ABSTRACT
Introduction
In patients at high cardiovascular risk, the rate of events remains elevated despite traditional, evidence-based lipid-lowering therapy. Residual hypertriglyceridemia is an important contributor to this risk. However, prior medications with triglyceride-lowering effects have not reduced adverse clinical outcomes in the statin era.
Areas covered
The present review summarizes evidence and recommendations related to triglyceride-lowering therapy in the primary and secondary preventive settings. We provide an overview of findings from recent meta-analyses, important observational studies, and a detailed description of landmark trials, including the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT). We further review recommendations from current guidelines.
Expert opinion
Icosapent ethyl is a stable, highly purified ethyl ester of eicosapentaenoic acid that safely and effectively reduces cardiovascular events in the contemporary setting. It is prescribed at a dose of 2 grams twice daily and is indicated in patients at high cardiovascular risk who have fasting or non-fasting triglyceride levels ≥150 mg/dl despite maximally tolerated statin treatment, or in individuals with triglyceride levels ≥500 mg/dl. Conversely, omega-3 fatty acid preparations containing a combination of eicosapentaenoic acid and docosahexaenoic acid are not indicated for reduction of cardiovascular risk and should be actively deprescribed.
1. Introduction
According to the 2017 Global Burden of Diseases, Injuries, and Risk Factors Study, atherosclerotic cardiovascular disease remains the most common cause of death worldwide and constitutes an increasingly important threat to global health [Citation1, Citation2]. In 2017, approximately 17.8 million deaths were attributed to cardiovascular disease, a number that is expected to exceed 22.2 million by 2030 [Citation1]. Among Americans ≥20 years of age, an estimated 18.2 million individuals have coronary artery disease, and 7.0 million have had a stroke [Citation3]. 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) play a pivotal role in reducing the risk of cardiovascular events in primary and secondary preventive settings and are unanimously recommended by international guidelines for both these purposes [Citation4-7]. However, a substantial residual cardiovascular risk may remain despite such evidence-based lipoprotein lowering treatment [Citation8–10].
Numerous studies have shown an independent and seemingly causal relationship between triglyceride concentrations and residual cardiovascular risk [Citation11–14], but prior medications targeting triglyceride or high-density lipoprotein cholesterol levels, e.g. fibrates and niacin, have not demonstrated an ability to reduce adverse clinical outcomes in the statin era [Citation15–20]. The importance of identifying a medication that effectively lowers triglyceride concentrations and, potentially via multifactorial mechanisms, subsequent cardiovascular events is further emphasized by the rising prevalence of obesity, a critical risk factor for hypertriglyceridemia [Citation21–23].
2. Observational evidence for omega-3 fatty acids
Several decades ago, it was postulated that high seal and whale consumption might explain the low rates of death from coronary artery disease among Greenland Inuit [Citation24–26]. These individuals had significantly lower plasma concentrations of total cholesterol and triglycerides, and significantly higher concentrations of high-density lipoprotein cholesterol than both Greenland Inuit living in Denmark and native Danes [Citation27–30]. Similar findings were since reported for other communities with a high fish intake [Citation31,Citation32]. Reduced platelet aggregation and longer bleeding times were also described among persons with a high consumption of fish [Citation33,Citation34].
In 1985, investigators from the Zutphen Study reported that there was an inverse dose-response relationship between fish consumption and death from coronary disease during 20 years of follow-up in 852 middle-aged men without baseline coronary heart disease [Citation35]. The degree of fish consumption correlated with intake of monounsaturated and polyunsaturated fat. In a subgroup of 552 individuals, the risk of stroke was also lower among those with persistent fish consumption [Citation36]. Likewise, data from the Chicago Western Electric Study showed that among 1822 middle-aged men free from cardiovascular disease at baseline, the 30-year risk of fatal myocardial infarction was significantly reduced among those with a higher degree of fish intake, though a beneficial effect on stroke was not seen [Citation37,Citation38].
Many observational studies corroborating the ability of n-3 (omega-3) fatty acids to reduce triglyceride levels and various types of cardiovascular events have since emerged [Citation39,Citation40]. The lowering of triglyceride levels appears to be dose-dependent and displays substantial interindividual variability, but with greater absolute risk reductions among those with higher baseline concentrations [Citation41]. Nevertheless, typical doses found in dietary supplements have not consistently been shown to produce substantial reductions in triglycerides or have anti-thrombotic effects [Citation40].
3. Chemistry and potential mechanisms of action for omega-3 fatty acids
The three key omega-3 fatty acids involved in human physiology are alpha-linolenic acid (ALA) derived from plant oils, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) derived largely from fish oils. The term omega-3 indicates the presence of a carbon-carbon double bond three carbons from the terminal or omega methyl group of the fatty acid chain [Citation41–43]. Proposed mechanisms for reduction of circulating triglyceride concentrations include reduced hepatic production of very low-density lipoprotein, increased chylomicron clearance, stimulation of lipoprotein lipase activity, reduced de novo lipogenesis, increased beta-oxidation, reduced delivery of fatty acids to the liver, reduced hepatic enzyme activity for triglyceride synthesis, and a relative increase in hepatic phospholipid synthesis [Citation44–50].
