ABSTRACT
Background
The extent to which adverse drug reactions (ADRs) of biologics differ per immune-mediated inflammatory disease (IMID), and the relevance of tailoring ADR information per IMID is not fully investigated. We aimed to compare patient-reported ADRs attributed to adalimumab and etanercept between different inflammatory rheumatic diseases (IRDs).
Research design and methods
ADR reports from IRD patients were extracted from the Dutch Biologic Monitor. ADR frequencies were compared using Fischer–Freeman–Halton exact test and the influence of covariates was assessed using binomial logistic regression.
Results
A total, of 729 participants were included, of which 354 participants reported 887 unique ADRs. ADR frequencies were not significantly different between the IRDs. Rheumatoid arthritis and ankylosing spondylitis including axial spondyloarthritis patients had an increased risk of ADRs related to ‘Respiratory, thoracic and mediastinal disorders’ and as compared to psoriatic arthritis patients. Etanercept use, combination therapy with methotrexate and/or corticosteroids, and age also influenced the risk of reporting specific ADRs.
Conclusions
There were no differences in frequencies and nature of patient-reported ADRs attributed to adalimumab and etanercept between different IRDs. However, more research is needed to align patients’ and health-care professionals’ perspectives to improve knowledge on disease-specific ADRs.
1. Introduction
Information on adverse drug reactions (ADRs) in the patient information leaflet and summary of product characteristics is generally clustered for all indications of a drug. For example, no distinction is made in the frequency and nature of injection site reactions, infections, cutaneous reactions, and induction of autoimmunity for the various indications of tumor necrosis factor alpha (TNF-α) inhibitors like adalimumab (ADA) and etanercept (ETN) [Citation1–3]. A previous study has shown that patients using a biologic DMARD (bDMARD) for an immune-mediated inflammatory disease (IMID) prefer to receive ADR information tailored to their own bDMARD and IMID [Citation4]. However, the extent to which ADRs differ per IMID, and the relevance of tailoring ADR information per IMID has not been fully investigated yet.
Previous research in a registry of rheumatic disease patients by Carmona et al. [Citation5] showed that the incidence rate ratio (IRR) of adverse events (AEs) during treatment with anti-TNF drugs were lower in psoriatic arthritis (PsA) (IRR = 0.81 (0.74, 0.89)), but higher in off-label uses in SpA-like chronic arthropathies (IRR = 1.33 (1.19, 1.49)) when compared to rheumatoid arthritis (RA). García-Doval et al. [Citation6] found similar results when comparing the safety profile of anti-TNF-α drugs in patients with RA and psoriasis. They concluded that published registry safety data of anti-TNF drugs for RA should not be fully extrapolated to psoriasis, because RA patients tend to have a higher risk of serious AEs (multivariate HR = 0.54 (0.47–0.61)) with a different pattern. Furthermore, comparison of the AE rate that occurred during clinical trials of ADA showed individual differences between six different therapeutic indications [Citation7]. Therefore, these studies suggest that, besides the possible influence of other factors associated with the occurrence of AEs, such as age, gender, comorbidities, and concomitant therapies, the underlying disease may also play a role in the incidence rates and safety profile of TNF-α inhibitors [Citation6–8].
Currently, there is limited data comparing the occurrence of ADRs during TNF-α inhibitor treatment between patients with different inflammatory rheumatic diseases (IRDs), such as RA, ankylosing spondylitis (AS) or axial spondyloarthritis (axSpA), and PsA. Additionally, the real-life patients’ perspective on ADRs is lacking as patient-centeredness and the focus on patients’ perspective are still upcoming in this field of research. This information is vital for patients themselves and for health-care providers (HCPs) in their patient guidance [Citation3,Citation9].
In this study, data of the Dutch Biologic Monitor (DBM), a prospective cohort event monitoring system for patient-reported ADRs attributed to bDMARDs, were used to obtain insight into the differences in the frequencies and nature of patient-reported ADRs attributed to TNF-α inhibitors between different IRDs (i.e. RA, PsA, AS/axSpA) [Citation10]. A secondary aim of this study was to assess the role of the covariates (i.e. indication, type of bDMARD (ADA/ETN), gender, and the most common combination therapies, i.e. methotrexate (MTX) and/or corticosteroids) in the reporting of ADRs.
1.1. Key messages
The underlying IRD does not seem to influence the occurrence of particular patient-reported ADRs attributed to the bDMARDs adalimumab and etanercept.
We recommend the use of a generic patient-reported outcome measure system in the systematic registration of patients’ perspectives on ADRs in future research.
