ABSTRACT
Background
Empagliflozin, a glucose-lowering drug licensed for type 2 diabetes (T2D), demonstrated tolerability and effectiveness overall in a post-marketing surveillance (PMS) study in Japan. However, the impact of body mass index (BMI) is unclear.
Research design and methods
This was a prespecified sub-analysis of the prospective, 3-year, PMS study of empagliflozin in Japan where the primary endpoint was adverse drug reactions (ADRs). We evaluated results according to BMI.
Results
We enrolled 7931 T2D patients treated with empagliflozin. Baseline mean age was 58.7 years; 63.01% were male. Baseline BMI was <20 kg/m2 in 2.06% of patients, while 21.28%, 37.35%, and 24.97% had BMI 20–<25, 25–<30 and ≥30 kg/m2, respectively. ADRs occurred in 19 (11.66%), 203 (12.03%), 411 (13.88%), and 295 (14.90%) patients with BMI <20, 20–<25, 25–<30 and ≥30 kg/m2, respectively. Excessive/frequent urination was the most frequent ADR of special interest in all BMI subgroups except 20–<25 kg/m2 (urinary tract infection). Mean change in glycated hemoglobin from baseline was –0.75%, with similar magnitude across BMI subgroups. Body-weight reduction seemed dependent on BMI, with almost no change in the <20 kg/m2 subgroup.
Conclusions
Empagliflozin appeared well tolerated and effective in Japanese T2D patients regardless of BMI, although the number of patients with BMI <20 kg/m2 was small in this study.
1. Introduction
Obesity, generally defined by body mass index (BMI), is an established risk factor for the development of type 2 diabetes (T2D) [Citation1], and the dramatically rising prevalence of T2D worldwide is associated with similar global increases in the number of people with obesity [Citation2]. However, BMI values in T2D patients in East Asian countries, including Japan, are generally lower than in Caucasian patients, and many East Asian patients with T2D are not obese [Citation3–6]. Consequently, East Asian-specific categories for BMI and waist circumference have been developed to assist the identification of obesity in the region. In Japan, although the prevalence of overweight and obesity among T2D patients has been increasing, most patients are not obese [Citation4,Citation6]. For example, the Shiga Diabetes Clinical Survey found that 83% of T2D patients aged less than 65 years and 95% of those aged 65 years or more were not obese in 2012, as defined by a BMI ≥30 kg/m2 [Citation4]. In addition, BMI was found to be an independent predictor of mortality in large cohorts of Japanese patients with diabetes [Citation7,Citation8]; specifically, those with low BMI (<18.5 kg/m2 [Citation8] or <20 kg/m2 [Citation7]) had a significantly increased risk of all-cause mortality compared to higher BMI subgroups.
Consequently, the effect of glucose-lowering drugs on body weight is an important consideration for selecting treatment to manage hyperglycemia. Although weight reduction is considered beneficial in patients with T2D who are overweight or obese, physicians may be concerned about weight loss in other individuals – especially elderly, lean patients – due to the potential risk of sarcopenia. Empagliflozin, a sodium-glucose co-transporter-2 (SGLT2) inhibitor, improves glycemic control in T2D patients by increasing urinary glucose excretion [Citation9,Citation10]. As with other SGLT2 inhibitors, empagliflozin also reduces body weight to a modest extent, possibly due to calorie loss from glucosuria [Citation11,Citation12]. As elderly patients typically have lower muscle mass than younger patients, Japanese experts recommend cautious use of SGLT2 inhibitors in certain elderly individuals [Citation13].
In addition to metabolic effects, SGLT2 inhibitors also reduce the risk for cardiorenal events, an effect that was first demonstrated in the landmark EMPA-REG OUTCOME study. In this multinational cardiovascular outcomes trial, empagliflozin significantly reduced the risk for cardiovascular death, all-cause mortality, hospitalization for heart failure, and progressive kidney disease in T2D patients with established cardiovascular disease [Citation14,Citation15]. These cardiorenal benefits were consistent in the subgroup of Asian patients [Citation16–18].
