ABSTRACT
Introduction
Medication errors are common events that compromise patient safety and are prevalent in all health-care settings. This umbrella review aims to systematically evaluate the evidence on contributory factors to medication errors in health-care settings in terms of the nature of these factors, methodologies and theories used to identify and classify them, and the terminologies and definitions used to describe them.
Areas covered
Medline, Cumulative Index to Nursing and Allied Health Literature, Embase, and Google Scholar were searched from inception to March 2022. The data extraction form was derived from the Joanna Briggs Institute (JBI) Reviewers’ Manual, and critical appraisal was conducted using the JBI quality assessment tool. A narrative approach to data synthesis was adopted.
Expert opinion
Twenty-seven systematic reviews were included, most of which focused on a specific health-care setting or clinical area. Decision-making mistakes such as non-consideration of patient risk factors most commonly led to error, followed by organizational and environmental factors (e.g. understaffing and distractions). Only 10 studies had a pre-specified methodology to classify contributory factors, among which the use of theory, specifically Reason’s theory was commonly used. None of the reviews evaluated the effectiveness of interventions in preventing errors. The collated contributory factors identified in this umbrella review can inform holistic theory-based intervention development.
1. Introduction
Medication errors are prevalent events that take place across the entire spectrum of the medication use process [Citation1,Citation2]. The National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) in the United States (U.S.) defines a medication error as ”any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer” [Citation3].
Consequences of medication errors can range from no or mild harm to severe harm and death [Citation4,Citation5]. For instance, in U.K., 237.3 million medication errors occur every year, with 66 million considered potentially harmful [Citation5]. The same report noted that medication errors caused 712 deaths and contributed to more than 1700 deaths in 1 year [Citation5]. The World Health Organization (WHO) estimated the global impact of medication errors to be approximately $42 billion annually [Citation6]. Additionally, medication errors can have a deleterious psychological impact on patients, families, and health practitioners [Citation7,Citation8].
Evidence suggests that up to 60% of medication errors are under-reported [Citation9,Citation10]. The practice of detecting and reporting medication errors by health-care providers, as well as investigating and analyzing such errors through rigorous research, is imperative to promote patient safety [Citation11].
A myriad of potential strategies have been proposed to decrease medication errors and improve patient outcomes, including pharmacist-led interventions, educational interventions, technology-driven interventions, and multidisciplinary team implementation. While a number of studies have demonstrated a reduction in the incidence of errors due to intervention, negative or no effects have also been reported [Citation12–17]. In addition, there is a dearth of the literature that describes the rationale and theoretical basis for intervention development targeting relevant contributory factors [Citation18,Citation19].
Several primary studies and systematic reviews have explored factors contributing to medication errors. Given the plethora of systematic reviews investigating contributory factors to medication errors, there is a need to identify, critically appraise, and synthesize these factors via an umbrella review. This will enhance access to high-quality evidence, provide recommendations to improve the robustness of future work, increase the understanding of contributory factors, and inform decision-making regarding the development of evidence-based and holistic interventions to reduce medication errors.
This study aimed to undertake an umbrella review of systematic reviews on contributory factors to medication errors in diverse health-care settings in terms of the nature of these factors, methodologies and theories used to identify and classify these factors, and the terminologies and definitions used to describe them.
2. Methods
2.1. Methodology reporting and registration
This umbrella review followed the recommendations provided by the Joanna Briggs Institute (JBI) reporting methodology manual and the Preferred Reporting Items for Overviews of Reviews (PRIOR) reporting guidelines-preprint (Supplementary material, ) [Citation20–22]. The review protocol was registered in PROSPERO (CRD42022321425) [Citation23].
Table 1. Search terms.
2.2. Data sources and search strategy
Searches were undertaken using four electronic databases and search engines from their inception to 29 March 2022: Ovid Medline, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Google Scholar (first 500 records). The process also included cross-referencing of included papers.
Search terms related to categories A (related to medication errors) and B (related to systematic reviews) were combined with Boolean operators (AND/OR). The search was limited to “English language,” “Human species,” “Systematic reviews,” and “Meta-analysis” as applicable to each database (). The detailed search strategy, MeSH, and other search terms were modified to suit each information source.
2.3. Eligibility criteria
Reviews were considered eligible if they met the following criteria: (1) reported factors contributing to medication errors, (2) systematic review with or without meta-analysis, (3) published in English language. For the purpose of this umbrella review, we included studies using different causation terms (e.g. contributory factors, causes, and risks). Systematic reviews focusing on adverse drug events (ADEs, i.e. harm experienced by a patient as a result of exposure to a medication. ADEs encompassing a wide range of incidents, such as adverse drug reactions and medication errors) with lack of clear relevance to medication errors were excluded [Citation24]. Narrative reviews, scoping reviews, or any other types of reviews were also excluded. No restriction on age, gender, or clinical specialty reported in the reviews was imposed.
2.4. Study selection
All retrieved articles were exported to EndNote 20® (2021 Clarivate), duplicates removed, and the remaining papers imported to Rayyan Qatar Computing Research Institute (QCRI) software for the titles and abstracts screening. This was followed by a full-text screening via Microsoft Excel. The two-phase screening process was conducted by two independent reviewers (L.N., R.A.A.), and discrepancies were resolved through a consensus discussion with a third reviewer (V.P.).
2.5. Data extraction
Data were extracted by one reviewer (L.N.) and verified by second reviewer (V.P.) using a Joanna Briggs Institute (JBI) Reviewers’ Manual informed data extraction form [Citation22]. Data on terminology, definitions, classifications, nature of contributory factors, methodologies, theories, models, and frameworks used to identify and classify these factors were extracted. In addition, information related to recommended interventions, characteristics of the interventions, and associated methods and outcomes was also extracted.
