ABSTRACT
Background
Some migraine treatments are contraindicated for patients with cardiovascular disease (CVD) or risk factors (CVRFs). We report safety and efficacy of lasmiditan, a new oral acute migraine treatment with no cardiovascular contraindication, in Japanese patients with CVRFs.
Research design and methods
MONONOFU was a multicenter, randomized, double-blind, placebo-controlled, phase 2 study of Japanese patients with migraine (met International Headache Society criteria, Migraine Disability Assessment score ≥11, disabling migraine for ≥1 year). Eligible patients were randomized (7:3:7:6) to placebo or lasmiditan 50, 100, 200 mg. This prespecified analysis described CVDs, CVRFs, and cardiovascular treatment-emergent adverse events (TEAEs). Efficacy (proportion pain-free, experienced pain relief, most bothersome symptom-free, or disability-free 2 hours post-dose) was evaluated within CVRF subgroups (≤1, ≥2).
Results
Of 846 randomized patients, 691 were analyzed (CVRF≤1: 375; CVRF≥2: 316). The proportion of lasmiditan-treated patients with ≥1 TEAE was not related to CVRF numbers. Eighteen (3.8%) lasmiditan-treated and three (1.4%) placebo-treated patients reported likely cardiovascular TEAEs. Lasmiditan was more effective than placebo at relieving pain, symptoms, and disability in both CVRF subgroups. There was no consistent relationship between CVRF subgroups and efficacy.
Conclusions
Lasmiditan was well tolerated and effective in Japanese patients with migraine and CVRFs.
Trial registration
ClinicalTrials.gov: NCT03962738.
1. Introduction
Migraine is a neurological disease that causes substantial disability and impact on quality of life. The prevalence of migraine in Japan is estimated to be 8.4–8.6% [Citation1,Citation2]. Japanese people with migraine experience substantial burden, including poorer quality of life, higher absenteeism, lower work productivity, and more regular physician visits than people without migraine [Citation3,Citation4].
Pharmacological treatment targeting a migraine attack (‘acute treatment’) comprises medications for symptom relief or to limit attack duration [Citation5]. However, as many as 42% of Japanese patients with migraine may be insufficient responders to acute treatment [Citation4]. In addition, some acute treatments, such as triptans, are contraindicated for patients with a history of cardiovascular disease (CVD) or with cardiovascular risk factors (CVRFs) [Citation6,Citation7].
This is because the triptan mechanism of action involves vasoconstriction, which can also occur in the coronary arteries [Citation6]. Safer and effective migraine treatment options are therefore needed for patients with migraine and a prior history of CVD or with CVRFs [Citation8]. These patients may be a substantial proportion of the migraine patient population. For example, in the American Migraine Prevalence and Prevention (AMPP) study, 69.5% of women and 73.4% of men had at least one CVRF and more patients aged ≥40 years were at high risk of a future CV event than those aged <40 years [Citation9].
Lasmiditan, an acute treatment for migraine with a novel mechanism of action, is a brain-penetrating high-affinity and highly selective 5-HT1F receptor agonist [Citation10]. Lasmiditan acts on the trigeminal nerve system to inhibit neurotransmitter release without causing vasoconstriction [Citation10,Citation11] and therefore is not contraindicated for patients with a history of CVD. Lasmiditan has been studied in two phase 2 [Citation12] and three phase 3 global studies [Citation13–16] and has been approved as an oral acute treatment for migraine in the United States. Consistent with the global studies, the phase 2 randomized clinical trial (MONONOFU study) demonstrated that lasmiditan is well tolerated and effective for acute treatment of Japanese patients with migraine [Citation17].
In an analysis of pooled data from two of the phase 3 global trials, the safety and efficacy of lasmiditan did not change with increasing numbers of CVRFs [Citation8]. This suggests that lasmiditan might be considered as a treatment option for patients with CVRFs. However, the global trials of lasmiditan mainly included non-Asian populations [Citation13–16], and it is not known if racial differences have any implications for the safety of lasmiditan in patients with CVRFs. In addition, the types of CVRFs may differ between Asian and non-Asian populations. In this analysis of data from the MONONOFU study, we evaluated the safety and efficacy of lasmiditan in acute treatment of migraine attacks in Japanese patients with CVRFs.