Empagliflozin was approved for treatment of T2D patients in Japan in 2014 and has subsequently demonstrated its overall glycemic effectiveness and safety profile in Japanese clinical practice in a routine post-marketing surveillance study [Citation19]. We evaluated the potential impact of BMI on the safety profile and effectiveness of empagliflozin in clinical practice in Japan by examining patient subgroups from this post-marketing surveillance study.
2. Patients and methods
The study design and methods are described briefly below; these have previously been reported in full [Citation19].
2.1. Study design and patient population
This study was conducted at 1,103 sites in Japan between June 2015 and December 2020 with 3 years of observation per patient (ClinicalTrials.gov identifier NCT02489942). The study adhered to the Ministry of Health, Labour and Welfare Good Post-Marketing Study Practice and Good Vigilance Practice ordinances. Therefore, it was not necessary to obtain either informed consent from patients or approval by an institutional review board or ethics committee.
The study population comprised T2D patients who had not previously received treatment with empagliflozin, although those who had previously been treated with another SGLT2 inhibitor were eligible. Patient data were de-identified prior to collection by electronic case report forms at months 3, 12, 24 and 36 after empagliflozin was initiated and at last observation if treatment was discontinued before month 36.
2.2. Assessments
The primary outcome was the incidence of adverse drug reactions (ADRs), defined as adverse events for which there was a possible relationship to empagliflozin treatment (or where that could not be judged due to insufficient or contradictory information) – as determined independently by either, or both, the study investigator and sponsor (the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance). The Medical Dictionary for Regulatory Activities (MedDRA) version 23.1 was used to categorize ADRs.
Other safety outcomes included serious ADRs and ADRs of special interest. Serious ADRs comprised those that were life-threatening or resulted in death, required or prolonged hospitalization, resulted in persistent or significant disability or incapacity, or were congenital anomalies/birth defects. Prespecified ADRs of special interest were adverse events relating to increases in ketones, bone fracture, cardiovascular event, diabetic ketoacidosis, genital infection, hypoglycemia, liver injury, malignancy, renal impairment, urinary tract infection, excessive urination/frequent urination, and volume depletion. After study initiation, lower limb amputations and muscle weakness (including sarcopenia) were also defined as ADRs of special interest.
Changes in levels of glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG) were the prespecified effectiveness endpoints. Changes in the following parameters were also assessed: body weight, blood pressure, pulse rate, hematocrit, hemoglobin, lipids, creatinine, and estimated glomerular filtration rate (eGFR) by the Modification of Diet in Renal Disease study equation modified for Japan [Citation20].
2.3. Statistical analysis
This study aimed to include at least 3000 patients completing 3 years of observation, which conferred 95% probability that an ADR with a true incidence of 0.10% would occur in ≥1 patient. For transparency on safety issues, all patients who received ≥1 dose of empagliflozin were included in the analysis of ADRs (the safety analysis set), except those with no visit after entry or those who did not have T2D, among other reasons. All patients in the safety analysis set were included in analysis of effectiveness data, except those without available HbA1c or FPG data – patients who initiated empagliflozin at 25 mg/day or had baseline eGFR <30 ml/min/1.73 m2 were also excluded, in order to discourage use that is off-label in Japan. Data were summarized descriptively without inferential analyses. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Statistical analyses were conducted by EPS Corporation (Tokyo, Japan) under contract by the sponsor.
3. Results
3.1. Patient disposition
As reported previously [Citation19], 8145 patients were enrolled and 8059 electronic case report forms were collected. The safety analysis set comprised 7931 patients while the effectiveness analysis set consisted of 7090 patients.
3.2. Patient characteristics at baseline
At baseline, BMI data were missing for 1138 of the 7931 patients (14.35%). A total of 2962 patients (37.35%) had BMI of 25 to <30 kg/m2 and 1980 (24.97%) had BMI ≥30, while 1688 (21.28%) had BMI of 20 to <25 and 163 (2.06%) had BMI <20 (). Overall, mean BMI was 28.12 kg/m2. Mean body weight was 75.56 kg overall, and 47.27 kg, 61.49 kg, 73.97 kg and 92.48 kg in the BMI subgroups of <20, 20 to <25, 25 to <30 and ≥30 kg/m2, respectively.