2.6. Quality assessment
The methodological robustness of the included systematic reviews was assessed by one reviewer (L.N.) and verified by a second reviewer (V.P.). The JBI 11-item critical appraisal tool for systematic reviews was utilized for the quality assessment [Citation22].
2.7. Data synthesis
Given that the outcomes of interest were qualitative, statistical pooling in meta-analysis was not appropriate. Synthesis of the findings was undertaken using a narrative approach. Narrative synthesis can be defined as “an approach to the systematic review and synthesis of findings from multiple studies that relies primarily on the use of words and texts to summarize and explain the findings of the synthesis” [Citation25]. Findings are presented in textual form and summary tables. Overlap between included systematic reviews was not assessed, as the primary aim was to assess the methodological quality of existing systematic reviews.
3. Results
3.1. Study selection
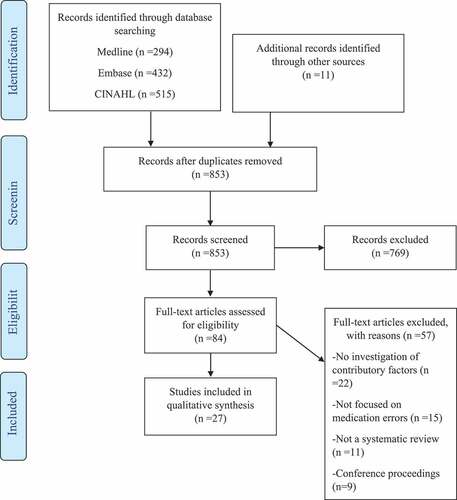
A total of 1252 citations were identified from the database searching and reference screening. Following duplicate removal, the remaining 853 articles were screened according to title and abstract. Twenty-seven systematic reviews were included in the final synthesis (). The most frequent reason for exclusion at the full-text screening stage was the lack of investigation into contributory factors to medication errors.
3.2. Characteristics of the included systematic reviews
summarizes the characteristics of the included reviews. The majority were published in the last 10 years, except for one each in 2007 [Citation26], 2009 [Citation27], and 2010 [Citation28]. It is noteworthy that the context of included reviews was not always the study setting. For example, in some cases, it was a certain geographical location or route of administration. Descriptions of the studied contexts are reported in and further described in this section. Of the 27 reviews, 16 focused on specific populations/settings including community-dwelling adults [Citation18,Citation29–31], home care setting [Citation32,Citation33], neonatal intensive care setting [Citation26,Citation34], inpatient setting [Citation27,Citation35], pediatrics [Citation36], elderly [Citation37], hematopoietic stem cell transplantation patients [Citation38], mental health patients [Citation39], perioperative setting [Citation40], and acute care setting [Citation28].
Table 2. Characteristics of included reviews.
Six reviews focused on the geographical location of the Middle East [Citation41,Citation42], Iran [Citation43,Citation44], Africa [Citation45], or Southeast Asia [Citation46] and systematically reviewed contributory factors in a variety of settings. Two reviews encompassed a specific pharmacological class or dosage form of direct oral anticoagulants [Citation47] and transdermal patches [Citation48]. One review included intern doctors only [Citation49], while another one examined a single prespecified contributory factor (shift work) in inpatient nurses [Citation50]. Schroers et al. (2020) were the only review that did not specify a population or a setting of interest [Citation51].
3.3. Search details
The number of databases reported in the systematic reviews ranged from 2 [Citation40,Citation48] to 21 [Citation28]. The most commonly recurring databases were Medline/PubMed, CINAHL, Embase, Cochrane, and British National Index (Supplementary material, ). Most reviews applied language as a filter, largely limiting their results to English language. Few reviews (n = 7) included studies of a number of languages, mainly those spoken by the research team.
Most reviews, except for four [Citation27,Citation35,Citation50,Citation51], did not integrate keywords specific to contributory factors in their search strategy. The keywords used were as follows: cause(s); causality; causalities; reason(s); etiology; etiology; factor(s); risk factor(s); contributing factor(s); determining factor(s); predictor(s); association(s); and determinants.
3.4. Quality assessment
All but six [Citation26,Citation27,Citation30,Citation34,Citation43,Citation48] of the reviews reported quality assessments of the included articles. The two most common quality assessment tools were the Allan and Barker instrument (with or without modifications) and the Critical Appraisal Skills Program checklist. As reported by the authors, the overall quality of primary studies included in the systematic reviews was variable (Supplementary material, ); with a considerable number reporting moderate overall quality.
Table 3. Data pertaining to medication errors.
According to the appraisal of the included systematic reviews using the JBI tool, the overall quality varied, with common areas of bias noted across reviews (Supplementary material, ). Most reviews described their aim (n = 19; 68%) and future research directions (n = 20; 74%), while 16 (59%) and 14 (52%) reviews lacked information about eligibility criteria and data extraction, respectively. Some reviews did not incorporate sufficient description of their search strategy (52%; n = 14) and resources (e.g. databases, gray literature, or reference lists) used to search for studies (41%; n = 11).
3.5. Medication errors: terminology, definitions, and classifications
Most reviews investigated medication errors without associating them with the stages of the medication use process (n= 17), of which one review used the terms ”medication errors” and ”adverse drug events” (ADEs) interchangeably [Citation50]. The remainder of the reviews focused on a single stage in the medication use process, specifically administration errors (n = 4), administration errors by patient or caregiver (n = 4), and prescribing errors (n= 2).
Of those reporting medication errors, four stated that they adopted the NCCMERP definition [Citation37,Citation39,Citation44,Citation46] and four adopted the definitions provided in the primary studies [Citation18,Citation26,Citation31,Citation47]. Two reviews on administration errors by patients or caregivers provided definitions in their methods section, of which one was suggested by the authors themselves [Citation29,Citation38]. Two different definitions were reported for administration errors [Citation33,Citation35]. Both were adopted from previous studies and entailed deviation between prescribed and administered medication. As for prescribing errors, one of the reviews reported that they adopted the working definitions in the original studies [Citation27].