Patients’ perspective should be combined with HCP reports to enrich drug safety profiles.
2. Methods
2.1. Study design
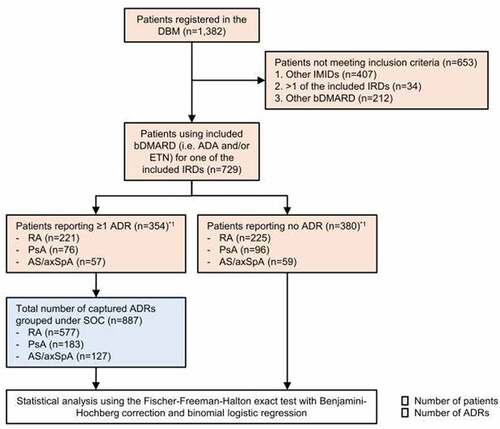
For this study, data of the DBM from January 2017 until December 2020 were used. The DBM is a prospective cohort event monitoring study for patient-reported ADRs attributed to bDMARDs. Patients that used ADA or ETN for one of the IRDs of interest, i.e. RA, PsA, or AS/SpA, were enrolled in this study according to the flowchart depicted in . We compared the frequencies and nature of patient-reported ADRs attributed to ETN and ADA between these diseases, which are the most used TNF-alpha inhibitors and most common IRDs in the DBM [Citation10]. This study was reported following the STROBE reporting guideline [Citation11].
Figure 1. Flowchart of the study design. Inclusion criteria were (1) one immune-mediated inflammatory disease (IMID), i.e. rheumatoid arthritis (RA), psoriatic arthritis (PsA) or ankylosing spondylitis/axial spondyloarthritis (AS/axSpA) and (2) the use of adalimumab (ADA) and/or etanercept (ETN) for their IMID. aThe combined number of patients from both groups (n = 734) exceeds the total number of included patients (n = 729) as 5 patients that had switched in bDMARD treatment reported an ADR with one, but not with the other bDMARD. Dutch Biologic Monitor (DBM); adverse drug reaction (ADR); System Organ Class (SOC); Inflammatory rheumatic diseases (IRDs).
2.2. Data source
The data of this study were obtained from the DBM database. Nine Dutch hospitals participated in the DBM between 1 January 2017 and 31 December 2020. Patients starting from the age of 18 that were proficient in Dutch and used one of the monitored bDMARDs, mainly for IMIDs, were selected consecutively until the end of the study. They were invited to participate via different recruitment strategies. Patients were contacted either by HCPs of the participating hospitals, via letters, at the outpatient pharmacy or during a visit at the ambulatory care unit for intravenous therapy [Citation10,Citation12]. After registration, a comprehensive web-based baseline questionnaire was sent to participating patients in which their demographic information (date of birth, height, weight, body mass index, gender, smoking habits), drug use (bDMARDs and combination therapy), start date, indication for bDMARD therapy and comorbidities at time of enrollment were covered. Furthermore, patients were asked to report detailed information about the ADRs they attributed to the used bDMARD. This included the type of ADR, start and stop date, course and burden (using a five-point Likert-type scale), but also whether the patient had been in contact with a HCP and which actions were taken in order to treat or relieve the ADR. Subsequent bimonthly follow-up questionnaires were focused exclusively on drug use and ADRs. When the previous questionnaire had expired (after 21 days) or if the patient withdrew from the study, no more questionnaires were sent. Withdrawal from the study was possible at any time. More in-depth information on the DBM can be found in previously published articles [Citation4,Citation9,Citation10,Citation12,Citation13].
After collection of ADR reports from DBM questionnaires, ADRs were coded by qualified pharmacovigilance assessors following Medical Dictionary for Regulatory Activities (MedDRA®) terminology (version 21.0), a standardized and internationally used set of medical terms used in pharmacovigilance databases among others [Citation14]. ADR reports were grouped under primary System Organ Class (SOC), High-Level Group Term (HLGT) and Preferred Term (PT). Recurring or persisting ADRs with the same PT that were repeated in the questionnaires by one patient were counted once. Multiple ADRs with different PTs, when reported by one patient, were counted separately.
2.3. Data selection
Patients enrolled in the DBM were selected for this study according to the flowchart depicted in . Patients using ADA or ETN for one of the IRDs of interest, i.e. RA, PsA, or AS/SpA, were included. Participants with other or more than one of the included diseases were excluded. Subsequently, patients who did not report an ADR and patients who reported one or more ADRs were separated. Reported ADRs were grouped under the primary SOC per indication.
