inferiority for the risk of 3P-MACE compared with placebo in Asians [Citation32], as did alogliptin in EXAMINE [Citation35], sitagliptin in TECOS [Citation39], and linagliptin in CARMELINA [Citation25,Citation27]. These data were for patients of Asian race in SAVOR-TIMI 53 and TECOS, and patients living in the Asia region in EXAMINE; in contrast, CARMELINA reported data both for individuals of Asian race living in Asia [Citation27] and for those of Asian race living anywhere [Citation25]. An active-comparator trial of linagliptin (CAROLINA) reported non-inferiority for risk of 3P-MACE compared with glimepiride, a sulfonylurea, for patients of Asian race [Citation29] and for patients living in Asia, all but one of whom was of Asian race [Citation30]. In all of these trials, the effects of the DPP-4 inhibitors on cardiovascular outcomes in the Asian subgroups were generally consistent with their effects in the overall trial cohorts.
Figure 2. Cardiovascular outcomes of Asian patients. 3P-MACE: 3-point major adverse CV events (CV death, non-fatal myocardial infarction, or non-fatal stroke); 4P-MACE: 4-point major adverse CV events (CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina); CI: confidence interval; CV: cardiovascular; DPP-4: dipeptidyl peptidase-4; GLP-1: glucagon-like peptide-1; SGLT2: sodium-glucose co-transporter-2. a95.47% CI. b95.6% CI. cNumerical data not provided but forest plot indicates no effect of treatment on outcome (CI crosses 1.00) [Citation61]. Point size is proportional to size of trial cohort (number of patients)
![Figure 2. Cardiovascular outcomes of Asian patients. 3P-MACE: 3-point major adverse CV events (CV death, non-fatal myocardial infarction, or non-fatal stroke); 4P-MACE: 4-point major adverse CV events (CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina); CI: confidence interval; CV: cardiovascular; DPP-4: dipeptidyl peptidase-4; GLP-1: glucagon-like peptide-1; SGLT2: sodium-glucose co-transporter-2. a95.47% CI. b95.6% CI. cNumerical data not provided but forest plot indicates no effect of treatment on outcome (CI crosses 1.00) [Citation61]. Point size is proportional to size of trial cohort (number of patients)](/cms/asset/d4ec65dd-a78a-4119-bd3d-6ac7746a2206/ieds_a_1898585_f0002_oc.jpg)
Four of these trials also reported data for Asian subgroups for other cardiovascular and mortality endpoints. In CARMELINA, linagliptin had a nominally reduced risk for hospitalization for heart failure compared with placebo in Asian patients (HR: 0.47; 95% CI: 0.24, 0.95, p = 0.0368 for treatment by region interaction), whereas it exhibited a safety profile in the overall trial cohort (HR: 0.90; 95% CI: 0.74, 1.08) [Citation27] (Supplemental Figure S1). It is unclear whether this is a chance finding, among the many subgroup analyses conducted, or a true treatment effect in Asian patients. Linagliptin did not increase the risk for other cardiovascular events or mortality in Asian patients, including: non-fatal myocardial infarction (HR: 0.87; 95% CI: 0.45, 1.69), stroke (HR: 0.60; 95% CI: 0.22, 1.66), cardiovascular death (HR: 0.70; 95% CI: 0.30, 1.64), and all-cause mortality (HR: 0.61; 95% CI: 0.30, 1.25), consistent with the neutral treatment effects for these outcomes in the overall trial cohort [Citation27]. In EXAMINE, alogliptin did not increase the risk for cardiovascular death in patients in the Asia/Pacific region (HR: 0.60; 95% CI: 0.34, 1.05), consistent with its treatment effect in the overall trial cohort (HR: 0.85; 95% CI: 0.66, 1.10) [Citation37]. Hospitalization for heart failure in Asian patients was not reported for either saxagliptin in the SAVOR-TIMI 53 trial or alogliptin in EXAMINE. However, in SAVOR-TIMI 53, there was an increased risk of hospitalization for heart failure with saxagliptin in the overall trial cohort (HR: 1.27; 95% CI: 1.07, 1.51) [Citation32]. In EXAMINE, the risk of hospitalization for heart failure in the overall trial cohort was not significantly different between the alogliptin and placebo groups (HR: 1.19; 95% CI: 0.90, 1.58) [Citation36]. In the TECOS trial, sitagliptin did not increase the risk of hospitalization for heart failure in Asian patients (HR: 0.89; 95% CI: 0.52, 1.53 versus HR: 1.00; 95% CI: 0.83, 1.19 in the overall trial cohort, p = 0.47 for treatment by race interaction) [Citation40] (Supplemental Figure S1). Myocardial infarction was reported in 2.3% and 2.6% of Asian patients receiving sitagliptin and placebo, respectively [Citation39,Citation41]. In Asian patients in CAROLINA, there was no significant difference between linagliptin and glimepiride for the risk of outcomes including hospitalization for heart failure (HR: 0.89; 95% CI: 0.36, 2.19) (Supplemental Figure S1), cardiovascular death (HR: 0.73; 95% CI: 0.38, 1.38), non-cardiovascular death (HR: 0.76; 95% CI: 0.37, 1.57), and all-cause mortality (HR: 0.74; 95% CI: 0.46, 1.20) – consistent with the overall trial cohort in all cases (all p-values > 0.05 for treatment by region interaction) [Citation30].
