ABSTRACT
Background
Fixed-dose combination (FDC) of the sodium-glucose co-transporter 2 inhibitor empagliflozin and the dipeptidyl peptidase-4 inhibitor linagliptin was approved for type 2 diabetes (T2D) treatment in Japan in 2018. We conducted a post-marketing surveillance study of empagliflozin/linagliptin FDC in routine clinical practice in Japan.
Research design and methods
This one-year, prospective, multicenter, observational study investigated the safety and effectiveness of empagliflozin/linagliptin FDC in Japanese patients with T2D. The primary outcome was incidence of adverse drug reactions (ADRs).
Results
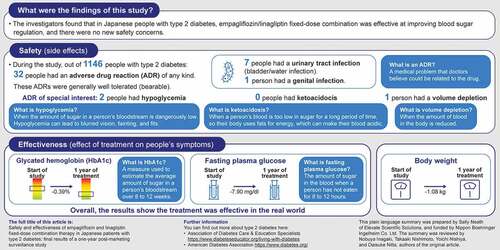
Among 1146 patients, mean (SD) age was 63.8 (12.8) years and 22.08% were aged ≥75 years. Mean (SD) glycated hemoglobin (HbA1c) was 7.66% (1.21); fasting plasma glucose (FPG) was 142.90 mg/dl (43.75). ADRs were experienced by 32 (2.79%) patients (1 serious ADR); ADRs of important identified risk included urinary tract infection (7 patients [0.61%]), hypoglycemia (2 [0.17%]), ketoacidosis (0), genital infection (1 [0.09%]), and volume depletion (1 [0.09%]). Overall mean (SD) change from baseline in body weight, HbA1c, and FPG were −1.08 kg (3.21), −0.39% (1.11), and −7.90 mg/dl (39.12), respectively.
Conclusions
Empagliflozin/linagliptin FDC was effective and generally well tolerated in Japanese patients with T2D; no new safety concerns were identified.
Trial Registration
The trial is registered at ClinicalTrials.gov (CT.gov identifier: NCT03761797)
KEYWORDS(MESH TERMS):
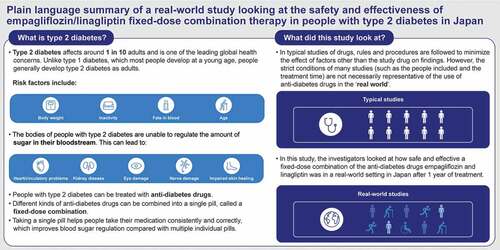
1. Introduction
Type 2 diabetes (T2D) is a leading cause of mortality and morbidity globally, and prevalence is increasing worldwide [Citation1]; in 2021, an estimated 1 in 10 adults were living with the disease [Citation2]. In Japan, the prevalence of T2D is increasing in line with the rapid aging of the population [Citation3]. It has been estimated that 45% of patients with T2D fail to meet adequate glycemic control, despite the availability of effective treatments [Citation4]. Among patients for whom one anti-diabetic drug fails to adequately control blood glucose, the American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), and Japanese Diabetes Society (JDS) guidelines recommend initiation of combination therapy [Citation5–7].
The sodium-glucose co-transporter 2 (SGLT2) inhibitor empagliflozin reduces reabsorption of glucose by the kidneys by blocking SGLT2 in the proximal tubule of the kidneys, eliciting glucosuria and thus improving glycemic control in T2D patients [Citation8]. In addition, the EMPA-REG OUTCOME trial showed that empagliflozin plus standard of care had cardioprotective and renoprotective benefits, reducing cardiovascular death, all-cause mortality, hospitalization for heart failure, and kidney disease progression among patients with T2D and established cardiovascular disease [Citation9,Citation10]; these results were consistent among the subset of Asian participants [Citation11,Citation12].
