ABSTRACT
Background
Studies on the efficacy of prescription omega-3 polyunsaturated fatty acids to reduce cardiovascular events have produced conflicting results.
Research design and methods
This 3-year prospective post-marketing surveillance study evaluated the effect of omega-3-acid ethyl esters (O3AEE; usual dosage 2 g/day) on cardiovascular events in high-risk statin-treated Japanese patients with hypertriglyceridemia. Statin-treated patients not receiving O3AEE were included as a reference cohort. The composite primary endpoint was cardiovascular death, myocardial infarction, stroke, angina requiring coronary revascularization, or peripheral arterial disease requiring surgery or peripheral arterial intervention.
Results
At 3 years, Kaplan–Meier estimated cumulative incidence of the primary endpoint was 2.5% (95% confidence interval, 2.1%–2.9%) in O3AEE-treated patients (N = 6,580) and 2.7% (2.4%–3.1%) in non-O3AEE-treated patients (N = 7,784; hazard ratio, 0.99; 95% confidence interval, 0.79–1.23). Incidence of heart failure requiring hospitalization was 0.4% with O3AEE versus 0.8% in non-O3AEE-treated patients (hazard ratio, 0.47; 95% confidence interval, 0.28–0.78; P < 0.05).
Conclusions
Among patients receiving statins, cardiovascular event incidence did not differ significantly between O3AEE-treated patients and non-O3AEE-treated patients. Further studies are required before definitive conclusions can be drawn on the effect of O3AEE on cardiovascular event incidence in high-risk patients with hypertriglyceridemia.
Trial registration
ClinicalTrials.gov, NCT02285166.
1. Introduction
Statin therapy, which targets low-density lipoprotein cholesterol (LDL-C) to prevent atherosclerosis, is the standard of care for the primary and secondary prevention of atherosclerotic cardiovascular (CV) disease events in patients with dyslipidemia [Citation1,Citation2]. However, even when target LDL-C levels are achieved with statin therapy, residual CV risk remains [Citation3,Citation4]. Elevated serum triglyceride (TG) levels are a marker of residual CV risk beyond LDL-C and are independently associated with increased risk of mortality and incidence of CV events [Citation5–8], even in patients receiving optimal statin therapy [Citation9]. Additional TG-lowering pharmacologic therapies may therefore be indicated to lower residual CV risk in patients with persistently elevated TG [Citation10,Citation11].
There is considerable interest in the potential CV benefits of omega-3 polyunsaturated fatty acids (n-3 PUFAs), which have been shown to significantly reduce serum TG concentrations in patients with hypertriglyceridemia, and are generally well tolerated with no clinically important safety concerns [Citation10–13].
Therapeutic levels and beneficial effects of n-3 PUFAs are, however, difficult to achieve from dietary sources of omega-3 fatty acids alone. Consumption of prescription omega-3 fatty acids at doses up to 4 g/day is recommended for the treatment of elevated serum TGs [Citation11,Citation12,Citation14,Citation15]. One such prescription drug is an oral capsule formulation of omega-3-acid ethyl esters (O3AEE) that was launched in Japan in January 2013 for use in patients with hypertriglyceridemia [Citation16–18], which is defined by the Japan Atherosclerosis Society as a fasting TG level ≥1.69 mmol/L (150 mg/dL) [Citation1]. O3AEE contains highly concentrated n-3 PUFAs, mainly eicosapentaenoic (also icosapentaenoic) acid ethyl ester (EPA EE) and docosahexaenoic acid ethyl ester (DHA EE) [Citation17].
A phase 3 clinical study comparing the TG-lowering effects of O3AEE 2 or 4 g/day with a 1.8 g/day dose of a product containing highly purified EPA EE alone in Japanese patients with hypertriglyceridemia following lifestyle modification recommendations showed O3AEE to have a dose-dependent effect, with the 2 g/day dose having a similar effect to EPA EE 1.8 g/day, and the 4 g/day dose resulting in a significantly greater effect than EPA EE 1.8 g/day [Citation17]. Changes in TG levels were independent of the presence or absence of statin use. A secondary analysis confirmed that the TG-lowering effect of O3AEE 2 g/day was noninferior to that of EPA EE 1.8 g/day [Citation17]. The EPA EE product was already available in Japan, and had previously been shown to significantly reduce the risk of major coronary events in the Japan EPA Lipid Intervention Study (JELIS), in which Japanese patients with hypercholesterolemia were randomized to low-intensity statin therapy plus EPA EE 1.8 g/day or statin therapy alone [Citation19]. Randomized clinical trials investigating the effect of O3AEE on CV outcomes in Japanese patients were therefore not required for approval of O3AEE in Japan.
Here, we report the results of the Outcome prevention on Cardiovascular Events by Antihyperlipidemic therapy with N3-fatty acid in Japan (OCEAN3) survey, a post-marketing surveillance study conducted to ascertain the effect of O3AEE on CV outcomes in patients with hypertriglyceridemia while undergoing treatment with statins in routine medical care in Japan.