2.4. Ethical considerations
The DBM received a waiver for the Dutch Medical Research Involving Human Subjects Act by the Dutch Medical Research Ethical Committee of Brabant (file number: NW2016-66). Moreover, the Dutch Biologic Monitor was approved by the medical ethics committees of the participating hospitals.
All participants received information about the study prior to participation and signed a digital informed consent form.
2.5. Data and statistical analysis
Baseline characteristics of the selected patients are presented in means with standard deviations (SD) or frequency with percentage, where appropriate. The frequencies (%) of the captured ADRs, grouped under primary SOC, was calculated per IRD. SOCs contributing for less than 1.5% to the total number of SOCs were grouped under ‘other SOC.’ Differences in ADR frequencies categorized by SOC were tested between the IRDs using the Fischer–Freeman–Halton (FFH) exact test (without simulation), which can be used when one or both variables have more than two categories [Citation15]. Subsequently, a correction was made for multiple comparisons using the Benjamini–Hochberg (BH) correction [Citation16,Citation17]. When a p-value <0.1 was found, a binomial logistic regression analysis was performed to assess the effect of selected potential covariates on the reporting of these SOCs. Therefore, a crude model was made with ‘indication’ as the independent variable. Subsequently, an adjusted model was made including indication, type of bDMARD (ADA/ETN), gender, and the most common combination therapies, i.e. MTX and/or corticosteroids, as independent variables [Citation18–22]. Due to limited data, other variables like comorbidities could not be included in this analysis. Results were presented as odds ratio (OR) with 95% confidence interval (CI) and p-values. Finally, the FFH exact test was used to evaluate the difference in the proportion of patients reporting no ADRs versus patients reporting one or more ADRs per included IRD.
Statistical analyses were performed using IBM SPSS Statistics (version 22), R software version 4.0.5, and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA). p-Values below 0.05 were considered statistically significant.
3. Results
In total, 729 inflammatory rheumatic patients met the selection criteria of whom 354 (48.6%) experienced at least one ADR. These patients reported 887 unique ADRs, grouped under SOC as depicted in . The overall mean age was 57.4 years (SD±12.8) at time of enrollment and the majority of patients was female (59.7%), as listed in . The mean age of patients who reported ADRs was 55.5 years (SD±12.9). The majority of these patients was female (68.4%).
Figure 2. The frequency (%) of patient-reported ADRs per IRD, attributed to the use of anti-TNF drugs. ADRs were grouped under MedDRA® system organ class (SOC). SOCs contributing for less than 1.5% to the total number of SOCs were grouped under ‘other.’ p-Values indicate the difference in frequencies of the ADRs between the different IRDs. Inflammatory rheumatic disease (IRD); rheumatoid arthritis (RA); psoriatic arthritis (PsA); ankylosing spondylitis (AS) including axial spondyloarthritis (axSpA).
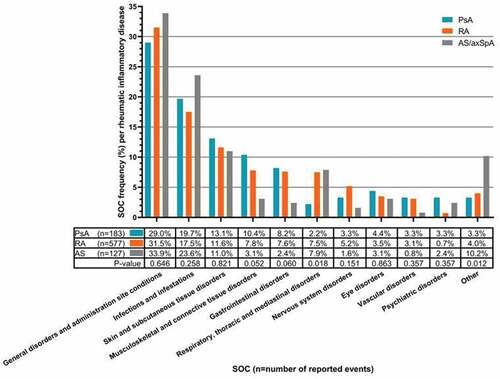
Table 1. Characteristics of patients registered in the DBM that use anti-TNF drugs for their IRD.
3.1. The frequency and nature of patient-reported ADRs attributed to TNF-α inhibitors
The frequency and nature of patient-reported ADRs attributed to ADA and ETN between the different IRDs is shown in . When comparing the proportion of reported events between the different IRDs for each SOC, a p-value below 0.1 was found for ‘musculoskeletal and connective tissue disorders’ (p = 0.052), ‘gastrointestinal disorders’ (p = 0.060), ‘respiratory, thoracic, and mediastinal disorders’ (p = 0.018), and ‘other SOC’ (p = 0.012) (). However, none of the outcomes were statistically significant after BH correction. Furthermore, no differences were found when comparing the ratio of patients reporting ADRs and no ADRs between the different IRDs (p = 0.486) ().
The most common HLGT reports in these groups of SOCs were ‘joint disorders’ such as arthralgia; ‘gastrointestinal signs and symptoms’ such as nausea; ‘respiratory disorders’ such as cough; and ‘benign cutaneous neoplasms’ such as skin papilloma, respectively.