The dipeptidyl peptidase-4 (DPP4) inhibitor linagliptin inhibits the proteolytic activity of the DPP4 enzyme, which results in decreased plasma glucose levels via enhanced insulin secretion from beta cells and decreased glucagon secretion from alpha cells in the pancreas [Citation13]. The cardiovascular safety of linagliptin among patients with T2D and elevated cardiovascular risk has been demonstrated in the CARMELINA and CAROLINA trials, including among the Asian population [Citation14–17].
Fixed-dose combination (FDC) therapies with complementary mechanisms of action can improve medication adherence by reducing pill burden [Citation18] and may improve glycemic control in T2D [Citation19–21]. Empagliflozin/linagliptin FDC, as well as empagliflozin/linagliptin/metformin FDC, have shown bioequivalence with the corresponding free combinations [Citation22,Citation23]. In Japan, empagliflozin/linagliptin FDC therapy was approved for the treatment of T2D in 2018 and is widely used. The glycemic efficacy and tolerability of the empagliflozin/linagliptin FDC in Japanese patients with T2D has been demonstrated in two randomized phase III trials of up to 52 weeks [Citation24,Citation25]. A pooled safety analysis of five randomized controlled trials showed that the safety profile of the empagliflozin/linagliptin FDC was similar to the safety profiles of the individual components, and no new safety signals were identified [Citation26].
Post-marketing surveillance (PMS) studies for empagliflozin and linagliptin in Japanese clinical settings have previously demonstrated consistency with the known safety profiles of the individual treatments [Citation27–29]. However, safety and efficacy data for the empagliflozin/linagliptin FDC in Japanese patients with T2D in routine clinical practice is lacking. Here we report the final results from a one-year PMS study of the safety and efficacy of the empagliflozin/linagliptin FDC in Japan.
2. Patients and methods
2.1. Study design
This PMS study was a prospective, observational, single-arm, one-year, multicenter study (ClinicalTrials.gov NCT03761797) conducted at 159 centers in Japan. Enrollment started on 15 January 2019 and was completed on 27 April 2021 (final data collection). Data were collected via electronic case report form (eCRF) at Weeks 12, 26, 40, and 52 of treatment with empagliflozin/linagliptin FDC, and at treatment discontinuation (last observation). Adverse event reports were collected continuously. The study was conducted in accordance with Japanese Ministry of Health and Welfare Ordinance Good Post-Marketing Study Practice and Good Vigilance Practice regulations, as well as the standard operating procedures of the sponsor. Accordingly, it was not necessary to obtain either informed consent from patients or approval by an institutional review board or ethics committee.
2.2. Study population
The safety analysis set included Japanese patients who initiated treatment with empagliflozin/linagliptin FDC AP (empagliflozin 10 mg/linagliptin 5 mg) or BP (empagliflozin 25 mg/linagliptin 5 mg) tablets and had not previously received empagliflozin/linagliptin FDC. The effectiveness analysis set included only those from the safety analysis set who had T2D, baseline and post-baseline HbA1c and FPG data available, eGFR ≥30 ml/min/1.73 m2, and received either empagliflozin or linagliptin pretreatment (in line with the approved use of empagliflozin/linagliptin FDC in Japan).
2.3. Assessments
The primary safety outcome in this study was the incidence of adverse drug reactions (ADRs), coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.0. The ADRs included in this study were defined as adverse events (AEs) that had at least a reasonable possibility of a causal relationship with the treatment, as determined by the investigator.
Further safety outcomes included the incidence of prespecified serious AEs and AEs of important identified and potential risk. Serious AEs were defined as those that resulted in death, were life-threatening, required hospitalization, prolonged hospitalization, resulted in persistent or substantial disability or incapacity, or were a congenital anomaly or birth defect. AEs of important identified and potential risk included hypoglycemia, ketoacidosis, and fluid volume decrease events. Prespecified efficacy outcomes included changes from baseline in glycated hemoglobin (HbA1c) and fasting plasma glucose (FPG).