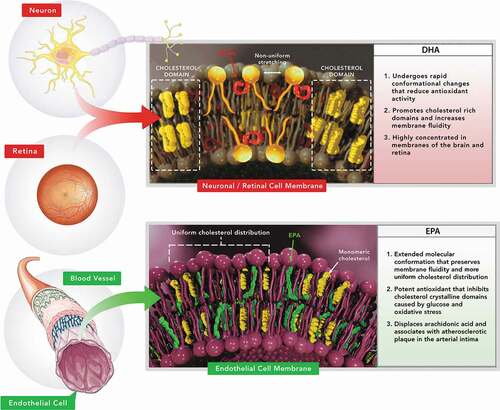
EPA serves as an enzymatic precursor for thromboxane A3 and prostaglandin I3, which have neutral and inhibitory effects on platelet aggregation, respectively [Citation51]. Both eicosanoids appear to be produced in subjects who consume at least 4 grams of EPA daily [Citation52,Citation53]. Individuals who ingest 4 grams or more of EPA daily may also have lower thromboxane A2 synthesis and increased prostaglandin I2 synthesis, augmenting the inhibitory effect on platelets [Citation53–57]. Other potentially beneficial effects of omega-3 fatty acids include those on blood pressure, endothelial function, atherosclerotic plaques, insulin resistance, and inflammation [Citation58]. New insights indicate differences among omega-3 fatty acids that may favor EPA over DHA with respect to plaque stabilization. In particular, EPA inhibits cholesterol crystal formation, stabilizes membrane structure, reverses endothelial dysfunction [Citation57,Citation58,Citation65], and produces sustained inhibition of lipoprotein and membrane lipid oxidation [Citation59,Citation60]. The benefits of EPA and its metabolites on triglyceride levels may also in part be due to activation of the peroxisome proliferator-activator receptor alpha (PPAR-α) [Citation61,Citation62]. The distinct membrane interactions, effects on oxidation, and tissue distributions of EPA and DHA are depicted in . In other words, there is no single unifying explanation for the potential benefits of omega-3 fatty acids on cardiovascular disease [Citation40].
4. Evidence from randomized controlled trials of omega-3 fatty acids
Many randomized controlled trials and subsequent meta-analyses have been conducted for various omega-3 fatty acid formulations [Citation63]. Nevertheless, results from clinical trials have not been entirely consistent, and contemporary data do not support routine use of omega-3 fatty acids for reduction of cardiovascular events [Citation64–67]. For example, a 2020 meta-analysis of 86 randomized controlled trials found no effect of increasing omega-3 fatty acid intake on all-cause mortality (risk ratio (RR) 0.97, 95% confidence interval (CI): 0.93 to 1.01), and only marginal evidence for reduction of cardiovascular mortality (RR 0.92, 95% CI: 0.86 to 0.99) and coronary heart disease mortality (RR 0.90, 95% CI: 0.81 to 1.00) [Citation67].
In the 2*2 factorial, randomized, controlled Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI-Prevenzione) trial, 11,324 patients with a myocardial infarction within the last 3 months were randomized to receive omega-3 fatty acids (1000 mg daily of EPA and DHA in an average ratio of 1:2), vitamin E (300 mg daily), both, or neither [Citation68]. At 3.5 years, treatment with omega-3 fatty acids was associated with a significant reduction in the risk of the primary composite endpoint of death, non-fatal myocardial infarction, or non-fatal stroke (RR 0.90, 95% CI: 0.82 to 0.99), but not the main secondary composite endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (RR 0.89, 95% CI: 0.80 to 1.01). Of note, since patients were recruited between 1993 and 1995, less than 5% were receiving traditional lipid-lowering therapy at baseline.
The more recently published A Study of Cardiovascular Events in Diabetes (ASCEND; n = 15,480) trial and Vitamin D and Omega-3 Trial (VITAL; n = 25,871) examined the use of omega-3 fatty acids (1000 mg daily of EPA and DHA in an average ratio of 1.3:1) in the primary preventive setting in individuals with diabetes and those predominantly without diabetes, respectively [Citation69,Citation70]. Neither trial was able to detect a significant reduction in the primary composite ischemic endpoint with omega-3 fatty acid supplementation (ASCEND: RR 0.97, 95% CI: 0.87 to 1.08; P = 0.55; VITAL: hazard ratio (HR) 0.92, 95% CI: 0.80 to 1.06; P = 0.24). However, the prevalence of lipid-lowering therapy was much higher than in GISSI-Prevenzione (~75% in ASCEND and ~38% in VITAL) [Citation68-70].
5. Differential effects and potential safety issues for dietary supplements
The above findings underscore the general lack of efficacy of mixed, low-dose omega-3 fatty acids for reduction of cardiovascular risk in contemporary clinical practice. While it is possible that negative study results may have been due to subtherapeutic doses, there appear to be potential differential effects between various preparations. The evidence base is weaker for ALA than for the predominantly marine-derived omega-3 fatty acids [Citation64,Citation67,Citation71]. Furthermore, while both EPA and DHA lower triglycerides, DHA may also increase low-density lipoprotein cholesterol [Citation72–77]. These considerations are particularly important as several mixed omega-3 formulations are approved as prescription medications which may hinder optimal treatment of patients with dyslipidemia [Citation78].
Qualitative and quantitative differences between prescription medications and dietary supplements have been well characterized [Citation79]. While dietary fish oil supplements are widely consumed [Citation80–82], they should not be used for treatment of medical conditions as they are not subjected to careful regulatory approval and oversight by the U.S. Food and Drug Administration (FDA) [Citation78,Citation83,Citation84]. Loosely regulated as food products, dietary fish oil supplements are considered safe until proven otherwise while prescription medications require proof of both clinical safety and efficacy with ongoing manufacturing oversight [Citation85]. Testing of fish oil supplements has identified issues with variable amounts of EPA and DHA, lipid oxidation products, and significant levels of saturated fat and other environmental contaminants that may actually be harmful to patients with cardiovascular risk [Citation86–90]. In the absence of clinical outcome evidence and FDA oversight, dietary supplements containing omega-3 fatty acids cannot be recommended to reduce triglycerides or cardiovascular risk.
