ABSTRACT
Introduction
Vortioxetine is a multimodal-acting antidepressant that provides improvements on cognitive function aside from antidepressants and anxiolytic effects. Vortioxetine has been found to be one of the most effective and best tolerated options for major depressive disorder (MDD) in head-to-head trials.
Areas covered
The present review intends to gather the most relevant and pragmatic data of vortioxetine in MDD, specially focusing on new studies that emerged between 2015 and 2020.
Expert opinion
Vortioxetine is the first antidepressant that has shown improvements both in depression and cognitive symptoms, due to the unique multimodal mechanism of action that combine the 5-HT reuptake inhibition with modulations of other key pre- and post-synaptic 5-HT receptors (agonism of 5-HT1A receptor, partial agonism of 5-HT1B receptor, and antagonism of 5-HT3, 5-HT1D and 5-HT7 receptors). This new mechanism of action can explain the dose-dependent effect and can be responsible for its effects on cognitive functioning and improved tolerability profile. Potential analgesic and anti-inflammatory properties observed in preclinical studies as well as interesting efficacy and tolerability results of clinical studies with specific target groups render it a promising therapeutic option for patients with MDD and concomitant conditions (as menopause symptoms, pain, inflammation, apathy, sleep and/or metabolic abnormalities).
1. Introduction
Major Depressive Disorder (MDD) is one of the most common psychiatric disorders worldwide [Citation1]. It encompasses a variety of changes in emotional, cognitive, vegetative and behavioral dimensions, including depressed mood, diminished interests and/or pleasure, concentration and/or memory problems, indecisiveness, guilty and hopelessness feelings, suicidal thoughts, anxiety, pain or psychosomatic complaints, fatigue, psychomotor inhibition and changes in appetite and sleep patterns, with a significant psychosocial impact [Citation2]. Impaired cognitive function ─one of the most common residual symptoms in MDD [Citation3], has been repeatedly related to negative outcomes [Citation4,Citation5]. Epidemiological studies suggest that less than a half of MDD patients will recover without suffering new episodes and at least 15% of them will have a chronic unremitting course [Citation6,Citation7]. Therefore, it is not surprising that MDD has become one of the leading contributors to disability worldwide and a non-negligible cause of premature mortality [Citation8].
Although other neurobiological systems are being studied as potential new therapeutic targets, nowadays, monoamine-based antidepressants represent the main therapeutic tool for MDD. These include the ‘classical’ tricyclic antidepressants and monoamine oxidase inhibitors (MAOI), which are assumed to have superior efficacy at least in severe, hospitalized and/or treatment-resistant patients [Citation9,Citation10] but are also associated with more tolerability and toxicity concerns. These problems are related to non-desired antagonistic effects on histaminergic, muscarinic and α-adrenergic receptors, and also to the increased tyramine sensitivity in the case of MAOI [Citation11,Citation12]. Thus, selective reuptake inhibitors (SSRIs), mainly targeting the serotonin (5-hydroxytryptamine; 5-HT) transporter (SERT), have become the most prescribed first-line antidepressants besides serotonin/norepinephrine reuptake inhibitors (SNRIs), which block both 5-HT and norepinephrine (NE) transporters [Citation13]. Others act by inhibiting NE or both NE and dopamine transporters (such as reboxetine and bupropion, respectively). By contrast, other atypical antidepressants include antagonizing actions on 5-HT postsynaptic receptors with very weak or no inhibiting effects on 5-HT transporter, such as trazodone -which blocks 5-HT2 and partially 5-HT1A receptors-, mirtazapine and mianserine—which block 5-HT2, 5-HT3 and pre-synaptic α2-adrenergic receptors- or even agomelatine—which is a melatoninergic agonist and a selective 5-HT2C antagonist-. Besides their effects enhancing monoaminergic synaptic transmission, all these antidepressants induce changes in intracellular signaling pathways, key gene expression and neural plasticity presumably related to their efficacy [Citation14]. Unfortunately, their mechanisms of action are not always so selective and they cause undesirable adverse-effects (AE). SSRIs and SNRIs, to varying degrees, are associated with sexual dysfunction, nausea, insomnia, sweating, somnolence, fatigue and weight-gain, emotional blunting and, in case of abrupt withdrawal, can produce dizziness, nausea, anxiety or irritability [Citation15]. Noradrenergic antidepressants are better tolerated in terms of sexual functioning; however, they can be associated with insomnia, anxiety, agitation, tachycardia or hypertension [Citation16]. Mirtazapine and trazodone, commonly used in combination with other antidepressants for their sleep-promoting and anxiolytic effects, can cause sedation, dizziness, weight-gain or even metabolic abnormalities [Citation17–19]. Therefore, there is a need for new treatments with more selective mechanisms of action, better efficacy, safety and tolerability profiles.
Vortioxetine is already approved for the treatment of MDD in 83 countries, including US, Europe, Canada, Chile, China, Japan, Mexico, Argentina, South Korea, Turkey, Australia, Hon Kong, Singapore, and South Africa. This differs from other antidepressants on their particular multimodal profile, combining the 5-HT reuptake inhibition with modulations of other key pre- and post-synaptic 5-HT receptors [Citation20]. These additional targets differentiate vortioxetine from other SSRIs and SNRIs and can be responsible for its further effects on cognitive functioning and improved tolerability profile [Citation20,Citation21].
Vortioxetine has been thoroughly investigated and more data are available each year supporting its efficacy and safety in MDD. In a meta-analysis comparing the 21 newest antidepressants for the acute treatment of MDD, vortioxetine was found to be one of the most effective and best tolerated option in head-to-head clinical trials (CT) [Citation22]. Other indirect comparisons have underlined its good balance of efficacy and tolerability [Citation21]. Current review intends to gather all relevant studies of vortioxetine in MDD, specially focusing on those emerged between 2015 and 2020.
2. Pharmacological profile and preclinical studies
Vortioxetine is orally administered once daily at 5–20 mg doses (either as tablets or drops) and is slowly absorbed with an absolute bioavailability of 75%. It has a linear and dose proportional pharmacokinetics, with a mean terminal half-life of approximately 66 h, and steady-state plasma concentration generally achieved within 2 weeks of dosing. No dose adjustments are needed for age, gender, any grade of renal or hepatic dysfunction [Citation23–25]. Food does not appear to affect vortioxetine’s absorption [Citation23,Citation24,Citation26]. Although alcohol intake is not advised during antidepressant treatment, coadministration with vortioxetine was well-tolerated with no relevant pharmacokinetic or pharmacodynamic interactions [Citation26].
Vortioxetine is extensively metabolized through oxidation via cytochrome P450 isozymes (CYP), mainly CYP2D6 but also CYP3A4/5, CYP2A6, CYP2C9, and CYP2C19. CYP2D6 metabolism converts vortioxetine to its principal inactive metabolite (Lu AA34443), which remains in plasma over 72 h [Citation23–25]. It is extensively metabolized by the liver (99%), so very small amounts of drug are excreted in urine (<1%) [Citation23–25].
Vortioxetine is the first so-called multimodal 5-HT agonist and antagonist AD, with different functional activities at different 5-HT neuronal targets including blockade of SERT, agonism of 5-HT1A receptor, partial agonism of 5-HT1B receptor, and antagonism of 5-HT3, 5-HT1D and 5-HT7 receptors [Citation27]. The potency rank order measured in rat brains was 5-HT3> SERT>5-HT1B>5-HT1A≈5-HT7. Therapeutic doses (5–20 mg/day) in humans correspond to 50 to >80% SERT occupancy. Considering 5-HT1A and 5-HT7 receptor affinities in humans are >10-fold higher than in rats, 5-HT3 receptors and SERT would be occupied at lower doses while also 5-HT1B, 5-HT1A and 5-HT7 receptors at higher doses [Citation27].
Preclinical studies suggest that vortioxetine may exert its antidepressant activity by modulating neurotransmission in multiple systems, including 5-HT, NE, dopamine, acetylcholine, histamine, glutamate, and gamma-aminobutyric acid (GABA) systems [Citation27]. Vortioxetine produced antidepressant-like responses and stimulated hippocampal neurogenesis in an SERT Met172 mouse model, in which 5-HT reuptake inhibitory potency for antidepressants is seriously impaired [Citation28]. It demonstrated that vortioxetine’s effects on other targets beyond SERT inhibition are crucial to explain its efficacy. This was also underscored by studies using the progesterone withdrawal [Citation29] and the tryptophan depletion [Citation30] models of depression in which regular 5-HT antidepressants have shown to be ineffective. Vortioxetine, but not an SSRI or an SNRI showed antidepressant-like effects in both models [Citation29,Citation30]. Moreover, even without having a recognizable affinity for glutamate receptors, vortioxetine reversed biochemical changes produced by tryptophan depletion, including several markers of glutamatergic activity [Citation30]. Vortioxetine’s antagonistic effects on 5-HT3 receptors in GABAergic interneurons can attenuate the inhibitory control over prefrontal pyramidal cells and may underlie these findings [Citation31].
Glutamatergic signaling and neuroplasticity attain significant roles in depression pathophysiology and antidepressant response [Citation32]. Postmortem studies revealed smaller size and density of neurons and loss of glial cells and dendrites in frontal cortex and hippocampus of MDD patients [Citation33], being an altered expression of synaptic-function-related genes a possible explanation [Citation34]. An acute administration of vortioxetine (but not fluoxetine or ketamine) transiently increased the expression of several genes involved in neuroplasticity and in serotoninergic and glutamatergic neurotransmission in the rat frontal cortex [Citation32]. In another study, vortioxetine, contrary to fluoxetine, induced positive changes in spine number and density and dendritic morphology in the rat hippocampus [Citation35].
A thorough review suggested that the blockade of 5-HT3 receptor is one of the crucial vortioxetine’s properties for the cognitive improvements observed in preclinical studies [Citation36]. This would increase glutamatergic and serotoninergic transmission as well as pyramidal neurons firing in the forebrain, which seems crucial in facilitating cognitive processing. The agonism of 5-HT1A receptors, the partial agonism of 5-HT1B receptors and the antagonism of 5-HT7 receptors by vortioxetine can also contribute to these effects. Moreover, downstream modulation of several neurotransmitters such as dopamine, NE, acetylcholine or histamine in the medial prefrontal cortex, as well as the enhancement in neurogenesis and neurotrophic processes in the hippocampus, can be additional mechanisms contributing to the vortioxetine’s procognitive profile. Vortioxetine has also shown to cause spectral changes in quantitative electroencephalography (qEEG) which are distinct from those of an SSRI and an SNRI. Specifically, vortioxetine, unlike escitalopram and duloxetine, has been shown to cause differential increases in the low and high cortical frequency ranges which can have a role in cognitive processing enhancements [Citation37].
