ABSTRACT
Background
A recent 3-year post-marketing surveillance (PMS) study reaffirmed the safety and effectiveness of linagliptin in linagliptin-naïve Japanese patients with type 2 diabetes (T2D). We present further analyses from this study by body mass index (BMI).
Research design and methods
Safety and effectiveness were assessed across BMI subgroups (<25, 25 to <30, and ≥30 kg/m2).
Results
Data were available for 876, 566, and 201 patients in the BMI subgroups, respectively. Incidence of adverse drug reactions [ADR] with linagliptin was 11.42%, 11.31%, 10.45%, respectively. The most common ADR of special interest was hepatic disorders (n [%]: 6 [0.68], 7 [1.24], and 3 [1.49], respectively). Additional use of glucose-lowering drugs (GLDs) increased with BMI (15.0%, 19.1%, 24.4% of patients; P < 0.001). In the overall population, HbA1c change (adjusted mean %±SE) until week 156 was –0.71±0.04, –0.68±0.04 and –0.74±0.09. In patients receiving linagliptin with no additional GLDs, HbA1c changes were –0.58%±0.04, –0.62%±0.04, and –0.77%±0.11.
Conclusions
In this study of linagliptin in Japanese patients with T2D, across BMI subgroups no new safety concerns were observed. The proportion of patients with additional GLD use increased with baseline BMI. Decreases in HbA1c were observed in all subgroups, including in patients with no additional GLD use.
ClinicalTrials.gov
NCT01650259
1. Introduction
Recent estimates suggest that over 450 million people globally have diabetes, including 7.4 million individuals in Japan [Citation1], although this figure may be as high as 10 million [Citation2]. Guidelines for the management of type 2 diabetes (T2D) in Japan recommend that treatment strategies may vary depending on factors such as underlying cause of T2D, patient age, metabolic abnormalities, and status of diabetic complications [Citation3].
Dipeptidyl peptidase-4 (DPP-4) inhibitors have practically become the first-line therapy in many Japanese patients with T2D [Citation4]. Linagliptin, a DPP-4 inhibitor indicated for the treatment of T2D [Citation5], has been shown in randomized, controlled trials to significantly improve glycemic control without increasing the risk of hypoglycemia or causing weight gain [Citation6,Citation7].
In general, there is evidence to suggest that Asian patients with T2D, when compared with Caucasian patients with T2D, have a lower body mass index (BMI) and reduced β-cell function (in particular individuals from East Asia) [Citation8–11]. A systematic review and meta-analysis of studies comparing the glucose-lowering efficacy of DPP-4 inhibitors between Asian and non-Asian patients with T2D demonstrated that the efficacy of DPP-4 inhibitors was greatest in Asian patients, and that differences in BMI across ethnic groups may mediate this difference in efficacy [Citation12]. The baseline BMI correlated significantly with the glycated hemoglobin (HbA1c)-lowering efficacy of DPP-4 inhibitors in patients with a baseline BMI <30 kg/m2, such that the lower the BMI, the greater the reduction in HbA1c achieved [Citation12]. In a 24-week, multicenter, comparative study of sitagliptin versus pioglitazone in Japanese patients with T2D, Takihata et al. demonstrated that sitagliptin was more effective in patients with a BMI <25 kg/m2 than in those with a BMI >25 kg/m2 [Citation13]. A Korean post-marketing surveillance (PMS) study of linagliptin as monotherapy or part of combination therapy with other glucose-lowering drugs (GLDs) found that lower BMI was independently associated with increased efficacy [Citation14].
By contrast, other studies with linagliptin have shown a consistent glucose-lowering effect independent of BMI, in a multinational pooled analysis of three, Phase 3, placebo-controlled studies [Citation15], and similarly in a pooled analysis in Asian patients from 11 randomized trials of at least 24 weeks’ duration [Citation16].
In a recent PMS study, the safety and effectiveness of linagliptin was determined in Japanese patients with T2D followed up for 3 years [Citation17]. The study reaffirmed the glucose-lowering effectiveness and safety profile of linagliptin in a real-world setting. Furthermore, the reduction in HbA1c over 3 years occurred regardless of age (<65, ≥65) [Citation17] and estimated glomerular filtration rate (eGFR) [Citation18]. The large majority of the patients in the PMS study received linagliptin monotherapy without additional GLDs [Citation17].