2. Materials and methods
2.1. Patients and study design
The study design and patient population for the MONONOFU study (ClinicalTrials.gov: NCT03962738) have been previously described [Citation17]. Briefly, the study was a prospective, multicenter, randomized, double-blind, placebo-controlled, phase 2 study of a single migraine attack. The study was conducted in accordance with the Declaration of Helsinki, the relevant laws and regulations in Japan, and the Guidance on Ethnic Factors in the Acceptability of Foreign Clinical Data (ICH-E5). Local institutional review boards approved the protocol for each site and all patients provided written informed consent.
As previously described [Citation17], patients included in the MONONOFU study were aged ≥18 years, had migraine with or without aura fulfilling the International Headache Society diagnostic criteria, had a history of disabling migraine for at least 1 year, had a history of 3–8 migraine attacks/month and less than 15 headache days/month during the past 3 months, and had a total Migraine Disability Assessment (MIDAS) score ≥11. This trial did not exclude patients with a known history of CVD or CVRFs such as coronary artery disease, clinically significant arrhythmia, and/or uncontrolled hypertension.
The primary objective of the MONONOFU study was to evaluate the efficacy of lasmiditan 200 mg on freedom from migraine headache pain at 2 hours post dose compared with placebo [Citation17]. Eligible patients were randomized to a single dose of placebo or lasmiditan 50, 100, or 200 mg in a 7:3:7:6 ratio. Patients were asked to treat a single qualifying migraine attack (moderate-to-severe, was not improving, and for which no other acute migraine treatments had been taken) within 4 hours of pain onset.
The objectives of this prespecified analysis of data from the MONONOFU study were to evaluate the cardiovascular safety of lasmiditan in the study population and to evaluate whether the safety and efficacy of lasmiditan were similar across population subgroups with ≤1 or ≥2 CVRFs.
2.1.1. Identification of baseline CVD
Identification of the subgroup of patients with baseline CVD was performed using medical history, reported by the patient at study entry. Participants were identified as having baseline CVD if they had one or more conditions that were part of the patients’ medical history or preexisting conditions in the narrow search terms of the following Standardized Medical Dictionary for Regulatory Activities 23.0 (MedDRA 23.0) Queries (SMQs): cardiac arrhythmias (including sub-SMQs bundle branch block [right or left], ventricular extrasystoles, arrhythmia, sinus node dysfunction, sinus tachycardia, and supraventricular tachycardia), cardiac failure, cardiomyopathy, central nervous system vascular disorders (this SMQ covers ischemic and hemorrhagic cerebrovascular accidents and includes the sub-SMQs of intracranial aneurysm, cerebral infarction, lacunar infarction, vertebral artery dissection, and cerebral endovascular aneurysm repair), embolic and thrombotic events (including sub-SMQs deep vein thrombosis, hemorrhoids thrombosed, and thrombophlebitis), hypertension, ischemic heart disease (including sub-SMQs angina pectoris and prinzmetal angina), pulmonary hypertension, and torsade de pointes/QT prolongation.
2.1.2. Identification of CVRFs
CVRFs were defined in accordance with the Japanese Comprehensive Risk Management Chart for Cerebro-Cardiovascular Diseases [Citation18] and were assessed at the baseline visit. A present/absent criterion was applied to each risk factor to assess the proportion of patients with each potential CVRF. Briefly, the risk factors were smoking habit, hypertension, diabetes, dyslipidemia, chronic kidney disease, obesity (body mass index ≥25 kg/m2), age (men ≥45 years, women ≥55 years), sex (male or postmenopausal female), and/or a family history of CVD [Citation18]. Full details of the risk factors are provided in Supplementary Table 1. Subgroup efficacy analyses compared patients with zero or one CVRF (CVRF≤1) with those with two or more CVRFs (CVRF≥2), following the method previously used in an analysis of global phase 3 trial data [Citation8]. This categorization was applied because many patients had one CVRF based on their age or sex alone.
