ABSTRACT
Background
Empagliflozin, a sodium-glucose co-transporter-2 inhibitor, was licensed for treating type 2 diabetes (T2D) in Japan and elsewhere in recent years. We conducted a post-marketing surveillance study of empagliflozin in Japan.
Research design and methods
This was a 3-year, prospective, multicenter, observational study of the safety and effectiveness of empagliflozin in T2D patients in Japanese clinical practice who had not previously received this medication. The primary endpoint was the incidence of adverse drug reactions (ADRs).
Results
Of 8145 patients enrolled from 1103 sites, 7931 received ≥1 dose of empagliflozin. Mean age was 58.7 years (10.5% aged ≥75), glycated hemoglobin (HbA1c) 8.0%, body mass index 28.1 kg/m2 (<20 kg/m2 in 2.1%); 63.0% were male and most had comorbidities (renal impairment in ~62%). Median treatment duration was 36.5 months. ADRs occurred in 1024 (12.91%) patients overall (serious ADRs in 2.09%) and 120 patients aged ≥75 years (14.46%). ADRs of special interest included hypoglycemia (0.44% of patients), urinary tract infections (1.07%), genital infections (0.66%), volume depletion (0.50%), diabetic ketoacidosis (0%), and lower limb amputation (0.04%). Overall mean change in HbA1c from baseline was –0.75%.
Conclusions
Empagliflozin is effective and generally well tolerated in Japanese patients, and ADRs are consistent with its known safety profile.
1. Introduction
Sodium-glucose co-transporter-2 (SGLT2) inhibitors are a class of glucose-lowering drugs first launched for treatment of type 2 diabetes (T2D) in 2013 in the United States (US) and 2014 in Japan. By blocking the SGLT2 protein in the proximal tubule of the kidneys, SGLT2 inhibitors reduce renal reabsorption of glucose, eliciting glucosuria and thus improving glycemic control in T2D patients [Citation1–3]. SGLT2 inhibitors also modestly reduce body weight and blood pressure [Citation4,Citation5]. In addition to these metabolic benefits, empagliflozin was the first SGLT2 inhibitor shown to reduce cardiorenal events, when reductions in cardiovascular and all-cause mortality, hospitalization for heart failure, and nephropathy were demonstrated in T2D patients with established cardiovascular disease in the landmark EMPA-REG OUTCOME trial in 2015 [Citation6,Citation7] – including the subgroup of Asian patients [Citation8–10].
In routine clinical practice, empagliflozin treatment of T2D patients has been associated with reduced risk of hospitalization for heart failure in the US [Citation11,Citation12] and reduced risk of hospitalization for heart failure, all-cause mortality and end-stage renal disease in East Asia (including Japan) [Citation13] compared with dipeptidyl peptidase-4 (DPP-4) inhibitors, another modern class of oral glucose-lowering drug. Treatment with empagliflozin in routine clinical practice in East Asia has also been associated with reduced healthcare resource utilization compared with DPP-4 inhibitors [Citation14]. Other SGLT2 inhibitors have now also demonstrated cardiorenal risk reduction in both clinical trials and clinical practice [Citation15–17].
Based on their mechanism of action, SGLT2 inhibitors could theoretically cause several types of adverse event, including urinary tract infections, genital infections, and diabetic ketoacidosis. However, empagliflozin has been generally well tolerated in clinical trials. Pooled analyses of safety data from placebo-controlled clinical trials of empagliflozin found no overall increased incidence of hypoglycemia, urinary tract infections, volume depletion, or diabetic ketoacidosis, but there was an increased incidence of genital infections [Citation18,Citation19].
Nevertheless, patients treated with empagliflozin in clinical practice may have different characteristics to those studied in clinical trials, such as older age, rendering them more vulnerable to adverse events as well as potentially affecting their glycemic response. In addition, there may be important pathophysiological differences between East Asian T2D patients and those from Western countries who predominated in the clinical trials [Citation20]. Therefore, it is important to confirm the long-term safety and effectiveness of empagliflozin in clinical practice in Japan – including in elderly individuals, as Japan is a super-aging country where it is estimated that more than 70% of T2D patients are over 65 years old [Citation21].
Consequently, we conducted a long-term post-marketing surveillance study of the safety and effectiveness of empagliflozin in Japan, which included elderly patients. Interim results have been reported [Citation22]. We report here the final results of this 3-year study.
2. Patients and methods
The methodology of this study has previously been reported in detail [Citation22] and is described here briefly.
2.1. Study design and patient population
This was a prospective, post-marketing surveillance study conducted at 1103 clinical practices in Japan between 12 June 2015 (first patient enrollment) and 4 December 2020 (final data collection) with an observation period of 3 years per patient (ClinicalTrials.gov identifier NCT02489942). The study met the requirements of the Japanese regulatory authority, i.e. the Ministry of Health, Labour and Welfare (MHLW), and was conducted in accordance with the MHLW Good Post-Marketing Study Practice and Good Vigilance Practice ordinances. Therefore, it was not necessary to obtain either informed consent from patients or approval by an institutional review board or ethics committee.
Eligible individuals were T2D patients who had not previously received treatment with empagliflozin; those who had previously received another SGLT2 inhibitor were eligible. Patient data were de-identified prior to collection. Data were collected via electronic case report forms at months 3, 12, 24 and 36 after initiating empagliflozin treatment and at treatment discontinuation (last observation).
