ABSTRACT
Background
Safety and tolerability of glucose-lowering drugs is a key consideration for use in type 2 diabetes (T2D). We evaluated the safety and tolerability of the dipeptidyl peptidase-4 inhibitor linagliptin in Asian patients with T2D.
Research design and methods
This was a post-hoc, descriptive pooled analysis of 21 randomized, double-blind, placebo-controlled clinical trials of linagliptin in T2D patients lasting ≤52 weeks. We evaluated adverse events (AEs) and laboratory parameters in Asian participants living in Asia, both overall and in the East Asian subgroup.
Results
This analysis included 4457 Asian patients overall (2712 receiving linagliptin; 1745 receiving placebo) and 3057 (68.6%) East Asians. AEs were reported in 1510 (55.7%) Asian patients receiving linagliptin and 1032 (59.1%) receiving placebo but were considered drug-related in only 13.0% of each group. Serious AEs occurred in 109 (4.0%) linagliptin patients and 90 (5.2%) placebo patients. The most common AEs were nasopharyngitis (6.4% linagliptin, 7.3% placebo), upper respiratory tract infection (5.7% linagliptin, 6.5% placebo), and hypoglycemia (7.3% linagliptin, 6.3% placebo). One linagliptin patient had pancreatitis; none had bullous pemphigoid. No clinically relevant mean changes in laboratory parameters occurred. These findings were consistent in East Asians.
Conclusions
Linagliptin is well tolerated in Asian T2D patients, including East Asians, with low risk for AEs.
1. Introduction
Diabetes mellitus has become highly prevalent in Asia where – like other regions – the vast majority of patients (~90%) have the type 2 form of the disease [Citation1]. Type 2 diabetes (T2D) is characterized by pancreatic β-cell dysfunction and insulin resistance rather than the overt loss of insulin secretion seen in type 1 diabetes. Together, the Western Pacific region (including Japan and China), with an estimated 163 million cases in 2019, and South-East Asia (including India), with 88 million cases, account for over half of the 463 million diabetes cases worldwide [Citation1].
Asian and Western guidelines on managing hyperglycemia in people with T2D indicate that most individuals require pharmacotherapy to achieve glycemic control, and many glucose-lowering drugs from novel molecular classes have been added to the therapeutic armamentarium over the past 15 years [Citation2–6]. Linagliptin is an oral drug from the dipeptidyl peptidase-4 (DPP-4) inhibitor class that is indicated in many countries as an adjunct to healthy diet and exercise to improve glycemic control in adults with T2D.
Linagliptin was launched between 2011 and 2013 in several Asian countries, where it is widely used as monotherapy or in combination with other glucose-lowering drugs. A pooled analysis in 2014 of 22 placebo-controlled, multinational clinical trials of the glycemic efficacy and tolerability of linagliptin treatment found that it had an acceptable overall safety and tolerability profile, with few adverse events occurring at a higher rate than with placebo treatment [Citation7]. Patients whose ethnicity was identified as Asian comprised approximately 37% of the cohort in this analysis. In 2018 and 2019, the results from the CARMELINA and CAROLINA cardiovascular outcome trials (CVOTs), respectively, demonstrated the cardiovascular and kidney safety profile of linagliptin added to standard of care in patients with high cardiovascular risk with [Citation8] or without high kidney risk [Citation9] (ClinicalTrials.gov, NCT01897532 and NCT01243424). Analyses of the Asian participants in these trials showed similar findings to the overall trial cohorts [Citation10,Citation11].
The pathophysiology of T2D in Asians may have clinically important differences compared with other ethnicities, and there may also be considerable heterogeneity between Asians from different geographical regions [Citation12–14]. Compared with white patients, Asians generally have greater abdominal obesity, but lower body mass index (BMI), and higher postprandial glucose excursions [Citation12,Citation13]. East Asian patients also tend to have lower pancreatic β-cell function while South Asians may have greater insulin resistance [Citation12–15]. However, amplification of prandial insulin secretion by gastrointestinal peptide hormones (i.e. the incretin effect) may not be reduced in East Asians with T2D as it is in white patients [Citation16], potentially explaining the greater glycemic response to incretin therapies such as DPP-4 inhibitors seen in Asians compared with non-Asians in some studies [Citation17–20]. Genetic factors in East Asians potentially contributing to T2D were identified in a recent meta-analysis of genome-wide association studies, with some differences compared with European populations [Citation21].