3.2. The role of the IRDs and covariates in the reporting of ADRs of interest
For the SOCs with a p-value <0.1, a binomial logistic regression analysis was performed to assess the role of the IRDs and potential covariates in the reporting of these ADRs (). Patients with RA and AS/axSpA showed an increased risk of ‘respiratory, thoracic and mediastinal disorders’ as compared to patients with PsA in a crude model (RA: OR = 4.69 (CI: 1.63–19.87); AS/axSpA: OR = 4.75 (CI: 1.34–22.21)) and when adjusted for covariates (RA: AOR = 6.24 (CI: 2.01–27.71); AS/axSpA: AOR = 5.17 (CI: 1.28–26.31)). The risk of reporting ‘respiratory, thoracic and mediastinal disorders’ was significantly influenced by the bDMARD used (ETN) (AOR = 0.37 (CI: 0.18–0.73)), combination therapy (MTX/corticosteroids) (AOR = 0.34 (CI: 0.15–0.74)/AOR = 2.49 (CI: 1.11–5.42)) and age (AOR = 1.04 (CI: 1.01–1.08)). Moreover, patients with AS/axSpA reported more ‘other SOCs’ as compared to patients with PsA in a crude model (OR = 3.59 (CI: 1.32–10.87)) and when adjusted for covariates (AOR = 4.33 (CI: 1.48–13.89)). There were no significant covariates that influenced this relationship.
Table 2. Logistic regression estimates of patient characteristics associated with the reporting of ADRs categorized by SOC.
Patients with RA and AS/axSpA showed similar chances on reporting adverse events in the SOCs ‘musculoskeletal and connective tissue disorders’ and ‘gastrointestinal disorders’ as compared to patients with PsA. There were no significant covariates that influenced this relationship, except for age, which significantly influenced the risk of reporting ‘musculoskeletal and connective tissue disorders’ (AOR = 1.04 (CI: 1.01–1.08)).
4. Discussion
In this study, we aimed to assess whether the frequency and nature of patient-reported ADRs attributed to anti-TNF drugs differed significantly between different IRDs, and whether these IRDs and other covariates influenced the reporting of these ADRs categorized by SOC. When compared to patients with PsA, both indications RA and AS/axSpA showed a significantly increased risk of ‘respiratory, thoracic, and mediastinal disorders’ (RA: AOR = 6.24 (CI: 2.01–27.71); AS/axSpA: AOR = 5.17 (CI: 1.28–26.31)), while AS/axSpA also showed a significantly increased risk of ‘other SOCs’ (AOR = 4.33 (CI: 1.48–13.89). Older patients that used adalimumab and corticosteroids were more prone to report respiratory, thoracic, and mediastinal disorders compared to etanercept users and patients without corticosteroids, whereas MTX use was associated with a lower chance of reporting this adverse event. Furthermore, older patients were more likely to report musculoskeletal and connective tissue disorders regardless of their IRD.
Although previous studies concluded that the underlying disease may play a crucial role in the incidence rates of AEs and safety profile of TNF-α inhibitors in patients with different IMIDs [Citation5–8], we could not draw the same conclusion. This might be due to a more limited database or the fact that IRDs are generally very closely related in pathophysiology and symptoms, except for the genetic disposition and the localization of the inflammation [Citation23,Citation24]. For example, García-Doval et al. [Citation6] showed differences in ADR profile of anti-TNF-α drugs in patients with RA and psoriasis, a non-rheumatic inflammatory disease. This is in accordance with preliminary findings in the DBM data when patient-reported ADR profiles of ADA were compared between patients with PsA, RA, AS/axSpA, and inflammatory bowel disease [Citation18].
Another explanation for the differences in findings in our study compared to previous studies is that our study was based on patient-reported ADRs, while the other studies were mainly based on HCP reports [Citation5–8]. A comparison between ADRs reported by patients and ADRs reported by HCPs in a patient registry showed that patients and HCPs attribute and report different ADRs to the use of bDMARDs [Citation25,Citation26].
Even though the frequency and nature of ADRs in this study did not differ significantly between the selected IRDs according to the FFH exact test, it is remarkable that the risk of reporting ADRs attributed to anti-TNF therapy in the categories ‘respiratory, thoracic, and mediastinal disorders’ and ‘other SOCs’ could partly be explained by the different IRDs after logistic regression. However, the causal relationship of this increased risk could not be investigated as the ADR reports were not verified by HCPs. Furthermore, RA, PsA, and AS/axSpA have all been associated with a higher risk of pulmonary diseases [Citation27–29]. Yet, also the type of bDMARD, combination therapy, and age also affected the chances of reporting an ADR in the SOC ‘respiratory, thoracic, and mediastinal disorders.’