2.2. Study evaluations and statistical analyses
2.2.1. Safety
All adverse events (AEs) were recorded by participants in a paper diary. A treatment-emergent AE (TEAE) was defined as an AE that newly occurred or that worsened in intensity within 48 hours post-dose and did not need to have a causal relationship with treatment. TEAEs were coded using the MedDRA 23.0.
Potential cardiovascular AEs (CV AEs) were identified using the SMQ broad and narrow terms cardiac arrhythmias, cardiac failure, cardiomyopathy, central nervous system vascular disorders, embolic and thrombotic events, hypertension, ischemic heart disease, pulmonary hypertension, and Torsade de pointes/QT prolongation. The list of potential CV AEs and individual patient data were medically reviewed to determine if the SMQ terms identified represented likely CV AEs. Medical review of potential CV AEs was conducted on blinded patient data by medical experts employed by the study sponsor. Treatment-emergent likely CV AEs were flagged as likely cardiovascular TEAEs (CV TEAEs).
Safety analyses were conducted using the safety population, which included all randomized patients who received at least one dose of study drug, regardless of whether they underwent any study assessments. Categorical safety measures are reported using descriptive statistics. TEAEs are presented across treatment groups and CVRF subgroups. Cardiovascular safety was assessed using likely CV TEAEs, presented across treatment groups.
2.2.2. Efficacy
Patients recorded their efficacy responses in an eDiary at set time points (pre-dose, and 0.5, 1, 1.5, 2, 3, 4, 24, and 48 hours post-dose). In the present analysis, comparisons were made between the CVRF subgroups (CVRF≤1, CVRF≥2) for the efficacy endpoints.
The primary efficacy endpoint was the proportion of participants in each group who were pain-free at 2 hours post-dose. Participants used the International Headache Society 4-point headache severity rating scale (0 = no pain, 1 = mild pain, 2 = moderate pain, and 3 = severe pain [Citation19]). Pain-free status was defined as moderate or severe pain at baseline becoming no pain. The key secondary endpoint was the proportion of participants with pain relief in each group at 2 hours post-dose. Pain relief was defined as moderate or severe pain at baseline becoming mild or no pain. Other secondary endpoints included the proportion of participants in each group who were free of their most bothersome symptom (MBS) associated with migraine at 2 hours post-dose, and the proportion of participants in each group who experienced no migraine-related disability at 2 hours post-dose. Participants’ MBS was selected by each individual at baseline (pre-dose) from the provided list of associated symptoms (nausea, phonophobia, or photophobia). Disability was assessed via the question ‘How much is your migraine interfering with your normal activities?’ (with four response options: not at all, mild interference, marked interference, and need complete bed rest).
Efficacy analyses were conducted using the modified intent-to-treat (mITT) population, who were treated with at least one dose of study drug, had at least one post-dose assessment, and were treated for a qualifying migraine attack within 4 hours (qualifying migraine attacks were moderate or severe). Logistic regression was used to model the relationship between the number of CVRFs and the efficacy of lasmiditan using the endpoints pain-free, pain relief, MBS-free, and disability. The model included treatment group, baseline usage of preventive migraine medications [Yes/No], subgroup (CVRFs [≤1, ≥2]), and treatment-by-subgroup interaction. For each of the efficacy outcomes pain-free, pain relief, MBS-free, and disability, odds ratios (ORs) were calculated between each lasmiditan dose and placebo in both CVRF subgroup categories (CVRF≤1, CVRF≥2). Results of these comparisons are presented as Forest plots.