The following variables, considered important baseline characteristics and potential risk factors for the outcomes of interest, were also measured: patient demographics; medical history/concomitant disease; previous/concomitant treatments; blood pressure and pulse rate; body weight; laboratory tests (hematology, blood chemistry, urinalysis); and estimated glomerular filtration rate (eGFR) according to the Japanese version of the Modification of Diet in Renal Disease study equation [Citation30].
2.4. Statistical analysis
Hypoglycemic AEs are an important factor for the evaluation of the risk-benefit balance for T2D treatments and are collected with high sensitivity and specificity in routine care. Consequently, hypoglycemic AEs are used as a reference event for sample size evaluations in Japanese PMS studies. As previously reported [Citation25], in a 2018 clinical trial of 433 adult Japanese patients, 1.1% experienced a hypoglycemic AE at Week 52 of empagliflozin/linagliptin FDC treatment. In the present study, to achieve >80% statistical power to show that the incidence of hypoglycemic AEs was not equal to 1.1% using a one-sample chi-square test with a 0.05 two-sided significance level (assuming that the true incidence of hypoglycemia AEs is two-fold higher than was found in the 2018 study [i.e. 2.2%]), a sample size of 1,000 patients was required.
The safety analysis set included all patients who received ≥1 dose of empagliflozin/linagliptin FDC, excluding those without valid registration or who had no observations after enrollment. The effectiveness analysis set included patients in the safety analysis set, excluding those without available effectiveness data.
As this PMS study was exploratory, all data are summarized descriptively (i.e. without inferential analyses). SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses.
3. Results
3.1. Patient disposition
Of 1,164 enrolled patients, eCRFs were collected for 1,159 patients (Supplemental Figure S1). The safety analysis set consisted of 1,146 patients, with 13 patients excluded as a result of no visits being recorded following enrollment. The effectiveness analysis set consisted of 994 patients, due to the exclusion of 152 patients from the safety analysis set.
3.2. Patient baseline characteristics
presents patient baseline characteristics for the overall population and by age group (<65 years, ≥65 to <75 years, and ≥75 years). The overall mean (standard deviation [SD]) age at baseline was 63.8 (12.8) years, with 572 patients (49.91%) aged <65 years, 321 (28.01%) aged ≥65 to <75 years, and 253 (22.08%) aged ≥75 years. At baseline, the overall mean (SD) HbA1c was 7.66% (1.21), FPG was 142.90 mg/dl (43.75) and eGFR was 74.43 ml/min/1.73 m2 (21.81). Most patients (80.10%) had ≥1 concomitant disease, most commonly dyslipidemia (56.20%) and hypertension (55.06%).
Table 1. Patient baseline characteristics by age group (safety analysis set).
3.3. Drug dose and exposure
Patients were treated with empagliflozin/linagliptin FDC for a mean duration of 52.68 weeks (median: 54.00) (Supplemental Table S1). Most patients (88.38%) received empagliflozin/linagliptin FDC for >52 weeks; this finding was consistent across age groups (<65 years, ≥65 to <75 years, and ≥75 years).
Among 121 (10.56%) patients who discontinued treatment during the study period, 37 (3.23%) discontinued treatment due to adverse events, 33 (2.88%) were lost to follow-up, 19 (1.66%) requested to discontinue treatment, 6 (0.52%) discontinued treatment due to improvement/remission, and 26 (2.27%) due to other reasons.
3.4. Safety and tolerability
3.4.1. Incidence of ADRs, serious ADRs, and deaths
Among all patients, 32 (2.79%) experienced ≥1 ADR during treatment with empagliflozin/linagliptin FDC (Supplemental Table S2); 2.45% of patients aged <65 years (14/572), 4.98% of those aged ≥65 to <75 years (16/321), and 0.79% of those aged ≥75 years (2/253) experienced ≥1 ADR. The only reported serious ADR was one case (i.e. 0.09% of all patients) of carotid artery occlusion in a 73-year-old man. The outcome was unknown. A total of five deaths were reported, all of which were considered to be unrelated to empagliflozin/linagliptin FDC treatment (Supplemental Table S3).