Therefore, we conducted a comprehensive analysis of the safety and tolerability of linagliptin as monotherapy or combination treatment in Asian patients, including a subgroup analysis of East Asians alone. Asian data from the CARMELINA and CAROLINA CVOTs were not included in this analysis, due to the substantial differences in study design compared with the glycemic efficacy trials; these included much greater study duration (as event-driven trials evaluating clinical outcomes), trial cohorts with increased cardiorenal risk, an active comparator in CAROLINA, and differences in the concomitant use of other glucose-lowering drugs. Safety data for the Asian patients in CARMELINA and CAROLINA have previously been published [Citation10,Citation11].
2. Patients and methods
2.1. Study design and patient population
This was a post hoc, descriptive analysis of patient-level safety and tolerability data from the 21 phase II, III or IV clinical trials in the linagliptin global development program that included Asian patients [Citation22–42]. In these trials, which were conducted in more than 40 countries, patients were randomized to daily oral treatment with linagliptin (generally 5 mg) or placebo administered double-blind. These trials were primarily designed to evaluate the glycemic efficacy of linagliptin as either monotherapy or in combination with other glucose-lowering drugs. The duration of the trials ranged from 12 weeks to 52 weeks but was most commonly 24 weeks (). Trials with only an active (non-placebo) comparator were excluded in order to focus on the inherent safety profile of linagliptin rather than its relative safety versus other glucose-lowering drugs.
Table 1. Randomized, double-blind, placebo-controlled phase II, III, and Ⅳ trials of linagliptin that included Asian patients
In general, these trials enrolled T2D patients aged ≥18 years (≥20 years in Japan) either naive to glucose-lowering pharmacotherapy or already receiving glucose-lowering drugs, with glycated hemoglobin (HbA1c) levels in the range of 6.5% to 11.0% (most commonly 7.0% to 10.0%), and BMI <40 kg/m2. Patients with impaired hepatic function, kidney failure, or recent cardiovascular event (myocardial infarction, stroke, transient ischemic attack) were generally excluded.
The trial protocols were approved by local institutional review boards or independent ethics committees at each trial site or country before commencement, and all participants provided written informed consent prior to enrollment. All trials were registered with ClinicalTrials.gov prior to commencement (), and were subsequently conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
The current pooled analysis was restricted to participants of Asian race living in a country in Asia, which included 11 territories in East Asia (Japan, China, Hong Kong, South Korea, Taiwan) and elsewhere in the region (India, Israel, Malaysia, Philippines, Thailand, Vietnam).
2.2. Assessments
In this pooled analysis, we evaluated the incidence of adverse events reported by patients and recorded by trial investigators, which were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1. Based on previous studies and theoretical considerations, we defined the following adverse events as being of special interest: angioedema, bullous pemphigoid, heart failure, hepatic injury, hypersensitivity, hypoglycemia, interstitial lung disease, intestinal obstruction, pancreatic cancer, pancreatitis, rhabdomyolysis, skin lesions, and worsening of kidney function. We also evaluated clinical laboratory test results, which were performed by central laboratories in each trial.
2.3. Statistical analysis
We pooled and analyzed individual patient data for all participants who received at least one dose of linagliptin 5 mg or placebo, i.e. the treated set. All analyses were descriptive in nature. We analyzed the number and percentage of patients with reported adverse events; we also calculated incidence rates (number of patients reporting adverse event per 100 patient-years) for adverse events of special interest. For analysis of laboratory parameters, we calculated means with standard deviations (SD) for baseline and change from baseline values; for amylase and lipase, we also calculated the number and percentage of patients with last value on treatment above or below the upper limit of normal.
3. Results
3.1. Patient disposition, baseline characteristics, and drug exposure
In the 21 eligible trials, 4457 Asian patients received at least one dose of study drug and were included in the treated set for this pooled analysis (2712 receiving linagliptin; 1745 receiving placebo). These 4457 Asian patients comprised 44% of the total population of the 21 phase II, III or IV clinical trials in the linagliptin global development program that included Asian patients.