Due to its potent 5-HT3 antagonism, vortioxetine has also been tested recently in animal models of neuropathic pain. Basic research has shown that serotonin-induced hyperalgesia involved in chronic pain is strongly mediated by 5-HT3 receptors [Citation38]. Vortioxetine, as opposed to fluoxetine, caused a robust analgesic effect, similar to that produced by an SNRI, so it may be effective in patients with neuropathic pain, particularly with comorbid depression [Citation39].
Vortioxetine has also shown potential immunomodulatory properties, demonstrating an antioxidant activity and anti-inflammatory effects that might result in additional clinical benefits [Citation40]. In a previously mentioned study [Citation30] vortioxetine outperformed paroxetine on normalizing tryptophan-induced rise of serum corticosterone and IL-6, a cytokine frequently elevated in MDD [Citation41]. Another study indicated that vortioxetine could have superior anti-inflammatory properties than other antidepressants. In another study, contrary to amitriptyline, escitalopram or tranylcypromine, vortioxetine increased brain levels of the anti-inflammatory cytokine IL-4 and decreased the hippocampal expression of the transcription factor NF-kB p65 that induces pro-inflammatory and oxidative effects [Citation42]. Vortioxetine could have superior anti-inflammatory properties than other antidepressants and its use in patients displaying inflammatory abnormalities are currently on-going [Citation43,Citation44].
In summary, its differential action on several 5-HT targets and the ability to modulate a wide range of neurotransmitters confers vortioxetine not only antidepressant but also pro-cognitive effects, as well as promising further analgesic and anti-inflammatory properties [Citation39,Citation40,Citation45].
3. Therapeutic efficacy
The efficacy of vortioxetine in MDD has been established in many short (6–8 weeks) and long-term (up to 52 weeks) studies, with broad beneficial effects on emotional, anxious, physical, and cognitive symptoms. Specifically, effects of vortioxetine have been evaluated against placebo and active comparators such as venlafaxine, duloxetine, agomelatine, escitalopram or paroxetine.
3.1. General antidepressant effects
A meta-analysis <of 11 short-term placebo-controlled studies evaluating the efficacy of vortioxetine (5–20 mg/day), supported the benefits of this drug in adults with MDD [Citation46]. Contrary to other commonly used antidepressants with which dose–response relationship is under debate [Citation47], increasing doses of vortioxetine were associated with increasing efficacy [Citation46].
Citrome published a study performing indirect comparisons of effect sizes reported in placebo-controlled CT of different antidepressants [Citation21]. As can be seen in , all antidepressants showed similar efficacy but vortioxetine achieved the best balance between efficacy and tolerability. Specifically, vortioxetine was 5.1 times more likely to be associated with response than with discontinuation [Citation21]. A posterior meta-analysis including up to 522 placebo-controlled and head-to-head studies of antidepressants in acute MDD, confirmed the same observations (see adapted from [Citation22]).
Figure 1. Dimensional graph about efficacy and acceptability in head-to-head studies.
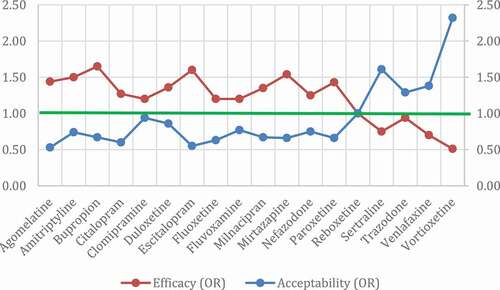
Table 1. Likelihood to be helped or harmed, and response vs. discontinuation because of an AE
3.2. Cognition
Treatments targeting a broader constellation of symptoms in MDD, including emotional but also cognitive symptoms are particularly needed [Citation48]. Cognitive symptoms in MDD include deficits in different domains: i.e. attention, executive functions, memory, and processing speed. These can be present among patients with recurrent episodes but also among those with their first depressive episode [Citation49] and frequently persist after remission [Citation3].
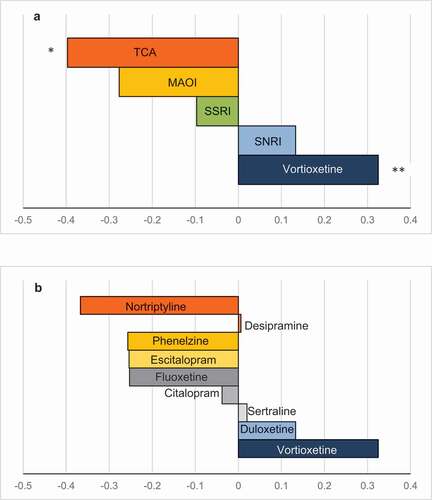
A meta-analysis [Citation50] showed that antidepressants overall had a positive effect on psychomotor speed domain[[and delayed recall, but, after removing vortioxetine from the analysis, statistical significance for the former vanished. In the analyses, vortioxetine displayed the largest effect size on psychomotor speed, executive control and cognitive control domains. In another meta-analysis, vortioxetine obtained the largest improvements in Digit Symbol Substitution Test (DSST), a neurocognitive test that integrates several domains affected in MDD patients. Among all investigated antidepressant classes, multimodal-acting vortioxetine was the only one that provoked significant changes on that task () [Citation51].
Figure 2. Standardized effect size relative to placebo.
Indeed, vortioxetine has shown direct cognitive-enhancing effects in a multi-domain fashion both in older and younger depressed adults with MDD. A post-hoc analysis of data from one of the largest placebo-controlled vortioxetine CT (FOCUS study, n = 602), showed that it resulted in general and multi-domain benefits on cognitive performance, including executive function, attention/speed of processing and memory domains [Citation52]. A meta-analysis revealed that vortioxetine, but not duloxetine, significantly improved cognitive function compared to placebo. Moreover, a post-hoc analysis of data from an CT including arms with vortioxetine and duloxetine [Citation53], showed that, although both outperformed placebo on depressive symptom improvements, only vortioxetine led to robust effects on functional capacity. The more the baseline functional impairment, the more the functional improvement with vortioxetine. Remarkably, changes in cognitive and functional capacity were direct and independent of antidepressant effects [Citation53,Citation54].
Cognitive dysfunction in MDD plays a key role in work-related disability. In the so-called AtWoRC study, vortioxetine yielded substantial long-term improvements in mood, cognitive and function in a large sample of gainfully employed patients with acute MDD [Citation55,Citation56]. Positive changes in subjective cognitive deficits at week 12 strongly correlated changes in work productivity at week 12 and 52 and independently predicted subsequent functioning outcomes. To wit, patients on vortioxetine who perceived cognitive function improvements also improved their workplace productivity [Citation56]. Interestingly, findings from previously mentioned FOCUS study, demonstrated that vortioxetine’s cognitive-enhancing effects in both subjective and objective functioning measures were even more pronounced on working patients, particularly in those whose job places higher demands on executive functioning [Citation57].
During the last few years, the pro-cognitive benefits of vortioxetine has been tested by other randomized CT focused on patients with cognitive residual symptoms and partially or fully remitted MDD [Citation48], as well as on patients with inadequate response to SSRI or SNRI and persisting cognitive complaints [Citation58]. In these cases, numerical differences favoring vortioxetine vs SSRI were seen across different secondary cognitive endpoints but primary outcomes were not met. A comprehensive review delves into it [Citation36].
As previously announced, enhanced glutamatergic transmission in prefrontal cortex through 5-HT3 or 5-HT7 antagonism, or 5-HT1A and 5-HT1B agonism, downstream effects on other neurotransmitter systems or hippocampal neuroplasticity elicited by vortioxetine can underlie positive cognitive effects observed in CT [Citation36]. Subtle spectral changes with vortioxetine have been described by means of qEEG [Citation59]. A functional MRI study demonstrated direct effects of vortioxetine on fronto-limbic networks and executive function, regardless of its antidepressant effects [Citation60]. Soon after 2 weeks, vortioxetine reduced neural activity in regions typically over-active in acute MDD (i.e. dorsolateral prefrontal cortex and hippocampus) in relation to a decrease on subjective cognitive complaints and executive functioning tasks. Overall, it showed direct effects on fronto-limbic networks and cognitive function, regardless of its antidepressant effects.
While age-related cognitive decline affects function and quality in the eldest, depressed mood is one of the most common neuropsychiatric symptoms in Alzheimer’s disease but data about antidepressants’ efficacy in those patients is scarce [Citation61]. Vortioxetine associated to a cognitive training program produced greater improvements on global cognitive performance compared with placebo and cognitive training in a CT with participants aged ≥65 years suffering from age-related cognitive decline without MDD, although differences only reached statistical significance at week 12 [Citation62]. In an open-label study of patients with Alzheimer’s disease and associated depressive symptomatology, vortioxetine showed superior improvements compared to other antidepressants on a variety of cognitive domains and depressive symptoms [Citation63]. Undoubtedly, larger double-blind randomized CT are required to confirm these promising observations.
Post-stroke depression is a common and serious complication [Citation64]. To our knowledge, there was only an exploratory study evaluating efficacy of vortioxetine in patients with ischemic stroke and mild-to-moderate depression [Citation65]. Patients on vortioxetine displayed robust and significantly higher improvements on depressive symptoms, motor deficits, functional assessments and on some cognitive outcome measures. They were compared to patients who refused to take antidepressants so findings have to be interpreted with the greatest caution.
Overall, vortioxetine is the first antidepressant with proved efficacy in improving multiple cognitive domains impaired in MDD and it should be particularly considered for the treatment of MDD with prominent cognitive symptoms [Citation66].
3.3. Other specific symptom domain effects
Although pro-cognitive and general antidepressant effects of vortioxetine in MDD have been already proved, several studies have focused on other specific symptom domains of considerable clinical importance such as anhedonia, physical symptoms, sleep disturbances or suicidal ideation.
3.3.1. Anhedonia
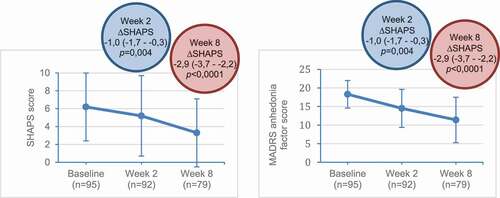
Anhedonia, defined as an impaired capacity to experience or anticipate pleasure, is, together with depressed mood, one of the core symptoms that can lead to the diagnosis of a MDD [Citation2]. It is estimated to be present up to 70–75% of patients with acute MDD and is associated with poor prognosis and suboptimal response [Citation67,Citation68]. An open-label CT of 100 patients with MDD treated with vortioxetine (10–20 mg/day, flexible doses) demonstrated a clear and significant improvement in this particular symptom domain. Improvement in anhedonia after vortioxetine treatment was correlated with functional recovery and quality of life () [Citation67]. In fact, amelioration of anhedonia with vortioxetine had a direct beneficial impact on social functioning, independent from global antidepressant effects.