6. Evidence from Japanese randomized controlled trials of icosapent ethyl
Few studies have examined the efficacy of EPA alone. The Japan EPA Lipid Intervention Study (JELIS) was a controlled trial in which 18,645 participants with a total cholesterol level ≥6.5 mmol/l were randomized to receive either highly purified EPA ethyl ester 600 mg thrice daily plus statin or statin alone [Citation91]. Mean daily statin doses were 10 mg for pravastatin and 5.6 mg for simvastatin. At a mean of 4.6 years, the risk of the primary composite endpoint of any major coronary event was significantly reduced in the EPA group (HR 0.81, 95% CI: 0.69 to 0.95; P = 0.01). Results were comparable between patients with or without a history of coronary artery disease. Low-density lipoprotein cholesterol reduction was similar in the two study groups (25%), but triglyceride reduction was more pronounced in those allocated to EPA (9% versus 4%, P < 0.001) [Citation90]. EPA appeared to be associated with a greater relative reduction of the primary endpoint among patients with a triglyceride concentration ≥150 mg/dl and a high-density lipoprotein concentration <40 mg/dl (HR 0.47, 95% CI: 0.23 to 0.98; P = 0.04) [Citation92]. The risk of incident events also correlated with on-treatment plasma EPA levels [Citation93].
The Combination Therapy of Eicosapentaenoic Acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography (CHERRY) study randomized 193 patients with stable coronary artery disease or an acute coronary syndrome who underwent percutaneous coronary intervention to either highly purified EPA 1800 mg daily plus pitavastatin 4 mg daily or pitavastatin alone and followed them for 6–8 months [Citation94]. The primary endpoint comprised changes in coronary plaque tissue characteristics as assessed by intravascular ultrasound. Several measures, including total atheroma volume regression and lipid volume regression were significantly more pronounced in the combination therapy group [Citation95].
7. Evidence from largely western randomized controlled trials of icosapent ethyl
The Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension (MARINE) and the Effect of AMR101 (Ethyl Icosapentate) on Triglyceride Levels in Patients on Statins With High Triglyceride Levels (ANCHOR) trials examined, in a randomized, placebo-controlled fashion, the efficacy of icosapent ethyl among 229 individuals with a triglyceride concentration 500–2000 mg/dl and 702 patients at high cardiovascular risk on statin therapy with a residual triglyceride concentration 200–499 mg/dl, respectively [Citation96,Citation97]. Icosapent ethyl, a stable, highly purified ethyl ester of EPA, was administered at doses of either 1 gram twice daily or 2 grams twice daily. Both doses resulted in significant reduction in triglyceride levels without affecting low-density lipoprotein cholesterol levels, but effects were more pronounced for the 2 grams twice daily regimen. The safety profile of icosapent ethyl was similar to that of placebo. High-sensitivity C-reactive protein concentrations were also reduced with icosapent ethyl versus placebo [Citation98].
These promising results paved the way for the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) [Citation99]. Patients with either established atherosclerosis (secondary prevention) or diabetes plus additional cardiovascular risk factors (primary prevention) who had received a statin for ≥4 weeks were eligible for inclusion. Furthermore, a fasting triglyceride concentration of 135–499 mg/dl and a low-density lipoprotein concentration of 41–100 mg/dl were required. A total of 8179 participants were enrolled and randomly assigned to either icosapent ethyl 2 grams twice daily or placebo. At a median of 4.9 years, the risk of the primary efficacy endpoint of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, or unstable angina was significantly reduced in the icosapent ethyl group (HR 0.75, 95% CI: 0.68 to 0.83; P < 0.001), as was the risk of the key secondary endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (HR 0.74, 95% CI: 0.65 to 0.83; P < 0.001) [Citation100–102]. In fact, all atherosclerotic cardiovascular endpoints were significantly reduced in patients randomized to icosapent ethyl, including cardiovascular death (HR 0.80, 95% CI: 0.66 to 0.98; P = 0.03) and total (first and subsequent) ischemic events (rate ratio 0.70; 95% CI: 0.62 to 0.78; P < 0.001) [Citation103,Citation104]. Considering the subgroup of 3146 patients enrolled in the United States, all-cause mortality was also reduced with icosapent ethyl (HR 0.70, 95% CI: 0.55 to 0.90; P = 0.004) [Citation105]. Finally, the benefits of icosapent ethyl were consistent across baseline triglyceride concentration tertiles [Citation106]. Interestingly, the efficacy of icosapent ethyl was greater than would be expected based on attained triglyceride levels, lending strength to the concept of EPA having multiple pleiotropic effects beyond triglyceride reduction [Citation100–103].
Adverse events were overall equally distributed between the two groups, irrespective of severity (). Rates of serious adverse bleeding events, including hemorrhagic stroke, other serious central nervous system bleeding, and gastrointestinal bleeding, were not significantly increased in the icosapent ethyl group (2.7% versus 2.1%, P = 0.06), though, when considering minor bleeding as well, all bleeding treatment-emergent adverse events were significantly increased (11.8% versus 9.9%, P = 0.006). Diarrhea (9.0% versus 11.1%, P = 0.002) and anemia (4.7% versus 5.8%, P = 0.03) were among the safety events more commonly observed in the placebo group. Conversely, constipation (5.4% versus 3.6%, P < 0.001) and peripheral edema (6.5% versus 5.0%, P = 0.002) were more common in the icosapent ethyl group. Furthermore, while the rate of hospitalization for atrial fibrillation or atrial flutter was significantly higher for icosapent ethyl (3.1% versus 2.1%, P = 0.004), the risk of stroke in the trial was not (HR 0.72, 95% CI: 0.55 to 0.93; P = 0.01) [Citation100]. Key features of completed randomized controlled trials of EPA products are summarized in .