2.2. Assessments
The primary safety outcome was the incidence of adverse drug reactions (ADRs), which were defined as adverse events with which empagliflozin had a possible causal relationship. Causality was considered to be established if either the investigator, study sponsor, or both, assessed the causal relationship of empagliflozin with the adverse event as either high possibility, low possibility, or unknown due to insufficient or contradictory information. The sponsor assessed the causal relationship independently from the investigator and did not influence the investigator’s assessment. ADRs were classified using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.1.
Other safety outcomes included the incidence of serious ADRs and ADRs of special interest. A serious ADR was defined as one that resulted in death, was life-threatening, required hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or was a congenital anomaly/birth defect. Prespecified ADRs of special interest were hypoglycemia, urinary tract infection, genital infection, volume depletion, cardiovascular event, diabetic ketoacidosis, renal impairment, liver injury, bone fracture, malignancy, excessive urination/frequent urination, and adverse events relating to increases in ketones. Lower limb amputations and muscle weakness, including sarcopenia, were defined as ADRs of special interest after the study had been initiated. The final clinical status of individual patients was based on information available at the end of the study.
The prespecified measures of glycemic effectiveness were change in glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) levels from baseline. Other assessments included changes in body weight, blood pressure, pulse rate, hematocrit, hemoglobin, lipids, creatinine, and estimated glomerular filtration rate (eGFR) according to the Japanese version of the Modification of Diet in Renal Disease study equation [Citation23].
2.3. Statistical analysis
At least 3000 patients completing 3 years of observation were required for 95% probability that an ADR with a true incidence of 0.10% would occur in ≥1 patient. For transparency on safety issues, safety data were evaluated for all patients who received ≥1 dose of empagliflozin, except those with no visit after entry or those who did not have T2D, among other reasons (Supplemental Figure S1). Effectiveness data were evaluated for all patients in the safety analysis set except those without available HbA1c or FPG data, those who initiated empagliflozin at 25 mg/day (although those who escalated from 10 mg/day to 25 mg/day were included), and those with baseline eGFR <30 ml/min/1.73 m2. The latter 2 groups were excluded from the effectiveness analysis to discourage use that is off-label according to Japanese prescribing information [Citation24]. As an exploratory study, data were summarized descriptively without inferential analyses. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient disposition
A total of 8145 patients were enrolled, from whom 8059 electronic case report forms were collected (Supplemental Figure S1). The safety analysis set comprised 7931 patients, with the most common reason for excluding the other 128 patients being no visit since the first visit (n = 100). The effectiveness analysis set consisted of 7090 patients, due to exclusion of 841 patients with either no baseline or no post-baseline HbA1c and FPG values, as well as those who initiated empagliflozin at 25 mg/day and those with baseline eGFR <30 ml/min/1.73 m2 (who were excluded for off-label use, as described above).
3.2. Patient characteristics at baseline
At baseline, mean age was 58.7 years, with 2077 individuals (26.2%) aged 65 to <75 and 830 (10.5%) aged ≥75 (). Furthermore, 325 patients (4.1%) were aged ≥80 years. Overall, 63% of patients were male and most were overweight (62.3% had body mass index [BMI] ≥25 kg/m2; mean BMI was 28.1 kg/m2) while 2.1% had BMI <20 kg/m2. More than a third of patients (34.8%) had had T2D for more than 5 years; mean HbA1c level was 8.0% while mean FPG level was 160 mg/dl. Most patients (76.9%) were also taking at least one other glucose-lowering drug, with the most common being DPP-4 inhibitors (52.9%), biguanides (41.7%), sulfonylureas (20.3%), and insulin (12.0%); 23.1% of patients were not receiving any other glucose-lowering drug at baseline. The large majority of patients (84.1%) had concomitant conditions, most commonly hypertension (58.2%) and dyslipidemia (57.5%). The prevalence of both cardiovascular/cerebrovascular disease (13.7% overall) and osteoporosis (1.7%) was higher in the older age groups. Approximately 62% of patients also had some kidney dysfunction, with only 30.2% having eGFR ≥90 ml/min/1.73 m2, while 0.3% had eGFR <30 ml/min/1.73 m2; mean eGFR was 82.4 ml/min/1.73 m2. Approximately 30% of patients had some degree of hepatic impairment.
Table 1. Patient demographics and baseline characteristics by age of patient (safety analysis set).
3.3. Drug dose and exposure
The starting dose of empagliflozin was 10 mg in 7525 patients (94.88%) and 25 mg in 353 patients (4.45%), while the final dose was 10 mg in 7068 patients (89.12%) and 25 mg in 812 patients (10.24%) (). Patients were treated with empagliflozin for a mean duration of 28.1 months (median: 36.5). More than half the patients (59.54%) received empagliflozin for >36 months. Of individuals who discontinued empagliflozin, the main reasons were patient request (n = 601, 7.58%), adverse event (n = 516, 6.51%), no change/progressive disease (n = 263, 3.32%), and improvement/remission (n = 157, 1.98%).
Table 2. Patient exposure to empagliflozin and discontinuation of empagliflozin (safety analysis set).