3.3.2. Cardiovascular outcomes with SGLT2 inhibitors in Asian patients
Five trials reported MACE outcomes for SGLT2 inhibitors (). The EMPA-REG OUTCOME trial demonstrated cardiovascular risk reduction with empagliflozin in T2D patients [Citation43]. Subgroup analyses showed this risk reduction was evident in Asian patients, with a HR for the primary endpoint (3P-MACE) of 0.68 (95% CI: 0.48, 0.95), which was consistent with the treatment effect in the overall trial cohort (HR: 0.86; 95% CI: 0.74, 0.99, p = 0.0872 for treatment by race interaction) [Citation43,Citation45]. Similarly, the CANVAS trial demonstrated reduced risk of 3P-MACE with canagliflozin versus placebo, with an HR of 0.86 (95% CI: 0.75, 0.97) for the overall population; although the HR was >1 for Asian patients (1.08; 95% CI: 0.72, 1.64), the p-value for treatment by race interaction (0.40) indicated a consistent treatment benefit across race subgroups [Citation48]. There was also a reduced risk of 3P-MACE with canagliflozin in the CREDENCE trial: HR of 0.80 (95% CI: 0.67, 0.95) in the overall trial cohort and 0.75 (95% CI: 0.49, 1.14) in patients of Asian race; p = 0.66 for treatment by race interaction). However, the primary endpoint in CREDENCE was a composite cardiorenal outcome and the study population had proteinuric diabetic nephropathy [Citation54]. The DECLARE-TIMI 58 trial reported no risk reduction with dapagliflozin for 3P-MACE in the Asia-Pacific region (HR: 0.96; 95% CI: 0.69, 1.33) [Citation58], consistent with the neutrality seen in the overall trial cohort (HR: 0.93; 95% CI: 0.84, 1.03) (p = 0.99 for treatment by region interaction) [Citation58]. Similarly, ertugliflozin did not reduce the risk of 3P-MACE in the VERTIS CV trial in either the overall trial cohort (HR: 0.97; 95.6% CI: 0.85, 1.11), the subgroup of patients of Asian race (HR: 0.89; 95% CI: 0.51, 1.56), or patients living in the Asian region (HR: 1.19; 95% CI: 0.72, 1.98) [Citation59].
In EMPA-REG OUTCOME, the reduced risk for hospitalization for heart failure with empagliflozin in the overall trial cohort (HR: 0.65; 95% CI: 0.50, 0.85) was consistent in the Asian subgroup (HR: 0.70; 95% CI: 0.37, 1.33, p = 0.82 for treatment by race interaction) (Supplemental Figure S1). Similarly, the reduced risk for all-cause mortality in the overall trial cohort (HR: 0.68; 95% CI: 0.57, 0.82) was consistent in the Asian subgroup (HR: 0.64; 95% CI: 0.40, 1.01; p = 0.28 for treatment by race interaction). Empagliflozin also reduced the risk for cardiovascular death in Asian patients (HR: 0.44; 95% CI: 0.25, 0.78; p = 0.43 for treatment by race interaction in the overall trial cohort [HR: 0.62; 95% CI: 0.49, 0.77]). Empagliflozin did not increase risk for myocardial infarction (HR: 0.62; 95% CI: 0.36, 1.08) or stroke (HR: 0.95; 95% CI: 0.55, 1.64) in Asian patients, consistent with its effects in the overall trial cohort [Citation45]. In the DECLARE-TIMI 58 trial, dapagliflozin reduced the risk of the composite endpoint of hospitalization for heart failure or cardiovascular death in the overall trial cohort (HR: 0.83; 95% CI: 0.73, 0.95), with consistent findings in the regional subgroups including the Asia/Pacific region (p = 0.53 for treatment by region interaction; HRs for regions were shown visually in the publication without accompanying data) [Citation58]. In the VERTIS-CV trial, the reduced risk of hospitalization for heart failure in the overall trial cohort (HR: 0.70; 95% CI: 0.54, 0.90) was consistent in the subgroups of patients of Asian race (HR: 0.68; 95% CI: 0.21, 2.13; p = 0.39 for treatment by race interaction) and patients living in the Asian region (HR: 0.90; 95% CI: 0.36, 2.26; p = 0.59 for treatment by region interaction) [Citation60] (Supplemental Figure S1).