Table 1. Treatment-emergent adverse events in Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) [Citation100]
Table 2. Key completed randomized controlled trials of eicosapentaenoic acid
8. Contemporary guidelines and recommendations
Several international societies have published updated clinical practice guidelines on the use of icosapent ethyl in individuals with hypertriglyceridemia who are at high risk for cardiovascular events [Citation6,Citation107–109]. The 2019 scientific statement from the National Lipid Association (NLA) recommends icosapent ethyl at a dose of 2 grams twice daily for risk reduction in patients aged ≥45 years with clinical atherosclerotic cardiovascular disease, or with medically treated diabetes mellitus plus at least one additional risk factor, who have fasting triglyceride concentrations 135–499 mg/dl despite high-intensity or maximally tolerated statin (class of recommendation: I) [Citation107]. Likewise, the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines advise that icosapent ethyl be considered in patients at high risk who have triglyceride levels 135–499 mg/dl despite statin treatment (class of recommendation: IIa) [Citation6]. The American Diabetes Association’s (ADA) 2020 Standards of Medical Care in Diabetes recommend consideration of icosapent ethyl in patients with atherosclerotic cardiovascular disease or other cardiovascular risk factors on a statin whose low-density lipoprotein cholesterol levels are controlled, but whose triglyceride concentrations are 135–499 mg/dl (class of recommendation: Level A) [Citation108]. Similar recommendations are provided by the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) [Citation109].
The medication label was expanded by the FDA from triglyceride reduction only in those with severe hypertriglyceridemia ≥500 mg/dl to include reduction of cardiovascular event risk in statin-treated patients with triglyceride concentrations ≥150 mg/dl who have either established cardiovascular disease or diabetes with at least two other cardiovascular risk factors [Citation110,Citation111]. While REDUCE-IT required fasting triglycerides, the FDA labeling does not require that the triglyceride measurement be in a fasting state. The trial also required statin use, while the FDA label states maximally tolerated statin therapy, which for some patients who are statin intolerant may mean no statin. Health Canada has approved icosapent ethyl to reduce the risk of cardiovascular events (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization, or hospitalization for unstable angina) in statin-treated patients with elevated triglycerides who are at high risk of cardiovascular events due to either established cardiovascular disease or diabetes and at least one other cardiovascular risk factor [Citation111]. Based on lack of positive clinical trial outcome data, the European Medicines Agency (EMA) recently concluded that other omega-3 fatty acid medications which contain a combination of EPA and DHA at a dose of 1000 mg daily were not effective in preventing recurrent cardiovascular events, an authorization these medications had otherwise held since 2000 [Citation112]. Icosapent ethyl has recently been approved by the EMA.
9. Other randomized controlled trials
Other trials of triglyceride lowering medications provide additional insights into the potential mechanisms of action for icosapent ethyl as well as different omega-3 fatty acid formulations. The randomized, double-blind, placebo-controlled Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) trial evaluated the effects of icosapent ethyl 4 g/day on coronary atherosclerotic plaque volume as assessed by coronary CT angiography [Citation113]. The prespecified interim analysis showed a significant reduction in total (non-calcified plus calcified) plaque volume at 9 months [Citation114], a finding that was confirmed by the final study results at 18 months [Citation115].The open-label Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy – Statin and Eicosapentaenoic Acid (RESPECT-EPA) has recruited 3900 patients with stable coronary artery disease receiving a statin, randomized them to EPA 1800 mg daily or no EPA, and is currently following them for incident atherosclerotic cardiovascular events [Citation116].
Considering mixed omega-3 fatty acid formulations, the Long‐Term Outcomes Study to Assess Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH) was prematurely terminated because of lack of any benefit with EPA (~550 mg) and DHA (~200 mg) free carboxylic acids in patients at high risk for cardiovascular events who were on optimal statin therapy, despite a significant reduction in triglycerides [Citation117,Citation118]. This underscores the importance of the type, not only quantity, of omega-3 fatty acid consumed, as the total dose of the preparation was 4 g/day. The randomized, double-blind, placebo-controlled OMega-3 fatty acids in Elderly patients with Myocardial Infarction (OMEMI) trial tested the utility of a supplement consisting of 1.8 g/day of mixed EPA and DHA to reduce cardiovascular outcomes, but in older individuals with a recent myocardial infarction [Citation119]. This trial was also negative.
Finally, the Pemafibrate to Reduce Cardiovascular OutcoMes by Reducing Triglycerides IN patiENts With diabeTes (PROMINENT) trial is examining whether the selective PPAR-α modulator (SPPARM-α) pemafibrate can reduce cardiovascular events in patients with type 2 diabetes, hypertriglyceridemia, and low high-density lipoprotein cholesterol [Citation120]. Detailed characteristics of these studies are summarized in .