3.4. Safety and tolerability
3.4.1. Incidence of ADRs, serious ADRs, and deaths
ADRs are summarized by MedDRA system organ class (SOC) in Supplemental Table S1, with the most common SOCs being Investigations (n = 203, 2.56%), Metabolism and nutrition disorders (n = 174, 2.19%), and Infections and infestations (n = 161, 2.03%).
Of the 7931 patients, 1024 (12.91%) experienced ≥1 ADR during treatment with empagliflozin: 594 (11.82%), 310 (14.93%), and 120 (14.46%) aged <65 years, 65 to <75, and ≥75, respectively (). A total of 166 (2.09%) patients experienced ≥1 serious ADR during empagliflozin treatment (Supplemental Table S2). Individual serious ADRs (i.e. MedDRA preferred terms) that occurred in ≥0.10% of patients were angina pectoris (n = 10, 0.13%) and cerebral infarction (n = 16, 0.20%). A total of 41 deaths were reported, including 28 considered unrelated to empagliflozin and 13 for which a relationship with empagliflozin could not be excluded. The cause of death in the latter cases were sudden death (n = 2), bile duct cancer (1), hypopharyngeal cancer (1), malignant ascites (1), hepatic cancer (1), malignant neoplasm of unknown primary site (1), cardiac failure (1), infection/multiple organ dysfunction syndrome (1), interstitial lung disease (1), intestinal obstruction (1), subdural hematoma (1), and unknown reason (1).
Table 3. Adverse drug reactions by age of patient (safety analysis set).
3.4.2. ADRs of special interest
The incidence of ADRs of special interest and serious ADRs of special interest are shown in and Supplemental Table S2, respectively. Time to onset of first episodes of ADRs of special interest is shown in Supplemental Table S3.
Hypoglycemia occurred in 35 (0.44%) patients overall. Of these 35 patients, 11 were also taking sulfonylureas, 21 were taking insulin, and 3 were taking both. Three patients experienced serious hypoglycemia: 1 receiving a sulfonylurea and 2 receiving insulin; all patients were reported to be recovered or recovering at the end of the study.
Urinary tract infections and genital infections occurred in 85 (1.07%) and 52 (0.66%) patients, respectively. Urinary tract infections occurred in 2.11% of women and 0.46% of men. Six patients experienced a serious urinary tract infection, including 4 with serious pyelonephritis, all of whom recovered or were recovering. Genital infections occurred in 34 (0.68%) patients aged <65 years, 12 (0.58%) aged ≥65 to <75, and 6 (0.72%) aged ≥75, but no cases were serious. Genital infections occurred in 1.33% of women and 0.26% of men.
Volume depletion occurred in 40 (0.50%) patients overall. Dehydration occurred in 34 cases, including 16 cases in summer. Five of the 40 volume-depletion events occurred in patients using diuretics, including 2 on loop diuretics. Three (0.04%) patients had serious volume depletion events: 1 with decreased blood pressure and 2 with dehydration. Excessive urination was reported in 102 patients (1.29%) but there were no serious cases, and only 15 cases of nocturia were reported (0.19%) with little difference between age groups (0.18%, 0.24%, 0.12% of patients aged <65, ≥65 to <75, ≥75 years, respectively).
Three patients (0.04%) had lower limb amputations, all toe amputations, 2 of which involved diabetic gangrene.
Adverse events relating to increased ketones occurred in 39 (0.49%) patients overall; none were deemed serious. No cases of diabetic ketoacidosis were reported.
Cardiovascular events occurred in 53 (0.67%) patients overall, including 25 (0.50%) aged <65 years, 18 (0.87%) aged ≥65 to <75, and 10 (1.20%) aged ≥75. A total of 21 of the 53 patients (39.62%) had cardiovascular/cerebrovascular disease at baseline: 8/25 (32.00%) patients aged <65 years, 6/18 (33.33%) patients aged ≥65 to <75, and 7/10 (70.00%) patients aged ≥75. Of the cardiovascular events, cardiac disorders occurred in 29 (0.37%) patients and cerebral events occurred in 24 (0.30%) patients. Cardiac disorders included 12 (0.15%) patients with angina pectoris, 7 (0.09%) with heart failure, 5 (0.06%) with acute myocardial infarction, and 1 each (0.01%) with unstable angina, chronic heart failure, myocardial infarction, silent myocardial infarction, myocardial ischemia, and acute coronary syndrome. The incidence of cardiac disorders was similar across age groups (0.34%, 0.43%, 0.36% of patients aged <65, ≥65 to <75, ≥75 years, respectively). Cerebrovascular events included 16 patients (0.20%) with cerebral infarction (i.e. stroke), 2 each (0.03%) with carotid artery stenosis, cerebellar infarction and lacunar infarction, and 1 each (0.01%) with brain stem infarction, cerebral hemorrhage, subarachnoid hemorrhage, cerebral hematoma, embolic cerebral infarction and thrombotic cerebral infarction. Cerebral infarction occurred in 6 (0.12%) patients aged <65 years, 5 (0.24%) aged ≥65 to <75, and 5 (0.60%) aged ≥75. Of these patients, hypertension and dyslipidemia were present at baseline in 6 (100%) and 5 (83.33%) individuals aged <65 years, 4 (80%) and 3 (60%) of those aged ≥65 to <75, and 5 (100%) and 3 (60%) of those aged ≥75, respectively. No patients had dehydration with cerebral infarction.