3.3.3. Cardiovascular outcomes with GLP-1 receptor agonists in Asian patients
Seven trials reported MACE outcomes for GLP-1 receptor agonists in Asian patients (), the results of which were consistent with their effects in the overall trial cohorts (p-values for treatment by region or race interaction were generally non-significant). In the overall trial cohort of LEADER, liraglutide significantly reduced the risk of 3P-MACE compared with placebo [Citation64], as did albiglutide in HARMONY Outcomes [Citation68], dulaglutide in REWIND [Citation71], and injectable semaglutide 0.5–1.0 mg weekly in SUSTAIN-6 [Citation73]. In PIONEER-6, there was no significant difference between oral semaglutide 14 mg daily and placebo for risk of 3P-MACE [Citation75]. The ELIXA trial of lixisenatide (primary endpoint: 4P-MACE) [Citation61] and the EXSCEL trial of exenatide once weekly (primary endpoint: 3P-MACE) [Citation66] also found no significant difference for risk of MACE with these GLP-1 receptor agonists compared with placebo.
The only trials to report other cardiovascular outcomes data for Asian patients were EXSCEL, in which hospitalization for heart failure occurred in 0.55% and 0.83% of patients treated with exenatide and placebo, respectively (effect size not reported), and REWIND, in which the reduced risk of stroke with dulaglutide in the overall trial cohort (HR: 0.76; 95% CI: 0.62, 0.94) was consistent in Asian patients (HR: 0.51; 95% CI: 0.13, 2.03, p = 0.26 for treatment by race interaction) [Citation72].
3.3.4. Cardiovascular outcomes with insulin in Asian patients
The only trial to report cardiovascular outcomes data for insulin in Asian patients was DEVOTE, which found no significant difference between insulin degludec and insulin glargine (a once-daily basal insulin analog) for the primary endpoint of 3P-MACE in the overall trial cohort (HR: 0.91; 95% CI: 0.78, 1.06) [Citation77]. There was a nominally significant interaction between treatment effect and region, which favored degludec in Asia (HR: 0.42; 95% CI: 0.22, 0.81; p = 0.0052 for treatment by region interaction). However, the interpretation of these data is unclear as – similar to most other trials – regional subgroup analyses were exploratory in nature and not adjusted for multiple testing.
3.4. Kidney outcomes in Asian patients
Only two of the outcomes trials were designed to robustly compare kidney outcomes with treatment versus comparator in the overall patient cohort: the CARMELINA trial of the DPP-4 inhibitor linagliptin [Citation24,Citation25] and the CREDENCE trial of the SGLT2 inhibitor canagliflozin [Citation52,Citation53]. However, other trials of DPP-4 inhibitors, SGLT2 inhibitors and GLP-1 receptor agonists reported kidney outcomes as additional endpoints, although none of the trials of GLP-1 receptor agonists reported kidney outcomes for Asian patients.
3.4.1. Kidney outcomes with DPP-4 inhibitors in Asian patients
In CARMELINA, linagliptin demonstrated no increased risk of adverse kidney outcomes in Asian patients with T2D, consistent with its effects in the overall trial cohort, as determined by the key secondary kidney endpoint, which was a composite of death from renal failure, progression to end-stage kidney disease, or a sustained decrease in eGFR of ≥40% () [Citation25,Citation27]. Furthermore, in the overall trial cohort, linagliptin was associated with a modest benefit for reducing progression of albuminuria (HR: 0.86; 95% CI: 0.78, 0.95), defined as progression from normoalbuminuria (UACR <30 mg/g) to either microalbuminuria (UACR 30–300 mg/g) or macroalbuminuria (UACR >300 mg/g), or from microalbuminuria to macroalbuminuria. This effect was also consistent in the Asian subgroup (HR: 0.95; 95% CI: 0.66, 1.36, p = 0.17 for treatment by region interaction). Data on kidney outcomes in Asian patients are not available for other DPP-4 inhibitors.