Table 1. Patient demographics and baseline characteristics by BMI of patient (safety analysis set)
Mean age was 58.7 years, with an inverse relationship between age and BMI subgroup whereby average age was higher in the lower BMI subgroups. Overall, 4996 patients (62.99%) were male, and females comprised a higher portion of the lower BMI subgroups than the higher BMI subgroups. Mean time since diagnosis of T2D was 8.23 years, while mean HbA1c was 8.02% and mean FPG was 160.2 mg/dl.
Most patients (84.13%) had comorbidities, most commonly hypertension (58.18%) and dyslipidemia (57.51%), both of which were more common in the higher BMI categories. Other comorbidities where the prevalence differed substantially between BMI categories included hepatic impairment (defined in ; 24.83% of patients overall) and gout/hyperuricemia (10.64%), which were more common at higher BMI. Conversely, osteoporosis (1.73% of patients overall) and malignant tumor (1.16%) were more common in the lower BMI categories.
Among patients taking at least 1 concomitant glucose-lowering drug (76.85%), dipeptidyl peptidase-4 (DPP-4) inhibitors (52.94% of patients overall) and alpha-glucosidase inhibitors (7.65% overall) were more commonly used in lower BMI subgroups while biguanides including metformin (41.66% overall) and glucagon-like peptide-1 (GLP-1) receptor agonists (4.75% overall) were more common in higher BMI subgroups. Statins (35.25% overall) and antihypertensive drugs were also more commonly used in higher BMI subgroups.
3.3. Drug exposure
Patients were treated with empagliflozin for a mean of 28.10 months (median 36.53; range: 0.0–57.4) (). Overall, 59.54% of patients received empagliflozin for >36 months: 48.47% of those with BMI <20 kg/m2, 59.00% with BMI 20 to <25, 61.41% with BMI 25 to <30 and 59.49% with BMI ≥30.
Table 2. Patient exposure to empagliflozin by BMI of patient (safety analysis set)
Among patients who discontinued empagliflozin, the main reasons overall were patient request (n = 601, 7.58%), adverse event (n = 516, 6.51%), no change/progressive disease (n = 263, 3.32%), and improvement/remission (n = 157, 1.98%). Adverse events and patient requests were also the most common reasons for discontinuation in patients with BMI <20 kg/m2.
3.4. Safety and tolerability
3.4.1. Incidence of ADRs and serious ADRs
Of the 7931 patients, 1024 (12.91%) experienced ≥1 ADR during treatment with empagliflozin: 19 (11.66%), 203 (12.03%), 411 (13.88%), and 295 (14.90%) of those with BMI <20, 20 to <25, 25 to <30 and ≥30 kg/m2, respectively ().
Table 3. Adverse drug reactions by BMI of patient (safety analysis set)
Excessive urination/frequent urination was the most common ADR of special interest in all BMI subgroups except 20 to <25 kg/m2 (<20 kg/m2: 1.84% of patients; 20 to <25: 0.95%; 25 to <30: 1.59%; ≥30: 1.31%). In patients with BMI of 20 to <25 kg/m2, the most common ADR of special interest was urinary tract infection (1.07%). Urinary tract infections also occurred in 1.11% and 1.31% of patients with BMI 25 to <30 and ≥30 kg/m2, respectively, but did not occur in any patients with BMI <20 kg/m2. Genital infections also tended to be more frequent in the higher BMI subgroups, occurring in 0%, 0.36%, 0.68% and 1.16% of patients with BMI <20, 20 to <25, 25 to <30, and ≥30 kg/m2, respectively. Muscle weakness occurred in only 1 patient, an individual in the BMI 25 to <30 subgroup, and was not serious (Supplemental Table S1).