3.4.2. ADRs of important identified and potential risk
Urinary tract infections occurred in seven (0.61%) patients (; Supplemental Table S4), only one of whom had a medical history of urinary tract infection. Three cases occurred among patients aged <65 years, three among patients aged ≥65 to <75 years, and one among patients aged ≥75 years; two cases occurred in male patients and five in female patients. Hypoglycemia occurred in two (0.17%) patients (; Supplemental Table S4): a 60-year-old male who was receiving no concomitant medications and a 65-year-old female who was receiving sulfonylurea treatment. Only one volume depletion event was reported. No cases of ketoacidosis were reported. Of all important identified risks, no ADRs were considered to be serious.
Table 2. Adverse drug reactions (ADRs) of important identified and potential risk by age group (safety analysis set).
3.5. Body weight, blood pressure, and laboratory parameters
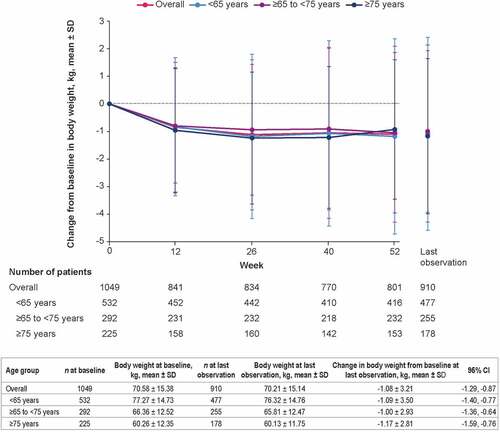
In the safety analysis population, empagliflozin/linagliptin FDC treatment was associated with reductions in body weight from baseline. At last observation, empagliflozin/linagliptin FDC reduced body weight by an overall mean (95% confidence interval [CI]) of −1.08 kg (−1.29, −0.87), with a similar magnitude of change across age groups ().
Figure 1. Change from baseline in body weight over time by age group (safety analysis set). Data are for patients who received ≥1 dose of empagliflozin/linagliptin FDC. CI: confidence interval; FDC: fixed-dose combination; SD: standard deviation.
The overall mean change in hematocrit, hemoglobin, serum lipids, markers of kidney function, and blood pressure at each data collection point are presented in Supplemental Table S5. Decreases in both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were observed at almost all time points. Increases in hematocrit and hemoglobin were observed at all time points.
3.6. Effectiveness
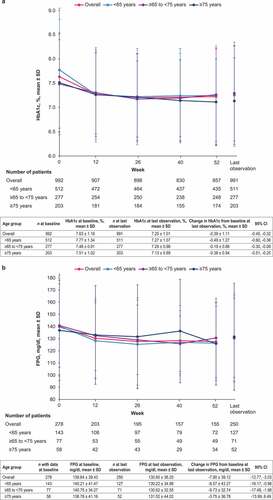
Among all patients, empagliflozin/linagliptin FDC reduced HbA1c by a mean (95% CI) of −0.39% (−0.45, −0.32) and FPG by −7.90 mg/dl (−12.77, −3.03) at last observation, with similar results among age categories ().
Figure 2. Mean change in a) HbA1c and b) FPG over time by age category (effectiveness analysis set). CI: confidence interval; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; SD: standard deviation.
When stratified by baseline HbA1c (<7%, ≥7 to <8%, ≥8 to <9%, and ≥9%), the reduction in HbA1c at final observation was greater with increasing baseline HbA1c category (Supplemental Figure S2).
Among 49 patients who received a change in dosage from empagliflozin/linagliptin FDC AP to BP tablets (empagliflozin 10 mg/linagliptin 5 mg to empagliflozin 25 mg/linagliptin 5 mg, respectively) during the study period, mean (SD) HbA1c was 8.04% (1.30) at baseline, 8.07% (0.99) before dose increase, and 7.87% (0.84) following dose increase at last observation. The mean (95% CI) change in HbA1c was −0.17% (−0.57, 0.23) from baseline to last observation and −0.20% (−0.44, 0.04) from the most recent measurement before dose increase to last observation (Supplemental Table S6).