Figure 3. Change from baseline in SHAPS and MADRS anhedonia factor scores with vortioxetine.
Although subjective measures of anhedonia and objective measures of motivation and reward processing could represent dissociable domains of the same psychopathological phenomenon, a study revealed that cognitive performance at the end of a treatment period with vortioxetine in a cohort of patients with MDD highly correlated with the willingness to expend effort for hard task rewards. Since it is just an analysis of cross-sectional data, inferences should be drawn with caution, but the current results would suggest that pro-cognitive properties of vortioxetine might be linked to putative positive effects on reward and hedonic processing [Citation69].
On the other hand, emotional blunting, a closely related concept that encompass feelings of detachment or reduced emotional responses, has also been described as a common subjective AE in patients treated with SSRIs with a significant impact on their daily functioning and quality of life [Citation70,Citation71]. A preliminary report showed that vortioxetine produced significant reductions of depressive symptoms in a sample of 143 patients with MDD and associated emotional blunting who had failed to respond to an adequate trial of SSRI o SNRI. After 8 weeks of treatment with vortioxetine, the mean change from baseline in OQD total score was −29.8 (p < 0.0001) with significant changes from week 1, and 50% of the patients reported no more blunting of their emotions in answer to the standardized screening question [Citation72].
3.3.2. Physical-symptoms and sleep disturbances
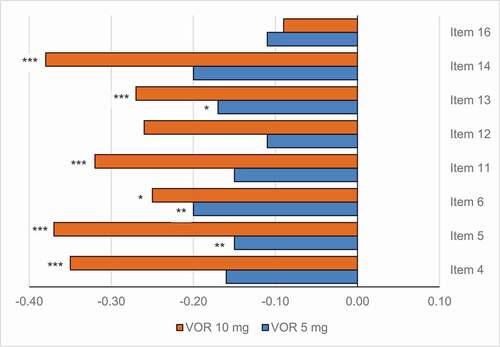
A meta-analysis using data from five short-term placebo-controlled studies was aimed to establish its potential benefits on depression-related physical symptoms, namely fatigue or low energy, somatic anxiety such muscle tension, headaches or other pain complaints, genital symptoms and sleep, appetite, or weight disturbances, among many others [Citation73]. These symptoms are frequently present in the acute episode of MDD but are also common residual chronic symptoms and they are often associated with comorbid anxiety disorders, exerting a negative impact on clinical and prognostic outcomes [Citation74]. Vortioxetine demonstrated a broad symptom relief in patients with MDD, including in those with high levels of anxiety, showing significant improvements in most of the physical symptoms assessed () [Citation73].
Figure 4. Change from baseline in HAM-D single items at week 6/8.
Effects of vortioxetine on sleep disturbances and sleep quality associated with MDD have been studied in more depth in another open-label 8-week clinical trial of 92 depressed patients treated with flexible doses of vortioxetine (10–20 mg/day) [Citation75]. Vortioxetine doubled and tripled the proportion of patients without insomnia and the proportion of good sleepers, respectively, at the end of the follow-up. By contrast, the percentage of patients with moderate-to-severe insomnia decreased after 8 weeks of treatment with vortioxetine. Of note, improvements in sleep quality were correlated with an overall improvement of depressive symptoms and they were predictive of antidepressant response. A retrospective analysis of the vortioxetine’s effects on sleep disturbances in MDD by another observational study found that benefits of vortioxetine on sleep quality and depressive symptoms were maintained after 6 months [Citation76].
In fact, treatment-emergent insomnia and treatment-emergent somnolence after treatment with vortioxetine seems to be rare and rates are much lower than with other antidepressants such as SSRIs or SNRIs [Citation77]. Clinical and preclinical polysomnographic studies have shown that SSRIs can facilitate wakefulness producing acute and long-term REM sleep suppression and reduction in REM onset latency [Citation78]. Both of these effects are believed to be related with the increased synaptic 5-HT levels promoted by reuptake inhibition and their actions on 5-HT1A receptors, but 5-HT3 receptors appear to play an additional crucial role. Two interrelated polysomnographic studies with healthy volunteers and rats, respectively, demonstrated that the antagonistic properties of vortioxetine on 5-HT3 receptors are crucial to explain the good profile of vortioxetine in sleep architecture in comparison with paroxetine [Citation78,Citation79]. Furthermore, vortioxetine’s blocking effects on 5-HT7 receptors can provide additional benefits since antagonist agents of these receptors have demonstrated to counteract SSRI-induced micro-arousals, a probable correlate of the sleep fragmentation induced by SSRI in some patients [Citation80,Citation81].
3.3.3. Suicidal ideation
Epidemiological and longitudinal observational studies with real-world patients and real-world clinical settings clearly support the beneficial effects of antidepressants on suicide ideation and suicide behavior in MDD [Citation82,Citation83]. However, a controversy over presumed suicidal effects of antidepressants persist since questionable interpretations of data from CT in children and adolescents led the U.S. Food and Drug Administration to issue a black box warning about a potential increased risk of suicidal thoughts and suicidal behaviors associated with antidepressants [Citation84,Citation85].
Nevertheless, post-hoc analyses of data from 10 short-term randomized, placebo-controlled studies and 3 additional long-term open-label extension studies of up to 52 weeks duration showed that vortioxetine was not associated with an increased risk of suicidal ideation or suicidal behavior in adults with MDD [Citation86]. It is also worth to mention that suicidal ideation is a common exclusion criterion in placebo-controlled CTs and it precludes the possibility of capture the possible anti-suicidal benefits of vortioxetine or any other antidepressant in such kind of studies, so data from epidemiological naturalistic studies will be greatly welcomed.
3.4. Anxiety and other psychiatric comorbidities
Coexistence of MDD and anxiety is common and more difficult to treat than MDD alone. A meta-analysis of data from up to 2165 depressed patients who presented with significant high levels of anxiety (i.e. Hamilton Anxiety Rating Scale score ≥ 20) in 10 randomized short-term CT of vortioxetine vs placebo, indicated that vortioxetine was also efficacious among this population [Citation87]. A dose-related effect favoring vortioxetine was observed in most sub-analyses, suggesting than increasing doses of vortioxetine can effectively reduce both depression and anxiety severity in patients with MDD and high anxiety levels.
MDD and social anxiety disorder (SAD) are often associated, causing higher disability and poorer prognostic outcomes than MDD alone with an increased risk of recurrence, chronic course and suicide behavior [Citation88]. Only a small double-blind, placebo-controlled CT of vortioxetine at (20 mg/day) with 40 patients with comorbid MDD and SAD has been published so far [Citation89]. Robust and significant reductions on dimensional measures for depression and social anxiety were observed although differences on response and remission rates did not reach the required statistical level.
Given the high prevalence of psychological trauma history in patients with MDD and the risk of suboptimal treatment response among them, effects of vortioxetine were also explored in a meta-analysis centered on adults with MDD who reported childhood or recent trauma [Citation90]. More than 60% of patients in the 5 CT included reported history of either childhood or recent trauma and almost 30% reported both. Vortioxetine still showed clear significant clinical benefits on depressive symptoms, anxiety, and overall functioning in this population. Patients with any trauma randomized to placebo were 2.8 times to relapse than patients that blindly continued vortioxetine.
On the other hand, depression is the most common psychiatric complication of alcohol use disorder and their co-occurrence is accompanied with greater clinical burden and poorer prognostic outcomes than either single disorder [Citation91]. Additionally, as previously mentioned, vortioxetine has proved to be safe and well-tolerated after alcohol consumption [Citation26] and could be even administered without restrictions in patients with any grade of hepatic dysfunction [Citation23,Citation24]. In this regard, preliminary clinical evidence proposes vortioxetine as a useful antidepressant for patients with both MDD and alcohol use disorder as part of an integrated therapeutic-rehabilitation program. In a retrospective analysis of two well-matched clinical samples, vortioxetine demonstrated similar antidepressant effects among depressed patients with and without alcohol use disorder [Citation92]. Depression remission rates after 6 months of treatment were 45.6% and 57.1%, respectively. Interestingly, significant improvements also emerged for both groups on anxiety scores, anhedonia, functioning and quality of life-related measures, cognitive performance and subjective cognitive complaints.
3.5. Older people, menopause, and non-psychiatric comorbidities
MDD among the older people is a public health problem of increasing concern that is more often presented with several psychiatric and nonpsychiatric disorders, along with higher rates of disability [Citation93]. Antidepressant’s efficacy has been repeatedly called into question in older patients [Citation93,Citation94]. A post-hoc analysis of data from 1508 patients aged between 55 and 88 years (mean age 62.4 years) who had participated in 12 placebo-controlled CT with vortioxetine showed that vortioxetine 5–20 mg/day is also efficacious and well-tolerated in such population [Citation95,Citation96].
Due to hormonal fluctuations, menopausal transition is a critical period for the onset of MDD in women. Menopause is often associated with vasomotor symptoms such as hot flashes and night sweats, but it could be also accompanied by fatigue, sleep disturbance, anxiety and depressive symptoms and cognitive complaints [Citation97]. In an open-label study conducted with perimenopausal and early postmenopausal women with MDD, antidepressant response to vortioxetine was remarkable, with statistically significant improvements of depression and anxiety symptoms, climacteric complaints, quality of life and cognitive performance. A more recent observational retrospective 6-months study comparing efficacy of vortioxetine vs paroxetine in postmenopausal women with MDD showed that both antidepressants were effective in significantly reducing depressive symptoms, but while paroxetine achieved greater severity reduction and, surprisingly, better performance on a cognitive screening tool at the end of the study, vortioxetine showed significantly superior efficacy in reducing menopausal symptoms [Citation98]. There were baseline differences that could affect the results, since all patients receiving vortioxetine had needed to switch from other previous antidepressant compared to only 12.5% of patients in paroxetine, which could explain superiority of paroxetine on cognitive performance. Apparently, vortioxetine offer a good profile to treat MDD during menopausal and perimenopausal period but further well-designed studies need to be conducted in this field.
Coincidence of MDD and general medical comorbidities is more the norm than the exception in current clinical practice, not only among older adult patients. It is associated with increased symptom severity, lower treatment adherence, decreased quality of life, poorer outcomes and even higher risk of mortality [Citation99].