Serious ADRs were reported for 166 patients overall (2.09%), occurring in 5 (3.07%), 42 (2.49%), 61 (2.06%) and 43 (2.17%) of patients with BMI <20, 20 to <25, 25 to <30 and ≥30 kg/m2, respectively (). The incidence of individual serious ADRs was <1% in all BMI subgroups. A total of 13 deaths (0.16% of patients overall) were reported as ADRs: none in the BMI <20 kg/m2 subgroup, 6 (0.36%), 3 (0.10%) and 1 (0.05%) in the subgroups of 20 to <25, 25 to <30 and ≥30 kg/m2, respectively, and 3 (0.26%) in the subgroup with missing BMI. Overall ADRs were also compared between baseline body weight categories and found to occur in 15.25%, 13.71%, 12.44% and 14.27% of those with body weight of ≤50, >50 to 70, >70 to 90 and >90 kg, respectively, and in 8.32% of those without data for body weight (Supplemental Table S2).
Table 4. Serious adverse drug reactions of special interest by BMI of patient (safety analysis set)
3.4.2. Body weight, eGFR, blood pressure and laboratory parameters
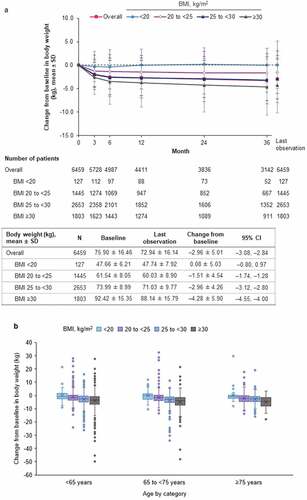
In the safety analysis set of patients, empagliflozin treatment was associated with significant overall reductions from baseline over time in body weight. At last observation, empagliflozin had reduced body weight by a mean of –2.96 kg overall (95% confidence interval [CI]: –3.08, –2.84) (). However, there was a positive association between magnitude of weight loss and BMI, with greater weight loss in patients with high BMI at baseline and little change in body weight in those with BMI <20 kg/m2. This association was consistent between subgroups of age <65 years, 65 to <75 years, and ≥75 years ().
Figure 1. (a) Change from baseline in body weight over time by BMI of patient (safety analysis set). (b) Change from baseline in bodyweight at last observation by BMI and age of patient (safety analysis set). BMI: body mass index; CI: confidence interval; N: number of patients; SD: standard deviation.
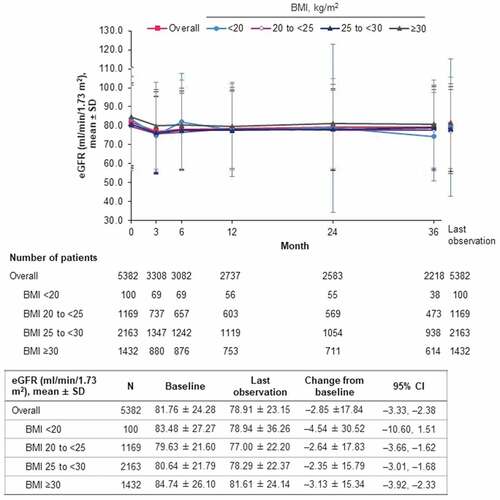
Overall, eGFR decreased from a baseline mean of 81.76 ml/min/1.73 m2 by a mean of –2.85 ml/min/1.73 m2 (95% CI: –3.33, –2.38) at last observation. Mean change was similar in patients with BMI 20 to <25, 25 to <30 and ≥30 (–2.64, –2.35, and –3.13 ml/min/1.73 m2, respectively) but higher in those with BMI <20 (–4.54 ml/min/1.73 m2) (). From baseline to month 3, mean change was –3.54 ml/min/1.73 m2 overall, and –3.78, –3.00, –3.46, –3.67 and –4.92 in those with BMI <20, 20 to <25, 25 to <30, ≥30 kg/m2 and missing data, respectively. From month 3 to month 36, mean change was 1.04 ml/min/1.73 m2 overall, and –2.82, 0.40, 1.75, 0.76 and 0.58 in those with BMI <20, 20 to <25, 25 to <30, ≥30 kg/m2 and missing data, respectively.
Figure 2. eGFR (J-MDRD) over time by BMI of patient (safety analysis set). BMI: body mass index; CI: confidence interval; eGFR: estimated glomerular filtration rate; J-MDRD: Japanese version of the Modification of Diet in Renal Disease study equation; N: number of patients; SD: standard deviation.