Medication errors were classified in eleven of the included reviews (), of which 10 reported errors according to the medication use process stages (e.g. prescribing errors). The remainder investigated self-administration errors and reported according to the incident type (e.g. wrong dose) [Citation29]. Among the 10 reviews, six further classified medication errors according to the incident type [Citation36,Citation39,Citation41,Citation45–47].
3.6. Contributory factors to medication errors: terminologies, definitions, methodologies, and classifications
The terms used to describe the contributory factor are presented in . The most common term was ‘contributory factor,’ with some reviews using ‘factor’ alone or proceeded with another terms such as “risk,” “associated,” “related,” “influencing,” “causal,” “causative,” or “etiological.” The other commonly reported term was “cause” and its derivatives including “root cause,” “causation,” and “causality.” Other less commonly used terms were “reason,” “etiology,” “predictor,” and “source.”
Table 4. Data pertaining to contributory factors to medication errors.
Two included reviews defined risks/contributory factors [Citation27,Citation36], while another adopted the definition of hazard/contributory factors suggested by the WHO [Citation31]. Tully et al. (2009) differentiated between the terms ”causes” and ”contributory factors”, in which the latter was suggested to refer to those assessed by the researcher, while the former referred to those identified by practitioners [Citation27].
All but three [Citation37,Citation39,Citation46] of the included reviews specifically aimed to explore contributory factors (). Ten systematic reviews had a prespecified methodology to identify and classify contributory factors, namely the use of theories/frameworks/models and thematic analysis. Four adopted Reason’s Accident Causation Model [Citation27,Citation35,Citation42,Citation47], of which three classified contributory factors into categories of active failures, error-producing conditions, and latent conditions. In their review of administration errors, Keers et al. (2013) adopted a version of the theory that had been modified for administration errors [Citation35]. Among the included reviews that used the Reason’s model, active failures, and decision-making mistakes were the most prevalent categories of contributory factors ().
Table 5. Contributing factors to medication errors.
The Framework for Analyzing Risk and Safety in Clinical Medicine was used by one review [Citation42]. This framework categorizes factors into six groups: individual, work environment, organization and management, team, tasks, and medications [Citation43,Citation52]. Another review utilized the Conceptual Framework for the International Classification for Patient Safety proposed by the WHO [Citation31]. This framework comprises 10 high-level classes and the contributing factors segment consists of a maximum of five levels () [Citation53].
Qualitative synthesis was used in four of the included reviews to categorize contributory factors. Of these, thematic analysis was applied in two, detailing the generation of codes and themes [Citation49,Citation51]. Other methods reported were meta-regression [Citation28] and inductive analysis [Citation33] but with little detail provided. The review of Assiri et al. (2018) had three prespecified categories [Citation18], while four reviews classified factors according to emerging themes [Citation30,Citation32,Citation40,Citation45].
Among those reviews that did not apply Reason’s theory, the most recurring themes were practitioner-related (n = 8), work environment-related (n = 7), patient-related (n = 5), and medication-related factors (n = 4) [].
gives the most commonly reported contributory factors. Decision-making mistakes (classified as active failure by Reason’s theory) such as failure to consider risk factors (e.g. chronic kidney disease and pediatrics) were reported in multiple systematic reviews. Other recurring factors were related to the organization or environment, including lack of knowledge, insufficient training, work overload, inadequate staffing levels, illegible prescriptions, distractions and interruptions, and poor communication. Polypharmacy, extreme age (elderly or pediatrics), and limited health literacy of patients were also common across reviews.
3.7. Interventions proposed to mitigate factors contributing to medication errors
None of the reviews aimed to evaluate interventions designed to mitigate contributory factors. Nevertheless, 21 included reviews discussed interventions without specifying the characteristics, method of development, and outcomes of these interventions. Multiple reviews emphasized the need for multifactorial interventions to holistically address contributory factors [Citation18,Citation27–29,Citation31–33,Citation38,Citation40,Citation44,Citation47]. Only one review suggested the use of theory to develop these interventions [Citation47].
Pharmacist-delivered [Citation18,Citation29,Citation32,Citation40,Citation45–47], educational [Citation18,Citation31,Citation33,Citation38,Citation41,Citation44,Citation45,Citation47,Citation48,Citation51] and technology-enabled interventions [Citation18,Citation30,Citation32,Citation33,Citation38,Citation40,Citation44,Citation45,Citation47] were most frequently suggested in the included reviews. Only two included reviews incorporated organization-level interventions (e.g. increase staffing) [Citation44,Citation51].
Among studies that recommended pharmacist-delivered interventions, four suggested full integration of the pharmacist in the health-care team [Citation18,Citation40,Citation45,Citation46] while three recommended initiating a pharmacist-led service (e.g. anticoagulation stewardship program) [Citation29,Citation32,Citation47]. Most reviews that suggested technology-enabled interventions highlighted the need for decision support systems to reduce prescribing errors [Citation44,Citation45]. Three studies proposed the development of innovative technological tools (e.g. mobile applications) that could be accessed by patients and tailored to their needs [Citation18,Citation30,Citation33].
Proposed educational interventions varied significantly among the included studies based on the context. For instance, studies that focused on administration errors, recommended distribution of educational material alongside the educational sessions for the nurses to refer to it when needed [Citation30,Citation51]. Nevertheless, a commonly suggested topic was the communication and interprofessional collaboration between different health-care providers [Citation27,Citation32,Citation33,Citation47,Citation51]. Few reviews reported that educational sessions should be conducted periodically [Citation38,Citation44].