Liver injury occurred in 44 (0.55%) patients. Two of these patients had serious liver injury: 1 (0.01%) was a 42-year-old man with bile duct stenosis and abnormal hepatic function due to exacerbation of autoimmune pancreatitis, who was recovering; the other was a 52-year-old woman with alcoholic hepatic cirrhosis who did not recover, which led to treatment discontinuation.
Renal impairment occurred in 22 (0.28%) patients. Of the 22 cases, 21 (0.26%) were renal impairment and 1 (0.01%) was prerenal failure; 2 cases were concomitant with dehydration, including the patient with prerenal failure. There were no serious cases.
Bone fractures occurred in 10 patients (0.13%). Malignancies occurred in 36 patients (0.45%).
An ADR of muscle weakness was reported for only 1 patient (0.01%): a non-serious case in a male aged 51 years at baseline with BMI 28.1 kg/m2, who had a body weight change from baseline of –3.4 kg during the study (Supplemental Table S4).
Only 496 of the 7931 patients (6.25%) up-titrated from empagliflozin 10 mg to 25 mg, and ADRs were reported for 97 (19.56%) of these individuals (Supplemental Table S5). Overall, the incidence of each ADR of special interest was low (<10 patients). Only increases in ketones occurred in >10 patients and was more frequent with 25 mg (n = 12, 2.42%) than 10 mg (n = 39, 0.49%); however, no cases of diabetic ketoacidosis occurred.
3.5. Body weight, eGFR, blood pressure and laboratory parameters
In the safety analysis set of patients, empagliflozin treatment was associated with significant reductions in body weight from baseline (). At last observation, empagliflozin reduced body weight by an overall mean of –2.96 kg (95% CI: –3.08, –2.84), with a similar magnitude of change across age groups ().
Figure 1. Change in body weight over time (safety analysis set). (a) Change from baseline in body weight; (b) bodyweight. CI: confidence interval; SD: standard deviation.
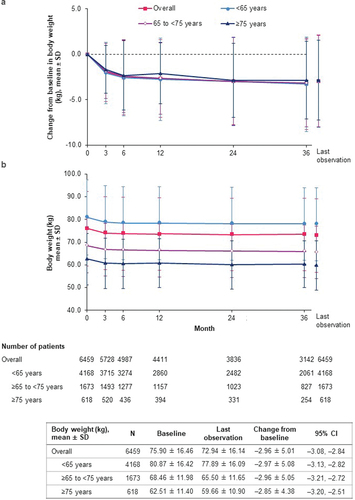
The overall mean change from baseline in eGFR at last observation was –2.85 ml/min/1.73 m2 (95% CI: –3.33, –2.38) from the baseline mean value of 81.76 ml/min/1.73 m2, with a similar magnitude of change across age groups ().
Figure 2. Change in eGFR (J-MDRD) over time (safety analysis set). (a) Change from baseline in eGFR; (b) eGFR. CI: confidence interval; eGFR: estimated glomerular filtration rate; J-MDRD: Japanese version of the Modification of Diet in Renal Disease study equation; SD: standard deviation.
Overall mean changes in hematocrit/hemoglobin, serum lipids, markers of kidney function, pulse rate and blood pressure are shown in . At baseline, pulse pressure was higher in older age groups but decreased slightly in all age groups soon after initiation of empagliflozin treatment, with reductions sustained over the course of the study (Supplemental Figure S2). The overall mean change in pulse pressure from baseline at last observation was –1.2 mmHg (95% CI: –1.5, –0.9).
Table 4. Change from baseline in laboratory parameters (safety analysis set).
3.6. Effectiveness
Over the course of the study, levels of both HbA1c and FPG were reduced (). The overall mean change in HbA1c from the baseline mean of 8.03% was –0.75% (95% CI: –0.78, –0.72) at last observation (). Both baseline levels of HbA1c and the magnitude of HbA1c reduction during the study were lower with increased age (). Across baseline eGFR categories of ≥90, 60 to <90, 45 to <60, and 30 to <45 ml/min/1.73 m2, mean HbA1c reductions at last observation were, respectively: –0.99% (95% CI: –1.05, –0.92) from baseline mean of 8.34%, –0.65% (–0.69, –0.61) from baseline 7.88%, –0.53% (–0.61, –0.45) from baseline 7.81%, and –0.54% (–0.80, –0.29) from baseline 8.02% (Supplemental Table S6).
Figure 3. Change in HbA1c (effectiveness analysis set). (a) Change from baseline in HbA1c; (b) HbA1c. CI: confidence interval; HbA1c: glycated hemoglobin; SD: standard deviation.
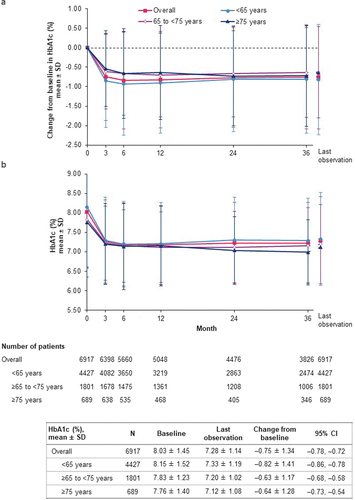
Figure 4. Change in FPG (effectiveness analysis set). (a) Change from baseline in FPG; (b) FPG. CI: confidence interval; FPG: fasting plasma glucose; SD: standard deviation.