Changes in blood pressure and laboratory parameters are shown in .
Table 5. Change from baseline in laboratory parameters by BMI of patient (safety analysis set)
3.5. Effectiveness
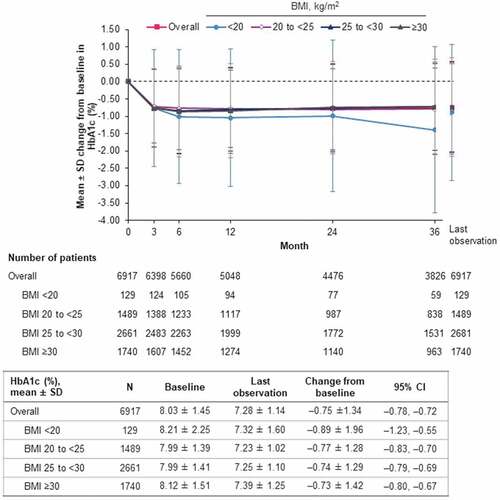
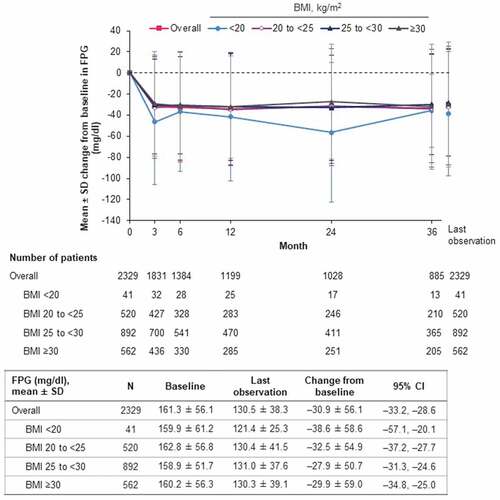
In the effectiveness analysis set, empagliflozin treatment was associated with reduction in levels of both HbA1c () and FPG (). Overall, mean HbA1c was reduced by –0.75% (95% CI: –0.78, –0.72) at last observation from a baseline level of 8.03%. The numerically largest reduction was in the BMI <20 kg/m2 subgroup, which also had higher mean baseline HbA1c than the other BMI subgroups (). Similarly, the reduction in mean FPG (overall: –30.9 mg/dl; 95% CI: –33.2, –28.6) was largest in the BMI <20 kg/m2 subgroup, although mean baseline levels were similar across subgroups ().
4. Discussion
In this study, we compared the safety and effectiveness of empagliflozin according to baseline BMI in Japanese T2D patients treated in routine clinical practice. We found that treatment with empagliflozin appeared to be well tolerated and effective over the long term, regardless of the BMI of patients.
Overall, the incidence of ADRs during the study tended to be slightly greater in the higher BMI subgroups compared with patients with lower BMI, who were older on average than those with higher BMI. The incidence of individual serious ADRs was low in all BMI subgroups (<1%). The low mortality rate observed during the study (0.16% of patients) might reflect the high quality of patient care as well as possibly a reduction in all-cause mortality with empagliflozin similar to that seen in the EMPA-REG OUTCOME trial (hazard ratio: 0.68 [95% CI: 0.57, 0.82]) [Citation16].
Excessive or frequent urination was the most frequent ADR of special interest in all BMI subgroups except those with BMI of 20 to <25 kg/m2 for whom it was the second most frequent ADR. Based on the mechanism of action of SGLT2 inhibitors, this is not a surprising finding, as glucosuria is accompanied by increased natriuresis and diuresis initially, as previously observed in Japanese [Citation21] and European patients [Citation22]. Overall, however, only a small minority of patients (1.29%) in this study reported excessive urination or frequent urination and there were no serious cases.
Interestingly, both urinary tract infections and genital infections tended to be more frequent in the higher BMI subgroups than in the lower BMI subgroups. Obesity is an established risk factor for certain types of infection such as surgical site and skin infections [Citation23], and the trend between urinary tract infections and increasing BMI in our study is consistent with a systematic review and meta-analysis of observational studies that found a positive association between BMI and incidence of urinary tract infection [Citation24].