Table 3. Additional randomized controlled trials of triglyceride lowering medications
10. Expert opinion
In patients at high cardiovascular risk, the rate of events remains elevated despite traditional, evidence-based lipid-lowering therapy. Residual hypertriglyceridemia is an important contributor to this risk and is a potent predictor of an elevated rate of ischemic events. However, the majority of medications aimed at reducing triglyceride levels have failed to demonstrate a reduction in adverse clinical outcomes in the statin era. Although various combinations of EPA and DHA have been tested in high-risk patients [Citation122], only icosapent ethyl, a stable, highly purified ethyl ester of EPA, safely and effectively reduces incident cardiovascular events in the contemporary setting.
Indeed, the large, randomized REDUCE-IT trial demonstrated considerable reductions in atherosclerotic cardiovascular events (both first and total), without an overall increase in the risk of adverse events with icosapent ethyl versus placebo. There were significant reductions in the individual endpoints of myocardial infarction, stroke, coronary revascularization, hospitalization for unstable angina, and cardiovascular death. Potential reductions in all-cause mortality were also seen. The medication improves the lipid profile, decreases coronary atherosclerotic plaque volume, and reduces markers of inflammation, providing mechanistic explanations for its clinical benefits. The salutary effects on plaque volume are evident within 9 months, with evidence of clinical reductions in cardiovascular events starting even before that timepoint. Several markers of unstable plaque are reduced as well. Accordingly, icosapent ethyl, prescribed at a dose of 2 grams twice daily, is warranted in adult patients at high cardiovascular risk, i.e., established atherosclerotic disease or diabetes with additional risk factors, who have triglyceride levels 135–499 mg/dl despite statin treatment (or who cannot take a statin because of intolerance), or in individuals with triglyceride levels ≥500 mg/dl, irrespective of additional risk factors. Conversely, omega-3 fatty acid preparations containing a combination of EPA and DHA at typical dietary supplement doses do not provide clinically meaningful lowering of triglyceride concentrations, are not indicated for reduction of cardiovascular risk, and should be actively deprescribed.
The body of evidence supporting icosapent ethyl is rapidly growing, with several mechanistic studies underway. Elegant experiments assessing the differential effects of various omega-3 fatty acids on cell membrane preparations will provide further insights into molecular mechanisms of EPA that produce downstream beneficial effects, such as attenuating inflammation. It is already clear that the benefits of icosapent ethyl are mediated largely through EPA and are independent of baseline triglyceride levels. Thus, while the REDUCE-IT trial used elevated triglycerides to enrich the rate of ischemic events, the results likely apply to patients with lower triglyceride levels than what was studied, assuming such patients are otherwise at high cardiovascular risk. In conclusion, icosapent ethyl will have an increasingly important impact on cardiovascular risk reduction given the growing population of patients with hypertriglyceridemia and other associated risk factors. The evaluations of cost-effectiveness in both primary and secondary prevention have been quite favorable, particularly when compared with more expensive therapies such as the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, alirocumab, and evolocumab [Citation123,Citation124]. Studies of the generalizability of REDUCE-IT have shown that tens of millions of people worldwide could derive benefit from this medication [Citation125–127]. Implementation of this effective and safe therapy should be a priority in healthcare systems worldwide.
Article highlights
In patients at high cardiovascular risk, the rate of events remains elevated despite traditional, evidence-based lipid-lowering therapy with statins, and residual hypertriglyceridemia is an important contributor to this risk
Icosapent ethyl safely and effectively reduces the risk of incident cardiovascular events in patients at high cardiovascular risk with fasting or non-fasting triglyceride levels 150-499 mg/dl despite statin treatment, and it is also indicated in individuals with triglyceride levels ≥500 mg/dl to lower triglycerides to try and reduce the risk of pancreatitis
Omega-3 fatty acid preparations containing a combination of eicosapentaenoic acid and docosahexaenoic acid are not indicated for reduction of cardiovascular risk and should be actively deprescribed
Declaration of interest
M Pareek discloses the following relationships – Advisory Board: AstraZeneca, Janssen-Cilag; Speaker Honorarium: AstraZeneca, Bayer, Boehringer Ingelheim, Janssen-Cilag. RP Mason has received consulting and research grants from Amarin Pharma Inc, Novartis, and Pfizer. Advisory Board: Cardax. DL Bhatt serves as the Chair and International Principal Investigator for REDUCE-IT, with research funding from Amarin to Brigham and Women’s Hospital. Dr. Bhatt discloses the following relationships - Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has received consulting fees from companies involved in omega-3 fatty acids including the preparation involved in REDUCE-IT. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Luke A. Groothoff of Elucida Research for his assistance in the preparation of the figure.
Additional information
Funding
References
- GBD 2017 Causes of death collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10. 2017;392(10159):1736–1788.
- Global Burden of Cardiovascular Diseases Collaboration, Roth GA, Johnson CO, Abate KH, et al. The burden of cardiovascular diseases among US States, 1990-2016. JAMA Cardiol. 2018 May 1;3(5):375–389.
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A report from the American Heart Association. Circulation. 2020 Mar 3;141(9):e139–e596.
- Piepoli MF, Hoes AW, Agewall S, et al. European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016 Aug 1;37(29):2315-2381.
- Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019 Jun 25;73(24):e285–e350.
- Mach F, Baigent C, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020 Jan 1;41(1):111–188.
- Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019 Sep 10;74(10):e177-e232.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005 Oct 8;366(9493):1267–1278.
- Baigent C, Blackwell L, Emberson J, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010 Nov 13;376(9753):1670–1681.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016 Nov 19;388(10059):2532–2561.
- Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007 Jan 30;115(4):450–458.
- Varbo A, Benn M, Tybjaerg-Hansen A, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013 Jan 29;61(4):427–436.