Several lines of research suggest a bidirectional relationship between MDD and diabetes. While diabetes is a risk factor for the development and recurrence of depression, MDD clearly increased the risk of developing type 2 diabetes and is associated with poorer glycemic control, lower treatment adherence and worse prognostic outcomes among patients with diabetes [Citation100]. Treating depression can improve treatment adherence and glycemic levels in patients suffering from both conditions but some antidepressants can negatively impact on glucose metabolism [Citation101]. Fifty subjects with type 2 diabetes and moderate-to-severe MDD were randomized to receive vortioxetine (10 mg/day) versus sertraline (75 mg/day) for 8 weeks in a single-blind pilot study. In accordance with common compliance issues in these patients, withdrawal rate was high (mainly due to non-adherence to antidepressants or to follow-up visits) and only data from 21 participants that finished the CT (12 assigned to vortioxetine and 9 to sertraline) was analyzed. Vortioxetine and sertraline lead to similar and consistent remission rates as well as improvements on diabetes-related stress at the end of the study. However, only patients treated with vortioxetine showed significant reductions in body weight, waist circumference, glycosylated hemoglobin and triglycerides while these remain unchanged or even increased in patients treated with sertraline [Citation102]. Perhaps, partial agonism of vortioxetine on 5-HT1B receptors, which can inhibit food intake and satiety and improve glucose tolerance [Citation103,Citation104].
Depressed mood is one of the most common non-motor symptoms in patients with Parkinson’s Disease, with a reported prevalence of 30–40% overall, and is associated with rapid progression of motor and cognitive symptoms, as well as with worse quality of life. A 3-month clinical trial was designed to assess the efficacy of vortioxetine (10–20 mg/day) in 150 patients with Parkinson’s Disease and comorbid MDD. Findings presented in a preliminary report showed that vortioxetine was able to improve depression severity without significant worsening of motor symptoms and with a good safety profile [Citation105]. Idiopathic REM sleep behavior disorder (RBD), a parasomnia characterized by loss of muscular atonia during REM sleep and the emergence of complex motor behaviors, has been described as a robust prodromal marker of Parkinson’s disease and other synucleinopathies. Antidepressants can also trigger RBD, but this can also be associated with an increased risk of this kind of neurodegenerative illnesses [Citation106]. Clonazepam and melatonin are two of the most recommended treatments in RBD disorder, although use of clonazepam in the older people and in patients with cognitive impairment has to be carefully considered due to safety concerns. By contrast, utility of paroxetine has also been suggested although is controversial since it has been useful in some cases, but it can even worsen symptoms in others [Citation107].
Burning mouth syndrome (BMS) is a disabling chronic idiopathic intraoral pain condition that is accompanied with a remarkable psychological distress increasing the risk of anxiety and depressive disorders. Since several neuropathic, central brain and inflammatory abnormalities have been described underlying BMS, the use of antidepressants is gaining momentum with these patients and vortioxetine’s profile make it particularly indicated for both the alleviation of pain and the psychiatric comorbidities. Adamo published a first open-label pilot study showing promising results in a sample of 30 patients with BMS and symptoms of anxiety and depression treated with vortioxetine. Posteriorly, the same group report the results of a larger and long-term (n = 150, 12 months follow-up) open-label randomized study comparing the effectiveness and tolerability of vortioxetine (15 mg/day) with other four commonly prescribed SSRIs and SNRIs (paroxetine sertraline, escitalopram, and duloxetine) [Citation108]. All antidepressants decreased pain, anxiety, and depressive symptomatology at follow-up but patients treated with vortioxetine showed faster improvements and significantly higher rates of response (96.6%) and remission (83.3%) at the end of the study, with very good tolerability.
Therefore, unique attributes of vortioxetine render it a promising therapeutic option for several non-psychiatric disorders that are accompanied by pain, inflammation, apathy, sleep and/or metabolic abnormalities besides prominent depressive and anxiety symptoms. Anyhow, most of these initial encouraging findings have to be confirmed in double-blind controlled studies.
3.6. Inadequate response to first-line antidepressant treatment
According to the evidence gathered, vortioxetine has become an additional first-line option for acute MDD in adults, as it can be found in a recently updated clinical guideline [Citation65]. But efficacy of vortioxetine has also been studied among patients who had failed to respond adequately to an SSRI or SNRI therapy in an 8-week double-blind CT published by Montgomery and colleagues [Citation109]. Two systematic reviews have evaluated the benefits of switching to vortioxetine by combining both direct and indirect comparisons with other antidepressants [Citation110,Citation111]. Vortioxetine as switch therapy showed significant advantages in terms of efficacy, functioning, and quality of life outcomes compared to agomelatine. Indirect analyses demonstrated that vortioxetine can also produce numerically higher remission rates and lower withdrawal rates due to AE compared to other antidepressants such as bupropion, citalopram, sertraline or venlafaxine ().
Figure 5. Risk difference for remission and for withdrawal due to AEs.
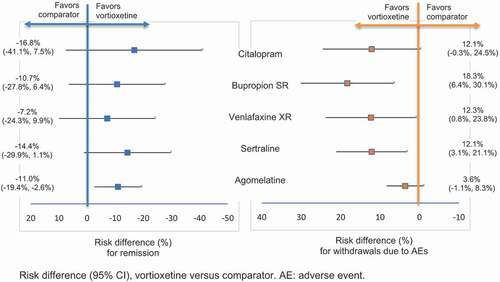
In a brief ecological study, Tegin found a positive correlation between minimal effective vortioxetine’s doses observed in CT and the defined daily doses (DDD) of antidepressants in the countries where these trials were performed [Citation112]. DDD can be assumed as a rough estimate of antidepressant exposure in the source populations but it may not reflect the real antidepressant use among studies participants. Therefore, findings should be interpreted with caution but, from a clinical practical perspective, it would imply that higher doses of vortioxetine are recommended in patients with previous antidepressant exposure.
Nevertheless, switching antidepressant is one of the most widely used strategy in patients who experience inadequate response or intolerable effects to SSRI or SNRI therapy [Citation113,Citation114] and converging evidence suggests vortioxetine as an appropriate second-line option in MDD.
3.7. Functioning outcomes and cost-utility
Several studies have investigated functional impairment in patients with MDD, which can negatively affect many aspects of daily life, including school, work, interpersonal relationships, and overall social functioning. A meta-analysis of the data from 9 short-term studies to determine the effect of vortioxetine on overall patient functioning using Sheehan Disability Scale (SDS), found that vortioxetine 5–20 mg for 6–8 weeks improved overall patient functioning, and vortioxetine 10 and 20 mg demonstrated significant improvement in SDS total score, and functional remission compared to placebo [Citation115]. In another study, evaluating the effect of vortioxetine on overall functionality using the University of California, San Diego Performance-Based Skills Assessment (UPSA, performance measures of functional capacity), found that vortioxetine demonstrated a significantly greater improvement compared with placebo, supporting the clinical effect of vortioxetine in functional capacity [Citation116].
Given that functional recovery together with symptomatic remission are the therapeutic objectives of antidepressant therapy, Cronquist and colleagues evaluated the combined effect of vortioxetine on depressive symptoms and functional capacity in 602 adult patients with MDD from a placebo-controlled, duloxetine-referenced study, using the Montgomery Åsberg Depression Rating Scale (MADRS) and UPSA score. They found that treatment with vortioxetine retrieved more patients classified as dual responders and therefore vortioxetine demonstrated a robust combined effect on depressive symptoms and functional capacity in patients with MDD [Citation117].
The disease burden of MDD is significant in some countries, and therefore a cost-utility analysis of treatments is needed to choose the optimal treatment. Soini assessed the cost-utility analyses of vortioxetine versus relevant comparators (agomelatine, bupropion SR, citalopram, duloxetine, escitalopram, sertraline, and venlafaxine XR) in MDD patients with inadequate response to previous antidepressants and found that vortioxetine was less costly and more effective versus all comparators. Therefore, vortioxetine was considered a cost-effective treatment option and the greater effectiveness associated with vortioxetine is an important driver for the cost savings and quality of life gains accrued with vortioxetine [Citation118,Citation119].
4. Safety/Tolerability
Side effects of vortioxetine are different from other commonly used antidepressants, according to its low incidence of sleep disturbances, sexual dysfunction, weight gain, cardiovascular alterations or discontinuation symptoms. As it has been discussed before, sleep disruptions (namely insomnia, initial insomnia, middle insomnia, hyposomnia, sleep disorder, dyssomnia, poor quality sleep or terminal insomnia) were uncommon in published CT among patients receiving vortioxetine [Citation77], and beneficial effects on sleep quality and continuity has been observed with vortioxetine in contrast to other conventional antidepressants [Citation75].
Treatment-emergent sexual dysfunction (TESD) is commonly associated with antidepressants such as SSRIs and SNRIs and a common reason for treatment withdrawal among patients with MDD [Citation120]. A pooled analysis of 7 short-term CT compared the general incidence of TESD between vortioxetine, placebo and/or duloxetine in patients without sexual dysfunction at baseline. The results found that vortioxetine has TESD rates not significantly different from placebo, while duloxetine presented a significantly higher TESD risk versus both placebo and vortioxetine () [Citation121].
Table 2. Spontaneously reported AEs related to sexual dysfunction
In order to avoid confounding effects of MDD or other medical comorbidities, independent effects of vortioxetine on sexual functioning were evaluated in a double-blind trial with a large sample of healthy volunteers receiving either vortioxetine 10 mg/day or 20 mg/day, paroxetine 20 mg/day or placebo. No significant differences of TESD were observed between placebo and vortioxetine at any dose although a trend toward dose-relationship was observed. Participants on paroxetine showed significantly more TESD than participants on placebo and a higher proportion of them experienced a clinically meaningful decline in sexual functioning (44.6%) compared to those treated with vortioxetine 10 mg/day (23.5%, p = 0.004) and vortioxetine 20 mg/day (38%, but only with a marginal significance p = 0.068) [Citation122].
Switching to another efficacious and better tolerated antidepressant is one of the most suitable strategies for patients with TESD who need to continue antidepressant treatment but there are very few controlled studies that have addressed this question [Citation123]. A double-blind randomized 8-week CT published by Jacobsen compared the benefits of switching from an SSRI (citalopram, paroxetine or sertraline) to flexible doses (10–20 mg) of either vortioxetine or escitalopram in a sample of 447 patients with MDD in remission who suffered from TESD [Citation121,Citation124]. Patients treated with vortioxetine achieved significantly greater improvements in sexual functioning compared to those treated with escitalopram (the SSRI with the safest profile in terms of sexual AE [Citation120]). Benefits were evident in all explored phases (i.e. desire, arousal and orgasm) and antidepressant efficacy was also maintained.
It is well known that increased levels of 5-HT after SERT blockade reduce sexual functioning, but not all 5-HT receptors play the same role in modulating sexual behavior [Citation125]. Preclinical studies showed that vortioxetine’s agonistic effects on 5-HT1A receptors could counteract the sexual AE induced by SERT inhibition and explain its reduced sexual side effects [Citation126].