Although multiplicity adjustments were made for both primary (lasmiditan 200 mg vs. placebo) and key secondary (lasmiditan 100 mg vs. placebo) analyses, no adjustments were made for the subgroup analyses reported here. Statistical analyses for the subgroup interactions were based on two-sided tests with a significance level of 0.05. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline clinical characteristics, CVRFs, and CVD
A total of 846 patients were randomized to placebo or lasmiditan dose groups. Of these, 691 treated a single migraine attack with a dose of study drug (safety population); 682 patients also provided postdose assessment data and treated their migraine within 4 h (mITT population) (). The distribution of the number and types of CVRFs was balanced between placebo- and lasmiditan-treated patients and generally balanced across the lasmiditan dose groups (). Most patients (78.1%) had ≥1 CVRF; 375 participants (54.3%) were classified in the CVRF≤1 subgroup and 316 participants (45.7%) in the CVRF≥2 subgroup (). The most common CVRFs were family history of CVD (43.4%) and sex (40.8% of participants were men or postmenopausal women). Other common CVRFs were obesity (21.7%), age (men ≥45 years, women ≥55 years; 20.5%), and hypertension (17.8%).
Figure 1. Patient disposition. aPatients who used at least one dose of study drug. bPatients with at least one dose of study drug and any post-dose headache severity or symptom assessments. cPatients treated with at least one dose of study drug, any post-dose assessments, and treated for a qualifying migraine attack within 4 hours. LTN lasmiditan, (m)ITT (modified) intent-to-treat.
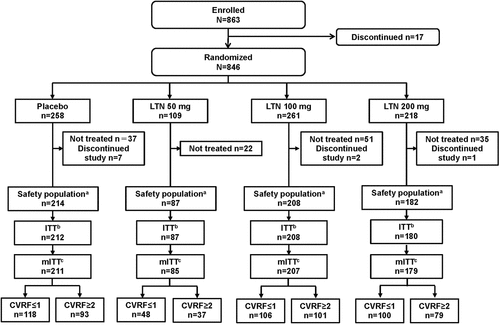
Table 1. Summary of cardiovascular risk factorsa
The distribution of baseline CVD in the safety population was generally similar across the treatment groups (Supplementary Table 2). Baseline CVD was reported in 23 participants (10.7%) in the placebo group, five participants (5.7%) in the lasmiditan 50-mg group, 20 participants (9.6%) in the lasmiditan 100-mg group, and 13 participants (7.1%) in the lasmiditan 200-mg group. The most frequently reported CVD type was hypertension, followed by cardiac arrhythmias and central nervous system vascular disorders.
In the mITT population, there were no apparent relationships between CVRF subgroup (CVRF≤1 or CVRF≥2) and baseline migraine clinical characteristics (attacks/month, migraine history, MIDAS score, and presence of aura) or use of preventive medication ().
Table 2. Baseline demographics and clinical characteristicsa, stratified by CVRF subgroup
3.2. Safety evaluations
The proportion of lasmiditan-treated patients who experienced ≥1 TEAE did not appear to be related to the number of CVRFs (). As previously reported [Citation17], the most common TEAE in lasmiditan-treated patients was dizziness. Although the incidence of dizziness differed by >15% among some lasmiditan-treated CVRF subgroups (e.g. 32.0% for CVRF≥4 vs. 49.2% for CVRF = 3), there was no trend of increasing incidence with increasing number of CVRFs. Similarly, no increasing trend with increasing number of CVRFs was observed for other TEAEs (; Supplementary Table 3; Supplementary Table 4).
Table 3. Most common TEAEsa (≥5% in all-lasmiditan group) and vertigob
Likely CV TEAEs were observed in three participants in the placebo group (1.4%) and 18 participants (3.8%) who received any dose of lasmiditan (all-lasmiditan group) (). The incidence of likely CV TEAEs was numerically higher in the lasmiditan-treated groups than in the placebo group. However, there was no consistent dose-relationship observed for any of the likely CV TEAEs among the lasmiditan-treated groups. The most common likely CV TEAE observed, across all treatment groups, was palpitations. There were no central nervous system vascular disorders, hypertension, embolic and thrombotic events, or ischemic heart disease TEAEs reported. All likely CV TEAEs were non-serious, and all participants recovered without intervention. One participant in the lasmiditan 200-mg group experienced a CV TEAE of palpitations considered to be of moderate severity; all other CV TEAEs were mild in severity. A single patient in the lasmiditan 50-mg group reported mild ‘faintness’ 2.3 hours post-dose that resolved immediately; this TEAE was coded as the preferred term ‘syncope’ under MedDRA 23.0. The patient had no history of any cardiovascular-related condition and this event resolved with no action.