Another aim of our study focused on the influence of these covariates on the reported ADRs. Our data suggest that there is a higher risk of reporting ‘respiratory, thoracic, and mediastinal disorders’ when using ADA compared to ETN. Furthermore, combination therapy with MTX showed a decreased risk. However, HCP reports in previous randomized controlled trials (RCTs) showed that combination therapy did not affect the risk of experiencing adverse events for ADA and ETN users [Citation30–32], except for infections [Citation33,Citation34].
It is known that the use of corticosteroids can lead to a variety of ADRs in patients with IRDs [Citation28,Citation35,Citation36]. This can potentially explain the increased risk of patient-reported ‘respiratory, thoracic, and mediastinal disorders’ with concomitant use of corticosteroids. Higher age increased this risk and the risk of musculoskeletal and connective tissue disorders as well, which is in line with previous research showing generally higher rates of AEs in elderly [Citation37,Citation38]. Interestingly, the use of MTX can cause respiratory, thoracic, and mediastinal disorders as described in its product characteristics [Citation39]. This somewhat contradicts our result that MTX use was associated with a decreased risk of reporting these side effects. However, it is possible that patients using MTX are aware it can cause respiratory, thoracic, and mediastinal disorders and may therefore not report them with the use of anti-TNF therapy. Combining patient reported ADRs and reports of HCPs could be of help to solve this issue.
This study has some limitations. First, selection bias cannot be ruled out completely when asking patients to report information, as patients experiencing (more) ADRs may be more willing to participate. Although literature on ADR prevalence varies significantly, the frequency found in this study seems to be relatively low compared to previous research and RCTs with a HCP perspective [Citation7,Citation30,Citation40,Citation41]. Therefore, we believe that the influence of selection bias was limited. Selection bias could also be introduced when patients prone to specific ADRs such as infections, which occur more often with ADA use, are less frequently treated with this specific drug [Citation42].
Another limitation is that the causality of the ADRs is not assessed, as the ADRs were reported by patients and not verified by HCPs. For this reason, it might be possible that some patient-reported ADRs in this study would not be considered as ADRs by HCPs. However, this approach is required to obtain an unbiased impression of patients’ perspective on ADRs. In the future, the use of similar questionnaires on ADRs for patients with all indications for a specific drug would be an effective and efficient method to collect information on ADRs, as it simultaneously facilitates the comparison of ADRs between different diseases. However, to enrich the safety profile of drugs, perspectives of both patient and HCP reports should ideally be combined.
Finally, this study focused on the frequency and nature of ADRs attributed to ADA and ETN combined, since these are the most used TNF-alpha inhibitors in the DBM and the Netherlands [Citation10]. Unfortunately, it was not possible to analyze ADA and ETN separately due to insufficient sample sizes. Therefore, future studies should also focus on separate anti-TNF-alpha drugs, as this contributes to tailored ADR information that is in line with patients’ preferences [Citation4,Citation43]. On the other hand, since HCPs consider TNF-inhibitors as a more or less homogenous group of drugs because of their comparable effectivity and safety profile [Citation44] and since they regularly decide between groups of bDMARDs [Citation45], this study can still be considered a valuable addition to current knowledge.
Moreover, it would be interesting to look into specific ADRs, because, besides SOCs mentioned in the patient information leaflets, the most common ADRs of bDMARDs are highlighted separately and more specifically as well [Citation1,Citation2]. This might also result in other outcomes or more outspoken differences as SOCs are a collection of ADRs with different pathophysiological mechanisms.
5. Conclusion
This is the first study comparing the frequency and nature of patient-reported ADRs attributed to bDMARDs between different IRDs. Although no significant differences in the frequency and nature were found, the underlying disease and several covariates like age and combination therapy cannot be excluded as contributing factors in the occurrence of particular ADRs.
Abbreviations
Author contributions
All authors were involved in the conception and design of the study. LH Roest, LJ Kosse, and NT Jessurun collected, analyzed, and interpreted the data. Furthermore, JA van Lint, HR Gosselt, and JHG Scholl contributed to the data analysis. All authors revised the paper for intellectual content, provided detailed feedback and critically reviewed and approved the final manuscript. All authors agreed to be accountable for all aspects of the work.