Safety and tolerability of higher dosages of vortioxetine was evaluated in a 52-week open-label study of patients with MDD. Long-term treatment with vortioxetine at dosages of 15 or 20 mg once daily was safe and well tolerated, being nausea and headache the most common treatment emergent AE (TEAE) (≥10%) [Citation127].
Several meta-analyses have evaluated general safety and tolerability profile of vortioxetine. Two of them focused on placebo-controlled CT using fixed doses of 10 mg/day or 20 mg/day of vortioxetine, respectively [Citation128,Citation129]. Significant differences compared to placebo were only evident for nausea and constipation in meta-analysis assessing safety of 10 mg/day, and for nausea and dry mouth in that assessing 20 mg/day. Another more comprehensive review included data from 11 short-term (6–8 weeks) and 5 long-term (up to 52 weeks) controlled-studies of vortioxetine 5–20 mg/day compared to both placebo and active-comparator arms, namely venlafaxine 225 mg/day and duloxetine 60 mg/day [Citation130]. As can be seen in , it showed that withdrawal rates due to TEAE for vortioxetine at different doses (4.5–7.1%) were slightly higher compared to placebo (3.6%) but lower compared to venlafaxine (14.2%) or duloxetine (8.8%) in short-term studies. Patients treated with vortioxetine showed no clinically relevant changes on body weight, heart rate, blood pressure and on clinical laboratory (including lipid and cholesterol) or electrocardiogram parameters, including QTcF interval (which can be prolonged by several antidepressants and psychotropics). In long-term treatment studies, no new types of TEAE emerged, and the mean weight gain was 0.7–0.8 kg. According to other previously referred reviews [Citation21,Citation22] vortioxetine showed a remarkable tolerability and safety profile compared to other antidepressants in the treatment of MDD. Meta-analyses that have used indirect treatment comparisons among antidepressants based on CT of switching therapy after a failed first-line treatment attempt in MDD, showed that withdrawal rates because of an AE were significantly lower for vortioxetine than for sertraline, venlafaxine or bupropion [Citation111]. In direct comparisons between vortioxetine and agomelatine, only nausea was significantly more associated to vortioxetine (16.2%) than agomelatine (9.1%), but the first still had a statistically significant lower probability of withdraw treatment due to TEAE compared with the latter (5.9% vs 9.5%, respectively) [Citation110].
Table 3. Analysis of treatment-emergent AEs
Although incidence of nausea with vortioxetine seems not to be higher than with other antidepressants (see ), it is the most common TEAE leading to withdrawal in CT and shows a dose–effect relationship [Citation130]. Nauseas are commonly transient, mild to moderate in intensity and typically occurred within the first week of treatment. But approximately 10% of patients on 10–20 mg/day dose reported nausea at the end of 6–8 week CT [Citation23]. It has also been reported that incidence of nausea in patients who had switched therapy to vortioxetine is lower than in treatment-naïve individuals who received vortioxetine for the first time [Citation110].
Antidepressant-induced nausea is thought to be mediated via 5-HT3 receptor activation in either the brain and/or the gut [Citation131], and eventual desensitization of these receptors would dissipate nauseas within few days. Vortioxetine’s potent antagonism of 5-HT3 receptors should provide it with a neutral gastrointestinal profile from the beginning. However, basic research has proven that vortioxetine’s inhibitory activity at 5-HT3 receptors differs from other competitive antagonists, and it might induce a brief initial agonistic response [Citation132]. Accordingly, some case-reports have suggested splitting daily dosage or even combining with a different 5-HT3 antagonist as mirtazapine to prevent the onset of nauseas [Citation133]. On the other hand, 5-HT3 antagonists such as ondansetron or granisetron, commonly used to treat chemotherapy-induced emesis, are actually much better preventing vomiting than nausea and the physiopathological mechanisms underlying nausea appear much harder to describe than those underlying vomiting [Citation134]. Beyond 5-HT3 receptors, there is evidence for an involvement of many other types of 5-HT receptors on emesis, including 5-HT1A, although their role is difficult to understand. In fact, some 5-HT1A agonists appear to have antiemetic properties but others, like buspirone or repinotan, can really cause nausea and vomiting [Citation135]. Interestingly, in post-hoc analyses of the study of Jacobson [Citation124], it was observed that nausea rates in patients previously treated with an SSRI, although transient, were significantly higher in those switching to vortioxetine (ranging 20–29.2%) than to escitalopram (ranging 3.3–6.1%). Desensitization of 5-HT3 receptors in such patients can be presupposed; therefore, it is reasonable to conclude that, besides its particular 5-HT3 binding pattern, other vortioxetine’s mechanisms of action, like 5-HT1A agonism, could be involved in transient treatment induced nausea associated with this multimodal acting AD.
Sudden interruption of treatment with SSRIs or SNRIs can yield the onset of several emotional (worsening mood and anxiety) and/or physical symptoms (flu-like and shock-like complaints, dizziness, sleep disturbances, etc.) which is often known as ‘discontinuation’ or ‘withdrawal’ syndrome [Citation136]. It is often mild and transient but sometimes is more intense and long-lasting and can be misidentified as signs of relapse. They are commonly related to agents with shorter half-lives and high potency, such as venlafaxine and paroxetine [Citation136]. Vortioxetine therapy can be interrupted abruptly since its long terminal half-life (approximately 66 h) makes it particularly safe to avoid the emergence of a discontinuation syndrome.
In summary, the more frequent TEAE of vortioxetine are nausea and vomiting, both usually transitory and dose dependent. Data from CT with vortioxetine showed a low incidence of sleep disruption, sexual dysfunction and discontinuation syndrome, with neither relevant cardiovascular nor weight alterations, and with beneficial effects on sleep quality. Therefore, vortioxetine displays fairly benign safety and tolerability profiles.
5. Interactions
Vortioxetine is primarily metabolized by the CYP2D6 enzyme [Citation24], but there are also other CYP enzymes involved in its metabolism. Therefore, there may be potential drug–drug interactions with drugs that are substrates of the same CYP enzymes [Citation24]. These effects have been evaluated in studies with healthy volunteers.
Co-administration of vortioxetine has no effects on the pharmacokinetics (PK) of warfarin, rifampicin, fluconazole, diazepam, midazolam, caffeine, tolbutamide, ethinyl estradiol, levonorgestrel, aspirin, ethanol, or lithium [Citation24,Citation26,Citation137]. Multiple doses of vortioxetine showed no substantial effects on the PK of caffeine (CYP1A2 substrate), tolbutamide (CYP2C9 substrate) and midazolam (CYP3A4/5 substrate), and minimal or negligible effects on the PK of dextromethorphan (CYP2D6 substrate), bupropion (CYP2B6 substrate) and omeprazole (CYP2C19 substrate), which indicates that vortioxetine was not a relevant inhibitor or inducer of any of these CYP enzymes [Citation24].
A study conducted with healthy subjects tried to determine the incidence of PK and pharmacodynamic alterations of vortioxetine co-administered with ethanol, diazepam, and lithium in three separated substudies. None of the PK parameters of any assayed substances suffered modifications due to the combined administration with vortioxetine. Regarding pharmacodynamics, the co-administration of vortioxetine together with ethanol or diazepam did not entail more deleterious effects on psychomotor or cognitive performance than administration of ethanol or diazepam alone [Citation26].
Another study with healthy subjects also explored the potential existence of interactions between vortioxetine and several inductors, inhibitors, and substrates of different type of P450 cytochrome (CYP). Vortioxetine did not modify the PK parameters of any of the other drugs, such as rifampicin, fluconazole, omeprazole, bupropion and ethinyl estradiol/levogestrel. However, maximum concentrations and AUC of vortioxetine significantly increased after co-administration with bupropion and were decreased after co-administration with rifampicin [Citation137]. It would mean that, if the combination of vortioxetine and bupropion is considered clinically appropriate for a certain patient with MDD, then the dose of vortioxetine should be reduced by half. Conversely, the combination of vortioxetine with rifampicin may require an increase of the vortioxetine dose up to three times the original dose [Citation24].
The increased risk of bleeding and hemorrhagic stroke associated to the use of SSRIs and SNRIs is well known [Citation138], being even higher in the older people and in combination with antithrombotic, platelet antiaggregant or nonsteroidal antiinflammatory drugs (NSAID). A placebo-controlled trial comparing co-administration of aspirin, warfarin and vortioxetine, did not show relevant modifications in the PK parameters, nor in the coagulation times or the international normalized ratio (INR). However, the absence of risks may not be ruled out considering the sample size used in this study, and the concomitant use should be done with caution until more safety studies are available [Citation139].
Antidepressant agents are often prescribed to women with breast cancer; however, as tamoxifen is a standard of care in breast cancer and some antidepressants are substrates or inhibitors of the tamoxifen metabolic pathway, there may be interactions when prescribed simultaneously, so that tamoxifen may become less effective or null. A review of basic and clinical evidence for choosing an antidepressant agent in women receiving tamoxifen, concluded that citalopram, desvenlafaxine, escitalopram, milnacipran and vortioxetine were the antidepressants with fewer AE and better global profile [Citation140].
On the other hand, effect of food intake on exposure to vortioxetine was also evaluated as co-administration of medications and food can affect by reducing, delaying, increasing, accelerating, or having no effect on drug absorption, and the results found no food effect on PK [Citation23].
6. Summary and Conclusions
Intrinsic factors such as age, gender, ethnicity, body size, and hepatic and renal impairment has no clinically meaningful effects on vortioxetine exposure. Food intake or alcohol consumption does not affect exposure to vortioxetine, although it is not recommended during treatment with antidepressants, as it may make some symptoms worse and impair evolution.
Vortioxetine is a multimodal-acting antidepressant specifically designed to inhibit serotonin reuptake and interact with several serotonin receptors (agonism of 5-HT1A receptor, partial agonism of 5-HT1B receptor, and antagonism of 5-HT3, 5-HT1D and 5-HT7 receptors), exerting modulatory effects on multiple neurotransmitter systems (as NE, dopamine, acetylcholine, histamine, glutamate, and GABA systems).
MDD is characterized by multiple debilitating symptoms, spanning emotional, physical, and cognitive domains, with serious consequences for patient’s psychosocial and occupational functioning. Efficacy has been proved for vortioxetine in short-term and long-term treatment of MDD, with broad beneficial effects on emotional, physical, and cognitive symptoms. Therefore, vortioxetine may enhance functional recovery which is the ultimate treatment goal for patients with MDD.
Vortioxetine is an efficacious treatment choice for depressed patients with anxious symptoms.
Vortioxetine has demonstrated its efficacy in MDD patients in several studies, being more effective than placebo and with similar efficacy to that observed in duloxetine and venlafaxine. In a systematic review and meta-analysis vortioxetine was found to be one of the most effective and best tolerated option in head-to-head CT.