Figure 3. Kidney outcomes of Asian patients. CI: confidence interval; CV: cardiovascular; DPP-4: dipeptidyl peptidase-4; eGFR: estimated glomerular filtration rate; ESKD: end-stage kidney disease; RRT: renal-replacement therapy; SCr: serum creatinine; SGLT2: sodium-glucose co-transporter-2. aeGFR was measured in ml/min/1.73 m2. bMacroalbuminuria was defined as urinary albumin-to-creatinine ratio >300 mg/g. Point size is proportional to size of trial cohort (number of patients)
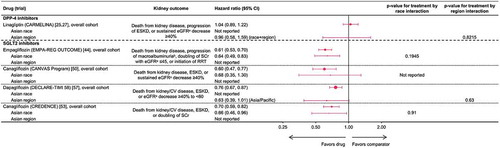
3.4.2. Kidney outcomes with SGLT2 inhibitors in Asian patients
Four trials reported reduced risk for adverse kidney outcomes with SGLT2 inhibitors in Asian patients. The first signal for renal risk reduction was observed in the EMPA-REG OUTCOME trial, where empagliflozin treatment was associated with reduced incidence of incident or worsening nephropathy, defined as progression to macroalbuminuria, doubling of serum creatinine level, initiation of renal-replacement therapy, or death from kidney disease () [Citation44,Citation46]. Empagliflozin was also associated with reduced risk in Asian patients for the composite of doubling of serum creatinine, initiation of renal-replacement therapy, or renal death (HR: 0.48, 95% CI: 0.25, 0.92) and progression to macroalbuminuria (HR: 0.64, 95% CI: 0.49, 0.85), both consistent with the overall trial cohort [Citation46]. Similarly, canagliflozin (in CANVAS [Citation48]) and dapagliflozin (in DECLARE-TIMI 58 [Citation57,Citation58]) reduced the risk of adverse kidney events in the overall trial cohorts with consistent effect in the Asian subgroups, as measured by various composite endpoints (). Subsequently, canagliflozin conclusively demonstrated significant renal risk reduction with SGLT2 inhibition in the CREDENCE trial population of patients with proteinuric diabetic nephropathy, where the primary endpoint was the composite of end-stage kidney disease, doubling of serum creatinine level from baseline, or death from kidney or cardiovascular disease () [Citation53]. Compared with placebo, canagliflozin reduced the risk for this composite endpoint in the overall trial cohort (HR: 0.70; 95% CI: 0.59, 0.82) and in the subgroup of Asian patients (HR: 0.66; 95% CI: 0.46, 0.96; p = 0.91 for treatment by race interaction) [Citation53].
4. Discussion
The key finding from this study is that there do not appear to be differences in cardiorenal outcomes between the subgroups of Asian patients and the overall trial cohorts in the CVOTs of DPP-4 inhibitors, SGLT2 inhibitors and GLP-1 receptor agonists, despite the potential differences in T2D pathophysiology and glycemic responses to glucose-lowering drugs between Asian and non-Asian patients.
None of the CVOTs of glucose-lowering drugs in T2D patients to date were conducted exclusively in Asian patients, despite Asia being an emerging epicenter of the T2D pandemic [Citation1]. Furthermore, only two randomized, placebo-controlled trials were specifically designed to evaluate kidney outcomes with glucose-lowering drugs in T2D patients [Citation25,Citation53]. However, most of these clinical trials did include patients of Asian race and/or from the Asian region within their multinational cohorts. In this analysis of those trials, data for patients of Asian race and/or patients living in Asia were found for the key outcomes, including the primary endpoints, within the manuscripts reporting data for the overall trial cohorts. In addition, separate publications for the CVOTs of the DPP-4 inhibitor linagliptin and the SGLT2 inhibitor empagliflozin presented detailed, stratified analyses of cardiovascular and kidney outcomes in the Asian subgroups; these demonstrated the cardiorenal safety of linagliptin and cardiorenal risk reduction with empagliflozin in Asian patients.