Here, we present a new and further detailed analysis from this 3-year PMS study to evaluate the safety and effectiveness of linagliptin therapy in Japanese patients according to their BMI status (<25, 25 to <30, and ≥30 kg/m2) in real-world clinical practice.
2. Patients and methods
2.1. Study design and patients
This was an exploratory post-hoc analysis from a 3-year prospective, observational, PMS study in Japan. The study has been described previously, and involved linagliptin-naïve patients with T2D who were initiated on linagliptin 5 mg once daily as monotherapy for the period July 2012–October 2017. Patients were enrolled from nearly 600 clinical sites and followed for 156 weeks or until linagliptin discontinuation. Data on all GLDs used previously/discontinued before study enrollment were collected at baseline and presented as ‘prior use of GLDs.’
Physicians made all treatment decisions, and other GLDs were permitted post-enrollment to reflect routine clinical use of linagliptin [Citation17]. Patients with available BMI data were included in this subgroup analysis. The protocol for this PMS study was approved by the Japanese Ministry of Health, Labour and Welfare, and was conducted in full compliance with Japanese Good Postmarketing Study Practice regulations. This study involved the collection of anonymous data from clinical settings and, therefore, it was not necessary to obtain informed consent from patients. The study was registered on ClinicalTrials.gov (NCT01650259).
The primary endpoint was the incidence of adverse drug reactions (ADRs), defined as adverse events for which the causal relationship with linagliptin was assessed as definite or probable, or for which a causal relationship with linagliptin could not be excluded by either the investigator or the sponsor (or both). The secondary endpoint was the change in HbA1c from baseline to the last available observation [Citation17].
Observational points were at baseline and at 12, 26, 40, 52, 64, 78, 104, 130, and 156 weeks after initiation of linagliptin therapy, or at the time of linagliptin discontinuation.
2.2. Data analysis
Subgroup analyses of safety and effectiveness were performed based on three categories of BMI: <25, 25 to <30, and ≥30 kg/m2 at initiation of linagliptin treatment. For the safety evaluations, we analyzed the safety data with descriptive statistics. The incidence of ADRs was assessed in the safety analysis set (SAS), which included all patients who received at least one treatment of linagliptin, except for those for whom no data were available after enrollment. ADRs of special interest, including hepatic disorders, hypersensitivity, worsening of renal function, hypoglycemia, intestinal obstruction, pancreatic cancer, cardiac failure, skin lesions, infections, interstitial lung disease, pancreatitis and pemphigoid.
Statistical tests were used for all other (exploratory) analyses. Baseline characteristics in the subgroups by baseline BMI were summarized using descriptive statistics, which included mean and standard deviation (SD), median, minimum, and maximum for continuous variables, and frequencies and proportions for categorical variables. Different statistical tests were conducted for the different baseline characteristics, specifically Fisher’s exact test for sex, concomitant diagnoses, cardiovascular history, and prior use of GLDs; the Chi-squared test for age groups, duration of diabetes, and number of prior GLDs; one-way analysis of variance (ANOVA) for age, body weight, BMI, HbA1c, fasting plasma glucose and eGFR. Differences between subgroups in the proportion of patients with additional use of GLDs were determined by the Cochran-Armitage test.
For the efficacy analysis, the change in HbA1c from baseline to the last available observation was assessed in the effectiveness analysis set, which included all patients who were also included in the SAS, except for those for whom there were no effectiveness data. The effectiveness of HbA1c reductions in the subgroups by baseline BMI were analyzed by descriptive statistics, which included the mean and SD, median, minimum, and maximum. One-way ANOVA tests were conducted for a descriptive analysis of the change in HbA1c.
To identify the statistically significant variables for the change from baseline in HbA1c, multivariate regression analysis in a stepwise approach was performed (significance level P < 0.15) in the overall population [Citation19]. The dependent variable was identified as change from baseline in HbA1c at last observation. The independent variables for the analysis were sex, age, baseline HbA1c, baseline BMI, baseline eGFR, duration of diabetes, additional use of GLDs and prior use of GLDs as general population characteristics or relevant factors for disease state and/or control in HbA1c.
HbA1c over time was analyzed using a mixed model for repeated measures (MMRM) approach by BMI subgroup. The statistical model included baseline HbA1c as covariate, visit, baseline BMI and statistically significant variables as fixed effect, and patients as random effect, in order to minimize the effect of cofounders identified in the multivariate regression analysis. P-values for the BMI group-by-time interaction were calculated for the MMRM analysis.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA; https://www.meddra.org/) version 20.1.