- Jorgensen AB, Frikke-Schmidt R, West AS, et al. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013 Jun;34(24):1826–1833.
- Ference BA, Kastelein JJP, Ray KK, et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR variants with risk of coronary heart disease. JAMA. 2019 Jan 29;321(4):364–373.
- Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987 Nov 12;317(20):1237–1245.
- Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999 Aug 5;341(6):410–418.
- Ginsberg HN, Elam MB, Lovato LC, et al. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010 Apr 29;362(17):1563–1574.
- Boden WE, Probstfield JL, Anderson T, et al. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011 Dec 15;365(24):2255–2267.
- Landray MJ, Haynes R, Hopewell JC, et al. HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014 Jul 17;371(3):203–212.
- Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018 Jul 17;72(3):330–343.
- Di CM, Bentham J, Stevens GA, et al. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016 Apr 2;387(10026):1377–1396.
- Justesen JM, Allin KH, Sandholt CH, et al. Interactions of lipid genetic risk scores with estimates of metabolic health in a danish population. Circ Cardiovasc Genet. 2015 Jun;8(3):465–472.
- Ali A, Varga TV, Stojkovic IA, et al. Do genetic factors modify the relationship between obesity and Hypertriglyceridemia? Findings from the GLACIER and the MDC Studies. Circ Cardiovasc Genet. 2016 Apr;9(2):162–171.
- Sinclair HM. Deficiency of essential fatty acids and atherosclerosis, etcetera. Lancet. 1956 Apr 7;270(6919):381–383.
- Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200(1–6):69–73.
- Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980 Dec;33(12):2657–2661.
- Bang HO, Dyerberg J, Nielsen AB. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971 Jun 5;297(7710):1143–1145.
- Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med Scand. 1972 Jul-Aug;192(1–6):85–94.
- Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma lipids in Greenland Eskimos. Am J Clin Nutr. 1975 Sep;28(9):958–966.
- Dyerberg J, Bang HO, Hjorne N. Plasma cholesterol concentration in Caucasian Danes and Greenland West-coast Eskimos. Dan Med Bull. 1977 Apr;24(2):52–55.
- Miller GJ, Koplan JP, Morgan P, et al. High-density lipoprotein cholesterol concentration and other serum lipids in an isolated island community free of coronary heart disease. Int J Epidemiol. 1979 Sep;8(3):219–225.
- Kagawa Y, Nishizawa M, Suzuki M, et al. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. J Nutr Sci Vitaminol (Tokyo). 1982;28(4):441–453.
- Dyerberg J, Bang HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979 Sep 1;28(8140):433–435.
- Hirai A, Hamazaki T, Terano T, et al. Eicosapentaenoic acid and platelet function in Japanese. Lancet. 1980 Nov 22;2(8204):1132–1133.
- Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985 May 9;312(19):1205–1209.
- Keli SO, Feskens EJ, Kromhout D. Fish consumption and risk of stroke. The Zutphen Study. Stroke. 1994 Feb;25(2):328–332.
- Orencia AJ, Daviglus ML, Dyer AR, et al. Fish consumption and stroke in men. 30-year findings of the Chicago Western ElectricStudy. Stroke. 1996 Feb;27(2):204–209.
- Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997 Apr 10;336(15):1046–1053.
- Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757.
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011 Nov 8;58(20):2047–2067.
- Harris WS. N-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997 May;65(5):1645S–1654S.
- Goyens PL, Mensink RP. Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur J Clin Nutr. 2006 Aug;60(8):978–984.
- Egert S, Kannenberg F, Somoza V, et al. Dietary alpha-linolenic Acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009 May;139(5):861–868.
- Rivellese AA, Maffettone A, Iovine C, et al. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996 Nov;19(11):1207–1213.
- Faeh D, Minehira K, Schwarz JM, et al. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005 Jul;54(7):1907–1913.
- Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006 Aug;17(4):387–393.
- Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006 Aug 21;98(4):27i–33i.
- Hein GJ, Bernasconi AM, Montanaro MA, et al. Nuclear receptors and hepatic lipidogenic enzyme response to a dyslipidemic sucrose-rich diet and its reversal by fish oil n-3 polyunsaturated fatty acids. Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E429–39.
- Sato A, Kawano H, Notsu T, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010 Oct;59(10):2495–2504.
- Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011 Mar;14(2):115–120.
- Dyerberg J, Bang HO, Stoffersen E, et al. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119.
- Fischer S, Weber PC. Thromboxane A3 (TXA3) is formed in human platelets after dietary eicosapentaenoic acid (C20:5 omega 3). Biochem Biophys Res Commun. 1983 Nov 15;116(3):1091–1099.
- Fischer S, Weber PC. Prostaglandin I3 is formed in vivo in man after dietary eicosapentaenoic acid. Nature. 1984 Jan 12-18;307(5947):165–168.
- Hornstra G, Haddeman E, ten Hoor F. Fish oils, prostaglandins, and arterial thrombosis. Lancet. 1979 Nov 17;2(8151):1080.
- Siess W, Roth P, Scherer B, et al. Platelet-membrane fatty acids, platelet aggregation, and thromboxane formation during a mackerel diet. Lancet. 1980 Mar 1;1(8166):441–444.
- Goodnight SH Jr, Harris WS, Connor WE, et al. Polyunsaturated fatty acids, hyperlipidemia, and thrombosis. Arteriosclerosis. 1982 Mar-Apr;2(2):87–113.