Declaration of interests
HE Vonkeman reports service on advisory boards, or as speaker, or consultant for AbbVie, Amgen, AstraZeneca, BMS, Celgene, Celltrion, Galapagos, Gilead, GSK, Janssen-Cilag, Lily, MSD, Novartis, Pfizer, Roche, Sanofi-Genzyme, all outside the submitted work. SW Tas reports grants and/or persona fees from AbbVie, Arthrogen, AstraZeneca, BMS, Celgene, Galapagos, GSK, MSD, Pfizer, Roche, Sanofi-Genzyme, all outside the submitted work. MT Nurmohamad reports grants and personal fees from AbbVie and Eli Lilly, and grants from BMS, Amgen, and Pfizer, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A peer reviewer on this manuscript has disclosed research grants from Pfizer, BMS, and AbbVie. All other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
The data are not publicly available due to legal restrictions related to data privacy protection. However, the data are available from the corresponding author upon reasonable request after authorization from the DBM board.
Additional information
Funding
References
- Package leaflet: information for the patient; Humira 40 mg solution for injection in pre-filled pen; Adalimumab [Internet]. [cited 2021 Apr 22]. Available from 2021 Apr 22: https://www.medicines.org.uk/emc/files/pil.272.pdf
- Package leaflet: information for the user enbrel 25 mg solution for injection in pre-filled syringe Enbrel 50 mg solution for injection in pre-filled syringe; etanercept [Internet]. [[cited 2021 Apr 22]. Available from: https://www.medicines.org.uk/emc/files/pil.272.pdf
- Kirkham B, Furst DE, Romain PLR. Tumor necrosis factor-alpha inhibitors: an overview of adverse effects [Internet]. UpToDate. 2020 [cited 2021 Apr 22]. Available from: https://www-uptodate-com.proxy.library.uu.nl/contents/tumor-necrosis-factor-alpha-inhibitors-an-overview-of-adverse-effects
- Kosse LJ, Weits G, Vonkeman HE, et al. Immune-mediated inflammatory disease patients’ preferences in adverse drug reaction information regarding biologics. Expert Opin Drug Saf [Internet]. 2020 [cited 2021 Apr 22];19(8):1–5. Available from: https://pubmed.ncbi.nlm.nih.gov/32524887/
- Carmona L, Descalzo MA, Ruiz-Montesinos D, et al. Safety and retention rate of off-label uses of TNF antagonists in rheumatic conditions: data from the Spanish registry BIOBADASER 2.0. Rheumatology [Internet]. 2011 [cited 2021 May 6];50(1):85–92. Available from: https://pubmed.ncbi.nlm.nih.gov/20601654/
- García-Doval I, Hernández MV, Vanaclocha F, et al. Should tumour necrosis factor antagonist safety information be applied from patients with rheumatoid arthritis to psoriasis? Rates of serious adverse events in the prospective rheumatoid arthritis BIOBADASER and psoriasis BIOBADADERM cohorts. Br J Dermatol [Internet]. 2017 [cited 2021 Apr 22];176(3):643–649. Available from: https://pubmed.ncbi.nlm.nih.gov/27258623/
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis [Internet]. 2013 [cited 2021 May 6];72(4):517–524. Available from: https://pubmed.ncbi.nlm.nih.gov/22562972/
- Baeten D, Van Hagen PM. Use of TNF blockers and other targeted therapies in rare refractory immune-mediated inflammatory diseases: evidence-based or rational? [Internet]. Ann. Rheum. Dis. Ann Rheum Dis; 2010 [cited 2021 May 6]. p. 2067–2073. Available from: https://pubmed.ncbi.nlm.nih.gov/20705637/
- Kosse LJ, Jessurun NT, Vonkeman HE, et al. Stakeholders’ perspectives on a patient-reported outcome measure-based drug safety monitoring system for immune-mediated inflammatory diseases. Expert Opin Drug Saf [Internet]. 2020 [cited 2021 Apr 22];19(11):1521–1528. Available from: https://pubmed.ncbi.nlm.nih.gov/32730115/
- Kosse LJ, Jessurun NT, Hebing RCF, et al. Patients with inflammatory rheumatic diseases: quality of self-reported medical information in a prospective cohort event monitoring system. Rheumatol (United Kingdom) [Internet]. 2020 [cited 2021 Apr 22];59:1253–1261. Available from: https://pubmed.ncbi.nlm.nih.gov/31566226/
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg [Internet]. 2014 [cited 2021 Jun 9];12(12):1500–1524. Available from: https://pubmed.ncbi.nlm.nih.gov/25046751/
- van Lint JA, Jessurun NT, Hebing RCF, et al. Patient-reported burden of adverse drug reactions attributed to biologics used for immune-mediated inflammatory diseases. Drug Saf [Internet]. 2020 [cited 2021 May 17];43(9):917–925. Available from: https://doi.org/10.1007/s40264-020-00946-z