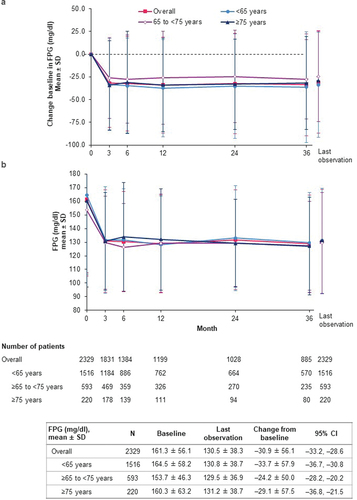
Overall mean change in FPG was –30.9 mg/dl (95% CI: –33.2, –28.6) at last observation from the baseline mean level of 161.3 mg/dl (). Baseline FPG levels and the magnitude of FPG reduction during the study were similar across age groups (). Mean FPG changes ranged from –36.9 mg/dl (95% CI: –41.2, –32.6) in those with baseline eGFR ≥90 ml/min/1.73 m2 to –22.7 mg/dl (–43.5, –2.0) in those with eGFR 30 to <45 ml/min/1.73 m2 (Supplemental Table S6).
Patients who up-titrated from 10 mg to 25 mg of empagliflozin during the study experienced an additional mean reduction in HbA1c of –0.21% (95% CI: –0.30, –0.11) for a total mean change from baseline of –0.64% (–0.77, –0.52). There was a similar incremental decrease in FPG level of –5.4 mg/dl (95% CI: –13.7, 2.8) after up-titration for a total mean change from baseline of –23.7 mg/dl (–33.3, –14.1) (Supplemental Table S7).
4. Discussion
These final results from the 3-year post-marketing surveillance study of empagliflozin in Japan (median treatment duration of 36.5 months) are consistent with the interim analysis (median ~25 months) [Citation22]. Empagliflozin was generally well tolerated, with ADRs occurring in 12.9% of patients overall compared with 8.5% in the interim analysis; the increase is probably related to the longer observation period. Furthermore, no new safety signals emerged, and the overall safety profile appears consistent with previous clinical trials of empagliflozin conducted in East Asia including Japan [Citation19,Citation25] and elsewhere [Citation18,Citation26]. Duration of treatment, safety and tolerability were all similar across age groups.
This study also provides new insights into ADRs of special interest. The incidence of hypoglycemia was low overall (0.45% of patients), which is consistent with randomized clinical trials and likely due to the insulin-independent mechanism of action of empagliflozin. The incidence of hypoglycemia was slightly higher in older patients, which may have been related to concomitant insulin use, as insulin was taken by only 9/18 patients aged <65 years who experienced hypoglycemia, compared with 6/9 and 6/8 patients aged 65 to <75 and ≥75 years, respectively. Expert recommendations on the use of SGLT2 inhibitors in Japan indicate to reduce their doses when used in combination with a sulfonylurea or insulin in order to minimize the risk of hypoglycemia [Citation27], as does the prescribing information for empagliflozin in Japan [Citation24].
Both urinary tract infections and genital infections are known risks of SGLT2 inhibitors, due to their mechanism of action (i.e. elicitation of glucosuria). In this study, the incidence of urinary tract infection was low overall (1.07% of patients), although slightly more common in the older age groups, with only 6 serious cases, all of whom had recovered or were recovering by the end of the study. Age-related increase in urinary tract infections was also observed in pooled analyses of multinational clinical trials where their incidence was similar with empagliflozin or placebo [Citation18]. The incidence of genital infections in this study was also low (0.66%) and did not increase across age groups. The higher incidence of urinary tract and genital infections in women than men in this study is consistent with pooled analyses of clinical trial data [Citation18,Citation19].
The potential for adverse events relating to volume depletion with SGLT2 inhibitors is also based on their mechanism of action, as glucosuria is accompanied by increased natriuresis and diuresis – at least transiently after drug initiation, as shown in studies in Japanese [Citation28] and European patients [Citation3]. In this study, however, only 1.29% of patients reported excessive urination generally and only 0.19% reported nocturia specifically, with low rates even in older individuals (0.24% and 0.12% of patients aged ≥65 to <75 and ≥75 years, respectively) – the latter is an important finding as nocturia is common in the elderly and negatively affects their general health and quality of life [Citation29,Citation30]. Furthermore, the rates of volume depletion and dehydration were low (0.50% and 0.43%, respectively). In Japan, increased fluid intake and monitoring for hydration in elderly patients is recommended during initial treatment with SGLT2 inhibitors, especially when administered in combination with diuretics [Citation27].