Vortioxetine is the first antidepressant drug with proven efficacy in improving cognitive symptoms of depression and is the only antidepressant agent showing evidence of a positive effect on multiple cognitive domains as attention, processing speed, executive function, learning, and memory.
Vortioxetine has shown be useful in symptoms as anhedonic, psychical symptoms, sleep disturbances and suicidal ideation.
Vortioxetine significantly improved cognition, independent of depressive symptoms. The improvements on cognition observed with vortioxetine were not apparent with duloxetine, and vortioxetine had the largest improvements in cognitive function versus other antidepressants (citalopram, escitalopram, nortriptyline, phenelzine, and sertraline). Vortioxetine demonstrated long-term benefits in working patients with MDD.
Vortioxetine showed significant improvements in depressive symptoms associated to improvements in sleep. Considering that sleep impairment is a frequent comorbid symptom in patients affected by MDD, vortioxetine could be considered for treating depressive symptoms and improving sleep quality in patients with MDD and insomnia.
Vortioxetine has demonstrated efficacy in the treatment of MDD associated with menopause, diabetes, Parkinson's, Alzheimer's and acute ischemic stroke.
Nausea was the most commonly associated AE with a dose–effect relationship. However, meta-analyses have shown that withdrawal rates because of an AE were significantly lower for vortioxetine than for sertraline, venlafaxine, or bupropion.
Vortioxetine has shown low rates of sexual dysfunction in MDD CT. Vortioxetine is one of the antidepressants with lowest incidence of TESD, with rates of sexual dysfunction not significantly different from placebo (for doses up to 15 mg), lower rates of TESD than duloxetine and paroxetine, and significantly greater improvements in sexual dysfunction when switching from SSRIs/SNRIs to vortioxetine than to escitalopram (possibly because the agonistic effects on 5-HT1A).
Both in short-term (6–8 weeks) and long-term (up to 52 weeks) studies, vortioxetine had no effect relative to placebo on clinical laboratory parameters, body weight, heart rate or blood pressure, and showed no clinically relevant effect on ECG parameters, including the QTcF interval.
Vortioxetine does not increase lipid or cholesterol, showed an improvement in biochemical parameters, and in type 2 diabetes patients showed a decrease in glycosylated hemoglobin, suggesting amelioration in metabolic control with vortioxetine.
Vortioxetine has a mean terminal half-life of approximately 66 h, which is long enough to avoid the presence of discontinuation symptoms following abrupt discontinuation of treatment with vortioxetine.
Vortioxetine is not an inducer or inhibitor for several CYP substrates, which means that it has a low potential for drug–drug interactions, and therefore can be used with most of the usual drugs. However, caution should be taken until more safety studies are available.
7. Expert opinion
Vortioxetine is a novel antidepressant with multimodal activity and high-affinity for SERT and several 5-HT receptor subtypes, which provide clinical advantages to other existing antidepressants.
This review, focused on the available evidences of vortioxetine, supports its short (6–8 weeks) and long-term (up to 52 weeks) antidepressant efficacy and tolerability in a broad range of settings in MDD patients. Efficacy has been proved for vortioxetine with broad beneficial effects on emotional, physical, and cognitive symptoms. Specifically, vortioxetine has evidenced improvements in cognitive domains of attention, processing speed, executive function, learning, and memory. Efficacy has also been suggested in patients with MDD for sexual dysfunction, physical symptoms (flu-like and shock-like complaints, dizziness, sleep disturbances, etc.), anxiety, social functioning, sleep disturbances, anhedonia, emotional blunting, and menopausal transition, and even in depressed patients with Parkinson’s Disease and with type 2 diabetes, and post-ictus among others. Vortioxetine has also shown analgesic and anti-inflammatory properties. The unique attributes of vortioxetine render it a promising therapeutic option for several non-psychiatric disorders that are accompanied by pain, inflammation, apathy, sleep and/or metabolic abnormalities besides prominent depressive and anxiety symptoms.
Although some antidepressants have shown improvements in cognitive function in patients with MDD, most of the antidepressants have not shown an effect on cognition. However, vortioxetine is the first antidepressant that has shown improvements both in depression and cognition. Furthermore, the positive effects of vortioxetine in cognition are independent of its beneficial effects on depressive symptoms, due to the unique multimodal mechanism of action of vortioxetine. Cognitive improvements observed with vortioxetine were not apparent with duloxetine in head-to-head CT, and vortioxetine had the largest cognitive-enhancing effects in indirect comparisons with other antidepressants (citalopram, escitalopram, nortriptyline, phenelzine, and sertraline).
8The long elimination half-life of vortioxetine allows administration of once daily doses which is more convenient for patients and promotes treatment compliance. It also allows abrupt discontinuation with no presence of discontinuation symptoms, and therefore vortioxetine can be discontinued whenever needed. Vortioxetine had shown lower withdrawal rates than other antidepressants.
Regarding tolerability, the side effects of vortioxetine are different from conventional antidepressants, being the most frequent AE nausea and vomiting, with a transitory and dose-dependent effect. Data from CT with vortioxetine showed a low incidence of sleep disruption, sexual dysfunction, and discontinuation syndrome, and beneficial effects on sleep quality. Importantly, vortioxetine does not increase lipid, cholesterol or glycemic levels and has a low incidence of cardiovascular and alterations, with no clinically relevant effect on ECG parameters, including the QTcF interval. The procognitive action together with the low incidence of somnolence and other cardiovascular alterations support that vortioxetine can be a good alternative to be considered in elder patients with MDD. Vortioxetine has also been suggested as an effective alternative for switching treatment in patients with MDD with inadequate response to SSRIs and SNRIs.
Vortioxetine has been demonstrated to be a cost-effective treatment option in patients with MDD versus other widely used antidepressant treatments such as agomelatine, bupropion XR, citalopram, duloxetine, escitalopram, sertraline, and venlafaxine XR. In summary, the benefits of vortioxetine relative to other antidepressants have been broadly evidenced in terms of efficacy, tolerability, and cost-effectiveness. Vortioxetine has been recently launched and there is a high expectation on future studies about its potential effects on other symptoms and comorbid pathologies.
Article highlights
Vortioxetine is a multimodal-acting antidepressant specifically designed to inhibit serotonin reuptake and interact with several serotonin receptors exerting modulatory effects on multiple neurotransmitter systems.
Is the first antidepressant drug with proven efficacy in improving cognitive symptoms of depression and is the only antidepressant agent showing evidence of a positive effect on multiple cognitive domains.
Is useful in symptoms as anhedonic, psychical symptoms, sleep disturbances, and suicidal ideation.
Vortioxetine has shown no clinically relevant effect on blood pressure, ECG parameters including the QTcF interval, does not increase lipid or cholesterol, and showed a decrease in glycosylated hemoglobin suggesting amelioration in metabolic control with vortioxetine.
Vortioxetine has a mean terminal half-life of approximately 66 h, which is long enough to avoid the presence of discontinuation symptoms following abrupt discontinuation of treatment with vortioxetine.
This box summarizes key points contained in the article.
Declaration of interests
J De Diego-Adeliño was supported by the Catalan Intensification Programme (PERIS, SLT008/18/00207; Generalitat de Catalunya). He has received consultancy and/or lecture honoraria from Lundbeck, Pfizer, Neuraxpharm, Janssen and Esteve in the last 5-years, none of them with direct relation to this work. JM Crespo has received consultancy and/or lecture honoraria from Lundbeck, Angelini, Casen Recordati, Esteve and Janssen in the last 5-years, none of them with direct relation with this work. F Mora has received consultancy and/or lecture honoraria from Lundbeck, Pfizer, Sandoz-Novartis, Baxter, Janssen, Neuraxpharm, Lilly, MSD and Takeda in the last 5-years, none of them with direct relation to this work. A Neyra has received consultancy and/or lecture honoraria from Lundbeck, Pfizer and Janssen in the last 5-years, none of them with direct relation to this work. P Iborra has been consultant or has received honoraria from Lundbeck. L Gutiérrez-Rojas has received consultancy and/or lecture honoraria from Lundbeck, Pfizer, Novartis, Janssen, Neuraxpharm, and Otsuka in the last 5-years, none of them with direct relation to this work. SF Salonia has been a consultant or has received honoraria from Servier, Lundbeck, Janssen, Esteve and Pfizer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are/have been a consultant and/or a speaker and/or have received research grants from Angelini, Apsen, Boheringer Ingelheim, Daiichi Sankyo, Doc Generici, Glaxo Smith Kline, Italfarmaco, Lundbeck,Janssen, Mylan, Neuraxpharm, Otsuka, Pfizer, Recordati, Sanofi Aventis, Sunovion, and Vifor. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Gutiérrez-Rojas L, Porras-Segovia A, Dunne H, et al. Prevalence and correlates of major depressive disorder: a systematic review. Braz J Psychiatry. 2020;42(6):657–672.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. APA. 2013. DSM–5. https://www.psychiatry.org/psychiatrists/practice/dsm
- Bora E, Harrison BJ, Yücel M, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. 2013;43(10):2017–2026.
- Etkin A, Patenaude B, Song YJ, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology. 2015;40(6):1332–1342.
- Jaeger J, Berns S, Uzelac S, et al. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006;145(1):39–48.
- Eaton WW, Shao H, Nestadt G, et al. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry. 2008;65(5):513–520.
- Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. 1999;156(7):1000–1006.
- Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382(9904):1575–1586.
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58(1):19–36.
- Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(Suppl 13):23–29.
- David DJ, Gourion D. Antidépresseurs et tolérance: déterminants et prise en charge des principaux effets indésirables[Antidepressant and tolerance: determinants and management of major side effects]. Encephale. 2016;42(6):553–561.
- Wille SM, Cooreman SG, Neels HM, et al. Relevant issues in the monitoring and the toxicology of antidepressants. Crit Rev Clin Lab Sci. 2008;45(1):25–89.
- Bauer M, Pfennig A, Severus E, et al. Task force on unipolar depressive disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334–385.
- Artigas F, Bortolozzi A, Celada P. Can we increase speed and efficacy of antidepressant treatments? Part I: general aspects and monoamine-based strategies. Eur Neuropsychopharmacol. 2018;28(4):445–456.
- Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–288.
- Whiskey E, Taylor D. A review of the adverse effects and safety of noradrenergic antidepressants. J Psychopharmacol. 2013;27(8):732–739.
- Henssler J, Bschor T, Baethge C. Combining antidepressants in acute treatment of depression: a meta-analysis of 38 studies including 4511 patients. Can J Psychiatry. 2016;61(1):29–43.