No other ADRs or serious ADRs of special interest occurred at high incidence in any particular BMI subgroup.
SGLT2 inhibitors are known to have a modest body weight-reducing effect on average, based on studies in T2D patients who were mostly overweight or obese, which is likely due to direct calorie loss from glucosuria as well as indirect mechanisms such as lipolysis accompanying gluconeogenesis [Citation25–27]. Although this weight reduction is primarily due to loss of adipose tissue, there may also be some reduction in lean body mass, although this is not firmly established [Citation28]. Therefore, there is some concern that reduction of body weight by SGLT2 inhibitors may lead to sarcopenia, particularly in lean, elderly T2D patients [Citation27]. Thus, an important finding of this post-marketing surveillance study is that weight loss was positively associated with BMI: the larger the baseline BMI, the greater the body-weight reduction, and there was almost no change in body weight in patients with baseline BMI <20 kg/m2. This phenomenon was consistent across age subgroups. A similar effect was observed in Asian patients in the EMPA-REG OUTCOME cardiovascular outcomes trial where empagliflozin, compared with placebo, elicited weight loss mostly in patients with high BMI [Citation29]. Furthermore, in our study, the only case of muscle weakness considered to be an ADR occurred in a patient younger than 65 years with baseline BMI 28 kg/m2. Although our study did not assess changes in body composition with empagliflozin treatment, there is an ongoing randomized clinical trial of empagliflozin in Japan (EMPA-ELDERLY) that is designed to assess the effects of this SGLT2 inhibitor on skeletal muscle mass, muscle strength, and physical performance [Citation30].
Mean eGFR declined slightly over the first 3 months of treatment with empagliflozin then subsequently stabilized over the remainder of the 3 years, both overall and in each BMI subgroup, as shown in , albeit the estimates had wide variability in the BMI <20 kg/m2 subgroup due to the small number of patients with long-term data (e.g. n = 38 at 36 months). There was an overall mean reduction in eGFR at month 36 of 2.50 ml/min/1.73 m2 from the baseline mean of 81.76 ml/min/1.73 m2; this absolute reduction of 3.1% over 3 years is consistent with pooled Japanese safety data from pre-marketing clinical trials [Citation31]. This slight reduction in eGFR is likely to reflect age- and/or disease-related decline in kidney function rather than a drug effect, as empagliflozin has been consistently shown to slow eGFR decline relative to placebo over the long term, notably in the EMPA-REG OUTCOME trial where eGFR in the empagliflozin group declined in the first few weeks after treatment initiation but subsequently remained stable, whereas eGFR in the placebo group declined over the long term to a lower average level than in the empagliflozin group [Citation32]. The reduction in eGFR was greater in patients with BMI <20 kg/m2, possibly because this subgroup had a greater proportion of patients with diabetic nephropathy and cardiac failure, and/or receiving loop diuretics. However, this finding is subject to uncertainty, given the limited number of patients with BMI <20 kg/m2 who had eGFR data and the fact that eGFR may overestimate true GFR in older patients with low muscle mass [Citation33].
With regards to the glycemic effects of empagliflozin treatment, clinically meaningful reductions in HbA1c and FPG occurred in this study irrespective of baseline BMI category.
The major limitation of this study is its observational design with no comparator, meaning that causality between empagliflozin and the reported ADRs cannot be conclusively established, as confounding factors such as concomitant medications and comorbidities may have contributed. Furthermore, analyses were descriptive in nature, BMI data were lacking for 1138 patients, and medication adherence and persistence were not measured. Conversely, its strengths include a larger sample of Japanese patients (n = 7931) than in pre-marketing clinical trials (n = 1403 [Citation34]), data from routine clinical practice that included a large number of elderly patients, and evaluation of the safety and effectiveness of empagliflozin in patients with BMI <20 kg/m2 in routine clinical settings in Japan (an under-investigated demographic). Although there was limited data for HbA1c, FPG, body weight and eGFR in patients with BMI <20 kg/m2, and consequently high variability, we believe these findings are informative, given the paucity of such data for SGLT2 inhibitors in patients with low BMI in Japan.