3. Results
3.1. Baseline characteristics
The PMS study enrolled 2513 patients, and case report forms were collected for 2415 patients. A total of 2235 patients were included in the safety analysis set, from which BMI data were available for 1643 patients; 2054 patients were included in the effectiveness analysis set, from which BMI data were available for 1541 patients [Citation17,Citation18]. Data on BMI were available for 876, 566, and 201 patients in the <25, 25 to <30, and ≥30 kg/m2 subgroups, respectively. In the overall population, baseline characteristics were generally balanced between the 3 BMI subgroups with regard to fasting plasma glucose, cardiovascular history, and prior use of GLDs. The highest BMI subgroup (≥30 kg/m2) had patients who were slightly younger, with higher HbA1c, more concomitant diagnoses, a shorter duration of diabetes, a higher eGFR value, and a higher waist circumference, compared with patients in the other subgroups ().
Table 1. Baseline characteristics by baseline BMI (overall population)
Baseline characteristics analyzed by baseline BMI in patients who had received linagliptin monotherapy with no additional GLDs, and in patients who had linagliptin monotherapy with additional GLDs, are also shown in . Baseline characteristics were again generally balanced between BMI subgroups, with similar trends to those seen in the overall population regardless of whether treatment with linagliptin monotherapy included additional GLDs. However, baseline HbA1c and fasting plasma glucose values were slightly higher in patients who received additional GLDs versus those not receiving additional GLDs. In addition, the patient population who received additional GLDs versus the population who did not (data shown for ≥30 kg/m2 BMI subgroup) had more males (61.2% vs 50.7%), were younger (mean age, years ± SD: 51.1 ± 12.3 vs 58.1 ± 14.1) and had a slightly higher eGFR (mean eGFR, mL/min/1.73 m2 ± SD: 86.2 ± 23.2 vs 73.4 ± 23.6).
Furthermore, comparing patients who had received linagliptin with versus without additional GLDs during the study, a difference was seen across BMI subgroups in the proportion of patients who had no prior GLD use before the study. In patients receiving linagliptin without additional GLDs during the study, the proportion of patients with no prior use of GLDs was similar across baseline BMI groups (89.1%, 90.0%, and 90.8% for <25 kg/m2, 25 to <30 kg/m2, and ≥30 kg/m2 groups, respectively; ). By contrast, in patients receiving linagliptin with additional GLDs during the study, the proportion of patients with no prior use of GLDs increased with increasing baseline BMI (84.7%, 91.7%, and 93.9% for <25 kg/m2, 25 to <30 kg/m2, and ≥30 kg/m2 groups, respectively; ).
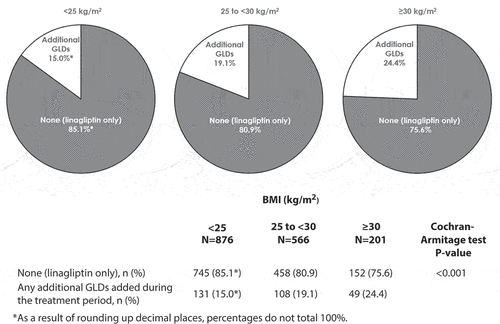
The proportion of patients in each BMI subgroup who received linagliptin only or additional GLDs during the study period is shown in . Across the three BMI subgroups, the proportion of patients who received linagliptin and no GLDs was 85.0% (n = 745), 80.9% (n = 458), and 75.6% (n = 152) in the <25, 25 to <30, and ≥30 kg/m2 subgroups, respectively (), indicating that the proportion of patients receiving additional GLDs increased with increasing BMI group, but even in the highest BMI group three-quarters of patients received only linagliptin. The proportion of patients with additional use of GLDs increased significantly (P < 0.001, Cochran-Armitage test) with increasing baseline BMI: <25 kg/m2, 15.0% of patients (n/N = 131/876); 25 to <30 kg/m2, 19.1% (n/N = 108/566); ≥30 kg/m2, 24.4% (n/N = 49/201).
Figure 1. Number (percentage) of patients receiving glucose-lowering drugs in addition to linagliptin during the study period by BMI subgroup. BMI, body mass index; GLD, glucose-lowering drug.