- Wichniak A, Wierzbicka A, Walęcka M, et al. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
- Song HR, Kwon YJ, Woo YS, et al. Effects of mirtazapine on patients undergoing naturalistic diabetes treatment: a follow-up study extended from 6 to 12 months. J Clin Psychopharmacol. 2015;35(6):730–731.
- Alvarez E, Perez V, Artigas F. Pharmacology and clinical potential of vortioxetine in the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2014;10:1297–1307.
- Citrome L. Vortioxetine for major depressive disorder: an indirect comparison with duloxetine, escitalopram, levomilnacipran, sertraline, venlafaxine, and vilazodone, using number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2016;196:225–233.
- Cipriani A, Furukawa TA, Salanti G, et al., Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 391(10128): 1357–1366. 2018.
- US Food and Drug Administration. Trintellix® (formerly Brintellix®) Medication guide (revised 07/2019). 2020. [cited 2021 Dec 20]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204447s018lbl.pdf
- Chen G, Højer AM, Areberg J, et al. Vortioxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2018;57(6):673–686.
- Chen G, Nomikos GG, Affinito J, et al. Effects of intrinsic factors on the clinical pharmacokinetics of vortioxetine. Clin Pharmacol Drug Dev. 2018;7(8):880–888.
- Chen G, Nomikos GG, Affinito J, et al. Lack of effect of vortioxetine on the pharmacokinetics and pharmacodynamics of Ethanol, Diazepam, and Lithium. Clin Pharmacokinet. 2016;55(9):1115‐1127.
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57.
- Nackenoff AG, Simmler LD, Baganz NL, et al. Serotonin transporter-independent actions of the antidepressant vortioxetine as revealed using the SERT Met172 mouse. ACS Chem Neurosci. 2017;8(5):1092–1100.
- Li Y, Raaby KF, Sánchez C, et al. Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res. 2013;256:520–528.
- Hlavacova N, Li Y, Pehrson A, et al. Effects of vortioxetine on biomarkers associated with glutamatergic activity in an SSRI insensitive model of depression in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2018;82:332–338.
- Riga MS, Sánchez C, Celada P, et al. Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology. 2016;108:73–81.
- Du Jardin KG, Müller HK, Sanchez C, et al. A single dose of vortioxetine, but not ketamine or fluoxetine, increases plasticity-related gene expression in the rat frontal cortex. Eur J Pharmacol. 2016;786:29–35.
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9):1085–1098.
- Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18(9):1413–1417.
- Chen F, Du Jardin KG, Waller JA, et al. Vortioxetine promotes early changes in dendritic morphology compared to fluoxetine in rat hippocampus. Eur Neuropsychopharmacol. 2016;26(2):234–245.
- Bennabi D, Haffen E, Van Waes V. Vortioxetine for cognitive enhancement in major depression: from animal models to clinical research. Front Psychiatry. 2019;10:771.
- Leiser SC, Pehrson AL, Robichaud PJ, et al. Multimodal antidepressant vortioxetine increases frontal cortical oscillations unlike escitalopram and duloxetine–a quantitative EEG study in rats. Br J Pharmacol. 2014;171(18):4255–4272.
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787.
- Zuena AR, Maftei D, Alemà GS, et al. Multimodal antidepressant vortioxetine causes analgesia in a mouse model of chronic neuropathic pain. Mol Pain. 2018;14:1744806918808987.
- Talmon M, Rossi S, Pastore A, et al. Vortioxetine exerts anti-inflammatory and immunomodulatory effects on human monocytes/macrophages. Br J Pharmacol. 2018;175(1):113‐124.
- Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373–387.
- Tomaz VS, Chaves Filho AJM, Cordeiro RC, et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J Affect Disord. 2020;268:188–200.
- Fourrier C, Sampson E, Mills NT, et al. Anti-inflammatory treatment of depression: study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. Trials. 2018;19(1):447.
- Talmon M, Chaudhari RD, Suryavanshi H, et al. Design, synthesis and biological evaluation of vortioxetine derivatives as new COX-1/2 inhibitors in human monocytes. Bioorg Med Chem. 2020;28(23):115760.
- Salagre E, Grande I, Solé B, et al. Vortioxetina: una nueva alternativa en el trastorno depresivo mayor.[Vortioxetine: a new alternative for the treatment of major depressive disorder]. Rev. Psiquiatr Salud Ment (Barc.). 2018;11(1):48–59.
- Thase ME, Mahableshwarkar AR, Dragheim M, et al., A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 26(6): 979–993. 2016.
- Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601–609.
- Nierenberg AA, Loft H, Olsen CK. Treatment effects on residual cognitive symptoms among partially or fully remitted patients with major depressive disorder: a randomized, double-blinded, exploratory study with vortioxetine. J Affect Disord. 2019;250:35‐42.
- Lee RS, Hermens DF, Porter MA, et al. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord. 2012;140(2):113–124.
- Rosenblat JD, Kakar R, McIntyre RS. The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol. 2015;19(2):yv082.
- Baune BT, Brignone M, Larsen KG. A network meta-analysis comparing effects of various antidepressant classes on the digit symbol substitution Test (DSST) as a measure of cognitive dysfunction in patients with major depressive disorder. Int J Neuropsychopharmacol. 2018;21(2):97–107.
- Harrison JE, Lophaven S, Olsen CK. Which Cognitive Domains are Improved by Treatment with Vortioxetine? Int J Neuropsychopharmacol. 2016 May 26;19(10):yw054.
- Jacobson W, Zhong W, Nomikos GG, et al. Effects of vortioxetine on functional capacity across different levels of functional impairment in patients with major depressive disorder: a University of California, San Diego Performance-based Skills Assessment (UPSA) analysis. Curr Med Res Opin. 2020;36(1):117–124.
- McIntyre RS, Harrison J, Loft H, et al., The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. 19(10): yw055. 2016.
- Chokka P, Bougie J, Rampakakis E, et al. Assessment in work productivity and the relationship with cognitive symptoms (AtWoRC): primary analysis from a Canadian open-label study of vortioxetine in patients with major depressive disorder (MDD). CNS Spectr. 2019;24(3):338–347.
- Chokka P, Bougie J, Proulx J, et al. Long-term functioning outcomes are predicted by cognitive symptoms in working patients with major depressive disorder treated with vortioxetine: results from the AtWoRC study. CNS Spectr. 2019;24(6):616–627.
- McIntyre RS, Florea I, Tonnoir B, et al. Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J Clin Psychiatry. 2017;78(1):115–121.
- Vieta E, Sluth LB, Olsen CK. The effects of vortioxetine on cognitive dysfunction in patients with inadequate response to current antidepressants in major depressive disorder: a short-term, randomized, double-blind, exploratory study versus escitalopram. J Affect Disord. 2018;227:803–809.
- Nissen TD, Laursen B, Viardot G, et al. Effects of vortioxetine and escitalopram on electroencephalographic recordings - a randomized, crossover trial in healthy males. Neuroscience. 2020;424:172‐181.
- Smith J, Browning M, Conen S, et al. Vortioxetine reduces BOLD signal during performance of the N-back working memory task: a randomised neuroimaging trial in remitted depressed patients and healthy controls. Mol Psychiatry. 2018;23(5):1127–1133.
- Orgeta V, Tabet N, Nilforooshan R, et al. efficacy of antidepressants for depression in Alzheimer’s Disease: systematic review and meta-analysis. J Alzheimers Dis. 2017;58(3):725–733.
- Lenze EJ, Stevens A, Waring JD, et al. Augmenting computerized cognitive training with vortioxetine for age-related cognitive decline: a randomized controlled trial. Am J Psychiatry. 2020;177(6):548–555.
- Cumbo E, Cumbo S, Torregrossa S, et al. Treatment Effects of Vortioxetine on Cognitive Functions in Mild Alzheimer’s Disease Patients with Depressive Symptoms: a 12 Month, Open-Label, Observational Study. J Prev Alzheimers Dis. 2019;6(3):192–197.
- Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert Opin Pharmacother. 2017;18(10):1011‐1017.
- Strelnikova IA, Svetkina AA, YuD M, et al. Experience with vortioxetine in the treatment of post-stroke depression. Nevrologiya, neiropsikhiatriya, psikhosomatika = Neurology. Neuropsychiatry, Psychosomatics. 2020;12(1):45–49.
- Kennedy SH, Lam RW, McIntyre RS, et al., Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. Can J Psychiatry. 61(9): 540–560. 2016.
- Cao B, Park C, Subramaniapillai M, et al. The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17.
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). J Affect Disord. 2007;99(1–3):83–89.
- Subramaniapillai M, Mansur RB, Zuckerman H, et al. Association between cognitive function and performance on effort based decision making in patients with major depressive disorder treated with Vortioxetine. Compr Psychiatry. 2019;94:152113.
- Opbroek A, Delgado PL, Laukes C, et al. Emotional blunting associated with SSRI-induced sexual dysfunction. Do SSRIs inhibit emotional responses? Int J Neuropsychopharmacol. 2002;5(2):147–151.
- Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211–217.
- Fagiolini A, Florea I, Loft H, et al. Effectiveness of vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to SSRI/SNRI treatment. J Affect Disord. 2021;283:472–479.
- Cronquist MC, Florea I, Lindsten A, et al. Efficacy of vortioxetine on the physical symptoms of major depressive disorder. J Psychopharmacol. 2018;32(10):1086‐1097.
- Jaracz J, Gattner K, Jaracz K, et al. Unexplained painful physical symptoms in patients with major depressive disorder: prevalence, pathophysiology and management. CNS Drugs. 2016;30(4):293–304.
- Cao B, Park C, Rosenblat JD, et al. Changes in sleep predict changes in depressive symptoms in depressed subjects receiving vortioxetine: an open-label clinical trial. J Psychopharmacol. 2019;33(11):1388–1394.
- Liguori C, Ferini-Strambi L, Izzi F, et al. Preliminary evidence that vortioxetine may improve sleep quality in depressed patients with insomnia: a retrospective questionnaire analysis. Br J Clin Pharmacol. 2019;85(1):240–244.
- Doghramji K, Jangro WC. Adverse effects of psychotropic medications on sleep. Psychiatr Clin North Am. 2016;39(3):487–502.
- Leiser SC, Iglesias-Bregna D, Westrich L, et al. Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: part 2, pharmacological interactions in rodents suggest a role of serotonin-3 receptor antagonism. J Psychopharmacol. 2015;29(10):1092–1105.
- Wilson S, Højer AM, Buchberg J, et al. Differentiated effects of the multimodal antidepressant vortioxetine on sleep architecture: part 1, a pharmacokinetic/pharmacodynamic comparison with paroxetine in healthy men. J Psychopharmacol. 2015;29(10):1085‐1091.
- Bonaventure P, Kelly L, Aluisio L, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. 2007;321(2):690–698.