2. Patients and methods
2.1. Survey design
The OCEAN3 survey was a 3-year, prospective, observational, multicenter post-marketing surveillance study (ClinicalTrials.gov identifier: NCT02285166; JapicCTI-142680). Patients receiving statin treatment who were prescribed O3AEE (Lotriga®; Takeda Pharmaceutical Company, Japan) as add-on therapy for the first time between October 2014 and December 2016 were enrolled in the O3AEE cohort by their prescribing physician.
In accordance with its approved label, O3AEE was administered orally immediately after a meal at the usual adult dosage of 2 g once daily, and this dosage could be increased to 2 g twice daily by the patient’s physician according to the patient’s TG level.
For comparison, CV event data were also collected in a reference cohort of patients receiving statin therapy who were not treated with O3AEE (non-O3AEE cohort), who were enrolled between October 2015 and December 2016. Patients were observed for 36 months after the start of O3AEE treatment or from the enrollment date for those in the non-O3AEE cohort. The investigators used electronic survey forms to record all data (inclusion/exclusion criteria, baseline demographic data, and CV outcomes data).
This survey was conducted in accordance with Japanese guidelines for Good Post-Marketing Study Practice. In accordance with Japanese regulations for post-marketing surveillance, it was not necessary to obtain independent review board/ethics committee approval or patient informed consent.
2.2. Patients
Male outpatients aged ≥50 years and female outpatients aged ≥60 years who had hyperlipidemia while receiving statin therapy and had ≥2 prespecified CV risk factors were eligible for inclusion. A fasting TG level ≥1.69 mmol/L (150 mg/dL) was required ≤3 months before the start of the observation period. The prespecified CV risk factors were hypertension (defined as presence of any of the following: systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or receiving antihypertensive medication), type 2 diabetes mellitus (glycated hemoglobin ≥6.5% and/or on antidiabetic medication), chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2 and/or proteinuria for ≥3 months before the start of observation), history of myocardial infarction (MI) or angina pectoris, history of cerebral infarction, and peripheral arterial disease (ankle brachial pressure index ≤0.9).
Patients with coronary artery disease or cerebrovascular disease events or who had undergone heart surgery or revascularization surgery ≤1 month before the start of the observation period were excluded, as were patients scheduled to undergo heart surgery or revascularization procedures. Other exclusion criteria included current treatment for malignant tumors, patients with hemorrhage (e.g. hemophilia, capillary fragility, gastrointestinal ulcer, urinary tract hemorrhage, hemoptysis, or vitreous hemorrhage), and patients who had previously received O3AEE. Prescription EPA products were not permitted ≤1 month before the start of the observation period or during the observation period. Use of over-the-counter or nutritional supplements containing n-3 PUFAs was allowed.
2.3. Study outcomes
The primary endpoint was the occurrence of any of the following CV events: major CV events (CV death, non-fatal MI, non-fatal ischemic or hemorrhagic stroke), angina pectoris requiring coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting), or peripheral arterial disease requiring surgery or peripheral arterial intervention. Secondary endpoints were: a) all-cause death; b) first occurrence of any of the component major CV events of the primary endpoint; c) first occurrence of any of the component major CV events of the primary endpoint or first occurrence of any of the following CV events: angina pectoris requiring hospitalization, angina pectoris requiring coronary revascularization, heart failure (HF) requiring hospitalization, transient ischemic attack requiring hospitalization, peripheral arterial disease requiring hospitalization, or peripheral arterial disease requiring surgery or peripheral arterial intervention; d) occurrence of each of the previously listed individual CV events (i.e. each as a separate secondary endpoint). Changes in serum TG and LDL-C levels (measured every 6 months) were included as exploratory endpoints. Adverse events other than CV events were not recorded in this study. Data on impaired glucose tolerance and waist circumference were not collected.
2.4. Statistical methods
Enrollment was planned of a cohort of 7,000 patients treated with O3AEE and a cohort of 7,000 patients not treated with O3AEE. The effectiveness analysis set consisted of all patients who met the eligibility criteria and were evaluable for efficacy. The cumulative incidence of CV events was estimated using the Kaplan–Meier method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional hazards model for the effectiveness analysis set, with treatment cohort as an independent variable and prespecified covariates (age, sex, body mass index, smoking history, fish consumption frequency, history or complications of cerebrovascular or CV disease, fasting TG level, antihypertensive drugs, antidiabetic drugs, antiplatelet drugs, anticoagulants, number of CV risk factors). Use of over-the-counter or nutritional supplements containing n-3 PUFAs was not included as a covariate, as these were taken by only around 1% of patients.
To check the robustness of the above analysis on the primary endpoint, two propensity score (PS)-based analyses were applied: inverse probability weighting (IPW) analysis and PS matching [Citation20]. The PS was calculated using a logistic regression model to establish each patient’s probability of receiving O3AEE according to baseline age, sex, body mass index, smoking history, fish consumption frequency, history or complications of cerebrovascular or CV disease, fasting TG level, concomitant medications (antihypertensive drugs, antidiabetic drugs, antiplatelet drugs, anticoagulants), and number of CV risk factors.