Table 4. Summary of likely CV TEAEsa
3.3. Efficacy subgroup analyses
In both CVRF subgroups, the proportion of patients who were pain-free, experienced pain relief, were most bothersome symptom-free, or were disability-free 2 hours post-dose was higher with lasmiditan than placebo (). However, across the lasmiditan doses, there was no consistent relationship between CVRF subgroup and efficacy response rates. There was no statistically significant treatment-by-subgroup interaction except a treatment-by-CVRF subgroup interaction (two-sided test with alpha = 0.05) in pain-free patients (); p=0.016).
Figure 2. Relationship between CVRF subgroup categories and: (a) Proportion of patients who were headache pain-free at 2 hours post-dose (interaction p = 0.016); (b) Proportion of patients who experienced headache pain relief at 2 hours post-dose (interaction p = 0.235); (c) Proportion of patients who were MBS-free at 2 hours post-dose (interaction p = 0.601); (d) Proportion of patients who reported no disability at 2 hours post-dose (interaction p = 0.051). P-values are for the overall treatment-by-subgroup interaction term of the logistic regression model (model included treatment group, baseline usage of preventive migraine medications [Yes/No], subgroup [CVRFs {≤1, ≥2}], and treatment-by-subgroup interaction). CVRF cardiovascular risk factor, LTN lasmiditan, MBS most bothersome symptom.
![Figure 2. Relationship between CVRF subgroup categories and: (a) Proportion of patients who were headache pain-free at 2 hours post-dose (interaction p = 0.016); (b) Proportion of patients who experienced headache pain relief at 2 hours post-dose (interaction p = 0.235); (c) Proportion of patients who were MBS-free at 2 hours post-dose (interaction p = 0.601); (d) Proportion of patients who reported no disability at 2 hours post-dose (interaction p = 0.051). P-values are for the overall treatment-by-subgroup interaction term of the logistic regression model (model included treatment group, baseline usage of preventive migraine medications [Yes/No], subgroup [CVRFs {≤1, ≥2}], and treatment-by-subgroup interaction). CVRF cardiovascular risk factor, LTN lasmiditan, MBS most bothersome symptom.](/cms/asset/b31e9bf8-e9d2-4e5a-a84a-4588860cea7a/ieds_a_2078302_f0002_b.gif)
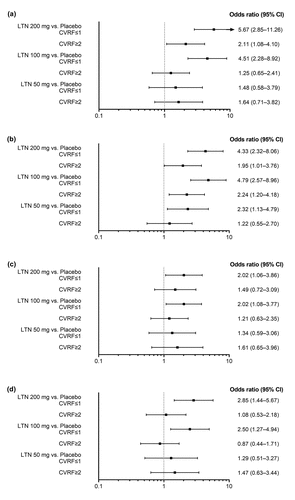
The proportion of participants who were pain-free at 2 hours post-dose was higher in all lasmiditan groups than in the placebo group (OR >1) for both the CVRF≤1 and the CVRF≥2 subgroups ()). Similar patterns were seen for patients who experienced pain relief, were MBS-free, and experienced no disability at 2 hours post-dose ()). Among participants who were pain-free and experienced pain relief, the CVRF≥2 subgroup had lower OR point estimates than the CVRF≤1 subgroup for lasmiditan 200 mg and 100 mg vs. placebo ().
Figure 3. Comparisons between each lasmiditan dose and placebo in each of the CVRF subgroup categories (Forest plots) for the following efficacy outcomes: (a) Patients who were headache pain-free at 2 hours post-dose (arrow indicates upper CI extending beyond the x-axis limit); (b) Patients who experienced headache pain relief at 2 hours post-dose; (c) Patients who were MBS-free at 2 hours post-dose; (d) Patients who reported no disability at 2 hours post-dose. CI confidence interval, CVRF cardiovascular risk factor, LTN lasmiditan, MBS most bothersome symptom.