At baseline, the study cohort was relatively young (mean age: 55.4 years) and slightly overweight, especially by Asian standards (mean BMI: 25.7 kg/m2) (). On average, patients had moderate hyperglycemia (mean HbA1c: 8.3%) and normal kidney function (mean estimated glomerular filtration rate [eGFR]: 89 ml/min/1.73 m2). Almost half the patients (45.6%) had been diagnosed with T2D over five years previously. Most patients (75.7%) were taking at least one oral glucose-lowering drug, but few were taking insulin (12.5%) (, Supplemental Table S1). More of the linagliptin group were taking sulfonylurea-containing regimens compared with the placebo group: metformin and sulfonylurea in 14.3% and 8.0% of patients, respectively, sulfonylurea monotherapy in 2.8% and 2.4%, and insulin and sulfonylurea (with or without other oral glucose-lowering drugs) in 0.5% and 0.5% (Supplemental Table S1).
Table 2. Demographic and baseline characteristics of the Asian patients included in this pooled analysis
Over two-thirds of the study cohort (n = 3057; 68.6%) was from East Asia and the baseline characteristics for these patients were similar to the overall cohort (Supplemental Table S2).
The median duration of exposure to study drug was 169 days in patients receiving linagliptin and 170 days in those receiving placebo, in both the overall cohort and the East Asian subgroup.
3.2. Summary of adverse events
Fewer Asian patients in the linagliptin group reported an adverse event compared with the placebo group (55.7% and 59.1%, respectively) (). Similarly, fewer linagliptin-treated patients had a serious adverse event compared with placebo-treated patients (4.0% and 5.2%, respectively) or an adverse event leading to discontinuation (2.8% and 3.9%) (). Drug-related adverse events were reported in 13.0% of patients in each group. Of the individual adverse events (i.e. MedDRA preferred terms) that occurred in more than 5% of either treatment group, only hypoglycemia was more common with linagliptin (7.3% of patients) than placebo (6.0%). This pattern of adverse events was similar in East Asians (Supplemental Table S3).
Table 3. Summary of adverse events in Asian patients
3.3. Adverse events of special interest
All adverse events of special interest, except hypoglycemia, occurred in <3% of Asian patients (). Angioedema, hypersensitivity events, and hypoglycemia were slightly more common in the linagliptin group than the placebo group. Very few events of heart failure were identified in this analysis (three patients in the linagliptin group and one patient in the placebo group). With regards to intestinal obstruction, two cases were identified in the linagliptin group and one in the placebo group.
Table 4. Adverse events of special interest in Asian patients
Worsening of renal function was also slightly more common in the linagliptin group (). Mean eGFR decreased slightly from baseline to study end in both the linagliptin group (–1.27 ml/min/1.73 m2 from a baseline of 89.19) and the placebo group (–0.13 ml/min/1.73 m2 from baseline 87.97). Mean uric acid increased slightly in both groups (by 0.38 and 0.17 μmol/l with linagliptin and placebo, respectively), as did serum creatinine (0.02 and 0.01 mg/dl, respectively) ().
Table 5. Changes in laboratory parameters in Asian patients
Hepatic injury was less common with linagliptin (1.99% of patients) than with placebo (2.92%) (). Mean changes from baseline in liver enzymes and bilirubin were small and generally similar in both groups ().
One patient experienced interstitial lung disease (an individual receiving placebo) and one experienced pancreatitis (an individual in the linagliptin group with acute pancreatitis) (). Mean changes in amylase and lipase levels were higher in the linagliptin group than the placebo group () but the percentage of patients with amylase or lipase levels greater than the upper limit of normal at end of treatment was low and similar in each group (). No patients experienced pancreatic cancer. Similarly, no patients in either treatment group experienced bullous pemphigoid or other skin lesions.
Figure 1. Proportion of Asian patients with last value on treatment for amylase and lipase relative to the upper limit of normal (ULN).
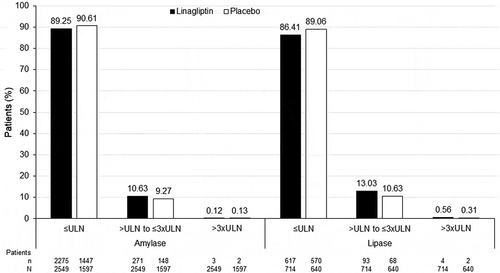
Overall, mean values at baseline and end of treatment were within the normal range for all laboratory parameters.