The IPW analysis was performed using an IPW-adjusted Kaplan–Meier method and IPW-adjusted Cox proportional hazards model with weight trimming at the 1st and the 99th percentiles. The PS matching analysis was performed using the Kaplan–Meier method and a Cox proportional hazards model in cohorts of patients who were PS-matched. The PS-matched cohorts were created by matching patients who received O3AEE according to their PS score to those who did not receive O3AEE using nearest-neighbor matching, with a 1:1 ratio and a minimum caliper width of 0.2 so as to minimize treatment selection bias. The balance between the PS-matched O3AEE and non-O3AEE cohorts for each covariate was assessed with standardized mean differences.
Prespecified subgroup analyses were conducted in six subgroups of patients considered to be at relatively high risk for CV events (history of MI, history of angina pectoris, history of cerebral infarction, history of cerebral hemorrhage, history of coronary revascularization, and secondary prevention patients). Post hoc subgroup analyses were also conducted in patients with TG levels <2.26 mmol/L (200 mg/dL) or ≥2.26 mmol/L (200 mg/dL) and patients with a TG level <1.69 mmol/L (150 mg/dL) or ≥1.69 mmol/L (150 mg/dL) at the final evaluation time point (last observation carried forward for patients who had missing data at 36 months). In addition, Kaplan–Meier estimates of the cumulative incidence of the primary endpoint at 36 months were calculated in subgroups defined by sex. Subgroup analyses based on diabetes status or BMI were not performed. Data on waist circumference were not collected in this study, so subgroup analyses according to metabolic syndrome were also not performed.
Least squares mean changes in TG levels were compared using analysis of covariance (ANCOVA) with baseline TG level as a covariate in the overall population as well as in O3AEE and non-O3AEE cohorts. Analyses of median changes in TG levels were conducted in the overall population and in the PS-matched cohorts. Adherence to treatment (expressed as a percentage) was calculated by dividing the duration of the treatment period by the duration of the observation period.
3. Results
3.1. Patient disposition
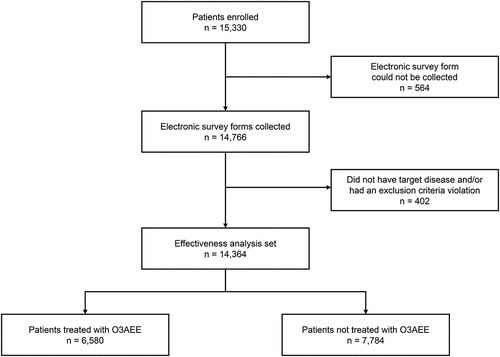
A total of 15,330 patients were enrolled from 1,076 medical institutions (). Electronic survey forms were collected from 14,766 patients, 14,364 of whom met eligibility criteria and were therefore included in the effectiveness analysis set. Within the effectiveness analysis set, 6,580 patients received O3AEE, and 7,784 were not treated with O3AEE.
With the exception of median TG level, patient demographics and characteristics at baseline were generally well balanced between the two cohorts (). The mean age of patients was 70.8 years, and 54.8% were male. The proportion of patients who were receiving statin therapy as primary prevention was 82.6% in the O3AEE cohort and 83.7% in the non-O3AEE cohort. Rosuvastatin was the most commonly used statin (37.6% of patients overall). Approximately 7% of patients in each cohort used additional drugs (other than statins, O3AEE, or EPA formulations) to treat hyperlipidemia (). Median TG level in the 3 months before the start of the observation period was higher in the O3AEE cohort than the non-O3AEE cohort (2.35 mmol/L [208.0 mg/dL] versus 2.10 mmol/L [186.0 mg/dL], respectively).
Table 1. Patient demographics and characteristics at baseline.
TG levels were well balanced between the O3AEE and non-O3AEE cohorts after PS matching (Supplementary Table 1). The standardized mean difference for baseline TG level was reduced from 0.276 in the pre-matching population to 0.026 in the post-matching population.