Overall, this synthesis of data for Asian T2D patients from the CVOTs suggests that DPP-4 inhibitors are not associated with increased risk of cardiovascular events, while SGLT2 inhibitors and GLP-1 receptor agonists seem to reduce risk – consistent with the effects seen in the overall trial cohorts. Furthermore, SGLT2 inhibitors appear to reduce renal risk in Asians; however, there is a lack of data on the effects of DPP-4 inhibitors (other than linagliptin) or GLP-1 receptor agonists on the kidneys in Asian T2D patients. The CAROLINA trial of linagliptin was notable for using an active comparator, namely glimepiride. Although linagliptin did not demonstrate cardiovascular benefit compared to the sulfonylurea, it did have much lower risk for hypoglycemia than glimepiride in Asian patients (HR: 0.25; 95% CI: 0.19, 0.33; p < 0.0001) despite similar glycemic control, and elicited modest relative weight loss (weighted average mean difference over 256 weeks of −1.82 kg; 95% CI: −2.28, −1.35) [Citation30]. There is a lack of information on cardiorenal outcomes with insulin in Asian T2D patients, as the only CVOT conducted to date used insulin analogs as both the treatment and comparator.
Reassuringly, data on clinical outcomes with these glucose-lowering drugs in Asian patients in routine clinical practice are now emerging from large database studies. In the CVD-Real 2/3 observational study, SGLT2 inhibitors were associated with significantly lower risk of cardiovascular events, major kidney events and mortality compared with other oral glucose-lowering drugs in six countries, including Japan, Singapore, and South Korea in CVD-REAL 2, and in five countries, including Japan and Taiwan in CVD-REAL 3 [Citation79,Citation80]. Similarly, in the EMPRISE East Asia study, empagliflozin was associated with a lower risk of hospitalization for heart failure, all-cause mortality and end-stage renal disease compared with DPP-4 inhibitors in Japan, South Korea, and Taiwan [Citation81].
The strengths of our study include the use of a well-defined methodology for rapid evidence assessment [Citation82]. In addition, the data retrieved were derived from high-quality randomized clinical trials with large sample sizes and very similar definitions of cardiovascular outcomes. The main limitation of the dataset analyzed was the lack of eligible trials conducted exclusively in Asian patients, which meant only exploratory subgroup analyses were included. Although randomization within each trial probably resulted in effect modifiers being evenly distributed between treatment groups, this cannot be automatically assumed, given the relatively small size of the Asian subgroups within the multinational CVOTs (typically 2000 patients or fewer), and baseline characteristics were rarely reported to demonstrate this. In addition, subgroups were not powered to detect treatment effects; thus, conclusions were based on effect sizes/directions and the reported tests for interaction between treatment effect and subgroup, rather than from the HR and CI for the subgroup in isolation. This approach is not as rigorous as a meta-analysis of individual patient data (which were not available to us). Furthermore, analyses of Asian patients by region were limited by the diversity of races found within Asia, as well as the inclusion in some analyses of non-Asian countries or patients of non-Asian races. In particular, we could not distinguish between East Asian and South Asian patients, who may have differences in T2D pathophysiology. Finally, renal outcomes were rarely assessed as primary or alpha-controlled endpoints. Given potential differences in T2D pathophysiology and glycemic responses to glucose-lowering drugs between Asian and non-Asian patients, the evidence base would be strengthened by future cardiorenal trials or further robust real-world studies of glucose-lowering drugs conducted exclusively in Asian T2D patients.
The SCORED [Citation83] and SOLOIST [Citation84] CVOTs of the SGLT1/SGLT2 inhibitor sotagliflozin in T2D patients were reported after the literature search and were not included in our rapid evidence assessment. SOLOIST enrolled T2D patients recently hospitalized for worsening heart failure, and found that sotagliflozin was associated with a significant 33% relative reduction in the risk of the primary end point (HR: 0.67; 95% CI: 0.52, 0.85), which was a composite of all cardiovascular deaths and hospitalizations and urgent visits for heart failure. Only 15 of the 1028 patients were of Asian race and a HR could not be calculated for this subgroup as there were no events in one treatment arm [Citation84]. The SCORED trial of sotagliflozin in T2D patients with chronic kidney disease used the same primary endpoint and found a 26% risk reduction in the overall trial cohort (HR: 0.74; 95% CI: 0.63, 0.88). Asian subgroup data were provided only for China (HR: 0.52; 95% CI: 0.12, 2.25), South Korea (HR could not be calculated due to zero events in one treatment arm), Taiwan (HR: 0.46; 95% CI: 0.12, 1.82), and India (HR: 4.66; 95% CI: 1.26, 17.20). No statistical testing for treatment by country interaction was reported [Citation83].