- Hornstra G, Haddeman E, Kloeze J, et al. Dietary-fat-induced changes in the formation of prostanoids of the 2 and 3 series in relation to arterial thrombosis (rat) and atherosclerosis (rabbit). Adv Prostaglandin Thromboxane Leukot Res. 1983;12:193–202.
- Nelson JR, True WS, Le V, et al. Can pleiotropic effects of eicosapentaenoic acid (EPA) impact residual cardiovascular risk? Postgrad Med. 2017 Nov;129(8):822–827.
- Mason RP. New insights into mechanisms of action for Omega-3 Fatty Acids in Atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019 Jan 12;21(1):2–019-0762-1.
- Mason RP, Libby P, Bhatt DL. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler Thromb Vasc Biol. 2020 May;40(5):1135–1147.
- Lefils-Lacourtablaise J, Socorro M, Geloen A, et al. The Eicosapentaenoic Acid Metabolite 15-Deoxy-delta(12,14)-Prostaglandin J3 Increases Adiponectin Secretion by Adipocytes Partly via a PPARgamma-Dependent Mechanism. PLoS One. 2013 May 29;8(5):e63997.
- Yamada H, Kikuchi S, Hakozaki M, et al. 8-Hydroxyeicosapentaenoic Acid Decreases Plasma and Hepatic Triglycerides via Activation of Peroxisome Proliferator-Activated Receptor Alpha in High-Fat Diet-Induced Obese Mice. J Lipids. 2016;2016:7498508.
- Patel PN, Patel SM, Bhatt DL. Cardiovascular risk reduction with icosapent ethyl. Curr Opin Cardiol. 2019 Nov;34(6):721–727.
- Wang C, Harris WS, Chung M, et al. N−3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 2006 Jul;84(1):5–17.
- Alexander DD, Miller PE, Van Elswyk ME, et al. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017 Jan;92(1):15–29.
- Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 Trials Involving 77917 Individuals. JAMA Cardiol. 2018 Mar 1;3(3):225–234.
- Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2020 Feb;29(3):CD003177.
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999 Aug 7;354(9177):447–455.
- Bowman L, Mafham M, Wallendszus K, et al. ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018 Oct 18;379(16):1540–1550.
- Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019 Jan 3;380(1):33–44.
- Pan A, Chen M, Chowdhury R, et al. Alpha-Linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am J Clin Nutr. 2012 Dec;96(6):1262–1273. .
- Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr Atheroscler Rep. 2011 Dec;13(6):474–483.
- Jacobson TA, Glickstein SB, Rowe JD, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J Clin Lipidol. 2012 Jan-Feb;6(1):5–18.
- Itakura H, Yokoyama M, Matsuzaki M, et al. The change in low-density lipoprotein cholesterol concentration is positively related to plasma docosahexaenoic acid but not eicosapentaenoic acid. J Atheroscler Thromb. 2012;19(7):673–679.
- Singh S, Arora RR, Singh M, et al. Eicosapentaenoic acid versus docosahexaenoic acid as options for vascular risk prevention: A fish story. Am J Ther. 2016 May-Jun;23(3):e905–10.
- Asztalos IB, Gleason JA, Sever S, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: A randomized clinical trial. Metabolism. 2016 Nov;65(11):1636–1645.
- Yang ZH, Amar M, Sampson M, et al. Comparison of Omega-3 Eicosapentaenoic Acid Versus Docosahexaenoic Acid-Rich fish oil supplementation on plasma lipids and Lipoproteins in Normolipidemic Adults. Nutrients. 2020 Mar 12;12(3):749.
- Fialkow J. Omega-3 fatty acid formulations in cardiovascular disease: Dietary supplements are not substitutes for prescription products. Am J Cardiovasc Drugs. 2016 Aug;16(4):229–239.
- Hilleman DE, Wiggins BS, Bottorff MB. Critical differences between dietary supplement and prescription omega-3 Fatty Acids: A narrative review. Adv Ther. 2020 Feb;37(2):656–670.
- Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016 Apr;176(4):473–482.
- Kantor ED, Rehm CD, Du M, et al. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016 Oct 11;316(14):1464–1474.
- Cohen PA. The supplement paradox: Negligible benefits, robust consumption. JAMA. 2016 Oct 11;316(14):1453–1454.
- Cohen PA. Hazards of Hindsight — monitoring the safety of nutritional supplements. N Engl J Med. 2014 Apr 3;370(14):1277–1280.
- Geller AI, Shehab N, Weidle NJ, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015 Oct 15;373(16):1531–1540.
- Collins N, Tighe AP, Brunton SA, et al. Differences between dietary supplement and prescription drug omega-3 fatty acid formulations: A legislative and regulatory perspective. J Am Coll Nutr. 2008 Dec;27(6):659–666.
- Ritter JC, Budge SM, Jovica F. Quality analysis of commercial fish oil preparations. J Sci Food Agric. 2013 Jun;93(8):1935–1939.
- Kleiner AC, Cladis DP, Santerre CR. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J Sci Food Agric. 2015 Apr;95(6):1260–1267.
- Albert BB, Derraik JG, Cameron-Smith D, et al. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. 2015 Jan 21;5(1):7928.
- Jackowski SA, Alvi AZ, Mirajkar A, et al. Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J Nutr Sci. 2015 Nov 4;4:e30.
- Mason RP, Sherratt SCR. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem Biophys Res Commun. 2017 Jan 29;483(1):425–429.
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007 Mar 31;369(9567):1090–1098.
- Saito Y, Yokoyama M, Origasa H, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008 Sep;200(1):135–140.
- Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18(2):99–107.