Concern about the risk of lower-limb amputations with SGTL2 inhibitors first arose from the CANVAS program in T2D patients where the incidence was almost doubled with canagliflozin compared with placebo (hazard ratio [HR]: 1.97; 95% CI: 1.41, 2.75) [Citation16]. However, no increased incidence of lower-limb amputation was seen with empagliflozin in the EMPA-REG OUTCOME trial (HR: 1.00; 95% CI: 0.70, 1.44) [Citation31] or in pooled safety analyses in either the overall [Citation18] or East Asian participants [Citation19]. In the current 3-year study, 3 patients had lower limb amputations, all involving toes, equating to an incidence rate of 0.16 per 1000 patient-years, which is lower than seen in a recent, prospective cohort study of Japanese T2D patients (0.47 per 1000 patient-years) [Citation32].
Diabetic ketoacidosis could plausibly result from SGLT2 inhibitor-mediated metabolic shift toward lipid utilization, leading to increased ketone body production [Citation33]. However, no cases of diabetic ketoacidosis were seen in this post-marketing study, although adverse events relating to an increase in ketones occurred in 39 patients (0.49%, none serious) – more commonly in those who up-titrated from 10 mg to 25 mg of empagliflozin, as consistent with a previous trial in Japanese T2D patients (in which ketone bodies remained in the physiological range) [Citation34]. Pooled analyses of clinical trials of empagliflozin found a low rate of diabetic ketoacidosis (<0.1%) in both the overall [Citation18] and East Asian participants [Citation19], which was no greater than the rate in patients receiving placebo.
The incidence of cardiovascular and cerebrovascular events was higher in older age groups in this study – unsurprisingly, given that age is a risk factor for such events. Approximately 40% of afflicted patients already had cardiovascular or cerebrovascular disease before initiating empagliflozin, with the highest baseline prevalence in those aged ≥75 years (70%). Most patients who had strokes during the study had hypertension and/or dyslipidemia at baseline, which are well-established risk factors for stroke in Japan [Citation35,Citation36], as in other countries. These findings should be interpreted in the context of the EMPA-REG OUTCOME trial, in which cardiovascular events were rigorously adjudicated by an independent Clinical Event Committee [Citation6,Citation37]. In that placebo-controlled trial in T2D patients with established cardiovascular disease, empagliflozin significantly reduced the risk for major adverse cardiovascular event [Citation6], as well as recurrent events [Citation38]. These findings were consistent in the subgroup of Asian participants [Citation8,Citation10].
In this study, liver injury, renal impairment, and bone fracture all occurred at a low rate overall (0.55%, 0.28% and 0.13% of patients, respectively). The higher incidence of liver injury in younger patients compared with older age groups is likely because more of the younger age group had preexisting hepatic impairment. Conversely, new cases of renal impairment during the study were more common in the older age groups, who also had higher rates of renal impairment at baseline – this is consistent with the fact that kidney function tends to deteriorate with age [Citation39]. In the EMPA-REG OUTCOME trial, empagliflozin treatment was associated with a significantly reduced risk for incident or worsening nephropathy in the overall and Asian participants [Citation7,Citation9]. Furthermore, eGFR initially decreased with empagliflozin compared with placebo in all age groups but subsequently stabilized after approximately 3 months, whereas eGFR in the placebo group continued to decline over the next 3 years [Citation40]. We observed a very similar trend in this post-marketing study, with an initial decline in eGFR after 3 months followed by stabilization of eGFR over the remaining 3 years. Notably, in EMPA-REG OUTCOME, an initial eGFR decrease over 10% with empagliflozin treatment was not associated with worse cardiorenal outcomes [Citation41].
The modest weight loss elicited by SGLT2 inhibitors is primarily due to calorie deficit from glucosuria and mostly involves reduction in adipose tissue [Citation1,Citation42,Citation43]. However, loss of adipose tissue may also be accompanied by some reductions in muscle mass [Citation44], which may lead to concern for sarcopenia, particularly in elderly T2D patients. In this study, the degree of weight loss was consistent across age groups. In addition, there were no cases of ADRs related to muscle weakness in the older age groups, with the only case reported during the study being in a patient younger than 65 years who fully recovered. To help elucidate this subject, the randomized EMPA-ELDERLY clinical trial is ongoing in Japan to specifically assess the effects of empagliflozin on skeletal muscle mass, muscle strength, and physical performance [Citation45].
Although pulse pressure, a surrogate marker for arterial stiffness, generally increases with age, it did not increase from baseline in any age group after initiation of empagliflozin, even in elderly patients. This finding is consistent with clinical trials of empagliflozin in patients with T2D or type 1 diabetes that either assessed pulse pressure or directly measured reductions in arterial stiffness using aortic pulse wave velocity [Citation46–49]. Taken together, these data suggest that empagliflozin may improve arterial stiffness in Japanese T2D patients, although further study is needed.
In the minority of patients who up-titrated from 10 mg to 25 mg during the study (6.25%), the incidence of ADRs was similar to the overall cohort. Of the ADRs of special interest in this subgroup, only increases in ketones occurred in more than 10 patients and at a higher rate than in patients receiving 10 mg, but there were no reported cases of diabetic ketoacidosis.
Finally, sustained and clinically meaningful reductions in HbA1c and FPG levels occurred in all age groups, irrespective of eGFR level (albeit those with baseline eGFR <30 ml/min/1.73 m2 were excluded from this analysis for off-label use). This finding suggests that the glycemic efficacy of empagliflozin in Japanese patients observed in clinical trials [Citation25,Citation50–54] translates to similar effectiveness in routine clinical practice.