The starting dosage of O3AEE was 2 g/day in 6,158 patients (93.6%) and 4 g/day in 421 patients (6.4%). One patient (0.02%) enrolled in the O3AEE cohort had their dosage recorded as ‘0 g’; this patient was handled as a patient treated with O3AEE and was not excluded from the effectiveness analysis set. During the 36-month observation period, 0.68% of patients (45/6,580) increased their O3AEE dosage from 2 to 4 g/day, and 4.4% of all patients (636/14,364) changed their statin dosage. An adherence rate to statin therapy of ≥90% was achieved by 6,215 patients (94.5%) in the O3AEE cohort and 7,406 patients (95.1%) in the non-O3AEE cohort. The mean overall adherence rate to statin therapy was 97.3% (standard deviation [SD] 15.5) in the O3AEE cohort and 97.8% (SD 16.8) in the non-O3AEE cohort. At the end of the 36-month observation period, 4,921 patients (74.8%) in the O3AEE cohort and 6,005 patients (77.1%) in the non-O3AEE cohort remained on statin treatment, and 5,690 patients (86.5%) in the O3AEE cohort continued to receive O3AEE treatment. Mean adherence to O3AEE treatment was 93.0% (SD 21.3%) in the overall O3AEE cohort and 97.3% (SD 10.4%) among patients in the O3AEE cohort who increased their O3AEE dosage from 2 to 4 g/day. Over-the-counter or nutritional supplements containing n-3 PUFAs were taken by 1.2% of all patients at the beginning of the study and by 0.8% at 36 months.
3.2. Primary endpoint
The Kaplan–Meier estimate of the cumulative incidence of CV events at 36 months from the start of observation was 2.5% (95% CI, 2.1%–2.9%) in the O3AEE cohort and 2.7% (2.4% to 3.1%) in the non-O3AEE cohort (). As shown in , the Cox proportional hazards analysis revealed no significant difference in the risk of CV events between the cohorts (HR, 0.99; 95% CI, 0.79–1.23).
Figure 2. Kaplan–Meier estimates of the incidence of the primary endpointa during the 36-month observation period.
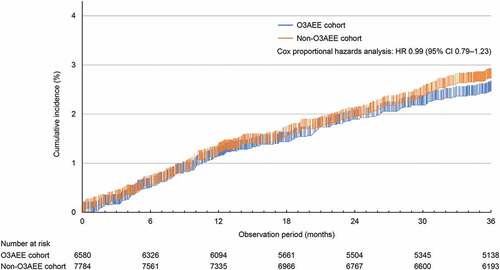
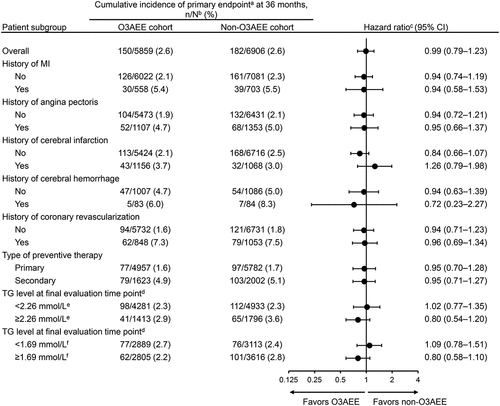
Figure 3. Cox proportional hazards analysis of CV events overall and in prespecified patient subgroups.
In the IPW analysis, the Kaplan–Meier estimate of the cumulative incidence of CV events at 36 months was 2.7% (95% CI, 2.3%–3.2%) in the O3AEE cohort and 2.9% (2.5% to 3.3%) in the non-O3AEE cohort, with no significant difference between the cohorts (HR, 0.96; 95% CI, 0.77–1.20; Supplementary Table 2).
The PS matching analysis was performed in a cohort of 5,342 O3AEE-treated patients and a cohort of 5,342 matched non-O3AEE-treated patients. In this analysis, the Kaplan–Meier estimate of the cumulative incidence of CV events at 36 months was 2.7% (95% CI, 2.3%–3.2%) in the O3AEE cohort and 2.8% (2.4% to 3.3%) in the non-O3AEE cohort, with no significant difference between the cohorts (HR, 1.00; 95% CI, 0.79–1.27; Supplementary Table 2).
3.3. Subgroup analyses of the primary endpoint
Cox proportional hazards analysis in subgroups did not reveal any significant differences in the risk of CV events for patients receiving O3AEE compared with patients not receiving O3AEE (), including in patients with a high TG level (≥2.26 mmol/L [200 mg/dL]) and in patients who still had a TG level above normal (≥1.69 mmol/L [150 mg/dL]) at their final evaluation.
Among men, the Kaplan–Meier estimate of the cumulative incidence of the primary endpoint at 36 months was numerically smaller in patients treated with O3AEE than in those not treated with O3AEE (2.7% versus 3.7%), but among women the reverse was observed (2.2% versus 1.5%; Supplementary Table 3, Supplementary Figure 1).
3.4. Secondary endpoints
The Kaplan–Meier estimate of the 36-month cumulative incidence of HF requiring hospitalization was 0.4% (95% CI, 0.27%–0.61%) for patients receiving O3AEE and 0.7% (95% CI, 0.55%–0.95%) for those not receiving O3AEE (Supplementary Table 4). Cox proportional hazards analysis of the first occurrence of HF requiring hospitalization showed a significantly reduced incidence rate with O3AEE (0.4% versus 0.8% for the non-O3AEE cohort; HR, 0.47; 95% CI, 0.28–0.78; P < 0.05). There were no significant differences between the groups in relation to other secondary endpoints (Supplementary Table 4).