- van Lint JA, Jessurun NT, Tas SW, et al. Gastrointestinal adverse drug reaction profile of etanercept: real world data from patients and healthcare professionals. J Rheumatol [Internet]. 2021 [cited 2021 Jun 20];201373. Available from: https://www.jrheum.org/content/early/2021/05/11/jrheum.201373
- Brown EG, Wood L, Wood S. The Medical Dictionary for Regulatory Activities (MEDDRA) [Internet]. Drug Saf. Drug Saf; 1999 [cited 2021 May 19]. p. 109–117. Available from: https://pubmed.ncbi.nlm.nih.gov/10082069/
- Fisher R. Tests of significance in harmonic analysis. 1929 [cited 2021 May 19]; Available from: https://hekyll.services.adelaide.edu.au/dspace/bitstream/2440/15201/1/75.pdf
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B [Internet]. 1995 [cited 2021 May 19];57:289–300. Available from: https://rss.onlinelibrary.wiley.com/doi/full/10.1111/j.2517-6161.1995.tb02031.x
- Jafari M, Ansari-Pour N. Why, when and how to adjust your P values? Cell J [Internet]. 2019 [cited 2021 May 19];20:604–607. Available from: https://pmc/articles/PMC6099145/
- Roest L, Kosse L, Van Lint J, et al. Disease-specific adverse drug reaction profiles of adalimumab and etanercept as reported by immune-mediated inflammatory disease patients. Ann Rheum Dis [Internet]. 2021 [cited 2021 Jun 21];80:548–549. Available from: http://ard.bmj.com/
- Patanè M, Ciriaco M, Chimirri S, et al. Interactions among low dose of methotrexate and drugs used in the treatment of rheumatoid arthritis. Adv Pharmacol Sci. 2013;2013. DOI:10.1155/2013/313858.
- Gibofsky A. Combination therapy for rheumatoid arthritis in the era of biologicals. HSS J [Internet]. 2006 [cited 2021 Jun 21];2(1):30–41. Available from: https://pmc/articles/PMC2504107/
- Wolfe F, Michaud K. Corticosteroids increase the risk of diabetes mellitus in RA and contribute to the risk of myocardial infarction and heart failure. Ann Rheum Dis - BMJ Publ Gr [Internet]. 2004 [cited 2021 Jun 21];63:495. Available from: https://www.researchgate.net/publication/216742242_Corticosteroids_increase_the_risk_of_diabetes_mellitus_in_RA_and_contribute_to_the_risk_of_myocardial_infarction_and_heart_failure
- Radovits BJ, Kievit W, Fransen J, et al. Influence of age on the outcome of antitumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis [Internet]. 2009 [cited 2021 May 19];68(9):1470–1473. Available from: https://pubmed.ncbi.nlm.nih.gov/19015210/
- Akhondi H, Varacallo M. Rheumatoid arthritis and ankylosing spondylitis [Internet]. StatPearls. StatPearls Publishing; 2018 [cited 2021 Jul 5]. Available from 2021 Jul 5: http://www.ncbi.nlm.nih.gov/pubmed/30335321
- Inman RD, El-Gabalawy HS. The immunology of ankylosing spondylitis and rheumatoid arthritis: a tale of similarities and dissimilarities. Clin Exp Rheumatol [Internet]. 2009 [cited 2021 Jul 5];27. Available from: https://pubmed.ncbi.nlm.nih.gov/19822042/
- Gäwert L, Hierse F, Zink A, et al. How well do patient reports reflect adverse drug reactions reported by rheumatologists? Agreement of physician- and patient-reported adverse events in patients with rheumatoid arthritis observed in the German biologics register. Rheumatology (Oxford) [Internet]. 2011 [cited 2021 Sep 12];50(1):152–160. Available from: https://pubmed.ncbi.nlm.nih.gov/20871128/
- Thomas PWA, Römkens TEH, West RL, et al. Discrepancy between patient- and healthcare provider-reported adverse drug reactions in inflammatory bowel disease patients on biological therapy. Uni Euro Gastro J [Internet]. 2021 [cited 2022 Jan 4];9(8):919–928. Available from: https://onlinelibrary-wiley-com.proxy.library.uu.nl/doi/full/10.1002/ueg2.12107
- Dejcman D, Skowasch D, Pizarro C, et al. Pulmonary manifestations of rheumatoid arthritis, psoriatic arthritis and peripheral spondyloarthritis: prevalence, diagnostic approach and treatment options. Curr Rheumatol Rev [Internet]. 2021 [cited 2021 Jul 16];17(1):17–28. Available from: https://pubmed.ncbi.nlm.nih.gov/32888273/
- Bluett J, Jani M, Symmons DPM. Practical management of respiratory comorbidities in patients with rheumatoid arthritis. Rheumatol Ther [Internet]. 2017 [cited 2021 Jul 16];4(2):309. Available from: https://pmc/articles/PMC5696283/
- Jani M, Hirani N, Matteson EL, et al. The safety of biologic therapies in RA-associated interstitial lung disease. Nat Rev Rheumatol [Internet]. 2013. [cited 2021 Jul 16];10(5):284–294. Available from: https://www-nature-com.proxy.library.uu.nl/articles/nrrheum.2013.197
- Furst DE, Schiff MH, Fleischmann RM, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety trial of adalimumab in rheumatoid arthritis). J Rheumatol. 2003;30(12):2563–2571.
- Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol [Internet]. 2019 [cited 2021 Jul 18];71(7):1112–1124. Available from: https://onlinelibrary-wiley-com.proxy.library.uu.nl/doi/full/10.1002/art.40851
- Klareskog L, van der Heijde D, de Jager J, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet [Internet]. 2004 [cited 2021 Jul 18];363(9410):675–681. Available from: https://pubmed.ncbi.nlm.nih.gov/15001324/
- Parakkal D, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the food and drug administration adverse event reporting system. J Gastrointestin Liver Dis [Internet]. 2013 [cited 2021 Jul 18];22:269–276. Available from: https://www.jgld.ro/jgld/index.php/jgld/article/view/2013.3.8/619
- Gerriets V, Bansal P, Goyal A, et al. Tumor Necrosis Factor Inhibitors. StatPearls [Internet]. 2021 [cited 2021 Jul 18]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK482425/.
- Li P, Zheng Y, Chen X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front Pharmacol. 2017:460.
- Ethgen O, de L EF, Bruyere O, et al. What do we know about the safety of corticosteroids in rheumatoid arthritis? Curr Med Res Opin. 2013 Jul 18;29:1147–1160.
- Vela P, Sanchez-Piedra C, Perez-Garcia C, et al. Influence of age on the occurrence of adverse events in rheumatic patients at the onset of biological treatment: data from the BIOBADASER III register. Arthritis Res Ther [Internet]. 2020 [cited 2021 Jul 18];22(1):1–9. Available from: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-020-02231-x
- Cho S-K, Sung Y-K, Kim D, et al. Drug retention and safety of TNF inhibitors in elderly patients with rheumatoid arthritis. BMC Musculoskelet Disord [Internet]. 2016 [cited 2021 Jul 18];17(1):1–8. Available from: https://pmc/articles/PMC4977640/
- Methotrexate 2.5 mg tablets - summary of product characteristics (smpc) - (emc) [internet]. Electron. Med. Compend. 2020 [cited 2022 May 26]. Available from 2022 May 26: https://www.medicines.org.uk/emc/product/511/smpc
- de CMC, Barros BCA, Fulone I, et al. Adverse events in patients with rheumatoid arthritis and psoriatic arthritis receiving long-term biological agents in a real-life setting. Front Pharmacol [Internet]. 2019 [cited 2021 Jul 19];10:965. Available from: https://pmc/articles/PMC6749844/
- van de PLBA, Atkins C, Malaise M, et al. Efficacy and safety of Adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis [Internet]. 2004 [cited 2021 Jul 19];63(5):508–516. Available from: https://ard.bmj.com/content/63/5/508
- Thalayasingam N, Isaacs JD. Anti-TNF therapy. Best Pract Res Clin Rheumatol [Internet]. 2011 [cited 2022 Jun 1];25:(4):549–567. Available from: https://pubmed.ncbi.nlm.nih.gov/22137924/
- Johnson KJ, Sanchez HN, Schoenbrunner N. Defining response to TNF-inhibitors in rheumatoid arthritis: the negative impact of anti-TNF cycling and the need for a personalized medicine approach to identify primary non-responders [Internet]. Clin Rheumatol Springer London. 2019 [cited 2021 Jun 30]. 2967–2976. Available from: https://pubmed.ncbi.nlm.nih.gov/31520227/
- Rubbert-Roth A, Atzeni F, Masala IF, et al. TNF inhibitors in rheumatoid arthritis and spondyloarthritis: are they the same? Autoimmun Rev. 2018;17(1):24–28.
- Atzeni F, Benucci M, Sallì S, et al. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev [Internet]. 2013 [cited 2021 Jul 17];12:(5):575–579.