The general trends for adverse events of special interest in the overall Asian cohort were very similar in the East Asian subgroup (Supplemental Table S4).
4. Discussion
Our comprehensive analysis demonstrates the safety and tolerability profile of the DPP-4 inhibitor linagliptin in Asian patients. This analysis pooled data for 4457 patients from 21 randomized, double-blind, placebo-controlled clinical trials of linagliptin published up to 2021, which includes all eligible trials from the now-completed, company-sponsored clinical development program for this medication. Overall, the percentage of Asian patients experiencing an adverse event with linagliptin was no greater than with placebo, as was also the case for serious adverse events, drug-related events, and events causing treatment discontinuation. Furthermore, there were no substantial differences in the incidence of most specific types of adverse event with linagliptin compared with placebo.
DPP-4 inhibitors are widely prescribed in Asian countries, in general, and are the most prescribed class of oral glucose-lowering drug in Japan in particular [Citation43–45]. Consequently, it is important to characterize their safety profiles in Asian patients, who have historically been a minority in multinational trials of glucose-lowering drugs. Unlike older classes of glucose-lowering drugs such as sulfonylureas and insulin, DPP-4 inhibitors have an inherently low risk for causing hypoglycemia, due to their glucose-dependent insulinotropic mechanism of action mediated via the incretin hormone glucagon-like peptide-1 (GLP-1) [Citation3–6]. Previous pooled analyses of clinical trials demonstrated the safety and tolerability profile of linagliptin in multinational patient cohorts [Citation7,Citation46–48] whereas a pooled analysis of Asian patients focused primarily on efficacy [Citation49] – our pooled analysis exclusively focuses on safety and tolerability findings in a larger cohort of Asian patients that includes the most recent trials. The safety and tolerability profile observed in our analysis is consistent with the adverse events listed in the current prescribing information for linagliptin [Citation17–19].
In our pooled analysis, the incidence of hypoglycemia was slightly higher with linagliptin (7.3%) than placebo (6.0%), which may reflect the greater use of sulfonylurea-containing regimens in the linagliptin group. This minor imbalance between treatment arms in the use of sulfonylureas was mainly due to the inclusion of Asian patients from one study: a multinational phase III trial in which patients with insufficient glycemic control despite using the maximum tolerated doses of metformin and a sulfonylurea were randomized in a 3:1 ratio to linagliptin or placebo [Citation25]. Increased incidence of hypoglycemia with concomitant use of sulfonylureas occurs with all DPP-4 inhibitors, as well as GLP-1 receptor agonists, and is thought to result from a pharmacodynamic interaction in which sulfonylureas uncouple the insulinotropic action of GLP-1 from its dependence on ambient glucose levels [Citation50]. For this reason, the prescribing information for linagliptin and other incretin therapies in most territories suggests reducing the dose of sulfonylurea when co-administered.
It is important to establish the cardiorenal safety of glucose-lowering drugs in Asian patients, given the high rates of cardiovascular and kidney complications in people with T2D, the latter of which are more common in Asian patients than white patients [Citation12,Citation13,Citation51–54]. In our pooled analysis, the incidence of cardiovascular and kidney-related adverse events was comparable between the linagliptin and placebo groups, as was the change in eGFR; however, the number of events was very low. Unlike the trials in our pooled analysis, the placebo-controlled CARMELINA trial was designed to robustly evaluate cardiovascular and kidney outcomes with linagliptin, and found no increased risk of cardiorenal events in either the overall trial cohort or the Asian subgroup. Notably, there was a modestly reduced risk for albuminuria progression and a numerically lower incidence of hospitalization for heart failure with linagliptin compared to placebo [Citation8,Citation10]. Furthermore, a three-year post-marketing surveillance study of linagliptin in routine clinical practice in Japan, which included over 2200 T2D patients, found that kidney function remained stable during the study period with no new safety concerns regardless of eGFR level [Citation55].