This study has certain limitations, as well as some strengths. The main limitation is that its observational design with no comparator means that causality between empagliflozin and the reported adverse events cannot be conclusively established. Although both the study investigators and sponsor did attempt to judge which adverse events were related to study drug, resulting in the ADRs analyzed here, confounding factors such as concomitant medications, comorbidities, and healthcare provider support may have affected the findings. In addition, all treatment decisions regarding the choice and dosage of concomitant glucose-lowering therapy were made at the investigator’s discretion, including any changes in dose, and were not dependent on whether the patient had achieved treatment targets. Finally, underreporting of certain adverse events by patients cannot be ruled out, particularly those where symptoms may be subtle or absent, such as hypoglycemia. The strengths of the study include a larger sample of Japanese patients (7931) than in pre-approval clinical trials (1403), and data from routine clinical practice that included a large number of elderly patients (2907 aged ≥65 years).
5. Conclusions
In this post-marketing surveillance study in 7931 Japanese patients with T2D, empagliflozin was well tolerated and effective over the long term in routine clinical practice. These findings were consistent across age groups. Clinically meaningful improvements in glycemic control were sustained across the 3-year observation period, and modest decreases in body weight also occurred that were consistent across age groups. Overall, the safety profile was consistent with previous observational studies and clinical trials, and no new safety concerns emerged.
Acknowledgments
The authors thank the physicians and patients who participated in this study. The authors also thank Kaori Ochiai (PMS Center, EPS Corporation, Tokyo, Japan) for statistical analyses, and Hisaka Saisho (Medicine Division, Nippon Boehringer Ingelheim Co. Ltd., Tokyo, Japan) and Seiko Mizuno (Eli Lilly Japan K.K, Kobe, Japan) for their contribution to design of the study and interpretation of the data.
Author contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
Role of the sponsor
The Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Data availability
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Empagliflozin_PMS_long-term_manuscript_Final_draft_17Feb2022_supplemental.docx
Download MS Word (216.1 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2054987
Disclosure statement
K. Kaku has acted in an advisory role for Astellas Pharma, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, and Novo Nordisk Pharma; received honoraria or fees for promotional materials from Astellas Pharma, AstraZeneca, Daiichi Sankyo, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, Nippon Boehringer Ingelheim, Taisho-Toyama Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, and Kowa Pharmaceutical; and received scholarships or donations from Nippon Boehringer Ingelheim, Taisho-Toyama Pharmaceutical, Mitsubishi Tanabe Pharma, and Kowa Pharmaceutical. K. Yamamoto has received speakers’ bureau/honorarium from Otsuka Pharmaceutical, Daiichi-Sankyo, Novartis Pharma K.K.; and research funds from Abbott, Otsuka Pharmaceutical, Daiichi-Sankyo, Biotronik Japan, Japan Lifeline, Fukuda Denshi, Takeda Pharmaceutical, Novo Nordisk Pharma, Ono Pharmaceutical, Nihon Kohden; and consultation fees from Novartis Pharma K.K. Y. Fukushima and A. Yasui are employees of Nippon Boehringer Ingelheim Co. Ltd. H. Iliev is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG.
Additional information
Funding
References
- Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015 Mar;12(2):78–89.
- Frampton JE. Empagliflozin: a review in type 2 diabetes. Drugs. 2018 Jul;78(10):1037–1048.
- Heise T, Jordan J, Wanner C, et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016 Oct;38(10):2265–2276.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018 Dec;41(12):2669–2701.
- Araki E, Goto A, Kondo T, et al. Japanese Clinical Practice Guideline for Diabetes 2019. J Diabetes Investig. 2020 Jul;11(4):1020–1076.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26 373(22):2117–2128.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016 Jul 28 375(4):323–334.
- Kaku K, Lee J, Mattheus M, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease - results from EMPA-REG OUTCOME. Circ J. 2017 Jan 25 81(2):227–234.
- Kadowaki T, Nangaku M, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME((R)) trial. J Diabetes Investig. 2019 May;10(3):760–770.
- Kaku K, Wanner C, Anker SD, et al. The effect of empagliflozin on the total burden of cardiovascular and hospitalization events in the Asian and non-Asian populations of the EMPA-REG OUTCOME trial of patients with type 2 diabetes and cardiovascular disease. Diabetes Obes Metab 2022 Apr; 24(4):662–674.
- Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019 Jun 18 139(25):2822–2830.
- Patorno E, Pawar A, Wexler DJ, et al. Effectiveness and safety of empagliflozin in routine care patients: results from the EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study. Diabetes Obes Metab. 2022 Mar;24(3): 442–454. DOI:10.1111/dom.14593. .
- Seino Y, Kim DJ, Yabe D, et al. Cardiovascular and renal effectiveness of empagliflozin in routine care in East Asia: results from the EMPRISE East Asia study. Endocrinol Diabetes Metab. 2021 Jan;4(1):e00183.
- Sheu WH, Seino Y, Tan EC, et al. Healthcare resource utilization in patients treated with empagliflozin in East Asia. J Diabetes Investig. 2021. 10.1111/jdi.13728. Online ahead of print Dec 3.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019 Jan 24 380(4):347–357.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017 Aug 17 377(7):644–657.
- Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017 Jul 18 136(3):249–259.
- Kinduryte Schorling O, Clark D, Zwiener I, et al. Pooled safety and tolerability analysis of empagliflozin in patients with type 2 diabetes mellitus. Adv Ther. 2020 Aug;37(8):3463–3484.
- Yabe D, Yasui A, Ji L, et al. Safety and tolerability of empagliflozin in East Asian patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. J Diabetes Investig. 2019 Mar;10(2):418–428.
- Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013 Apr;1281(1):64–91.
- Ministry of Health, Labour, and Welfare, Japan. Summary of Patient Survey 2017 [Accessed 2021 Aug 16]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/17/dl/toukei.pdf
- Kaku K, Chin R, Naito Y, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: interim analysis from a post-marketing surveillance study. Expert Opin Drug Saf. 2020 Feb;19(2):211–221.
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009 Jun;53(6):982–992.
- Nippon Boehringer Ingelheim. Jardiance (empagliflozin) Japanese package insert. [cited 2021 Jul 21]. Available at: https://www.pmda.go.jp/
- Shiba T, Ishii S, Okamura T, et al. Efficacy and safety of empagliflozin in Japanese patients with type 2 diabetes mellitus: a sub-analysis by body mass index and age of pooled data from three clinical trials. Diabetes Res Clin Pract. 2017 Sep;131:169–178.
- Kohler S, Zeller C, Iliev H, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017 Jul;34(7):1707–1726.
- Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig. 2020 Jan;11(1):257–261.
- Yasui A, Lee G, Hirase T, et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018 Apr;9(2):863–871.
- Hirayama A, Torimoto K, Mastusita C, et al. Evaluation of factors influencing the natural history of nocturia in elderly subjects: results of the Fujiwara-kyo Study. J Urol. 2013 Mar;189(3):980–986.
- Suekane S, Ueda K, Suyama S, et al. Comprehensive health-related quality of life is influenced by nocturia and sleep disturbance: investigation based on the SF-8. Kurume Med J. 2016;62(1–2):9–16.
- Inzucchi SE, Iliev H, Pfarr E, et al. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018 Jan;41(1):e4–e5.
- Iwase M, Fujii H, Nakamura U, et al. Incidence of diabetic foot ulcer in Japanese patients with type 2 diabetes mellitus: the Fukuoka diabetes registry. Diabetes Res Clin Pract. 2018 Mar;137:183–189.
- Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016 May;65(5):1190–1195.
- Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin on free fatty acids and ketone bodies in Japanese patients with type 2 diabetes mellitus: a randomized controlled trial. Adv Ther. 2019 Oct;36(10):2769–2782.
- Tanizaki Y, Kiyohara Y, Kato I, et al. Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama study. Stroke. 2000 Nov;31(11):2616–2622.
- Ohta Y, Takao Y, Harada K, et al. Metabolic syndrome is a risk factor for acute cerebral infarction in a younger elderly Kurashiki population. J Stroke Cerebrovasc Dis. 2012 Apr;21(3):231–239.
- Zinman B, Inzucchi SE, Lachin JM, et al. Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. 2017 May;48(5):1218–1225.
- McGuire DK, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on first and recurrent clinical events in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a secondary analysis of the EMPA-REG OUTCOME trial. Lancet Diabetes Endocrinol. 2020 Dec;8(12):949–959.
- Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009 Dec;13(6):621–630.
- Monteiro P, Bergenstal RM, Toural E, et al. Efficacy and safety of empagliflozin in older patients in the EMPA-REG OUTCOME(R) trial. Age Ageing. 2019 Nov 1 48(6):859–866.
- Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. 2021 Mar;99(3):750–762.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2015 Dec 1; 309(11):F889–900.
- Sargeant JA, Henson J, King JA, et al. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab (Seoul). 2019 Sep;34(3):247–262.
- Sasaki T, Sugawara M, Fukuda M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig. 2019 Jan;10(1):108–117.
- Yabe D, Shiki K, Suzaki K, et al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021 Apr 7;11(4):e045844.
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015 Dec;17(12):1180–1193.
- Striepe K, Jumar A, Ott C, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017 Sep 19 136(12):1167–1169.
- Lunder M, Janic M, Japelj M, et al. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol. 2018 Dec 3 17(1):153.
- Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014 Jan 29 13(1):28.
- Kadowaki T, Haneda M, Inagaki N, et al. Efficacy and safety of empagliflozin monotherapy for 52 weeks in Japanese patients with type 2 diabetes: a randomized, double-blind, parallel-group study. Adv Ther. 2015 Apr;32(4):306–318.
- Sone H, Kaneko T, Shiki K, et al. Efficacy and safety of empagliflozin as add-on to insulin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020 Mar;22(3):417–426.
- Terauchi Y, Utsunomiya K, Yasui A, et al. Safety and efficacy of empagliflozin as add-on therapy to GLP-1 receptor agonist (liraglutide) in Japanese patients with type 2 diabetes mellitus: a randomised, double-blind, parallel-group phase 4 study. Diabetes Ther. 2019 Jun;10(3):951–963.
- Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018 Sep;20(9):2200–2209.
- Araki E, Tanizawa Y, Tanaka Y, et al. Long-term treatment with empagliflozin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015 Jul;17(7):665–674.