The proportion of patients receiving GLDs in addition to linagliptin during the study period by BMI subgroup and by class of GLD is shown in . The GLDs most frequently received by patients were biguanides and sulfonylureas. The proportion of patients receiving each type of GLD was broadly similar across each of the 3 BMI subgroups, although the proportion of patients receiving biguanides (5.3%, 11.1%, 15.4%) and sodium-glucose transport protein 2 inhibitors (0.5%, 2.3%, 7.5%) increased across the <25 kg/m2, 25 to <30 kg/m2, and ≥30 kg/m2 BMI subgroups, respectively.
Table 2. Breakdown of glucose-lowering drugs received in addition to linagliptin during the study period: Subgroup by BMI
3.2. Duration of treatment
In the overall population, the median (minimum–maximum) number of weeks of exposure to linagliptin was 154.0 (0.9–231.4), 155.3 (0.7–239.4), and 153.1 (3.1–212.3) in the <25, 25 to <30, and ≥30 kg/m2 BMI subgroups, respectively, with the majority of patients receiving >144 weeks of treatment, and a comparable proportion of patients within BMI subgroups across the different durations of treatment (Supplementary Figure S1A). A similar pattern was observed in duration of treatment in patients who received linagliptin with no additional GLDs (Supplementary Figure S1B), and in patients who received linagliptin monotherapy with additional GLDs (Supplementary Figure S1C).
3.3. Safety: ADRs
The incidence of ADRs, serious ADRs, and ADRs of special interest are shown in . The incidence of ADRs and serious ADRs were generally low and balanced across the 3 BMI subgroups, and similarly for each of the ADRs of special interest, including pancreatitis, pancreatic cancer and bullous pemphigoid. There were no cases of biliary cancer in the study.
Table 3. ADRs, serious ADRs, and ADRs of special interest
The incidence of hepatic disorders was low in the <25 kg/m2 group (n = 6 [0.68%]), but slightly elevated in the other BMI subgroups. In the 25 to <30 kg/m2 group, hepatic disorders were reported in 1.24% of patients (n = 7), comprising <1% of patients (n = 5) with ‘hepatic function abnormal’ and <1% of patients (n = 2) with ‘hepatic enzymes increased.’ In the ≥30 kg/m2 subgroup, hepatic disorders were reported in 1.49% of patients (n = 3), comprising 1% of patients (n = 2) with ‘hepatic function abnormal’ and 0.5% of patients (n = 1) with increased gamma-glutamyltransferase level. For all other ADRs of special interest, the incidence across all subgroups was <1% ().
3.4. Effectiveness: change in HbA1c
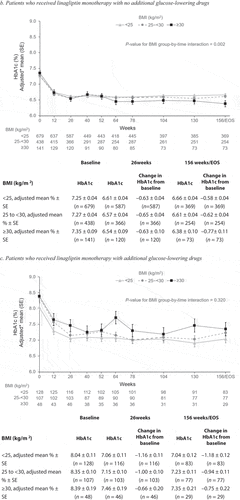
shows the change in HbA1c from baseline to week 26 and to week 156/end of study by BMI subgroup in the overall population, and in patients who received linagliptin with no additional GLDs (descriptive statistics). In the overall population, the change in HbA1c (mean % ± SD) from baseline to week 156/end of study was –0.66 ± 1.14 in the <25 kg/m2 BMI subgroup, –0.71 ± 1.25 in the 25 to <30 kg/m2 subgroup, and –1.09 ± 1.50 in the ≥30 kg/m2 subgroup (P = 0.006). A similar pattern was seen in patients who received linagliptin with no additional GLDs (), and in patients who received linagliptin monotherapy with additional GLDs (), although the decreases in HbA1c from baseline were slightly greater in patients receiving linagliptin and additional GLDs compared with the decreases seen in overall population.