3.5. Lipid parameters and other indicators of cardiovascular risk
While serum LDL-C levels remained relatively stable over the 36-month observation period (mean reductions of 0.11 mmol/L [4.1 mg/dL] and 0.06 mmol/L [2.2 mg/dL] in the O3AEE and non-O3AEE cohorts, respectively, at 36 months), there was a progressive reduction in serum TG level in the O3AEE cohort ( and Supplementary Table 5). At the final evaluation, TG levels were reduced from baseline by a median of 0.67 mmol/L (59.0 mg/dL) in the O3AEE cohort and by 0.36 mmol/L (32.0 mg/dL) in the non-O3AEE cohort, resulting in median TG levels that were similar between the two cohorts. After adjusting for baseline TG levels, the least squares mean reduction in TG level at the final evaluation was 0.64 mmol/L (56.8 mg/dL) in the O3AEE cohort and significantly higher than 0.50 mmol/L (44.0 mg/dL) in the non-O3AEE cohort (P < 0.0001). After baseline TG levels between the O3AEE and non-O3AEE cohorts were balanced using PS matching, median reductions in TG levels at the final evaluation were 0.62 mmol/L (55.0 mg/dL) and 0.38 mmol/L (34.0 mg/dL) in the PS-matched O3AEE and non-O3AEE cohorts, respectively.
Table 2. TG and LDL-C levels during the 36-month observation period in patients treated or not treated with O3AEE.
Median TG/high-density lipoprotein cholesterol (HDL-C) ratio was higher in the O3AEE cohort than in the non-O3AEE cohort at baseline (4.38 versus 3.76) but was similar in both cohorts at the final evaluation (2.94 versus 3.00; ). In both cohorts, changes in HDL-C levels over the 36-month observation period were minimal (), as were changes in glycated hemoglobin (Supplementary Table 6) and blood pressure (Supplementary Table 7).
Table 3. HDL-C levels and TG/HDL-C ratios during the 36-month observation period in patients treated or not treated with O3AEE.
4. Discussion
This study evaluated the effect of long-term use of O3AEE on the incidence of CV events among high-risk patients with hypertriglyceridemia during statin therapy in routine medical care in Japan. A cohort of statin-treated patients not receiving O3AEE was also included in this study for reference. The incidence of the composite primary endpoint did not differ significantly between patients who received O3AEE and the reference cohort of patients who were not treated with O3AEE. There was a significantly reduced incidence of HF requiring hospitalization, a secondary outcome, in O3AEE-treated patients compared with the reference cohort. However, results from secondary outcomes need to be interpreted with caution because of the risk of reporting a false-positive result [Citation21], but our observation contributes to accumulating evidence that EPA EE + DHA EE may be beneficial in patients with or at risk of HF [Citation22–25].
The patients in the O3AEE and non-O3AEE cohorts had similar TG levels at the end of the study, which may have contributed to the non-significant difference between cohorts that was seen in the incidence of CV events [Citation6,Citation9,Citation26]. However, the key limitation of our study is that the non-randomized study design means that it is not possible to make definitive conclusions on whether or not O3AEE is effective in reducing the incidence of CV events when added to statin therapy. Although IPW and PS matching analyses, as well as the primary Cox proportional hazards model, all found that the cumulative incidence of CV events was similar between the O3AEE and non-O3AEE cohorts, factors such as changes in statin dosage could have acted as confounders. In addition, it is more difficult to detect the preventive effects of n-3 PUFAs on CV events in patients receiving pharmacologic therapy involving statins, the effects of which can confound the beneficial effects of n-3 PUFA supplementation [Citation22,Citation27]. Because most patients in the non-O3AEE cohort did not change their statin dosage during the study, the reasons for the reduction in TG levels seen in this cohort are not clear.
Caution is required when comparing the results of this study with other studies of n-3 PUFAs, because of the differences in the study designs (e.g. randomized or observational), study populations (e.g. primary or secondary prevention; Asian or Western countries), and n-3 PUFA formulations (e.g. ethyl ester or carboxylic acid formulations). Over 20 years ago, the GISSI-Prevenzione (GISSI-P) investigators reported that EPA EE + DHA EE 1 g/day decreased major CV events in patients with a recent MI [Citation28]; however, the rate of statin use was very low in the GISSI-P study, and it is unlikely that the GISSI-P results are generalizable to contemporary patient populations [Citation29]. The randomized, controlled OMEMI trial which enrolled patients in 2012 to 2013 found that treatment with EPA (930 mg) + DHA (660 mg) in addition to standard of care did not reduce clinical events over 2 years compared with placebo in elderly patients with a recent acute MI [Citation30].
In the Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH), a carboxylic acid formulation of EPA + DHA 4 g/day did not reduce the incidence of CV events versus corn oil placebo after a median follow-up of 42 months in a largely Western population [Citation31]. As opposed to neutral effects on composite CV event outcomes, evidence from STRENGTH and other studies suggests that EPA + DHA formulations may prevent coronary heart disease (CHD) events [Citation31–33].