In addition to the cardiorenal outcomes trials conducted exclusively in T2D patients, three multinational trials have now investigated SGLT2 inhibitors for preventing cardiorenal events in patients with heart failure with reduced ejection fraction or patients with chronic kidney disease, regardless of diabetes status, namely the DAPA-HF [Citation85] and DAPA-CKD [Citation86] trials of dapagliflozin and the EMPEROR-Reduced trial of empagliflozin [Citation87]. As DAPA-CKD and EMPEROR-Reduced were published after our rapid evidence assessment and none of these trials were conducted exclusively in T2D patients, they were not included. In the DAPA-HF trial in 4744 patients with heart failure with reduced ejection fraction, dapagliflozin significantly reduced the risk of the primary composite endpoint (cardiovascular death or hospitalization for heart failure) in the overall trial cohort (HR: 0.70; 95% CI: 0.59, 0.83), and had a seemingly consistent treatment effect in the 1116 patients of Asian race (HR: 0.64; 95% CI: 0.48, 0.86, p-value for treatment by race interaction not reported) [Citation85]. Similarly in EMPEROR-Reduced (n = 3730), which was also conducted in heart failure patients with reduced ejection fraction, empagliflozin significantly reduced the risk of cardiovascular death or hospitalization for heart failure in the overall trial cohort (HR: 0.75; 95% CI: 0.65, 0.86) with a consistent treatment effect in the 672 patients of Asian race (HR: 0.57; 95% CI: 0.41, 0.78) and the 493 patients living in Asia (HR: 0.55; 95% CI: 0.38, 0.78) [Citation87]. In the DAPA-CKD trial in 4304 patients with chronic kidney disease, dapagliflozin significantly reduced the risk of the primary composite endpoint of eGFR decline of ≥50%, end-stage kidney disease, or death from kidney or cardiovascular causes in the overall trial cohort (HR: 0.61; 95% CI: 0.51, 0.72), with a similar treatment effect in the 1467 patients of Asian race (HR: 0.66; 95% CI: 0.46, 0.93) and the 1346 patients living in Asia (HR: 0.70; 95% CI: 0.48, 1.00) [Citation86]. Thus, these trials showed consistent cardiorenal risk reductions in both the overall trial cohorts and subgroups defined by race (including Asian) and region (including Asia), as well as by presence or absence of diabetes [Citation85–87].
5. Conclusions
This analysis of multinational CVOTs found that glucose-lowering drugs from the modern classes of SGLT2 inhibitors and GLP-1 receptor agonists appear to reduce cardiovascular risk in Asian T2D patients, while DPP-4 inhibitors appear to have cardiovascular safety. In addition, SGLT2 inhibitors appear to reduce the risk for kidney disease in Asian patients. Although the power to detect treatment differences compared with other races/ethnicities was limited, these cardio renal effects seem similar to those in the overall multinational cohorts of these CVOTs.
Declaration of interest
T. Kadowaki reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kowa Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., SANWA KAGAKU KENKYUSHO CO., LTD., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited., and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Limited.; contracted research from AstraZeneca K.K. and Takeda Pharmaceutical Company Limited.; joint research from Daiichi Sankyo Company, Limited.; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited. F. Yamamoto, Y. Taneda, Y. Naito, and T. Okamura are employees of Nippon Boehringer Ingelheim Co. Ltd. D. Clark and S.S. Lund are employees of Boehringer Ingelheim International GmbH; S.S. Lund owns shares in Novo Nordisk A/S and shares in dynamically traded investment funds, which may own stocks from pharmaceutical companies. K. Kaku has acted in an advisory role for Astellas Pharma, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, and Novo Nordisk Pharma; received honoraria or fees for promotional materials from Astellas Pharma, AstraZeneca, Daiichi Sankyo, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, Nippon Boehringer Ingelheim, Taisho-Toyama Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, and Kowa Pharmaceutical; and received scholarships or donations from Nippon Boehringer Ingelheim, Taisho-Toyama Pharmaceutical, Mitsubishi Tanabe Pharma, and Kowa Pharmaceutical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Role of the sponsor
The Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Supplemental Material
Download MS Word (343.8 KB)Acknowledgments
Victoria Young, PhD, of CURO Payer Evidence (part of the Envision Pharma Group) was involved in the design and development of the rapid evidence assessment, and was funded by Nippon Boehringer Ingelheim Co. Ltd.
Data availability
The data that support the findings of this study are available from the corresponding author, F.Y., upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2021.1898585.
Additional information
Funding
References
- International Diabetes Federation. IDF diabetes atlas. Brussels, Belgium: International Diabetes Federation; 2019. [cited 2020 Sep 30]. Available from: https://www.diabetesatlas.org/en/resources/.
- Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019 Apr 16;139(16):e840–e878.