Table 4. HbA1c change from baseline subgroup by BMI (descriptive statistics)
A multivariate regression analysis for change in HbA1c from baseline was conducted in the overall population, as well as in patients who received linagliptin with no additional GLDs and in patients who received linagliptin with additional GLDs, since the descriptive statistics suggest that differences in patient background may have influenced the effect of lowering HbA1c. The following variables were identified in the overall population and in patients receiving linagliptin with no additional GLDs as statistically significant variables for the change in HbA1c: age, baseline HbA1c, duration of diabetes, prior use of GLDs. In addition, for the overall population, additional use of GLDs was identified as a variable (Supplementary Table S1). In patients who received linagliptin with additional GLDs, age and baseline HbA1c were identified as statistically significant variables for the change in HbA1c. The multivariate analysis showed that the variable that most influenced the decrease in HbA1c was baseline HbA1c in all populations, based on the P and F values (overall population: P value < 0.001, F value = 1450.585; linagliptin with no GLDs, P value < 0.001, F value = 1059.550; linagliptin with additional GLDs, P value < 0.001, F value = 376.103). The F value is used in the stepwise approach for determining the independent variables. This multivariate regression analysis did not show a statistical difference of BMI associated with the change in HbA1c, in the overall population (P = 0.686), in patients who received linagliptin with no additional GLDs (P = 0.920) or in patients who received linagliptin with additional GLDs (P = 0.372) (Supplementary Table S1). Of note, neither gender nor baseline eGFR were found to be statistically significant variables for the change in HbA1c.
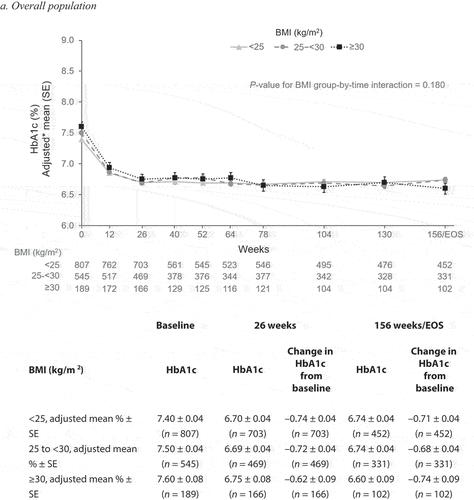
To minimize the effect of factors that influenced the reduction in HbA1c, an MMRM analysis was performed considering the statistically significant (P < 0.15) variables identified in the multivariate regression analysis for HbA1c change from baseline by baseline BMI. The change in HbA1c from baseline over time by BMI subgroup is shown in . In the overall population, across all three subgroups there was a decrease in HbA1c from baseline to week 26, with HbA1c levels remaining generally stable to week 156/end of study (). The change in HbA1c (adjusted mean % ± standard error [SE]) from baseline to week 26 was –0.74 ± 0.04 in the <25 kg/m2 BMI subgroup, –0.72 ± 0.04 in the 25 to <30 kg/m2 subgroup, and –0.62 ± 0.09 in the ≥30 kg/m2 subgroup. The corresponding change from baseline to week 156/end of study was –0.71 ± 0.04 in the <25 kg/m2 BMI subgroup, –0.68 ± 0.04 in the 25 to <30 kg/ m2 subgroup, and –0.74 ± 0.09 in the ≥30 kg/m2 subgroup (P-value for BMI group-by-time interaction = 0.180). A similar pattern was seen across all three subgroups in patients who received linagliptin with no additional GLDs (). The change in HbA1c (adjusted mean % ± SE) from baseline to week 26 was –0.63 ± 0.04 in the <25 kg/m2 BMI subgroup, –0.65 ± 0.04 in the 25 to <30 kg/m2 subgroup, and –0.63 ± 0.10 in the ≥30 kg/m2 subgroup. The corresponding change from baseline to week 156/end of study was –0.58 ± 0.04 in the <25 kg/m2 BMI subgroup, –0.62 ± 0.04 in the 25 to <30 kg/m2 subgroup, and –0.77 ± 0.11 in the ≥30 kg/m2 subgroup (P-value for BMI group-by-time interaction = 0.002). These trends were also seen across all three subgroups in patients who received linagliptin with additional GLDs (P-value for BMI group-by-time interaction = 0.320), although the changes in HbA1c from baseline were slightly greater at weeks 26 and 156/end of study across the three subgroups compared with the overall population and patients who received linagliptin with no additional GLDs ().
Figure 2. Change in HbA1c from baseline over time by BMI. Figures 2a,2b,2c *Adjusted mean ± SE is shown. MMRM analysis was performed. Tables show change in HbA1c from baseline MMRM results over time using the statistically significant (P < 0.05) variables identified in the multivariate regression analysis for HbA1c change from baseline by baseline BMI – efficacy set. BMI, body mass index; EOS, end of study; HbA1c, glycated hemoglobin; MMRM, mixed model repeated measures; SE, standard error. a. Overall population b. Patients who received linagliptin monotherapy with no additional glucose-lowering drugs c. Patients who received linagliptin monotherapy with additional glucose-lowering drugs.