Pancreatitis is a known but rare adverse event associated with DPP-4 inhibitors [Citation56]. In our pooled analysis, there was one case of pancreatitis (an acute episode) with linagliptin among the 2712 treated patients and none with placebo. Observational studies in Japan, Taiwan and South Korea have not found an increased risk for pancreatitis with DPP-4 inhibitors in clinical practice [Citation57–59]. Descriptive mean changes in lipase and amylase levels were higher in the linagliptin group compared with the placebo group. In addition, the percentage of patients in the linagliptin group who had levels more than three times greater than the upper limit of normal at last observation was similar or slightly higher numerically than in the placebo group (amylase: linagliptin 0.12% [n/N = 3/2549], placebo 0.13% [n/N = 2/1597]; lipase: linagliptin 0.56% [n/N = 4/714], placebo 0.31% [n/N = 2/640]).
DPP-4 is a ubiquitous multifunctional protein whose diverse biological activities include enzymatic cleavage of various growth factors and chemokines in addition to GLP-1, as well as non-enzymatic roles in immunomodulation, inflammation, and antioxidative response – together, these suggest a potential role in cancer biology [Citation60]. Some preclinical studies have found an association between DPP-4 inhibitors and cancer, but clinical evidence to date has not found a clear association between cancer incidence or prognosis in T2D patients [Citation60]. Potential effects on pancreatic cancer are of particular interest, given the activity of GLP-1 in the pancreas. In our pooled analysis, there were no cases of pancreatic cancer; however, the observation period was too short to evaluate cancer risk. The CARMELINA and CAROLINA trials were much longer (median durations of 2.2. and 6.3 years, respectively). In CARMELINA, there were no cases of pancreatic cancer with linagliptin and one case with placebo (0.4%) among the Asian participants [Citation10]; in CAROLINA, there were three cases with linagliptin (0.6%) and two with glimepiride (0.4%) in Asians [Citation11]. Overall, the risk of pancreatic cancer with DPP-4 inhibitors remains uncertain [Citation61], especially given the lengthy latency period of this tumor type and the complexity of the potential association, with some risk factors for pancreatic cancer being common in T2D patients.
In 2015, a safety signal for bullous pemphigoid with DPP-4 inhibitors was identified from a disproportionality analysis of the US Food and Drug Administration Adverse Event Reporting System. Consequently, US prescribing information for DPP-4 inhibitors was updated to include bullous pemphigoid, an autoimmune skin disorder characterized by blisters. Japanese prescribing information was updated similarly in 2016 following a request from the Pharmaceuticals and Medical Devices Agency. In our analysis, we did not detect any cases of bullous pemphigoid or other types of skin lesions. However, bullous pemphigoid occurs mainly in patients over the age of 70, whereas patients in our analysis were younger on average (mean of 55 years). Notably, no cases of bullous pemphigoid were detected in Asian participants in the CARMELINA trial where the average age was higher (65 years), despite a numerical imbalance in the overall trial cohort with seven cases in the linagliptin group (0.2%) and none in the placebo group [Citation10]. There were no cases of pemphigoid with linagliptin monotherapy in 2235 patients in a three-year post-marketing surveillance study in Japan [Citation62], while a similar three-year post-marketing surveillance study in Japan observed two cases in 3372 patients (0.06%) receiving linagliptin added to other glucose-lowering drugs [Citation63].
In our pooled analysis, we also did not observe any liver safety signal, with hepatic injury reported for ~2% of the linagliptin group and ~3% of the placebo group, and no clinically meaningful changes in liver enzymes or bilirubin. This is an important observation, given the high prevalence of liver disease in both T2D patients generally and the Asian region in particular, and it is consistent with a pooled analysis of patients with hepatic disorders receiving linagliptin [Citation64].
The strengths and limitations of our analysis should be noted. The major limitation is that the safety and tolerability data are derived from clinical trials lasting less than 52 weeks that were primarily designed to evaluate the glycemic efficacy of linagliptin. Hence, the data are derived from descriptive, exploratory analyses, and do not provide insights into long-term safety. Conversely, the main strength is that the underlying trials were robustly controlled with randomization, placebo comparators and double-blinding, which enables confidence that the findings reflect the inherent safety and tolerability profile of linagliptin, albeit there were minor imbalances in some patient characteristics between treatment groups.