The results of a prespecified subgroup analysis from the STRENGTH study did, however, reveal a reduction in CV event risk with EPA + DHA carboxylic acids among Asians (HR 0.72; 95% CI, 0.54–0.96) and in the Asia region (HR, 0.78; 95% CI, 0.62–0.98) [Citation31]. This raises the possibility that EPA + DHA has CV benefits in some Asian patients [Citation34]. Given that greater risk reduction may occur in subpopulations who eat less fish [Citation35], the Japanese diet, with its relatively high n-3 PUFAs intake from fatty fish, may have influenced our findings with regard to CV outcomes. For example, the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP) study found that total fish intake among adults aged 40–59 years was 40 g/1000 kcal in Japan but only 9 g/1000 kcal in the UK and USA [Citation36]. A separate population-based cross-sectional survey found that Japanese men living in Japan had serum levels of marine-derived n-3 PUFAs that were approximately two times those of Japanese American men and White men born and living in the United States [Citation37]. Dietary intake of n-3 PUFAs has also been found to be significantly higher in Japanese men and women living in Japan than in Japanese American men and women living in Hawaii [Citation38].
In contrast to the outcomes of the STRENGTH trial, the results of two large randomized controlled trials point to potential CV benefits of highly purified formulations of EPA alone [Citation19,Citation29,Citation39]. The Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT), which studied the effect of EPA EE 4 g/day in statin-treated patients with hypertriglyceridemia and established CV disease or elevated CV risk, demonstrated a significant reduction in CV events with EPA versus mineral oil placebo over a median follow-up of 4.9 years in a largely Western population [Citation39]. REDUCE-IT followed JELIS, which found a significant benefit related to major coronary events of EPA EE 1.8 g/day versus statin therapy alone in a Japanese population [Citation19]. The JELIS findings should, however, be interpreted with caution because this open-label study did not specifically enroll patients with hypertriglyceridemia and statin intensity was low, with a mean attained LDL-C level of approximately 3.5 mmol/L (136 mg/dL) in contrast to a median attained LDL-C level of approximately 2.0 mmol/L (76–77 mg/dL) in REDUCE-IT and STRENGTH [Citation19,Citation29,Citation31,Citation39]. The mean attained LDL-C levels in our study (approximately 2.5 mmol/L [96 mg/dL]) were also markedly lower than those obtained in JELIS.
A meta-analysis of 13 randomized controlled trials involving 127,477 participants has also found that supplementation with n-3 PUFAs lowers risk for MI, CHD mortality, total CHD, CV mortality, and total CV disease, and that the risk reductions appeared to be linearly related to n-3 PUFA dose [Citation40].
As was also the case in our pre- and post-PS matching populations, TG levels decreased to a greater extent in the n-3 PUFA treatment groups than the control groups in the JELIS, REDUCE-IT and STRENGTH studies, with modest changes in LDL-C [Citation19,Citation31,Citation39]. TG levels were reduced by 18–19% in the REDUCE-IT and STRENGTH trials after 12 months, indicating that improvements in CV outcomes observed with EPA in REDUCE-IT may not have been attributed to TG-lowering effects alone [Citation31,Citation39]. Antithrombotic effects, membrane-stabilizing effects, and stabilization or regression of coronary plaque may all play a part [Citation39,Citation41,Citation42].
The reasons for the differing results obtained with high dose n-3 PUFAs in the REDUCE-IT and STRENGTH studies remain uncertain, but several hypotheses have been proposed, including different effects of carboxylic acid versus ethyl ester formulations of n-3 PUFAs; differences in placebo, with REDUCE-IT using a mineral oil placebo that may have elevated CV risk as opposed to the more neutral corn oil placebo used in STRENGTH; moderately higher plasma levels of EPA achieved in REDUCE-IT; and possible harm from the DHA included in the n-3 PUFA formulation tested in STRENGTH [Citation43–45].
Although increases in LDL-C and high-sensitivity C-reactive protein seen with the mineral oil placebo comparator may have increased the risk of CV events in the placebo arm of REDUCE-IT, this cannot entirely explain the observed differences in outcomes between the two studies [Citation29,Citation46,Citation47]. A post-hoc analysis of the STRENGTH trial of EPA + DHA has shown the highest achieved tertiles of plasma EPA and DHA were associated with neither benefit nor harm [Citation43], suggesting that it is unlikely that a beneficial effect of EPA could have been offset by a detrimental effect of DHA, or that there was a threshold EPA effect. Furthermore, compared with EPA EE alone, EPA EE + DHA EE shifts LDL particles to a larger, less atherogenic profile [Citation48]. Given that increases in plasma EPA and DHA levels were lower than expected in STRENGTH, concerns have also been raised regarding treatment adherence in that study [Citation49]. It is also possible that adherence to O3AEE therapy may have been suboptimal in the clinical practice setting of our post-marketing surveillance study.