4. Discussion
4.1. Statement of key findings
This umbrella review shows that decision-making mistakes, which include non-consideration of risk factors (e.g. chronic kidney disease and pediatrics), were the most common contributory factor, followed by factors related to the organization and environment such as the lack of knowledge/training, understaffing, and distractions. Most reviews did not pre-specify a methodology in relation to classification of contributory factors. Among the reviews that followed a structured method to classify contributory factors, the use of the theory and Reason’s model was most commonly used. The included reviews were of variable quality due to issues primarily related to search strategy, quality assessment, and data extraction processes. A range of terminologies and definitions were used to refer to contributory factors. To target the contributory factors and subsequently reduce the errors, several interventions were suggested in the included reviews. These included pharmacist-provided, educational, and technology-based interventions. The discussion of interventions lacked details on the development, evaluation, and implementation.
4.2. Interpretation of findings
Decision-making mistakes (also known as errors of judgment), which include failure to consider risk factors (e.g. chronic kidney disease and pediatrics), were the predominant contributory factor to medication errors across diverse health-care settings. Decision-making mistakes and other types of human errors are foreseeable in the context of the complex and often challenging clinical practices [Citation54]. Additionally, healthcare is dynamic in nature, with a great deal of uncertainty and potential subjectivity surrounding clinical decisions [Citation54,Citation55]. Therefore, although it is imperative to attempt at mitigating these mistakes, it is unrealistic to expect an error-free system. However, innovative theory-based interventions that promote multidisciplinary team working, blame-free culture, use of technology, and expertise of pharmacists can minimize errors.
Another common contributory factor identified in our umbrella review is related to organizational and environmental factors. These factors have been poorly reported in the previous literature as less attention has been given to error-prone systems [Citation56].
Although the use of the theoretical framework has been strongly recommended to undertak exploratory and interventional research to identify and target different behaviors [Citation57,Citation58], most of the reviews did not report a prespecified method to synthesize contributory factors, with only six using a theory-based approach. One recurring model to classify contributory factors was Reason’s model. This model shifts the focus of human error investigation from person-centered to system approach considering errors occurring at both the sharp (active failures) and blunt (latent conditions) ends of the system [Citation59,Citation60]. The model also moves away from blame culture while still being easy-to-use; thus, it has been extensively utilized in the safety field [Citation60,Citation61]. Nonetheless, Reason’s model has limitations that should be considered by researchers who use it as well as practitioners who interpret findings from studies that have used it. The model is considered a complex linear model, which assumes that accidents are the result of a series of events that interact sequentially in a linear fashion [Citation62]. This approach may overlook the complexity of the system and the interrelations between its components, particularly when the contributory factors are far from the incident in terms of time or location [Citation62,Citation63]. Furthermore, some researchers argue that Reason’s theory may not account sufficiently for the interactions between defense layers and the errors produced by the defense mechanisms [Citation59].
Seventeen different terms and five definitions were used by the reviews to describe contributory factors. Variations in the definition of medication error (and subclasses) were also noted among the reviews. This reinforces findings from previous arguments suggesting multiplicity in the use of patient safety practice-related terminologies [Citation31,Citation47,Citation64,Citation65]. It is likely that an array of definitions for both medication errors and contributing factors used in other literature may not be captured by reviews included in our study.
It is worth noting that the primary studies that focused on interventions to mitigate errors were prospective/retrospective cohort studies or cross-sectional studies [Citation66–73]. A definitive evaluation utilizing randomized controlled trials was missing. Additionally, the majority of studies had a short follow-up duration, a small sample size, and were conducted in a single center [Citation66–73]. The primary outcome measure evaluated in these studies mainly related to the number of interventions offered, such as changing one of the components of a medication regimen (e.g. dose and duration) or highlighting the interaction between prescribed drugs. Another outcome measure was the total number of errors that were assessed to be potentially preventable upon implementing the interventions [Citation66–73].
4.3. Strengths and limitations
To our knowledge, this is the first attempt to systematically report the terminology, methodology, and classes of contributory factors to medication errors via an umbrella review. A comprehensive search of several databases followed by citation checking allowed retrieval of all relevant systematic reviews.
This review was limited by the lack of assessment for the potential overlap of individual studies within the included reviews. In addition, our summary of terms and definitions of contributory factors and medication errors relied on what has been reported by the included reviews. Lastly, only publications in the English language were included.
4.4. Implication for practice and research
Although the context of existing systematic reviews varied, several contributory factors were common across the reviews. A comprehensive synthesis of these factors could enable the development of holistic theory-informed interventions to target the identified factors. The contributory factors identified included decision-making mistakes and organizational factors. Accordingly, multifaceted theory-based interventions are required to prevent medication errors. These interventions should target contributory factors from the organizational level to specific tasks at the individual level.
Failure to account for risk factors was a common example of decision-making mistakes. Previous studies have shown that pharmacist-led and technology-enabled interventions minimize medication errors, including those occurring in high-risk cohorts [Citation74–76]. Although the role of pharmacists and technology has expanded in recent years [Citation74–76], their expertise remain underutilized [Citation77–80].
System failures due to top-level management decisions were also identified among the most recurring contributory factors. Inadequate training and knowledge was the predominant latent condition. This indicates that limited continuing professional development activities alone might be insufficient in terms of quantity or quality. A previous systematic review showed that pharmacist-conducted educational interventions led to a significant reduction in medication error rates [Citation12]. Accordingly, implementation of educational sessions that are based on a structured need assessment to address the exact gaps in knowledge are likely to impact positive changes [Citation81].
Despite the continuous growth of health-care costs, issues related to understaffing and poor work environment were still prominent in our review. Hence, strategic allocation of available resources and implementation of cost-effective mitigation mechanisms are recommended. Moreover, organizational and environmental factors that lead to breakdowns in communication and collaboration between health-care providers have been repeatedly reported across the included reviews. Thus, interdisciplinary collaborations could be considered in future interventions as they represent an important facet of facilitating communication [Citation82]. This is particularly important as medication errors are a complex problem affecting diverse health-care disciplines and contexts.