- Oishi Y, Manabe I. Organ system crosstalk in cardiometabolic disease in the age of multimorbidity. Front Cardiovasc Med. 2020;7:64.
- American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020 Jan;43(Suppl1):S111–S134.
- Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016 Feb;12(2):73–81.
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005 Nov 23;294(20):2581–2586.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007 Jun 14;356(24):2457–2471.
- Meinert CL, Knatterud GL, Prout TE, et al. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 1970;19(Suppl):789–830.
- Kilo C, Miller JP, Williamson JR. The achilles heel of the university group diabetes program. JAMA. 1980 Feb 1;243(5):450–457.
- O’Sullivan JB, D’Agostino RB. Decisive factors in the tolbutamide controversy. JAMA. 1975 May 26;232(8):825–829.
- Center for Drug Evaluation and Research. Guidance for industry. Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Internet], December 2008. Silver Spring, MD, U.S. Food and Drug Administration. [cited 2019 Nov 17]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf.
- Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013 Apr;1281:64–91.
- Gujral UP, Pradeepa R, Weber MB, et al. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013;1281:51–63.
- Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004 Dec;66(Suppl 1):S37–43.
- Spracklen CN, Horikoshi M, Kim YJ, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020 Jun;582(7811):240–245.
- Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015 Sep;6(5):495–507.
- Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013 Apr;56(4):696–708.
- Kim YG, Hahn S, Oh TJ, et al. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014 Oct;16(10):900–909.
- Ito Y, Ambe K, Kobayashi M, et al. Ethnic difference in the pharmacodynamics-efficacy relationship of dipeptidyl peptidase-4 inhibitors between Japanese and non-Japanese patients: a systematic review. Clin Pharmacol Ther. 2017 Oct;102(4):701–708.
- Gan S, Dawed AY, Donnelly LA, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in white and Asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(8):1948–1957.
- Scheen AJ. SGLT2 inhibitors as add-on therapy to metformin for people with type 2 diabetes: a review of placebo-controlled trials in Asian versus non-Asian patients. Diabetes Metab Syndr Obes. 2020;13:2765–2779.
- Scheen AJ. Pharmacokinetic/pharmacodynamic properties and clinical use of SGLT2 inhibitors in non-Asian and Asian patients with type 2 diabetes and chronic kidney disease. Clin Pharmacokinet. 2020 Aug;59(8):981–994.
- PRISMA. PRISMA 2009 checklist 2009 [cited 2020 May 29]. Available from: http://prisma-statement.org/prismastatement/Checklist.aspx
- Rosenstock J, Perkovic V, Alexander JH, et al. Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol. 2018;17(1):39.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019 Jan 1;321(1):69–79.
- McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation. 2019 Jan 15;139(3):351–361.
- Inagaki N, Yang W, Watada H, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA((R)) trial. Diabetol Int. 2020 Apr;11(2):129–141.
- Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the CARdiovascular outcome trial of LINAgliptin versus glimepiride in type 2 diabetes (CAROLINA(R)). Diab Vasc Dis Res. 2015 May;12(3):164–174.
- Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019 Sep 19;322(12):1155–1166.
- Kadowaki T, Wang G, Rosenstock J, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA(R) trial. Diabetol Int. 2021 Jan;12(1):87–100.
- Mosenzon O, Raz I, Scirica BM, et al. Baseline characteristics of the patient population in the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus (SAVOR)-TIMI 53 trial. Diabetes Metab Res Rev. 2013;29(5):417–426.
- Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326.
- Scirica BM, Bhatt DL, Braunwald E, et al. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J. 2011;162(5):818–825.e6.
- White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011 Oct;162(4):620–626 e1.
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013 Oct 3;369(14):1327–1335.
- Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015 May 23;385(9982):2067–2076.
- White WB, Kupfer S, Zannad F, et al. Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care. 2016;39(7):1267–1273.
- Green JB, Bethel MA, Paul SK, et al. Rationale, design, and organization of a randomized, controlled trial evaluating cardiovascular outcomes with sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J. 2013;166(6):983–989.e7.
- Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242.
- McGuire DK, Van De Werf F, Armstrong PW, et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016 May 1;1(2):126–135.
- Nauck MA, McGuire DK, Pieper KS, et al. Sitagliptin does not reduce the risk of cardiovascular death or hospitalization for heart failure following myocardial infarction in patients with diabetes: observations from TECOS. Cardiovasc Diabetol. 2019 Sep 3;18(1):116.
- Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol. 2014 Jun;19(13):102.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26;373(22):2117–2128.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016 Jul 28;375(4):323–334.