In order to detect a preventive effect of O3AEE against CV events in a Japanese population receiving statin therapy, a randomized controlled trial and/or a larger sample size and longer observation period may be required. Given that the 4 g/day dosage of O3AEE was found to lower TG to a significantly greater extent than EPA EE in a phase 3 clinical trial in Japanese patients with hypertriglyceridemia [Citation17], future clinical trials are also warranted to investigate the effect of this higher dosage on CV events.
5. Conclusions
In this post marketing surveillance study which was conducted to examine the incidence of cardiovascular events in patients with hypertriglyceridemia who received O3AEE as an add-on to statin therapy, the cumulative incidence of CV events after 3 years was similar to that observed in a cohort of statin-treated patients not receiving O3AEE which was included in this study for reference. This finding may reflect the similar TG levels in both cohorts at 3 years. However, because of the limitations of the non-randomized study design, definitive conclusions cannot be drawn on whether use of O3AEE in high-risk patients with hypertriglyceridemia has a significant effect on the incidence of a composite of CV events. Additional clinical studies are needed to further explore this topic.
Author contributions
All authors were involved in the conception and design of the study, the analysis and interpretation of the data, and development of the manuscript. All authors approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Declaration of interest
T Teramoto reports consulting fees from Takeda; payment or honoraria for lectures from Bayer Yakuhin; and scholarship grants from Daiichi Sankyo and Sanofi K.K. H Ogawa reports personal fees from Bayer Yakuhin, Takeda Pharmaceutical Company Limited, Novartis Pharma, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Eisai, Teijin, Boehringer Ingelheim, Towa Yakuhin, Toa Eiyo, and Abbott Medical Japan LLC outside of the submitted work. K Haze reports consulting fees from Takeda Pharmaceutical Company Limited. K Fujikawa, T Hashimoto, S Sakui, K Nishimura and M Kajita are employees of Takeda Pharmaceutical Company Limited. J Fernandez was an employee of Takeda Pharmaceutical Company Limited at the time of this study and reports stock or stock options in GSK and Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One reviewer has received research funding and consulting fees from Acasti Pharma, Matinas BioPharma, GOED, Pharmavite, and ACH Foods and consulting fees from 89bio, AlaskOmega and Amarin in the past 5 years.
All peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Safety for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (333.4 KB)Acknowledgments
Medical writing support was provided by Joanne Dalton and Reza Sayeed on behalf of MIMS Co., Ltd., funded by Takeda Pharmaceutical Company Limited, in accordance with GPP3.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article will be made available, within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. For submitting your request, please visit https://vivli.org/ourmember/takeda/
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2094914
Additional information
Funding
References
- Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018 Sep 1;25(9):846–984.
- Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep 7;42(34):3227–3337.
- Averna M, Stroes E. How to assess and manage cardiovascular risk associated with lipid alterations beyond LDL. Atheroscler Suppl. 2017 Apr;26:16–24.
- Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012 Feb;14(1):1–10.
- Klempfner R, Erez A, Sagit BZ, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two–year follow-up of the Bezafibrate Infarction Prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016 Mar;9(2):100–108.
- Iso H, Imano H, Yamagishi K, et al. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis. 2014 Nov;237(1):361–368.
- Okamura T, Kokubo Y, Watanabe M, et al. A revised definition of the metabolic syndrome predicts coronary artery disease and ischemic stroke after adjusting for low density lipoprotein cholesterol in a 13-year cohort study of Japanese: the Suita study. Atherosclerosis. 2011 Jul;217(1):201–206.
- Noda H, Iso H, Saito I, et al. The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res. 2009 Apr;32(4):289–298.
- Nichols GA, Philip S, Reynolds K, et al. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J Clin Endocrinol Metab. 2018 Aug 1;103(8):3019–3027.
- Backes J, Anzalone D, Hilleman D, et al. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016 Jul 22;15(1):118.
- Skulas-Ray AC, Wilson PWF, Harris WS, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019 Sep 17;140(12):e673–e691.
- Watanabe Y, Tatsuno I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: present, past and future. Expert Rev Clin Pharmacol. 2017 Aug;10(8):865–873.
- Albert CM, Cook NR, Pester J, et al. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. 2021 Mar 16;325(11):1061–1073.
- Bhatt DL, Budoff MJ, Mason RP. A revolution in omega-3 fatty acid research. J Am Coll Cardiol. 2020 Nov 3;76(18):2098–2101.
- Pradhan A, Bhandari M, Vishwakarma P, et al. Triglycerides and cardiovascular outcomes—can we REDUCE-IT? Int J Angiol. 2020 Mar;29(1):2–11.
- Watanabe Y, Tatsuno I. Prevention of cardiovascular events with omega-3 polyunsaturated fatty acids and the mechanism involved. J Atheroscler Thromb. 2020 Mar 1;27(3):183–198.