- Watanabe T, Miyamoto T, Miyasita T, et al. Combination therapy of eicosapentaenoic acid and pitavastatin for coronary plaque regression evaluated by integrated backscatter intravascular ultrasonography (CHERRY study)—Rationale and design. J Cardiol. 2014 Sep;64(3):236–239.
- Watanabe T, Ando K, Daidoji H, et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017 Dec;70(6):537–544.
- Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011 Sep 1;108(5):682–690.
- Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012 Oct 1;110(7):984–992.
- Bays HE, Ballantyne CM, Braeckman RA, et al. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013 Feb;13(1):37–46.
- Bhatt DL, Steg PG, Brinton EA, et al. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol. 2017 Mar;40(3):138–148.
- Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019 Jan 3;380(1):11–22.
- Kastelein JJP, Stroes ESG. FISHing for the Miracle of Eicosapentaenoic Acid. N Engl J Med. 2019 Jan 3;380(1):89–90.
- Bhatt DL, Steg PG, Miller M. Cardiovascular Risk Reduction with Icosapent Ethyl. Reply. N Engl J Med. 2019 Apr 25;380(17):1678.
- Bhatt DL. REDUCE-IT. Eur Heart J. 2019 Apr 14;40(15):1174–1175.
- Bhatt DL, Steg PG, Miller M, et al. Effects of Icosapent Ethyl on Total Ischemic Events: from REDUCE-IT. J Am Coll Cardiol. 2019 Jun 11;73(22):2791–2802.
- Bhatt DL, Miller M, Brinton EA, et al. REDUCE-IT USA: Results From the 3146 Patients Randomized in the United States. Circulation. 2020 Feb 4;141(5):367–375.
- Bhatt DL, Steg PG, Miller M, et al. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am Coll Cardiol. 2019 Aug 27;74(8):1159–1161.
- Orringer CE, Jacobson TA, Maki KC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J Clin Lipidol. 2019 Nov - Dec;13(6):860–872.
- American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of medical care in diabetes-2020. Diabetes Care. 2020 Jan;43(Suppl1):S111–S134.
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2020 Executive Summary. Endocr Pract. 2020 Jan;26(1):107–139.
- U.S. Food and Drug Administration. FDA News Release: FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups. December 13, 2019; Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-use-drug-reduce-risk-cardiovascular-events-certain-adult-patient-groups. Cited 2020 May 20
- Health Canada. Notice: Prescription Drug List (PDL): multiple additions. February 13, 2020; Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/prescription-drug-list/multiple-additions-2020-02-13.html. Cited 2020 May 20
- European Medicines Agency. EMA confirms omega-3 fatty acid medicines are not effective in preventing further heart problems after a heart attack. March 29, 2019; Available at: https://www.ema.europa.eu/en/news/ema-confirms-omega-3-fatty-acid-medicines-are-not-effective-preventing-further-heart-problems-after. Cited 2020 May 20
- Budoff M, Brent Muhlestein J, Le VT, et al. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: Rationale and design of the EVAPORATE study. Clin Cardiol. 2018 Jan;41(1):13–19.
- Budoff MJ, Muhlestein JB, Bhatt DL, et al. Effect of Icosapent Ethyl on progression of coronary atherosclerosis in patients with elevated Triglycerides on Statin Therapy: a prospective, placebo-controlled randomized trial (EVAPORATE): interim Results. Cardiovasc Res. 2021 Mar 21;117(4):1070-1077.
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: Final results of the EVAPORATE trial. Eur Heart J. 2020 Oct 21;41(40):3925–3932.
- UMIN-CTR Clinical Trial. Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy - Statin and Eicosapentaenoic Acid. April 27, 2018; Available at: https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014051. Cited 2020 May 20
- Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol. 2018 Oct;41(10):1281–1288.
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA. 2020 Dec 8;324(22):2268–2280.
- Kalstad AA, Myhre PL, Laake K, et al. Effects of n-3 Fatty Acid supplements in elderly patients after myocardial infarction: A randomized, controlled trial. Circulation. 2021 Feb 9;143(6):528–539.
- Pradhan AD, Paynter NP, Everett BM, et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am Heart J. 2018 Dec;206:80–93.
- Laake K, Myhre P, Nordby LM, et al. Effects of omega3 supplementation in elderly patients with acute myocardial infarction: design of a prospective randomized placebo controlled study. BMC Geriatr. 2014 Jun 13;14(1):74.
- Skulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation. 2019 Sep 17;140(12):e673–e691.
- Weintraub WS, Bhatt DL, Zhang Z, et al. Cost-effectiveness of icosapent ethyl in US REDUCE-IT patients. 2020; Available at: https://www.onlinejacc.org/content/75/11_Supplement_1/1914. Cited 2020 Sept 17
- Bhatt DL, Briggs AH, Reed SD, et al. Cost-Effectiveness of Alirocumab in patients with acute coronary syndromes: The ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2020 May 12;75(18):2297–2308.
- Jia X, Akeroyd JM, Nasir K, et al. Eligibility and Cost for Icosapent Ethyl Based on the REDUCE-IT Trial. Circulation. 2019 Mar 5;139(10):1341–1343.
- Picard F, Bhatt DL, Ducrocq G, et al. Generalizability of the REDUCE-IT trial in patients with stable coronary artery disease. J Am Coll Cardiol. 2019 Mar 26;73(11):1362–1364.
- Case BC, Bress AP, Kolm P, et al. The economic burden of hypertriglyceridemia among US adults with diabetes or atherosclerotic cardiovascular disease on statin therapy. J Clin Lipidol. 2019 Sep - Oct;13(5):754–761.