It is evident from the findings of this review that there are certain populations/settings for which contributory factors to medication errors have not been systematically synthesized yet. Thus, future systematic reviews should focus on these clinical areas, such as oncology patients or outpatient and ambulatory settings.
This study has also identified a dearth of reviews incorporating theories in classifying contributory factors and developing interventions. This issue has been discussed before in the literature after some interventions that were implemented on a wide-scale have been proven ineffective or sometimes even had negative effect [Citation83]. The first crucial step to prevent an undesirable event is to explore and diagnose the behaviors and mediating pathways leading to it, which in our case would be contributory factors. This could be achieved through the explicit use of behavioral theories [Citation84,Citation85]. Accordingly, we strongly encourage future researchers to utilize behavior theoretical frameworks, such as the Theoretical Domain Framework (TDF) for both understanding contributory factors and developing interventions that address these factors [Citation86].
Given the range of terminologies used to refer to contributory factors to medication errors, future research should utilize consistent terminology. Based on our findings, the consistent use of ‘contributory factors’ is recommended. Although the term “causes” and “reasons” might be acceptable, we advise against their use. This is important to avoid confusion as these two terms have been used in different contexts in the literature. For example, some reviews represented fundamentally different concepts between ‘contributory factors’ and ‘causes’ [Citation27,Citation87]. Others used the terms ”reasons” and ”causes” interchangeably with ”type” or ”nature” of medication errors [Citation13,Citation88].
It is pivotal to remove ambiguity and reach international consensus on all patient safety terminology, including contributory factors and their subclasses. This will enable the accurate quantification of the burden of each factor, analysis of data, and comparison of research outcomes [Citation1,Citation64,Citation89,Citation90]. We also suggest maintaining consistency in the terms used across each study and to provide definitions for each term. This is of particular importance, as variation might lead to the inclusion of papers that may not actually be studying the phenomenon of interest. This could enhance the reliability of the outcomes and subsequently facilitate the development of possibly effective interventions.
Similarly, multiple definitions for contributory factors have emerged in the included reviews; however, our summary does not reflect all proposed definitions in the literature. Therefore, future research should focus on developing and validating definitions of key terminologies used in research related to patient safety such as medication errors and contributory factors.
5. Conclusion
This umbrella review highlights a significant variation in terminology and definitions used to describe contributory factors in the published literature. Decision-making mistakes, which included failure to consider risk factors (e.g. chronic kidney disease and pediatrics) were the most common contributory factors, followed by factors related to the organization and environment such as understaffing and distractions. However, a lack of prespecified methodology to identify and classify contributory factors was noted. Additionally, none of the reviews evaluated the effectiveness of interventions to prevent errors.
The recommendations offered in this review have the potential to enhance consistency in the use of terminology, definitions, and methodology used in contributory factors to medication error research. This will subsequently enable practitioners, policymakers, and other stakeholders to develop theory-informed interventions to promote patient safety. In addition, the comprehensive network of contributory factors synthesized in this review will inform future evaluations and classification of contributory factors and assist in the development of holistic interventions that target different levels of the healthcare system.
6. Expert opinion
Our umbrella review provides a comprehensive synthesis of the network of contributory factors to medication errors across diverse health-care settings. Decision-making mistakes, which included failure to consider risk factors (e.g. chronic kidney disease and pediatrics), particularly in cohorts requiring dose adjustments, were the most common contributors to medication error. This was followed by organizational and environmental factors, including insufficient knowledge/training, work overload, inadequate staffing levels, and suboptimal work environment. There is a need for theory-driven holistic interventions that incorporate pharmacist services, effective use of technology, multidisciplinary teamwork, educational sessions, and organizational-level strategies (such as effective allocation of resources and promoting blame-free culture).
Medication errors pose a substantial threat to patient safety, creating a serious public health problem, yet they are a common occurrence. Several interventions have been implemented to reduce medication errors previously, however some of these interventions have been proven ineffective. The development of these interventions was based mainly on a pragmatic approach or ISLAGIATT (It Seemed Like A Good Idea At The Time) principle, which lack the theoretical basis at the design stage [Citation83,Citation85,Citation91–94]. Our findings suggest a paucity of research that used theory to diagnose and classify contributory factors and to develop interventions. Thus, future research needs to be undertaken through the explicit use of theoretical frameworks. Undertaking research utilizing frameworks for complex interventions can be a substantial undertaking. However, in the long run, such interventions have the potential to deliver important influence on medication errors.
Additionally, it is pivotal that health-care systems move to a blame-free and non-punitive culture. It is also important that subject matter and safety experts provide timely and system-oriented solutions and feedback to the reported errors in a confidential manner [Citation95]. This will encourage health-care providers to report and disclose medication errors, which will allow policymakers to accurately estimate the extent of the problem and understand the exact contributory factors and offer support.
Several terms have been utilized to refer to the factors contributing to medication errors in the included systematic reviews. Additionally, some reviews have used multiple terms (e.g. contributory factors, reasons, and causes) interchangeably. This practice creates confusion about the phenomenon of interest and subsequently could lead to the development of ineffective interventions. Based on the findings from this review, the consistent use of the term ‘contributory factors’ is encouraged. Future research should attempt to define the term ‘contributory factors’ as well as other terms reported in the included reviews through consensus methodology.
Article highlights
The dominant contributory factors were decision-making mistakes, which include failure to consider risk factors (e.g. chronic kidney disease and pediatrics), and system failures, such as inadequate opportunities for training, work overload, inadequate staffing levels, and suboptimal work environment.
Among studies that had a prespecified methodology to identify and classify contributory factors, the use of theory, specifically Reason’s Accident Causation Model, was predominant.
Methodological limitations were mainly related to search strategy, quality assessment, and data extraction processes. The lack of a predetermined methodology to classify contributory factors was also noted.
”Contributory factors” and ”causes” were the most frequently used terms to refer to contributory factors.