3.7. Safety and effectiveness by prior treatment with SGLT2 and/or DPP4 inhibitors
ADRs were experienced by seven (1.94%) of the 360 patients who received an SGLT2 inhibitor but not a DPP4 inhibitor before the study start; nine (3.21%) of the 280 patients who received a DPP4 inhibitor but not an SGLT2 inhibitor; and 16 (3.44%) of the 465 patients who received both an SGLT2 and a DPP4 inhibitor. No ADRs were reported among the 41 patients who received neither SGLT2 nor DPP4 inhibitors before the study start. The patient who experienced a serious ADR during the study had received an SGLT2 inhibitor but not a DPP4 inhibitor before the study start.
Patients who had not received an SGLT2 inhibitor prior to the study start experienced a greater reduction in body weight and blood pressure from baseline at last observation compared with those who had. Mean (95% CI) change in body weight was −1.95 kg (−2.42, −1.48) among those who received a DPP4 inhibitor but not an SGLT2 inhibitor prior to the study start and −0.71 kg (−1.01, −0.40) among those who had received both SGLT2 and DPP4 inhibitors. Body weight decreased consistently among all age groups regardless of the presence or absence of SGLT2 inhibitor use at baseline. Mean (95% CI) changes in SBP and DBP were −2.61 mmHg (−4.65, −0.57) and −1.68 mmHg (−2.92, −0.44), respectively, among those who received a DPP4 inhibitor but not an SGLT2 inhibitor prior to the study start, and −1.44 mmHg (−2.94, 0.05) and −0.67 mmHg (−1.69, 0.36), respectively, among those who had received both an SGLT2 and a DPP4 inhibitor.
Increases in hematocrit and hemoglobin from baseline at last observation were greater among patients who had not received an SGLT2 inhibitor prior to the study start compared with those who had. Mean (95% CI) increase in hematocrit was 1.77% (1.25, 2.29) among patients who received a DPP4 inhibitor but not an SGLT2 inhibitor and 0.61% (0.27, 0.95) among those who received both a DPP4 and an SGLT2 inhibitor. Mean (95% CI) increase in hemoglobin was 0.40 g/dl (0.19, 0.61) among patients who received a DPP4 inhibitor but not an SGLT2 inhibitor and 0.16 g/dl (0.04, 0.28) among those who received both a DPP4 and an SGLT2 inhibitor.
4. Discussion
The present PMS study is the first study demonstrating the long-term safety and effectiveness of empagliflozin/linagliptin FDC in routine clinical practice in Japan. Consistent with the broad, real-world use of empagliflozin/linagliptin FDC in Japan, 22.08% of patients in this study were aged ≥75 years. Empagliflozin/linagliptin FDC was generally well tolerated, and no new AEs were identified. The overall incidence of AEs with empagliflozin/linagliptin FDC was moderately reduced in comparison to previous clinical studies in Japan [Citation24,Citation25]. Safety results were consistent across age categories.
The association between SGLT2 inhibitor treatment and urinary and genital infections is well known [Citation31]. In this study, the incidence of urinary tract infections was low, and no serious cases were reported. Incidence was consistent across age categories and there was a slightly higher occurrence among females compared with males, in line with previous studies [Citation32,Citation33]. Genital infection was reported in only one patient and was not considered to be a serious event. Hypoglycemia is a major concern in the treatment of T2D, and is associated with increased risk of cardiovascular events, as well as poor treatment satisfaction and health-related quality of life [Citation34,Citation35]. Reassuringly, only two cases of hypoglycemia were reported in the present study (incidence of 0.17%). No cases of ketoacidosis were reported in this study, consistent with previous studies of SGLT2 inhibitor and DPP4 inhibitor combination therapy in Japanese patients [Citation24,Citation25,Citation36–38].