- Kaku K, Lee J, Mattheus M, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease - results from EMPA-REG OUTCOME((R)). Circ J. 2017 Jan 25;81(2):227–234.
- Kadowaki T, Nangaku M, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME((R)) trial. J Diabetes Investig. 2019 May;10(3):760–770.
- Perkovic V, Koitka-Weber A, Cooper ME, et al. Choice of endpoint in kidney outcome trials: considerations from the EMPA-REG OUTCOME(R) trial. Nephrol Dial Transplant. 2020 Dec 4;35(12):2103–2111.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017 Aug 17;377(7):644–657.
- Neal B, Perkovic V, Mahaffey KW, et al. Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017 Jul;19(7):926–935.
- Perkovic V, De Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018 Sep;6(9):691–704.
- Neal B, Perkovic V, De Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin cardiovascular assessment study (CANVAS)–a randomized placebo-controlled trial. Am Heart J. 2013 Aug;166(2):217–223 e11.
- Jardine MJ, Mahaffey KW, Neal B, et al. The Canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017 Dec 13;46(6):462–472.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295–2306.
- Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019 Aug 27;140(9):739–750.
- Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: participants’ baseline characteristics. Diabetes Obes Metab. 2018 May;20(5):1102–1110.
- Wiviott SD, Raz I, Bonaca MP, et al. The design and rationale for the Dapagliflozin effect on cardiovascular events (DECLARE)-TIMI 58 Trial. Am Heart J. 2018;200:83–89.
- Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019 Aug;7(8):606–617.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019 Jan 24;380(4):347–357.
- Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020 Oct 8;383(15):1425–1435.
- Cosentino F, Cannon CP, Cherney DZI, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020 Dec 8;142(23):2205–2215.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015 Dec 3;373(23):2247–2257.
- Bentley-Lewis R, Aguilar D, Riddle MC, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J. 2015 May;169(5):631–638 e7.
- Marso SP, Poulter NR, Nissen SE, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013 Nov;166(5):823–30 e5.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016 Jul 28;375(4):311–322.
- Mentz RJ, Bethel MA, Gustavson S, et al. Baseline characteristics of patients enrolled in the exenatide study of cardiovascular event lowering (EXSCEL). Am Heart J. 2017;187:1–9.
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017 Sep 28;377(13):1228–1239.
- Fudim M, White J, Pagidipati NJ, et al. Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure and heart failure-related outcomes: insights from the EXSCEL trial. Circulation. 2019 Nov 12;140(20):1613–1622.
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018 Oct 27;392(10157):1519–1529.
- Green JB, Hernandez AF, D’Agostino RB, et al. Harmony outcomes: a randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus-Rationale, design, and baseline characteristics. Am Heart J. 2018;203:30–38.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Design and baseline characteristics of participants in the researching cardiovascular events with a weekly INcretin in diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018 Jan;20(1):42–49.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019 Jul 13;394(10193):121–130.
- Gerstein HC, Hart R, Colhoun HM, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 2020 Feb;8(2):106–114.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016 Nov 10;375(19):1834–1844.
- Bain SC, Mosenzon O, Arechavaleta R, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019 Mar;21(3):499–508.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019 Aug 29;381(9):841–851.
- Marso SP, McGuire DK, Zinman B, et al. Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events) - DEVOTE 1. Am Heart J. 2016;179:175–183.
- Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017 Aug 24;377(8):723–732.
- Davis TME, Mulder H, Lokhnygina Y, et al. Effect of race on the glycaemic response to sitagliptin: insights from the trial evaluating cardiovascular outcomes with sitagliptin (TECOS). Diabetes Obes Metab. 2018 Jun;20(6):1427–1434.
- Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018 Jun 12;71(23):2628–2639.
- Koh ES, Han K, Nam YS, et al. Renal outcomes and all-cause death associated with sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL 3 Korea). Diabetes Obes Metab. 2021 Feb;23(2):455–466.
- Seino Y, Kim DJ, Yabe D, et al. Cardiovascular and renal effectiveness of empagliflozin in routine care in East Asia: results from the EMPRISE East Asia study. Endocrinol Diabetes Metab. 2021 Jan;4(1):e00183.
- Garritty C, Gartlehner G, Nussbaumer-Streit B, et al. Cochrane Rapid Reviews Methods Group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2020 Oct;15(130):13–22.
- Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021 Jan 14;384(2):129–139.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021 Jan 14;384(2):117–128.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019 Nov 21;381(21):1995–2008.
- Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020 Oct 8;383(15):1436–1446.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020 Oct 8;383(15):1413–1424.