Multiple definitions for contributory factors have emerged in the included reviews; however, the summary presented in our review does not reflect all proposed definitions in the literature.
The findings of this review will inform the development of holistic theory-based interventions that target different levels of the healthcare system. Such theory-based interventions have the potential to reduce the occurrence of medication errors and promote patient safety.
Our findings emphasize the need for consistent use of terminology, definitions, and methodology used in research aiming to identify and quantify contributory factors to medication errors.
This box summarizes key points contained in the article.
Declaration of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution
L Naseralallah contributed to: study design, screening, developing data extraction tool, piloting the data extraction tool, data extraction, quality assessment, data synthesis, writing original draft, writing (review and editing). D Stewart contributed to: study design, developing data extraction tool, writing (review and editing), supervising. RA Ali contributed to: screening, piloting the data extraction tool, writing (review and editing). V Paudyal contributed to: study design, developing data extraction tool, verifying data extraction and quality assessment results, writing (review and editing), supervising.
Data availability
All relevant data are within the manuscript and its supplementary material.
Revised_Supplementary_material_v4.docx
Download MS Word (54.5 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2022.2147921
Additional information
Funding
References
- Lisby M, Nielsen LP, Brock B, et al. How are medication errors defined? A systematic literature review of definitions and characteristics. Int J Qual Health Care. 2010;22(6):507–518.
- da Silva BA, Krishnamurthy M. The alarming reality of medication error: a patient case and review of Pennsylvania and national data. J Community Hosp Intern Med Perspect. 2016;6(4):31758.
- About medication errors: national coordinating council for medication error reporting and prevention; [cited 2021 Nov 27]. Available from 2021 Nov 27: https://www.nccmerp.org/about-medication-errors.
- Bates DW, Boyle DL, Vander Vliet MB, et al. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10(4):199–205.
- Elliott RA, Camacho E, Jankovic D, et al. Economic analysis of the prevalence and clinical and economic burden of medication error in England. BMJ Qual Saf. 2021;30(2):96–105.
- Donaldson LJ, Kelley ET, Dhingra-Kumar N, et al. Medication without harm: WHO’s third global patient safety challenge. Lancet. 2017;389(10080):1680–1681.
- Cohen H, Mandrack MM. Application of the 80/20 rule in safeguarding the use of high-alert medications. Crit Care Nurs Clin North Am. 2002;14(4):369–374.
- Waterman AD, Garbutt J, Hazel E, et al. The emotional impact of medical errors on practicing physicians in the United States and Canada. Jt Comm J Qual Patient Saf. 2007;33(8):467–476.
- Matin BK, Hajizadeh M, Nouri B, et al. Period prevalence and reporting rate of medication errors among nurses in Iran: a systematic review and meta-analysis. J Nurs Manag. 2018;26(5):498–508.
- Barach P, Small SD. Reporting and preventing medical mishaps: lessons from non-medical near miss reporting systems. BMJ. 2000;320(7237):759–763.
- Anderson DJ, Webster CS. A systems approach to the reduction of medication error on the hospital ward. J Adv Nurs. 2001;35(1):34–41.
- Jaam M, Naseralallah LM, Hussain TA, et al. Pharmacist-led educational interventions provided to healthcare providers to reduce medication errors: a systematic review and meta-analysis. PLoS One. 2021;16(6):e0253588.
- Naseralallah LM, Hussain TA, Jaam M, et al. Impact of pharmacist interventions on medication errors in hospitalized pediatric patients: a systematic review and meta-analysis. Int J Clin Pharm. 2020;42(4):979–994.
- de Araújo BC, de Melo RC, de Bortoli MC, et al. How to prevent or reduce prescribing errors: an evidence brief for policy. Front Pharmacol. 2019;10:439.
- El-Awaisi A, Al-Shaibi S, Al-Ansari R, et al. A systematic review on the impact of pharmacist-provided services on patients’ health outcomes in Arab countries. J Clin Pharm Ther. 2022;47(7):879–896.
- Berdot S, Roudot M, Schramm C, et al. Interventions to reduce nurses’ medication administration errors in inpatient settings: a systematic review and meta-analysis. Int J Nurs Stud. 2016;53:342–350.
- Fletcher KE, Davis SQ, Underwood W, et al. Systematic review: effects of resident work hours on patient safety. Ann Intern Med. 2004;141(11):851–857.
- Assiri GA, Shebl NA, Mahmoud MA, et al. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open. 2018;8(5):e019101.
- Alghamdi AA, Keers RN, Sutherland A, et al. Prevalence and nature of medication errors and preventable adverse drug events in paediatric and neonatal intensive care settings: a systematic review. Drug Saf. 2019;42(12):1423–1436.
- Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: the Preferred Reporting Items for Overviews of Reviews (PRIOR) statement. BMJ. 2022;378:e070849 .
- Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: The Preferred Reporting Items for Overviews of Reviews (PRIOR) Explanation & Elaboration. 2022;378:e070849.
- Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–140.
- Naseralallah L, Paudyal V, Stewart D, et al. Synthesizing and critically appraising the evidence on factors contributing to medication errors: umbrella review. PROSPERO 2022 CRD42022321425 [ Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=321425.
- Howard I, Howland I, Castle N, et al. Retrospective identification of medication related adverse events in the emergency medical services through the analysis of a patient safety register. Sci Rep. 2022;12(1):2622.
- Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods programme. 2006.
- Snijders C, van Lingen RA, Molendijk A, et al. Incidents and errors in neonatal intensive care: a review of the literature. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F391–8.
- Tully MP, Ashcroft DM, Dornan T, et al. The causes of and factors associated with prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(10):819–836.
- Wimpenny P, Kirkpatrick P. Roles and systems for routine medication administration to prevent medication errors in hospital-based, acute care settings: a systematic review. JBI Libr Syst Rev. 2010;8(10):405–446.