5. Conclusions
This comprehensive, up-to-date pooled analysis of clinical trial data demonstrates the safety and tolerability profile of linagliptin in Asian patients, including a large subgroup of East Asians. The results are consistent with older pooled analyses of overall trial populations including several races/ethnicities [Citation7,Citation46] and the current prescribing information for linagliptin [Citation17–19], with no new safety signals identified. These findings complement recent subgroup analyses of the CARMELINA and CAROLINA outcomes trials demonstrating the cardiorenal safety profile of linagliptin in Asian patients [Citation10,Citation11], as well as post-marketing surveillance studies in Japan [Citation55,Citation62,Citation63]. Taken together, linagliptin appears to be well tolerated in Asian patients with T2D, including East Asians.
Declaration of interests
K. Kanasaki has received lecture fees from Boehringer Ingelheim, Eli Lilly and Sanofi, and is under contract for consultancy with Boehringer Ingelheim; Mitsubishi Tanabe Pharma, Boehringer Ingelheim, and Ono Pharmaceutical contributed to establishing the Division of Anticipatory Molecular Food Science and Technology, Medical Research Institute, Kanazawa Medical University. F. Yamamoto is an employee of Nippon Boehringer Ingelheim Co. Ltd. C. Schepers and R. Sani Simões are employees of Boehringer Ingelheim. D. Yabe has received consulting/lecture fees from Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Company Limited, and grants from Arkray Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd., Taisho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Company Limited, and Terumo Corporation during the conduct of the study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Author contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
Role of the sponsor
The Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Data availability
The sponsor of the clinical trials (Boehringer Ingelheim) is committed to responsible sharing of clinical study reports, related clinical documents, and patient-level clinical study data. Researchers are invited to submit inquiries via the following website (https://trials.boehringer-ingelheim.com/).
Linagliptin_pooled_safety_in_Asians_manuscript_Revision_28Sep2021_supplemental.docx
Download MS Word (57.3 KB)Acknowledgments
The authors thank the physicians and patients who participated in the clinical trials analyzed in this manuscript. The authors also thank the following individuals for their assistance and advice: Tomoo Okamura (Nippon Boehringer Ingelheim Co, Ltd, Tokyo, Japan), Atsushi Taniguchi, MSc (Nippon Boehringer Ingelheim Co, Ltd, Tokyo, Japan), Søren S. Lund (Boehringer Ingelheim International GmbH, Ingelheim, Germany), and Thomas Meinicke (Boehringer Ingelheim International GmbH, Ingelheim, Germany).
Supplementary materials
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.1999409.
Additional information
Funding
References
- International Diabetes Federation. IDF Diabetes Atlas. Belgium (BRU): International Diabetes Federation; 2019. Available from: https://www.diabetesatlas.org/en/resources/
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020 Feb;43(2):487–493.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018 Dec;41(12):2669–2701.
- Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020 Jul;11(4):1020–1076.
- Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019 Sep;35(6):e3158.
- Chawla R, Madhu SV, Makkar BM, et al. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab. 2020 Jan-Feb;24(1):1–122.
- Lehrke M, Marx N, Patel S, et al. Safety and tolerability of linagliptin in patients with type 2 diabetes: a comprehensive pooled analysis of 22 placebo-controlled studies. Clin Ther. 2014 Aug 1;36(8):1130–1146.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019 Jan 1;321(1):69–79.
- Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019 Sep 19;322(12):1155–1166.
- Inagaki N, Yang W, Watada H, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA((R)) trial. Diabetol Int. 2020 Apr;11(2):129–141.
- Kadowaki T, Wang G, Rosenstock J, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA(R) trial. Diabetol Int. 2021 Jan;12(1):87–100.
- Gujral UP, Pradeepa R, Weber MB, et al. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci. 2013 Apr;1281:51–63.
- Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013 Apr;1281:64–91.
- Yabe D, Seino Y, Fukushima M, et al. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015 Jun;15(6):602.
- Yabe D, Seino Y. Type 2 diabetes via beta-cell dysfunction in east Asian people. Lancet Diabetes Endocrinol. 2016 Jan;4(1):2–3.
- Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Investig. 2015 Sep;6(5): 495–507.
- Ito Y, Ambe K, Kobayashi M, et al. Ethnic difference in the pharmacodynamics-efficacy relationship of dipeptidyl peptidase-4 inhibitors between Japanese and non-Japanese patients: a systematic review. Clin Pharmacol Ther. 2017 Oct;102(4):701–708.
- Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013 Apr;56(4):696–708.
- Kim YG, Hahn S, Oh TJ, et al. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. 2014 Oct;16(10):900–909.
- Gan S, Dawed AY, Donnelly LA, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in white and Asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(8):1948–1957.
- Spracklen CN, Horikoshi M, Kim YJ, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020 Jun;582(7811):240–245.
- Gomis R, Espadero RM, Jones R, et al. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011 Jul;13(7):653–661.
- Del Prato S, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of beta-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011 Mar;13(3):258–267.
- Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011 Jan;13(1):65–74.
- Owens DR, Swallow R, Dugi KA, et al. Efficacy and safety of linagliptin in persons with type 2 diabetes inadequately controlled by a combination of metformin and sulphonylurea: a 24-week randomized study. Diabet Med. 2011 Nov;28(11):1352–1361.
- Kawamori R, Inagaki N, Araki E, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012 Apr;14(4):348–357.
- Lewin AJ, Arvay L, Liu D, et al. Efficacy and tolerability of linagliptin added to a sulfonylurea regimen in patients with inadequately controlled type 2 diabetes mellitus: an 18-week, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2012 Sep;34(9):1909–19 e15.
- Haak T, Meinicke T, Jones R, et al. Initial combination of linagliptin and metformin improves glycaemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2012 Jun;14(6):565–574.
- Barnett AH, Patel S, Harper R, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes Obes Metab. 2012 Dec;14(12):1145–1154.
- Ross SA, Rafeiro E, Meinicke T, et al. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin. 2012 Sep;28(9):1465–1474.
- Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a >/=52-week randomized, double-blind study. Diabetes Care. 2013 Dec;36(12):3875–3881.
- McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013 Feb;36(2):237–244.
- Bajaj M, Gilman R, Patel S, et al. Linagliptin improved glycaemic control without weight gain or hypoglycaemia in patients with type 2 diabetes inadequately controlled by a combination of metformin and pioglitazone: a 24-week randomized, double-blind study. Diabet Med. 2014 Dec;31(12):1505–1514.
- Lewin A, DeFronzo RA, Patel S, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015 Mar;38(3):394–402.
- Laakso M, Rosenstock J, Groop PH, et al. Treatment with the dipeptidyl peptidase-4 inhibitor linagliptin or placebo followed by glimepiride in patients with type 2 diabetes with moderate to severe renal impairment: a 52-week, randomized, double-blind clinical trial. Diabetes Care. 2015 Feb;38(2):e15–7.
- Chen Y, Ning G, Wang C, et al. Efficacy and safety of linagliptin monotherapy in Asian patients with inadequately controlled type 2 diabetes mellitus: a multinational, 24-week, randomized, clinical trial. J Diabetes Investig. 2015 Nov;6(6):692–698.
- Wang W, Yang J, Yang G, et al. Efficacy and safety of linagliptin in Asian patients with type 2 diabetes mellitus inadequately controlled by metformin: a multinational 24-week, randomized clinical trial. J Diabetes. 2016 Mar;8(2):229–237.
- Mu Y, Pan C, Fan B, et al. Efficacy and safety of linagliptin/metformin single-pill combination as initial therapy in drug-naive Asian patients with type 2 diabetes. Diabetes Res Clin Pract. 2017 Feb;124:48–56.
- Groop PH, Cooper ME, Perkovic V, et al. Linagliptin and its effects on hyperglycaemia and albuminuria in patients with type 2 diabetes and renal dysfunction: the randomized MARLINA-T2D trial. Diabetes Obes Metab. 2017 Nov;19(11):1610–1619.
- Araki E, Unno Y, Tanaka Y, et al. Long-term efficacy and safety of linagliptin in a Japanese population with type 2 diabetes aged >/= 60 years treated with basal insulin: a randomised trial. Adv Ther. 2019 Oct;36(10):2697–2711.
- Kaku K, Haneda M, Tanaka Y, et al. Linagliptin as add-on to empagliflozin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a two-part, randomized, placebo-controlled trial. Diabetes Obes Metab. 2019 Jan;21(1):136–145.
- Yang W, Xu X, Lei T, et al. Efficacy and safety of linagliptin as add-on therapy to insulin in Chinese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2021 Feb;23(2):642–647.