4. Discussion
This analysis of data from the MONONOFU study is the first assessment of the safety and efficacy of lasmiditan in Japanese patients with migraine and CVRFs. Lasmiditan safety and efficacy were not affected by the number of CVRFs. The proportion of participants with one or more TEAEs and the types of TEAEs were similar across all CVRF subgroups, and no clinically relevant subgroup effects were observed. These subgroup analysis results suggest that lasmiditan is well tolerated in Japanese patients who have CVRFs.
Migraine patients often have comorbid CVD and/or CVRFs [Citation8,Citation9,Citation20]. In the present study, 8.8% of participants had baseline CVD. This was slightly lower than the incidence of CV events, conditions, or procedures (13.1%) among those surveyed in the AMPP study [Citation20]; this may be related to population-level differences in CVD prevalence between the United States and Japan [Citation21], as well as to the different study designs (i.e. interventional clinical trial vs. observational survey). However, the baseline incidence of two or more CVRFs in the present study (45.7%) was consistent with results from global phase 3 trials of lasmiditan, in which 41.3% had two or more CVRFs [Citation8]. Baseline incidence of two or more CVRFs was slightly higher than in the AMPP (39.5% [Citation9]), which may be related to the inclusion of the CVRF of family history, which was not included in the AMPP assessment of CVRFs [Citation9].
Apart from the risk factors of sex and age, the most common CVRFs in the MONONOFU patients were a family history of CVD (43.4%), obesity (21.7%), hypertension (17.8%), and dyslipidemia (10.3%). In Japanese patients with migraine included in the Adelphi Migraine Disease Specific Programme study, physicians reported frequencies of 27.4% for hypertension and 20.6% for hyperlipidemia [Citation4]; obesity and family history of CVD were not reported. The differences between the Adelphi data and the present study are likely due to intrinsic differences between real-world data (patient and physician surveys) and results from a clinical trial.
As previously described [Citation17,Citation22], the types of TEAEs reported with lasmiditan in Japanese patients are consistent with those reported in non-Japanese patients [Citation13–15,Citation23,Citation24]. The overall incidence of TEAEs experienced by patients in the MONONOFU study and the proportion of lasmiditan-treated patients who experienced ≥1 TEAE were not affected by the number of CVRFs. There was no increasing trend with increasing number of CVRFs observed for any of the common TEAEs, including central TEAEs, such as dizziness, that have been linked to possible short-term driving impairment [Citation25,Citation26]. The proportion of Japanese patients who experienced at least one likely CV TEAE was 3.8% in all lasmiditan-treated patients; this was higher than the proportion observed in the global pooled analysis (0.9%) [Citation8]. The difference is likely due to the overall rate of TEAEs being generally higher in the MONONOFU study (51–81%) than in the global clinical trials (25–61%) [Citation13–15,Citation17,Citation23]. Although the proportion of likely CV TEAEs was higher in lasmiditan-treated than in placebo-treated patients (1.4%), there was no clear relationship between lasmiditan dose and presence of CV TEAEs. Whereas the global analysis reported an OR for likely CV TEAEs of 2.46 (95% confidence interval [CI], 0.95–6.39; p = 0.06) [Citation8], in a post hoc analysis of the MONONOFU study, the OR was 3.23 (95% CI, 0.95–17.12; p = 0.07 [the Fisher exact test]). Therefore, the risk of likely CV TEAEs was not statistically significantly higher with lasmiditan treatment compared with placebo in either the global or the Japanese analyses. Palpitations were the most common likely CV TEAE reported in the present study; they were reported in patients across all treatment groups, including placebo, and in both CVRF subgroups. In the global analysis, palpitations were similarly reported across all CVRF subgroups [Citation8]. However, concomitant electrocardiogram or vital signs were not recorded during the post-dose period when AEs occurred, so it is not known whether the descriptor ‘palpitations’ reflects true changes in heart rate in all cases. Palpitations can also be associated with non-cardiac conditions such as anxiety or panic [Citation8]. The mechanism of action by which lasmiditan might cause palpitations or changes in heart rate is unknown [Citation8], but nevertheless it is advisable to be cautious in prescribing lasmiditan for concomitant use with other drugs that may affect heart rate.