4. Discussion
This subgroup analysis from a 3-year real-world PMS study of Japanese patients with T2D examined the safety and effectiveness of long-term linagliptin therapy according to baseline BMI status (<25, 25 to <30, and ≥30 kg/m2). The incidences of ADRs, serious ADRs, and ADRs of special interest were generally low and balanced across BMI subgroups in the overall population. No new safety findings were observed with linagliptin.
The data from our study demonstrate that linagliptin has a consistent glucose-lowering effect across all baseline BMI categories in patients with T2D. This is supported by previous studies of linagliptin, which have also shown a consistent glucose-lowering effect in patients with T2D regardless of their baseline BMI. For instance, in a pooled analysis of three Phase 3, 24-week, placebo-controlled multinational trials by Del Prato et al., which compared the efficacy and safety of linagliptin by patient baseline characteristics, linagliptin produced consistent efficacy across three subgroups of BMI (<25, 25 to 30, and ≥30 kg/m2), which included White, Asian and Black patients with T2D, albeit with a slightly greater magnitude of effect for patients in the lowest BMI group [Citation15]. Therefore, baseline BMI did not appear to largely affect the effectiveness in these clinical trial data of linagliptin.
In the current PMS study, the proportion of patients with additional use of GLDs increased significantly (P < 0.001) with increasing baseline BMI from 15.0% of patients in the <25 kg/m2 BMI subgroup to 24.4% of patients in the ≥30 kg/m2 BMI subgroup. This observation suggests that additional GLDs were required more frequently among patients in the highest baseline BMI subgroup in order to help achieve HbA1c treatment goals. Such trends have also been reported in previous studies with other DPP-4 inhibitors: for instance, in studies comparing the glucose-lowering effectiveness of DPP-4 inhibitors in Asian patients with T2D, the treatment effect was relatively lower in patients with a high baseline BMI versus patients with a low baseline BMI [Citation12,Citation13,Citation19–21]. It is therefore not surprising that the population of patients with the use of additional GLDs in this study showed a slightly greater glucose-lowering effect compared with the decreases in HbA1c seen in the overall population given that multiple anti-glycemic mechanisms were at work in patients with the use of additional GLDs. The findings from the multivariate regression analysis showed that baseline BMI was not a statistically significant factor for the change in HbA1c in linagliptin monotherapy population with no additional GLDs. Furthermore, from the MMRM analysis, which considered the significant variables identified in the multivariate analysis, baseline BMI did not largely affect the effectiveness of linagliptin in the population with no additional GLDs, which is similar to findings from clinical trials of linagliptin. Accordingly, by adjusting for statistically significant variables in the MMRM analysis, including baseline HbA1c as the variable with the largest influence on the change in HbA1c, the differences in the adjusted changes in HbA1c from baseline in the population with no additional GLDs were smaller between the BMI subgroups than the HbA1c changes seen in the descriptive statistics.
There are limitations to the current study. Some possible limitations of this study are shared with the primary analysis [Citation17]. Primarily, this was a post-hoc analysis using a single-arm observational study, and was not designed to compare linagliptin with other therapeutic interventions in terms of the safety and effectiveness, allowing alterations to diet, exercise, and the use of additional medications. Furthermore, potential non-adherence to diet and exercise recommendations in routine clinical practice should be considered as this may impact the success of treating to HbA1c targets, and may have affected the current findings. A specific limitation of this subgroup analysis relates to the unequal distribution of patients in each BMI group. In addition, our data represent obesity-related analyses according to BMI, but may not be representative of other adiposity or metabolism markers, which could form a topic for further research. These limitations should be taken into account when interpreting the findings in the present report.
5. Conclusions
The results of this subgroup analysis of a 3-year PMS study reaffirm the safety profile and effectiveness of long-term treatment with linagliptin in Japanese patients with T2D by baseline BMI in a real-world clinical practice setting. No new safety concerns were observed. The proportion of patients with additional use of GLDs increased with baseline BMI, and decreases in HbA1c were observed in all BMI subgroups, including in patients with no additional use of GLDs.
Author contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. F Yamamoto and T Okamura were involved in the study design, and F Yamamoto was involved in data collection. All authors agree to be accountable for all aspects of this work.