- Tatsuno I, Saito Y, Kudou K, et al. Efficacy and safety of TAK-085 compared with eicosapentaenoic acid in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega-3 fatty acids randomized double-blind (ORD) study. J Clin Lipidol. 2013 May-Jun;7(3):199–207.
- Tatsuno I, Saito Y, Kudou K, et al. Long-term safety and efficacy of TAK-085 in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega-3 fatty acids randomized long-term (ORL) study. J Clin Lipidol. 2013 Nov-Dec;7(6):615–625.
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007 Mar 31;369(9567):1090–1098.
- Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014 Mar 30;33(7):1242–1258.
- Keaney JF Jr., Rosen CJ. VITAL signs for dietary supplementation to prevent cancer and heart disease. N Engl J Med. 2019 Jan 3;380(1):91–93.
- Elagizi A, Lavie CJ, O’Keefe E, et al. An update on omega-3 polyunsaturated fatty acids and cardiovascular health. Nutrients. 2021 Jan 12;13(1):204.
- Matsuo N, Miyoshi T, Takaishi A, et al. High plasma docosahexaenoic acid associated to better prognoses of patients with acute decompensated heart failure with preserved ejection fraction. Nutrients. 2021 Jan 26;13(2):371.
- Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Oct 4;372(9645):1223–1230.
- Toko H, Morita H, Katakura M, et al. Omega-3 fatty acid prevents the development of heart failure by changing fatty acid composition in the heart. Sci Rep. 2020 Sep 23;10(1):15553.
- Marston NA, Giugliano RP, Im K, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation. 2019 Oct 15;140(16):1308–1317.
- Visioli F, Poli A. Fatty acids and cardiovascular risk. evidence, lack of evidence, and diligence. Nutrients. 2020 Dec 9;12(12):3782.
- Marchioli R. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999 Aug 7;354(9177):447–455.
- Boden WE, Baum S, Toth PP, et al. Impact of expanded FDA indication for icosapent ethyl on enhanced cardiovascular residual risk reduction. Future Cardiol. 2021 Jan;17(1):155–174.
- Kalstad AA, Myhre PL, Laake K, et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. 2021 Feb 9;143(6):528–539.
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020 Dec 8;324(22):2268–2280.
- Bernasconi AA, Wiest MM, Lavie CJ, et al. Effect of omega-3 dosage on cardiovascular outcomes: an updated meta-analysis and meta-regression of interventional trials. Mayo Clin Proc. 2021 Feb;96(2):304–313.
- Farukhi ZM, Mora S, Manson JE. Marine omega-3 fatty acids and cardiovascular disease prevention: seeking clearer water. Mayo Clin Proc. 2021 Feb;96(2):277–279.
- Park KY, Park HK, Hwang HS. Omega-3 fatty acids effect on major cardiovascular events in patients at high cardiovascular risk. JAMA. 2021 Apr 6;325(13):1334.
- Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019 Jan 3;380(1):23–32.
- Gibson R, Lau CE, Loo RL, et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am J Clin Nutr. 2020 Feb 1;111(2):280–290.
- Sekikawa A, Curb JD, Ueshima H, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008 Aug 5;52(6):417–424.
- Ueshima H, Okayama A, Saitoh S, et al. Differences in cardiovascular disease risk factors between Japanese in Japan and Japanese-Americans in Hawaii: the INTERLIPID study. J Hum Hypertens. 2003 Sep;17(9):631–639.
- Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019 Jan 3;380(1):11–22.
- Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019 Oct;8(19):e013543.
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020 Oct 21;41(40):3925–3932.
- Ruscica M, Ferri N, Santos RD, et al. Lipid lowering drugs: present status and future developments. Curr Atheroscler Rep. 2021 Mar 10;23(5):17.
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk: a secondary analysis of the STRENGTH trial. JAMA Cardiol. 2021 May 16;6(8):1–8.
- Olshansky B, Bhatt DL, Chung MK. Omega-3 fatty acids effect on major cardiovascular events in patients at high cardiovascular risk. JAMA. 2021 Apr 6;325(13):1332–1333.
- Volpe M, Patrono C. The REDUCE-IT verdict on eicosapentaenoic acid and cardiovascular outcome challenged with STRENGTH. Eur Heart J. 2021 Feb 1;42(5):370–371.
- Curfman G. Do omega-3 fatty acids benefit health? JAMA. 2020 Dec 8;324(22):2280–2281.
- Sharma G, Martin SS, Blumenthal RS. Effects of omega-3 fatty acids on major adverse cardiovascular events: what matters most: the drug, the dose, or the placebo? JAMA. 2020 Dec 8;324(22):2262–2264.
- Tatsuno I, Kudou K, Kagawa T. Effect of TAK-085 on low-density lipoprotein particle si0ze in patients with hypertriglyceridemia: a double-blind randomized clinical study. Cardiovasc Ther. 2015 Dec;33(6):317–323.
- Wang Y. Omega-3 fatty acids effect on major cardiovascular events in patients at high cardiovascular risk. JAMA. 2021 Apr 6;325(13):1333.