- Shelton J, Bonaventure P, Li X, et al. 5-HT7 receptor deletion enhances REM sleep suppression induced by selective serotonin reuptake inhibitors, but not by direct stimulation of 5-HT1A receptor. Neuropharmacology. 2009;56(2):448–454.
- Simon GE, Savarino J. Suicide attempts among patients starting depression treatment with medications or psychotherapy. Am J Psychiatry. 2007;164(7):1029–1034.
- Simon GE, Savarino J, Operskalski B, et al. Suicide risk during antidepressant treatment. Am J Psychiatry. 2006;163(1):41–47.
- Khan A, Khan S, Kolts R, et al. Suicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reports. Am J Psychiatry. 2003;160(4):790–792.
- Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ. 2005;330(7488):385.
- Mahableshwarkar AR, Affinito J, Reines EH, et al. Suicidal ideation and behavior in adults with major depressive disorder treated with vortioxetine: post hoc pooled analyses of randomized, placebo-controlled, short-term and open-label, long-term extension trials. CNS Spectr. 2019;14:1–11.
- Baldwin DS, Florea I, Jacobsen PL, et al. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord. 2016;206:140–150.
- Blanco C, Nissenson K, Liebowitz MR. Social anxiety disorder: recent findings in the areas of epidemiology, etiology, and treatment. Curr Psychiatry Rep. 2001;3(4):273–280.
- Liebowitz MR, Careri J, Blatt K, et al. Vortioxetine versus placebo in major depressive disorder comorbid with social anxiety disorder. Depress Anxiety. 2017;34(12):1164–1172.
- Cronquist MC, Florea I, Loft H, et al. Efficacy of vortioxetine in patients with major depressive disorder reporting childhood or recent trauma. J Affect Disord. 2020;263:258–266.
- Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(8):807–816.
- Di Nicola M, Pepe M, Panaccione I, et al. Effect of vortioxetine in subjects with major depressive and alcohol use disorders: a 6-month retrospective analysis. CNS Spectr 2020 1–9 https://doi.org/10.1017/S109285292000173X
- Oslin DW, Datto CJ, Kallan MJ, et al. Association between medical comorbidity and treatment outcomes in late-life depression. J Am Geriatr Soc. 2002;50(5):823–828.
- Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord. 2012;141(2–3):103–115.
- Nomikos GG, Tomori D, Zhong W, et al. Efficacy, safety, and tolerability of vortioxetine for the treatment of major depressive disorder in patients aged 55 years or older. CNS Spectr. 2017;22(4):348‐362.
- Agüera-Ortiz LF. Tratamiento actual de la depresión en el anciano: un foco en la vortioxetina.[Current treatment of depression in the elderly: a focus on vortioxetine]. Psicogeriatria. 2020;10(Supl 1):S1–S9.
- Freeman MP, Cheng LJ, Moustafa D, et al. Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition. Ann Clin Psychiatry. 2017;29(4):249–257.
- Callegari C, Ielmini M, Caselli I, et al. Paroxetine versus vortioxetine for depressive symptoms in postmenopausal transition: a preliminary study. Psychopharmacol Bull. 2019;49(1):28–43.
- Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858.
- Fiore V, Marci M, Poggi A, et al. The association between diabetes and depression: a very disabling condition. Endocrine. 2015;48(1):14–24.
- Roopan S, Larsen ER. Use of antidepressants in patients with depression and comorbid diabetes mellitus: a systematic review. Acta Neuropsychiatr. 2017;29(3):127–139.
- Tovilla-Zárate CA, Pérez-Mandujano A, Ramírez-González IR, et al. Vortioxetine versus sertraline in metabolic control, distress and depression in Mexican patients with type 2 diabetes. Ann Transl Med. 2019;7(22):656.
- Lee MD, Kennett GA, Dourish CT, et al. 5-HT1B receptors modulate components of satiety in the rat: behavioural and pharmacological analyses of the selective serotonin1B agonist CP-94,253. Psychopharmacology (Berl). 2002;164(1):49–60.
- Nonogaki K, Kaji T. Pharmacological stimulation of serotonin 5-HT1B receptors enhances increases in plasma active glucagon-like peptide-1 levels induced by dipeptidyl peptidase-4 inhibition independently of feeding in mice. Diabetes Metab. 2015;41(5):425–428.
- Russo M, Carrarini C, Dono F, et al. Vortioxetine treatment of depression in Parkinson’s disease[abstract]. Mov Disord. 2019; 34(suppl 2). [cited 2021 Dec 20]. Available from https://www.mdsabstracts.org/abstract/vortioxetine-treatment-of-depression-in-parkinsons-disease/
- Postuma RB, Gagnon JF, Tuineaig M, et al. Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal? Sleep. 2013;36(11):1579–1585.
- Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. 2010;6(1):85–95.
- Adamo D, Pecoraro G, Coppola N, et al. Vortioxetine versus other antidepressants in the treatment of burning mouth syndrome: an open-label randomized trial. Oral Dis. 2020;27(4):1022–1041.
- Montgomery SA, Nielsen RZ, Poulsen LH, et al. A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol. 2014;29(5):470–482.
- Thase ME, Danchenko N, Brignone M, et al. Comparative evaluation of vortioxetine as a switch therapy in patients with major depressive disorder. Eur Neuropsychopharmacol. 2017;27(8):773‐781.
- Brignone M, Diamand F, Painchault C, et al. Efficacy and tolerability of switching therapy to vortioxetine versus other antidepressants in patients with major depressive disorder. Curr Med Res Opin. 2016;32(2):351–366.
- Tegin C, Tegin G, El-Mallakh RS. Effective vortioxetine dose varies with extent of antidepressant use across countries. Psychopharmacol Bull. 2018;48(1):26‐39.
- Garcia-Toro M, Medina E, Galan JL, et al. Treatment patterns in major depressive disorder after an inadequate response to first-line antidepressant treatment. BMC Psychiatry. 2012;12(1):143.
- Goldberg JF, Freeman MP, Balon R, et al. The American society of clinical psychopharmacology survey of psychopharmacologists’ practice patterns for the treatment of mood disorders. Depress Anxiety. 2015;32(8):605–613.
- Florea I, Loft H, Danchenko N, et al. The effect of vortioxetine on overall patient functioning in patients with major depressive disorder. Brain Behav. 2017;7(3):e00622.
- Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025–2037.
- Cronquist MC, Loft H, McIntyre RS. Vortioxetine improves symptomatic and functional outcomes in major depressive disorder: a novel dual outcome measure in depressive disorders. J Affect Disord. 2018;227:787–794.
- Soini E, Hallinen T, Brignone M, et al. Cost-utility analysis of vortioxetine versus agomelatine, bupropion SR, sertraline and venlafaxine XR after treatment switch in major depressive disorder in Finland. Expert Rev Pharmacoecon Outcomes Res. 2017;17(3):293–302.
- Young AH, Evitt L, Brignone M, et al. Cost-utility evaluation of vortioxetine in patients with major depressive disorder experiencing inadequate response to alternative antidepressants in the United Kingdom. J Affect Disord. 2017;218:291–298.
- Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29(3):259–266.
- Jacobsen PL, Mahableshwarkar AR, Palo WA, et al. Treatment-emergent sexual dysfunction in randomized trials of vortioxetine for major depressive disorder or generalized anxiety disorder: a pooled analysis. CNS Spectr. 2016;21(5):367–378.
- Jacobsen P, Zhong W, Nomikos G, et al. Paroxetine, but not vortioxetine, impairs sexual functioning compared with placebo in healthy adults: a randomized, controlled trial. J Sex Med. 2019;16(10):1638–1649.
- Montejo AL, Prieto N, de Alarcón R, et al. Management strategies for antidepressant-related sexual dysfunction: a clinical approach. J Clin Med. 2019;8(10):1640.
- Jacobsen PL, Mahableshwarkar AR, Chen Y, et al. Effect of vortioxetine vs. escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing ssri-induced sexual dysfunction. J Sex Med. 2015;12(10):2036–2048.
- Olivier JDA, Olivier B. Antidepressants and sexual dysfunctions: a translational perspective. Curr Sex Health Rep. 2019;11(3):156–166.
- Li Y, Pehrson AL, Oosting RS, et al. A study of time- and sex-dependent effects of vortioxetine on rat sexual behavior: possible roles of direct receptor modulation. Neuropharmacology. 2017;121:89–99.
- Jacobsen PL, Harper L, Chrones L, et al. Safety and tolerability of vortioxetine (15 and 20 mg) in patients with major depressive disorder: results of an open-label, flexible-dose, 52-week extension study. Int Clin Psychopharmacol. 2015;30(5):255–264.
- Zheng J, Wang Z, Li E. The efficacy and safety of 10 mg/day vortioxetine compared to placebo for adult major depressive disorder: a meta-analysis. Afr Health Sci. 2019;19(1):1716–1726.
- Behzadifar M, Dehghan H, Saki K, et al. Evaluation efficacy and safety of Vortioxetine 20 mg/d versus placebo for treatment major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. Pharmacology & Pharmacy. 2015;6(4):221.
- Baldwin DS, Chrones L, Florea I, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30(3):242–252.
- Coupland NJ, Bailey JE, Potokar JP, et al. 5-HT3 receptors, nausea, and serotonin reuptake inhibition. J Clin Psychopharmacol. 1997;17(2):142–143.
- Ladefoged LK, Munro L, Pedersen AJ, et al. Modeling and mutational analysis of the binding mode for the multimodal antidepressant drug vortioxetine to the human 5-HT3A receptor. Mol Pharmacol. 2018;94(6):1421–1434.
- Crapanzano C, Politano A, Amendola C, et al. Vortioxetine-induced nausea and its treatment: a case report. Archives of Clinical Psychiatry (São Paulo). 2021;47(5):160–161.
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163(7):1411–1422.
- Johnston KD, Lu Z, Rudd JA. Looking beyond 5-HT(3) receptors: a review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur J Pharmacol. 2014;722:13–25.
- Fava GA, Benasi G, Lucente M, et al. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother Psychosom. 2018;87(4):195–203.
- Chen G, Lee R, Højer AM, et al. Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013;33(10):727–736.
- Schäfer W, Princk C, Kollhorst B, et al. Antidepressants and the risk of hemorrhagic stroke in the elderly: a nested case-control study. Drug Saf. 2019;42(9):1081–1089.
- Chen G, Zhang W, Serenko M. Lack of effect of multiple doses of vortioxetine on the pharmacokinetics and pharmacodynamics of aspirin and warfarin. J Clin Pharmacol. 2015;55(6):671–679.
- Me IO, Gaete GL. Antidepressants agents in breast cancer patients using tamoxifen: review of basic and clinical evidence. Rev Med Chil. 2016;144(10):1326–1335.