The question of whether there is an imbalance in the incidence of ADRs between patients who have been treated with SGLT2 inhibitors and those treated with DPP4 inhibitors prior to combination therapy may be of interest to some physicians. In the present study, the incidence of total ADRs was low, and did not appear to differ based on patient history of treatment with SGLT2 and/or DPP4 inhibitors.
Empagliflozin/linagliptin FDC treatment was associated with reductions in body weight and SBP, which is characteristic of SGLT2 inhibitors [Citation27,Citation39]. Linagliptin is known to be neutral for these variables [Citation40], and changes were not expected as a result of adding linagliptin to empagliflozin treatment [Citation24]. Accordingly, in the present study there was a trend toward greater reduction in body weight and blood pressure among patients who had not been treated with an SGLT2 inhibitor prior to initiation of empagliflozin/linagliptin FDC compared with those who had.
Previous studies have shown that SGLT2-inhibitor treatment is associated with a modest increase in hematocrit relative to placebo and that changes in hematocrit appear to be important mediators of the reduction in mortality risk with SGLT2 inhibitors in univariable and multivariable models [Citation41]. In this study, increases in hematocrit and hemoglobin were observed with empagliflozin/linagliptin FDC, and the magnitude of increase was greater among patients who had not been treated with an SGLT2 inhibitor prior to initiation of empagliflozin/linagliptin FDC compared with those who had.
One case of carotid artery occlusion was reported, which was included as an ADR in this study because the possibility of a causal relationship cannot be ruled out considering that the increase in hematocrit (an important determinant of blood viscosity) associated with empagliflozin treatment may have some influence on the carotid artery. However, since empagliflozin has also been reported to have beneficial cardiovascular effects, such as a decrease in intima-media thickness [Citation42], further consideration is required regarding the possibility of a causal relationship.
The present study included 297 patients with baseline HbA1c below 7.0%. This could be because the target HbA1c in the Japanese diabetes treatment guidelines is <7.0% for complication prevention and <6.0% for normalization of blood glucose [Citation43]; therefore, some physicians might have aimed to achieve the latter target in patients with baseline HbA1c <7.0%. Additionally, approximately 24% of patients included in this study were previously treated with empagliflozin and linagliptin. The reason that these patients were included is because empagliflozin/linagliptin FDC is distinct from the individual medications. For instance, patient medication adherence is improved with FDC versus individual therapies [Citation18], which may improve glycemic control [Citation19–21], and the financial burden on patients is reduced. Also, these patients could have received empagliflozin and linagliptin on separate occasions prior to enrollment.
Although data between the studies cannot be directly compared, the degree to which HbA1c was decreased with empagliflozin/linagliptin FDC treatment appeared to be moderately lower in the present study compared with decreases observed in previous Japanese PMS studies investigating the safety and effectiveness of empagliflozin monotherapy and empagliflozin/linagliptin FDC treatment (mean change in HbA1c from baseline at last observation −0.39% [SD 1.11] vs −0.75% [SD 1.34] to −0.94% [standard error 0.05], respectively) and similar to that of a recent PMS in patients receiving linagliptin monotherapy (−0.49% [SD 1.33]) [Citation24,Citation25,Citation27,Citation29,Citation44]. The perceived differences in outcomes might be due to the lower baseline HbA1c among the patient population included in the present study compared with populations included in previous empagliflozin monotherapy and empagliflozin/linagliptin FDC studies. Furthermore, most patients in the present study received empagliflozin or linagliptin prior to empagliflozin/linagliptin FDC treatment and approximately one quarter received both empagliflozin and linagliptin prior to empagliflozin/linagliptin FDC treatment, as such, blood glucose might have been well controlled at baseline in these patients.
5. Conclusions
This is the first study demonstrating the safety and effectiveness of empagliflozin/linagliptin FDC among patients with T2D in routine clinical practice in Japan. The incidence of ADRs was relatively low and no new safety concerns were identified. Safety and efficacy outcomes were consistent across age groups (<65, ≥65 to <75, and ≥75 years).