- Kadowaki T, Sarai N, Hirakawa T, et al. Persistence of oral antidiabetic treatment for type 2 diabetes characterized by drug class, patient characteristics and severity of renal impairment: a Japanese database analysis. Diabetes Obes Metab. 2018 Dec;20(12):2830–2839.
- Kameda T, Kumamaru H, Nishimura S, et al. Use of oral antidiabetic drugs in Japanese working-age patients with type 2 diabetes mellitus: dosing pattern for metformin initiators. Curr Med Res Opin. 2020 May;36(5):749–756.
- Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019 Mar 1;9(3):e025806.
- Schernthaner G, Barnett AH, Emser A, et al. Safety and tolerability of linagliptin: a pooled analysis of data from randomized controlled trials in 3572 patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012 May;14(5):470–478.
- Gomes GKA, de Camargos Ramos AI, de Sousa CT, et al. Linagliptin safety profile: a systematic review. Prim Care Diabetes. 2018 Dec;12(6):477–490.
- Del Prato S, Patel S, Crowe S, et al. Efficacy and safety of linagliptin according to patient baseline characteristics: a pooled analysis of three phase 3 trials. Nutr Metab Cardiovasc Dis. 2016 Oct;26(10):886–892.
- Ning G, Bandgar T, Hehnke U, et al. Efficacy and safety of linagliptin in 2681 Asian patients stratified by age, obesity, and renal function: a pooled analysis of randomized clinical trials. Adv Ther. 2017 Sep;34(9):2150–2162.
- de Heer J, Holst JJ. Sulfonylurea compounds uncouple the glucose dependence of the insulinotropic effect of glucagon-like peptide 1. Diabetes. 2007 Feb;56(2):438–443.
- American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021 Jan;44(Suppl 1):S125–S150.
- Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77(8):1923–1932.
- Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016 Feb;12(2):73–81.
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology [Review]. JAMA. 2009 May 27;301(20):2129–2140.
- Yamamoto F, Ikeda R, Ochiai K, et al. Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes and renal dysfunction: a post-marketing surveillance study. Diabetes Ther. 2020 Feb;11(2):523–533.
- DeVries JH, Rosenstock J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017 Feb;40(2):161–163.
- Yabe D, Kuwata H, Kaneko M, et al. Use of the Japanese health insurance claims database to assess the risk of acute pancreatitis in patients with diabetes: comparison of DPP-4 inhibitors with other oral antidiabetic drugs. Diabetes Obes Metab. 2015 Apr;17(4):430–434.
- Kim YG, Kim S, Han SJ, et al. Dipeptidyl peptidase-4 inhibitors and the risk of pancreatitis in patients with type 2 diabetes mellitus: a population-based cohort study. J Diabetes Res. 2018;2018:5246976.
- Chou HC, Chen WW, Hsiao FY. Acute pancreatitis in patients with type 2 diabetes mellitus treated with dipeptidyl peptidase-4 inhibitors: a population-based nested case-control study. Drug Saf. 2014 Jul;37(7):521–528.
- Kawakita E, Koya D, Kanasaki K. CD26/DPP-4: type 2 diabetes drug target with potential influence on cancer biology. Cancers (Basel). 2021 May 2;13(9). doi:https://doi.org/10.3390/cancers13092191.
- Dicembrini I, Montereggi C, Nreu B, et al. Pancreatitis and pancreatic cancer in patients treated with dipeptidyl peptidase-4 inhibitors: an extensive and updated meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2020 Jan;159:107981.
- Yamamoto F, Unno Y, Okamura T, et al. Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes mellitus: a 3-year post-marketing surveillance study. Diabetes Ther. 2020 Jan;11(1):107–117.
- Ito T, Naito Y, Shimmoto N, et al. Long-term safety and effectiveness of linagliptin as add-on therapy in Japanese patients with type 2 diabetes: final results of a 3-year post-marketing surveillance. Expert Opin Drug Saf. 2021 Mar;20(3):363–372.
- Inagaki N, Sheu WH, Owens DR, et al. Efficacy and safety of linagliptin in type 2 diabetes patients with self-reported hepatic disorders: a retrospective pooled analysis of 17 randomized, double-blind, placebo-controlled clinical trials. J Diabetes Complications. 2016 Nov - Dec;30(8):1622–1630.