5. Conclusions
In this 3-year post-marketing surveillance study, empagliflozin appeared well tolerated and effective over the long term in routine clinical practice in Japan, regardless of the BMI of patients. ADRs were consistent with previous studies and the prescribing information for empagliflozin in Japan, and no new safety signals emerged. Furthermore, clinically meaningful improvements in glycemic control were sustained over the 3-year observation period in all BMI subgroups. Mean body weight did not change significantly in patients with BMI <20 kg/m2, but those with higher BMI experienced modest reductions in body weight, on average. These findings complement the recent subgroup analysis of the EMPA-REG OUTCOME cardiovascular outcomes trial showing that empagliflozin reduced cardiorenal and mortality risk in Asians regardless of BMI, including those with a lower BMI [Citation29].
Declaration of interests
K Kaku has acted in an advisory role for Astellas Pharma, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, and Novo Nordisk Pharma; received honoraria or fees for promotional materials from Astellas Pharma, AstraZeneca, Daiichi Sankyo, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, Nippon Boehringer Ingelheim, Taisho-Toyama Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, and Kowa Pharmaceutical; and received scholarships or donations from Nippon Boehringer Ingelheim, Taisho-Toyama Pharmaceutical, Mitsubishi Tanabe Pharma, and Kowa Pharmaceutical. K Yamamoto has received speakers’ bureau/honorarium from Otsuka Pharmaceutical, Daiichi-Sankyo, Novartis Pharma K.K.; and research funds from Abbott, Otsuka Pharmaceutical, Daiichi-Sankyo, Biotronik Japan, Japan Lifeline, Fukuda Denshi, Takeda Pharmaceutical, Novo Nordisk Pharma, Ono Pharmaceutical, Nihon Kohden; and consultation fees from Novartis Pharma K.K. Y Fukushima and D Nitta are employees of Nippon Boehringer Ingelheim Co. Ltd. S Mizuno is an employee of Eli Lilly Japan K.K. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Safety for their review work but have no other relevant financial relationships to disclose.
Author contributions
All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
Role of the sponsor
The Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Data availability
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Empagliflozin_PMS_BMI_manuscript_Revision_31Mar2022_supplemental_clean.docx
Download MS Word (61 KB)Acknowledgments
The authors thank the physicians and patients who participated in this study. The authors also thank Kaori Ochiai (PMS Center, EPS Corporation, Tokyo, Japan) for statistical analyses, and Hisaka Saisho (Medicine Division, Nippon Boehringer Ingelheim Co. Ltd., Tokyo, Japan) for contribution to design of the study and interpretation of the data. Medical writing assistance was provided by Giles Brooke, PhD, of Elevate Scientific Solutions during the preparation of this article, and was funded by Nippon Boehringer Ingelheim Co. Ltd.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2062322
Additional information
Funding
References
- van Dam RM. The epidemiology of lifestyle and risk for type 2 diabetes. Eur J Epidemiol. 2003;18(12):1115–1125.
- Sun H, Saeedi P, Karuranga S, et al. IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021;24:109–119.
- Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the Diabetes Study of Northern California (DISTANCE). Diabetes Care. 2013 Mar;36(3):574–579.
- Miyazawa I, Kadota A, Miura K, et al. Twelve-year trends of increasing overweight and obesity in patients with diabetes: the Shiga Diabetes Clinical Survey. Endocr J. 2018 May 28;65(5):527–536.
- Zhou X, Ji L, Ran X, et al. Prevalence of obesity and its influence on achievement of cardiometabolic therapeutic goals in Chinese type 2 diabetes patients: an analysis of the nationwide, cross-sectional 3B study. PLoS One. 2016;11(1):e0144179.
- Sone H, Ito H, Ohashi Y, et al. Obesity and type 2 diabetes in Japanese patients. Lancet. 2003 Jan 4;361(9351):85.