Acknowledgments
The authors thank the physicians and patients who participated in this study. The authors would also like to thank Naoki Shimmoto of Nippon Boehringer Ingelheim Co. Ltd. pharmacovigilance and Junichi Wakayama of EPS Corporation for their contributions to the data analyses. Medical writing assistance was provided by Sally Neath of Elevate Scientific Solutions during the preparation of this manuscript, and was funded by Nippon Boehringer Ingelheim Co. Ltd.
Author contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
Declaration of interest
N Inagaki has received clinical commissioned/joint research grants from Terumo, Drawbridge Inc., and Asken; honoraria for lectures from Kyowa Kirin, MSD, Astellas Pharma, Novo Nordisk, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Takeda, Mitsubishi Tanabe Pharma, Sumitomo Dainippon Pharma, Sanofi, Eli Lilly Japan; scholarship grants from Kissei Pharmaceutical, Sanofi, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Takeda, Japan Tobacco, Kyowa Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, MSD, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, Novo Nordisk, Kowa, Novartis, and LifeScan Japan. T Nishimoto is an employee of Eli Lilly Japan. Y Nishiya and D Nitta are employees of Nippon Boehringer Ingelheim Co. Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Data availability
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with Boehringer Ingelheim’s Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on Vivli - Center for Global Clinical Research Data, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Drug Safety for their review work but have no other relevant financial relationships to disclose.
Role of the sponsor
The Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Supplemental Material
Download MS Word (188.9 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2107200
Additional information
Funding
References
- Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018 Apr;138:271–281.
- International Diabetes Federation. IDF diabetes atlas. 10th. Belgium: Brussels; 2021. [cited 2022 May 05]. Available from: https://www.diabetesatlas.org
- Ministry of Health Labour and Welfare. The National Health and Nutrition Survey in Japan 2016.
- Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299–1307.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020 Feb;43(2):487–493.
- Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int. 2018 Feb;9(1):1–45.
- Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018 Mar 26;9:657–697.
- Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012 Feb 7;8(8):495–502.
- Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016 Jul 28;375(4):323–334.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26;373(22):2117–2128.
- Kaku K, Lee J, Mattheus M, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease- results from EMPA-REG OUTCOME®. Circ J. 2017 Jan 25;81(2):227–234.
- Kadowaki T, Nangaku M, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME® trial. J Diabetes Investig. 2019 May;10(3):760–770.
- McKeage K. Linagliptin: an update of its use in patients with type 2 diabetes mellitus. Drugs. 2014 Oct;74(16):1927–1946.
- Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019 Sep 24;322(12):1155–1166.
- Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019 Jan 1;321(1):69–79.
- Kadowaki T, Wang G, Rosenstock J, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA(R) trial. Diabetol Int. 2021 Jan;12(1):87–100.
- Inagaki N, Yang W, Watada H, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA® trial. Diabetol Int. 2020 Apr;11(2):129–141.
- Vijayakumar TM, Jayram J, and Meghana Cheekireddy V, et al. Safety, efficacy, and bioavailability of fixed-dose combinations in type 2 diabetes mellitus: a systematic updated review. Curr Ther Res Clin Exp. 2017;84:4–9.
- Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016 Jun 7;164(11):740–751.
- Wang J-S, Huang C-N, Hung Y-J, et al. Acarbose plus metformin fixed-dose combination outperforms acarbose monotherapy for type 2 diabetes. Diabetes Res Clin Pract. 2013 2013/10/01/;102(1):16–24.
- Khaladkar K, Mohan B, Khaladkar K, et al. Efficacy and safety of a fixed dose combination of remogliflozin etabonate and vildagliptin in patients with type-2 diabetes mellitus: a randomized, active-controlled, double-blind, Phase III study. J Assoc Physicians India. 2022 Apr;70(4):11–12.
- Glund S, Mattheus M, Runge F, et al. Relative bioavailability of an empagliflozin 25-mg/linagliptin 5-mg fixed-dose combination tablet. Int J Clin Pharmacol Ther. 2017 Apr;55(4):355–367.