In Japanese migraine patients with CVRFs, there was no consistent relationship between CVRF subgroups (≤1, ≥2) and the proportions of patients who were headache pain-free, experienced headache pain relief, or were MBS-free 2 hours post-dose. In the analysis of pooled CVRF subgroups of patients from global phase 3 trials of lasmiditan, the proportion of patients who were pain-free and MBS-free was similarly unaffected by the number of CVRFs [Citation8]. In the present study, although a treatment-by-CVRF subgroup interaction was observed for patients who were pain-free at 2 hours post-dose, this appeared not to be clinically meaningful because the proportion of pain-free participants was higher in all lasmiditan groups than in the placebo group in both CVRF ≤1 and CVRF ≥2 subgroups. In addition, given that the response differences between CVRF subgroups for the placebo arm (~11%) and for each lasmiditan arm were of similar magnitudes (~5–16%), these differences appear not to be clinically meaningful. The proportions of participants who experienced pain relief and those who were MBS-free at 2 hours post-dose were also higher in all lasmiditan groups than in the placebo group for both CVRF≤1 and CVRF≥2 subgroups. Among pain-free and pain relief participants, there was a trend toward higher ORs for the CVRF≤1 subgroup than for the CVRF≥2 subgroup, but this might be explained by the efficacy responses in the placebo group. For all efficacy outcomes, the placebo CVRF≥2 subgroup had higher response rates than the placebo CVRF≤1 subgroup, making the CVRF≥2 subgroup ORs relatively smaller than the CVRF≤1 ORs. Thus, the presence of CVRFs did not have an apparent consistent effect on the response rate of each lasmiditan dose group.
Strengths of this study include its prospective, multicenter, randomized, placebo-controlled design and its inclusion of patients with at least moderate migraine disability and a range of CVRFs. This was the first study to examine the safety and efficacy of lasmiditan in Asian patients with CVD and CVRFs. Limitations of this study include fewer patients in the lasmiditan 50-mg dose group than in the other dose groups – limiting interpretation of findings from this treatment group – and a small sample size of patients with a history of CVD. The subgroup analyses were, by nature, based on small sample sizes in each subgroup, which led to relatively wide CIs, and the study was not powered for these subgroup analyses. However, our purpose was to describe the overall trend for the subgroups, without making statistical comparisons between study arms. Despite these limitations, this study provides insight into the safety and efficacy of lasmiditan in Japanese patients with CVRFs, which may be verified in larger studies or by evaluation of post-marketing surveillance data.
5. Conclusions
In Japanese patients with migraine who also had CVRFs, lasmiditan was both well tolerated and effective. The relatively high proportion of Japanese patients with migraine who had multiple CVRFs in the MONONOFU study highlights the importance of providing medications that are not contraindicated for patients with CVRFs.
Declaration of interest
Y Hashimoto has received personal fees from Daiichi Sankyo Company, Limited and Pfizer Japan Inc. K Hirata received research funding from the Japanese Ministry of Health, Labour and Welfare and the Japan Agency for Medical Research and Development and has received personal fees from Amgen K.K., Eisai Co., Ltd., Eli Lilly Japan K.K., MSD K.K., Otsuka Pharmaceutical Co., Ltd., and Pfizer Japan Inc. Y Tanji, A Ozeki, and M Komori are employees of Eli Lilly Japan K.K. and have minor shareholdings in Eli Lilly and Company.
Reviewer disclosures
One reviewer is employed by Lundbeck. The remaining reviewers have no relevant financial relationships or otherwise to disclose.
Author contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. Y Hashimoto and K Hirata were investigators in the study. Y Tanji was involved in data curation and validation. A Ozeki conducted the statistical analysis and was involved in data curation and validation. M Komori was involved in study conceptualization, data curation, and validation.
Role of the sponsor
Eli Lilly Japan K.K. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Supplementary_Lasmiditan_CVRFs.docx
Download MS Word (46.6 KB)Acknowledgements
The authors would like to thank all study participants.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2078302
Additional information
Funding
References
- Sakai F, Igarashi H. Prevalence of migraine in Japan: a nationwide survey. Cephalalgia. 1997;17(1):15–22.