- Kubota Y, Iso H, Tamakoshi A, et al. Association of body mass index and mortality in Japanese diabetic men and women based on self-reports: the Japan Collaborative Cohort (JACC) study. J Epidemiol. 2015;25(8): 553–558.
- Tanaka S, Tanaka S, Iimuro S, et al. Body mass index and mortality among Japanese patients with type 2 diabetes: pooled analysis of the Japan Diabetes Complications Study and the Japanese Elderly Diabetes Intervention Trial. J Clin Endocrinol Metab. 2014 Dec;99(12):E2692–6.
- Michel MC, Mayoux E, Vallon V. A comprehensive review of the pharmacodynamics of the SGLT2 inhibitor empagliflozin in animals and humans. Naunyn Schmiedebergs Arch Pharmacol. 2015 Aug;388(8):801–816.
- Frampton JE. Empagliflozin: a review in type 2 diabetes. Drugs. 2018 Jul;78(10):1037–1048.
- Giaccari A. Sodium-glucose co-transporter inhibitors: medications that mimic fasting for cardiovascular prevention. Diabetes Obes Metab. 2019 Oct;21(10):2211–2218.
- Barnett AH. Impact of sodium glucose cotransporter 2 inhibitors on weight in patients with type 2 diabetes mellitus. Postgrad Med. 2013 Sep;125(5):92–100.
- Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig. 2020 Jan;11(1):257–261.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26;373(22):2117–2128.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016 Jul 28;375(4):323–334.
- Kaku K, Lee J, Mattheus M, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease - results from EMPA-REG OUTCOME. Circ J. 2017 Jan 25;81(2):227–234.
- Kadowaki T, Nangaku M, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME((R)) trial. J Diabetes Investig. 2019 May;10(3):760–770.
- Kaku K, Wanner C, Anker SD, et al. The effect of empagliflozin on the total burden of cardiovascular and hospitalization events in the Asian and non-Asian populations of the EMPA-REG OUTCOME trial of patients with type 2 diabetes and cardiovascular disease. Diabetes Obes Metab. 2022 Apr;24(4):662–674.
- Kaku K, Yamamoto K, Fukushima Y, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: final results of a 3-year post-marketing surveillance study. Expert Opin Drug Saf. 2022 Mar 22;1–14. Online ahead of print. doi:10.1080/14740338.2022.2054987.
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009 Jun;53(6):982–992.
- Yasui A, Lee G, Hirase T, et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018 Apr;9(2):863–871.
- Heise T, Jordan J, Wanner C, et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016 Oct;38(10):2265–2276.
- Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013 Mar;37(3):333–340.
- Alhabeeb H, Baradwan S, Kord-Varkaneh H, et al. Association between body mass index and urinary tract infection: a systematic review and meta-analysis of observational cohort studies. Eat Weight Disord. 2021 Oct;26(7):2117–2125.
- Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2015 Dec 1;309(11):F889–900.
- Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015 Mar;12(2):78–89.
- Sasaki T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig. 2019 Mar;10(2):193–195.
- Sargeant JA, Henson J, King JA, et al. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab (Seoul). 2019 Sep;34(3):247–262.
- Ji Q, Ji L, Mu Y, et al. Effect of empagliflozin on cardiorenal outcomes and mortality according to body mass index: a subgroup analysis of the EMPA-REG OUTCOME trial with a focus on Asia. Diabetes Obes Metab. 2021 Aug;23(8):1886–1891.
- Yabe D, Shiki K, Suzaki K, et al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021 Apr 7;11(4):e045844.
- Boehringer Ingelheim. Data on file.
- Wanner C, Heerspink HJL, Zinman B, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018 Nov;29(11):2755–2769.
- Pavkov ME, Nelson RG. Estimating GFR in the elderly - new approaches to an old problem. Kidney Int Rep. 2019 Jun;4(6):763–765.
- Shiba T, Ishii S, Okamura T, et al. Efficacy and safety of empagliflozin in Japanese patients with type 2 diabetes mellitus: a sub-analysis by body mass index and age of pooled data from three clinical trials. Diabetes Res Clin Pract. 2017 Sep;131:169–178.