- Aldila F, Walpola RL. Medicine self-administration errors in the older adult population: a systematic review. Res Social Adm Pharm. 2021;17(11):1877–1886.
- Mira JJ, Lorenzo S, Guilabert M, et al. A systematic review of patient medication error on self-administering medication at home. Expert Opin Drug Saf. 2015;14(6):815–838.
- Sears K, Ross-White A, Godfrey CM. The incidence, prevalence and contributing factors associated with the occurrence of medication errors for children and adults in the community setting: a systematic review. JBI Libr Syst Rev. 2012;10(35):2350–2464.
- Dionisi S, Di Simone E, Liquori G, et al. Medication errors’ causes analysis in home care setting: a systematic review. Public Health Nurs. 2022;39(4):876–897.
- Parand A, Garfield S, Vincent C, et al. Carers’ medication administration errors in the domiciliary setting: a systematic review. PLoS One. 2016;11(12):e0167204.
- Santesteban E, Arenas S, Campino A. Medication errors in neonatal care: a systematic review of types of errors and effectiveness of preventive strategies. J Neonatal Nurs. 2015;21(5):200–208.
- Keers RN, Williams SD, Cooke J, et al. Causes of medication administration errors in hospitals: a systematic review of quantitative and qualitative evidence. Drug Saf. 2013;36(11):1045–1067.
- Lopez-Pineda A, Gonzalez de Dios J, Guilabert Mora M, et al. A systematic review on pediatric medication errors by parents or caregivers at home. Expert Opin Drug Saf. 2022;21(1):95–105.
- Salmasi S, Wimmer BC, Khan TM, et al. Quantitative exploration of medication errors among older people: a systematic review. Drugs Therapy Perspect. 2017;34(3):129–137.
- Pereira Lermontov S, Carreiro Brasil S, Rezende de Carvalho M. Medication errors in the context of hematopoietic stem cell transplantation: a systematic review. Cancer Nurs. 2019;42(5):365–372.
- Alshehri GH, Keers RN, Ashcroft DM. Frequency and nature of medication errors and adverse drug events in mental health hospitals: a systematic review. Drug Saf. 2017;40(10):871–886.
- Boytim J, Ulrich B. Factors contributing to perioperative medication errors: a systematic literature review: 2.1. AORN J. 2018;107(1):91–107. www.aornjournal.org/content/cme
- Alsulami Z, Conroy S, Choonara I. Medication errors in the Middle East countries: a systematic review of the literature. Eur J Clin Pharmacol. 2013;69(4):995–1008.
- Thomas B, Paudyal V, MacLure K, et al. Medication errors in hospitals in the Middle East: a systematic review of prevalence, nature, severity and contributory factors. Eur J Clin Pharmacol. 2019;75(9):1269–1282.
- Mansouri A, Ahmadvand A, Hadjibabaie M, et al. A review of medication errors in Iran: sources, underreporting reasons and preventive measures. Iran J Pharm Res. 2014;13(1):3–17.
- Marznaki Z, Pouy S, Salisu J, et al. Medication errors among Iranian emergency nurses: a systematic review. Epidemiol Health. 2020;42:e2020030.
- Mekonnen AB, Alhawassi TM, McLachlan AJ, et al. Adverse drug events and medication errors in African hospitals: a systematic review. Drugs Real World Outcomes. 2018;5(1):1–24.
- Salmasi S, Khan TM, Hong YH, et al. Medication errors in the Southeast Asian Countries: a systematic review. PLoS One. 2015;10(9):e0136545.
- Al Rowily A, Jalal Z, Price MJ, et al. Prevalence, contributory factors and severity of medication errors associated with direct-acting oral anticoagulants in adult patients: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78(4):623–645.
- Lampert A, Seiberth J, Haefeli WE, et al. A systematic review of medication administration errors with transdermal patches. Expert Opin Drug Saf. 2014;13(8):1101–1114.
- Hansen C, Bradley P, S J. Factors influencing successful prescribing by intern doctors: a qualitative systematic review. Pharmacy (Basel). 2016;4(3):24.
- Di Muzio M, Dionisi S, Di Simone E, et al. Can nurses’ shift work jeopardize the patient safety? A systematic review. Eur Rev Med Pharmacol Sci. 2019;23(10):4507–4519.
- Schroers G, Ross JG, Moriarty H. Nurses’ perceived causes of medication administration errors: a qualitative systematic review. Jt Comm J Qual Patient Saf. 2020. DOI:10.1016/j.jcjq.2020.09.010
- Vincent C, Taylor-Adams S, Stanhope N. Framework for analysing risk and safety in clinical medicine. BMJ. 1998;316(7138):1154–1157.
- McElroy LM, Woods DM, Yanes AF, et al. Applying the WHO conceptual framework for the International classification for patient safety to a surgical population. Int J Qual Health Care. 2016;28(2):166–174.
- Bucknall TK. Medical error and decision making: learning from the past and present in intensive care. Aust Crit Care. 2010;23(3):150–156.
- Henry RE. Risk factors, subjectivity, and truth in healthcare. Am Health Drug Benefits. 2008;1(2):5–6.
- Rogers E, Griffin E, Carnie W, et al. A just culture approach to managing medication errors. Hosp Pharm. 2017;52(4):308–315.
- Reason J. Human error: models and management. BMJ. 2000;320(7237):768–770.
- Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of medical research council guidance. BMJ. 2021;374:n2061.
- Li Y, Thimbleby H. Hot cheese: a processed Swiss cheese model. J R Coll Physicians Edinb. 2014;44(2):116–121.
- Elliott M, Page K, Worrall-Carter L. Reason’s accident causation model: application to adverse events in acute care. Contemp Nurse. 2012;43(1):22–28.
- Hinton Walker P, Carlton G, Holden L, et al. The intersection of patient safety and nursing research. Annu Rev Nurs Res. 2006;24(1):3–15.