- Lingvay I, Beetz N, Sennewald R, et al. Triple fixed-dose combination empagliflozin, linagliptin, and metformin for patients with type 2 diabetes. Postgrad Med. 2020 May;132(4):337–345.
- Kaku K, Haneda M, Tanaka Y, et al. Linagliptin as add-on to empagliflozin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a two-part, randomized, placebo-controlled trial. Diabetes Obes Metab. 2019 Jan;21(1):136–145.
- Kawamori R, Haneda M, Suzaki K, et al. Empagliflozin as add-on to linagliptin in a fixed-dose combination in Japanese patients with type 2 diabetes: glycaemic efficacy and safety profile in a 52-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2018 Sep;20(9):2200–2209.
- Watada H, Yamauchi T, and Yamamoto F, et al. Safety and tolerability of empagliflozin and linagliptin combination therapy in patients with type 2 diabetes mellitus: a pooled analysis of data from five randomized, controlled clinical trials. Expert Opin Drug Saf. 2020;19(9):1193–1202.
- Kaku K, Chin R, and Naito Y, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: interim analysis from a post-marketing surveillance study. Expert Opin Drug Saf. 2020 Feb;19(2):211–221.
- Yamamoto F, Unno Y, and Okamura T, et al. Long-Term safety and effectiveness of linagliptin in Japanese patients with type 2 diabetes mellitus: a 3-year post-marketing surveillance study. Diabetes Ther. 2020 Jan;11(1):107–117.
- Ito T, Naito Y, and Shimmoto N, et al. Long-term safety and effectiveness of linagliptin as add-on therapy in Japanese patients with type 2 diabetes: final results of a 3-year post-marketing surveillance. Expert Opin Drug Saf. 2021 Mar;20(3):363–372.
- Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009 Jun;53(6):982–992.
- Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014 Mar;103(3):373–381.
- Kinduryte Schorling O, Clark D, Zwiener I, et al. Pooled safety and tolerability analysis of empagliflozin in patients with type 2 diabetes mellitus. Adv Ther. 2020 Aug;37(8):3463–3484.
- Yabe D, Yasui A, Ji L, et al. Safety and tolerability of empagliflozin in East Asian patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. J Diabetes Investig. 2019 Mar;10(2):418–428.
- Amiel SA, Aschner P, Childs B. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019 May;7(5):385–396.
- Hendrieckx C, Ivory N, Singh H, et al. Impact of severe hypoglycaemia on psychological outcomes in adults with Type 2 diabetes: a systematic review. Diabet Med. 2019 Sep;36(9):1082–1091.
- Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017 Jun;19(6):874–882.
- Kadowaki T, Inagaki N, Kondo K, et al. Long-term safety and efficacy of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2018 Jan;20(1):77–84.
- Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of teneligliptin added to canagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a multicentre, randomized, double-blind, placebo-controlled, parallel-group comparative study. Diabetes Obes Metab. 2018 Feb;20(2):453–457.
- Ji Q, Ji L, Mu Y, et al. Effect of empagliflozin on cardiorenal outcomes and mortality according to body mass index: a subgroup analysis of the EMPA-REG OUTCOME trial with a focus on Asia. Diabetes Obes Metab. 2021 Aug;23(8):1886–1891.
- Inagaki N, Yang W, Watada H, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA(®) trial. Diabetol Int. 2020 Apr;11(2):129–141.
- Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018 Feb;41(2):356–363.
- Irace C, Casciaro F, Scavelli FB, et al. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc Diabetol. 2018 Apr 9;17(1):52.
- Araki E, Haneda M, Kasuga M, et al. New glycemic targets for patients with diabetes from the Japan Diabetes Society. J Diabetes Investig. 2017 Jan;8(1):123–125.
- Kaku K, Yamamoto K, and Fukushima Y, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: final results of a 3-year post-marketing surveillance study. Expert Opin Drug Saf. 2022 Mar 30;1–14 DOI:10.1080/14740338.2022.2054987. Online ahead of print.