- Hirata K, Ueda K, Komori M, et al. Comprehensive population-based survey of migraine in Japan: results of the ObserVational Survey of the Epidemiology, tReatment, and Care of MigrainE (OVERCOME [Japan]) study. Curr Med Res Opin. 2021;37(11):1945–1955.
- Igarashi H, Ueda K, Jung S, et al. Social burden of people with the migraine diagnosis in Japan: evidence from a population-based cross-sectional survey. BMJ Open. 2020;10(11):e038987.
- Hirata K, Ueda K, Ye W, et al., Factors associated with insufficient response to acute treatment of migraine in Japan: analysis of real-world data from the Adelphi Migraine Disease Specific Programme. BMC Neurol. 20(1): 274. 2020.
- Japanese Society of Neurology and the Japanese Headache Society. Clinical practice guideline for chronic headache. 2013 [cited 2021 Mar 30]. Available from: https://www.neurology-jp.org/guidelinem/ch/documents/preface.pdf
- González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, et al. Side effects associated with current and prospective antimigraine pharmacotherapies. Expert Opin Drug Metab Toxicol. 2018;14(1):25–41.
- Dodick D, Lipton RB, Martin V, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414–425.
- Shapiro RE, Hochstetler HM, Dennehy EB, et al., Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain. 20(1): 90. 2019.
- Lipton RB, Reed ML, Kurth T, et al., Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 57(10): 1507–1521. 2017.
- Clemow DB, Johnson KW, Hochstetler HM, et al. Lasmiditan mechanism of action - review of a selective 5-HT1F agonist. J Headache Pain. 2020;21(1):71.
- Nelson DL, Phebus LA, Johnson KW, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30(10):1159–1169.
- Tfelt-Hansen PC, Olesen J. The 5-HT1F receptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled phase II trials. J Headache Pain. 2012;13(4):271–275.
- Brandes JL, Klise S, Krege JH, et al. Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study). Cephalalgia. 2019;39(11):1343–1357.
- Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–1904.
- Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222–e2232.
- Lipton RB, Lombard L, Ruff DD, et al. Trajectory of migraine-related disability following long-term treatment with lasmiditan: results of the GLADIATOR study. J Headache Pain. 2020;21(1):20.
- Sakai F, Takeshima T, Homma G, et al., Phase 2 randomized placebo-controlled study of lasmiditan for the acute treatment of migraine in Japanese patients. Headache. 61(5): 755–765. 2021.
- Joint Committee for Comprehensive Risk Management Chart for the Prevention of Cerebro-Cardiovascular Diseases. Comprehensive risk management for the prevention of cerebro-cardiovascular diseases in Japan. J Atheroscler Thromb. 2017;24(7):749–764.
- Diener H-C, Tassorelli C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: fourth edition. Cephalalgia. 2019;39(6):687–710.
- Buse DC, Reed ML, Fanning KM, et al. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2017;57(1):31–44.
- Iso H. Lifestyle and cardiovascular disease in Japan. J Atheroscler Thromb. 2011;18(2):83–88.
- Hirata K, Matsumori Y, Tanji Y, et al. Safety profile of lasmiditan: phase 2 study for a single migraine attack in Japanese patients. Expert Opin Drug Saf. 2021;20:721–733. in review
- Ashina M, Reuter U, Smith T, et al. Randomized, controlled trial of lasmiditan over four migraine attacks: findings from the CENTURION study. Cephalalgia. 2021;41(3):294–304.
- Hou M, Xing H, Li C, et al. Short-term efficacy and safety of lasmiditan, a novel 5-HT1F receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J Headache Pain. 2020;21(1):66.
- Food and Drug Administration. Evaluating drug effects on the ability to operate a motor vehicle: guidance for industry 2017. 2017. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/evaluating-drug-effects-ability-operate-motor-vehicle
- Pearlman EM, Wilbraham D, Dennehy EB, et al. Effects of lasmiditan on simulated driving performance: results of two randomized, blinded, crossover studies with placebo and active controls. Hum Psychopharmacol. 2020;35(5):e2732–e2732.