Role of the sponsor
Employees of Nippon Boehringer Ingelheim Co. Ltd were involved in the study design, data collection, data analysis, and preparation of the manuscript.
Declaration of interest
D Yabe has received consulting/lecture fees from Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Company Limited, and grants from Arkray Inc., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd., Taisho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Company Limited, and Terumo Corporation during the conduct of the study. F Yamamoto and T Okamura are employees of Nippon Boehringer Ingelheim Co. Ltd. SS Lund is an employee of Boehringer Ingelheim International GmbH. SS Lund owns shares in Novo Nordisk and shares in dynamically traded investment funds, which might own stocks from pharmaceutical companies. T Kadowaki reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, Fujirebio, Johnson & Johnson Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kowa Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., Sanwa Kagaku Kenkyusho Co., Ltd., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited and Terumo, grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Company Limited, contracted research from AstraZeneca K.K. and Takeda Pharmaceutical Company Limited, joint research from Daiichi Sankyo Company, Limited, and endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd and Takeda Pharmaceutical Company Limited. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Safety for their review work but have no other relevant financial relationships to disclose.
Data availability
The data that support the findings of this study are available from the corresponding author, Fumiko Yamamoto, upon reasonable request.
Supplemental Material
Download MS Word (356.9 KB)Acknowledgments
The authors thank all of the participants in the study analyzed in this manuscript, and Kaori Ochiai (EPS Corporation) for statistical support and expertise with the analyses.
Supplementary materials
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2057948
Additional information
Funding
References
- International Diabetes Federation. IDF diabetes atlas. Belgium: International Diabetes Federation; 2019. [cited 2022 Apr 5]. Available from: https://www.diabetesatlas.org/en/resources/.
- Ministry of Health, Labour, and Welfare, Japan. National Health and Nutrition Survey. 2016. [cited 2022 Apr 5]. Available from: https://www.mhlw.go.jp/english/
- Araki E, Goto A, Kondo T, et al., Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 11(4): 1020–1076. 2020.
- Yabe D, Higashiyama H, Kadowaki T, et al., Real-world observational study on patient outcomes in diabetes (RESPOND): study design and baseline characteristics of patients with type 2 diabetes newly initiating oral antidiabetic drug monotherapy in Japan. BMJ Open Diabetes Res Care. 8(2): e001361. 2020.
- Deeks ED. Linagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2012;72(13):1793–1824.
- Barnett AH, Huisman H, Jones R, et al. Linagliptin for patients aged 70 years or older with type 2 diabetes inadequately controlled with common antidiabetes treatments: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9902):1413–1423.
- Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–483.
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140.
- Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281(1):64–91.
- Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–1688.
- Yabe D, Seino Y, Fukushima M, et al. Beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015;15(6):602.
- Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56(4):696–708.
- Takihata M, Nakamura A, Tajima K, et al. Comparative study of sitagliptin with pioglitazone in Japanese type 2 diabetic patients: the COMPASS randomized controlled trial. Diabetes Obes Metab. 2013;15(5):455–462.
- Bae J, Song KH, Park JS, et al. Safety and effectiveness of linagliptin in Korean patients with type 2 diabetes: a postmarketing surveillance study. Diabetes Obes Metab. 2021;23(5):1208–1212.
- Del Prato S, Patel S, Crowe S, et al. Efficacy and safety of linagliptin according to patient baseline characteristics: a pooled analysis of three phase 3 trials. Nutr Metab Cardiovasc Dis. 2016;26(10):886–892.
- Ning G, Bandgar T, Hehnke U, et al., Efficacy and safety of linagliptin in 2681 Asian patients stratified by age, obesity, and renal function: a pooled analysis of randomized clinical trials. Adv Ther. 34(9): 2150–2162. 2017.
- Yamamoto F, Unno Y, Okamura T, et al., Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes mellitus: a 3-year post-marketing surveillance study. Diabetes Ther. 11(1): 107–117. 2020.
- Yamamoto F, Ikeda R, Ochiai K, et al., Long-term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes and renal dysfunction: a post-marketing surveillance study. Diabetes Ther. 11(2): 523–533. 2020.
- Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Community Health. 2020;8(1):e000262.
- Aso Y, Ozeki N, Terasawa T, et al. Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res. 2012;159(1):25–31.
- Nomiyama T, Akehi Y, Takenoshita H, et al. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;95(2):e27–28.