- Carthey J. Understanding safety in healthcare: the system evolution, erosion and enhancement model. J Public Health Res. 2013;2(3):e25–e.
- Reason J. Managing the risk of organisational accidents. 1 ed. Farnham United Kingdom: Ashgate; 1997.
- Yu KH, Nation RL, Dooley MJ. Multiplicity of medication safety terms, definitions and functional meanings: when is enough enough? Qual Saf Health Care. 2005;14(5):358–363.
- Ferner RE, Aronson JK. Clarification of terminology in medication errors: definitions and classification. Drug Saf. 2006;29(11):1011–1022.
- Dean GE, Scott LD, Rogers AE. Infants at risk: when nurse fatigue jeopardizes quality care. Adv Neonatal Care. 2006;6(3):120–126.
- Johnson AL, Jung L, Song Y, et al. Sleep deprivation and error in nurses who work the night shift. J Nurs Adm. 2014;44(1):17–22.
- Sarzynski E, Ensberg M, Parkinson A, et al. Eliciting nurses’ perspectives to improve health information exchange between hospital and home health care. Geriatr Nurs. 2019;40(3):277–283.
- Berland A, Bentsen SB. Medication errors in home care: a qualitative focus group study. J Clin Nurs. 2017;26(21–22):3734–3741.
- Giannetta N, Cianciulli A, Dionisi S, et al. Orphan drugs: an European production, research and development policies. Farmaci orfani: uno sguardo sulle politiche di produzione e ricerca in ambito europeo. Giornale Italiano di Farmacia Clinica. 2019;33:29–34.
- Holmqvist M, Ekstedt M, Walter SR, et al. Medication management in municipality-based healthcare: a time and motion study of nurses. Home Healthc Now. 2018;36(4):238–246.
- Dionisi S, Di Simone E, Alicastro GM, et al. Nursing summary: designing a nursing section in the electronic health record. Acta Biomed. 2019;90(3):293–299.
- Champion C, Sockolow PS, Bowles KH, et al. Getting to complete and accurate medication lists during the transition to home health care. J Am Med Dir Assoc. 2021;22(5):1003–1008.
- Akbar Z, Saeed H, Saleem Z, et al. Dosing errors in total parenteral nutrition prescriptions at a specialized cancer care hospital of Lahore: the role of clinical pharmacist. J Oncol Pharm Pract. 2021;27(3):531–540.
- Bassett E, Frantzen L, Zabel K. Evaluation of pharmacist renal dose adjustments and planning for future evaluations of pharmacist services. Hosp Pharm. 2021;56(5):416–423.
- Gillaizeau F, Chan E, Trinquart L, et al. Computerized advice on drug dosage to improve prescribing practice. Cochrane Database Syst Rev. 2013; (11):Cd002894. DOI:10.1002/14651858.CD002894.pub3.
- Glaser JP, Drazen EL, Cohen LA. Maximizing the benefits of health care information systems. J Med Syst. 1986;10(1):51–56.
- Kibicho J, Pinkerton SD, Owczarzak J, et al. Are community-based pharmacists underused in the care of persons living with HIV? A need for structural and policy changes. J Am Pharm Assoc (2003). 2015;55(1):19–30.
- Wiebe C. Pharmacists: a “secret weapon” for reducing drug errors. MedGenMed. 2007;9(2):10.
- Teerawattananon Y, Painter C, Dabak S, et al. Avoiding health technology assessment: a global survey of reasons for not using health technology assessment in decision making. Cost Eff Resour Alloc. 2021;19(1):62.
- Al-Ismail MS, Naseralallah LM, Hussain TA, et al. Learning needs assessments in continuing professional development: a scoping review. Med Teach 2022 1–9 10.1080/0142159X.2022.2126756
- Manias E. Effects of interdisciplinary collaboration in hospitals on medication errors: an integrative review. Expert Opin Drug Saf. 2018;17(3):259–275.
- Hughes CM, Cadogan CA, Ryan CA. Development of a pharmacy practice intervention: lessons from the literature. Int J Clin Pharm. 2016;38(3):601–606.
- Davies P, Walker AE, Grimshaw JM. A systematic review of the use of theory in the design of guideline dissemination and implementation strategies and interpretation of the results of rigorous evaluations. Implement Sci. 2010;5(1):14.
- Atkins L. Using the Behaviour Change Wheel in infection prevention and control practice. J Infect Prev. 2016;17(2):74–78.
- Godin G, Kok G. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot. 1996;11(2):87–98.
- Aidah S, Gillani SW, Alderazi A, et al. Medication error trends in Middle Eastern countries: a systematic review on healthcare services. J Educ Health Promot. 2021;10:227.
- Kuitunen S, Niittynen I, Airaksinen M, et al. Systemic causes of in-hospital intravenous medication errors: a systematic review. J Patient Saf. 2021;17(8):e1660–e8.
- Falconer N, Barras M, Martin J, et al. Defining and classifying terminology for medication harm: a call for consensus. Eur J Clin Pharmacol. 2019;75(2):137–145.
- Biro J, Rucks M, Neyens DM, et al. Medication errors, critical incidents, adverse drug events, and more: a review examining patient safety-related terminology in anaesthesia. Br J Anaesth. 2022;128(3):535–545.
- Michie S. Behaviour change wheel. United Kingdom (UK): Silverback Publishing; 2018.
- Steinmo SH, Michie S, Fuller C, et al. Bridging the gap between pragmatic intervention design and theory: using behavioural science tools to modify an existing quality improvement programme to implement “Sepsis Six.” Implement Sci. 2016;11(1):14.
- ICEtBRG I. Designing theoretically-informed implementation interventions. Implement Sci. 2006;1:4.
- Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005;14(1):26.
- Leape LL. Reporting of adverse events. N Engl J Med. 2002;347(20):1633–1638.