ABSTRACT
Introduction
Neo/adjuvant therapy for early-stage breast cancer has become increasingly common in the last few decades; as a consequence, the number of breast cancer survivors experiencing often debilitating long-term side effects has increased, and thus the need for a comprehensive approach to the variety of symptoms involved.
Areas covered and methods
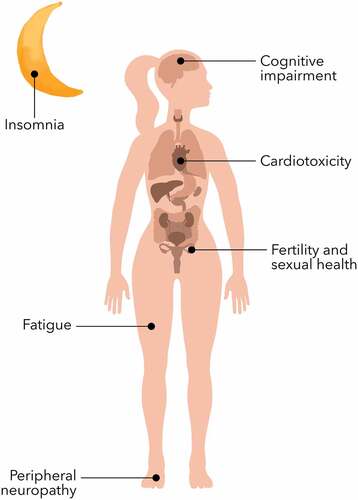
We performed a literature search on the main public scientific databases (PubMed, Embase, Cochrane, and CrossRef) from 2000 to April 2022 to identify prevention and management strategies for the most common long-term side effects, including fatigue, insomnia, peripheral neuropathy, cognitive impairment, estrogen deprivation, cardiotoxicity, and second cancers.
Expert opinion
Long-term toxicities may affect a majority of breast cancer survivors, significantly interfering with their quality of life. Although there are guidelines for the management of isolated side effects, such as peripheral neuropathy, we aim to provide a more inclusive clinical-oriented approach, focusing on both prevention and therapeutic strategies
1. Introduction
Breast cancer (BC) is the most common type of cancer in women worldwide; through the last two decades, the use of (neo)adjuvant therapies in early stage became state of the art and played a relevant role in improving survival rates [Citation1]. Thus, the ever-increasing number of breast cancer survivors experience unique long-term physical, psychological, and psychosocial changes, which can strongly affect their quality of life (.) [Citation2] Given the dearth of evidence regarding prevention or treatment of long-term side effects, there is an urgent need for the identification and development of useful strategies to manage this vast array of debilitating symptoms. Herein, we provide a comprehensive review of management strategies for the most common long-term chemotherapy toxicities experienced by breast cancer survivors; these are summarized in .
Table 1. Summary of main management strategies.
2. Methods
We performed a literature search on the main public scientific databases (PubMed, Cochrane, Embase, and CrossRef), for manuscripts published from 2000 to April 2022, using combinations of the following keywords: ‘early breast cancer,’ ‘(neo)adjuvant chemotherapy,’ ‘fatigue,’ ‘insomnia,’ ”neurotoxicity” ”CIPN” ”peripheral neuropathy”, “cognitive impairment,” “chemobrain,” “fertility,” “bone health,” “menopause,” “menopausal symptoms,” ”cardiac toxicity”, and “second cancer.” Furthermore, we identified additional data checking the references of other relevant reviews, guidelines, and published papers in this field, and through experts’ advice.
3. Fatigue
Cancer-related fatigue (CRF) is defined as ‘a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness, related to cancer or cancer treatment, that is not proportional to recent activity and interferes with usual functioning’ [Citation3]. Fatigue is the most common side effect experienced by BC survivors; its incidence can reach 50% of patients and persist for at least 5 years after completion of treatment. These patients usually show worse scores for all quality of life (QoL) functions [Citation4].
Adjuvant chemotherapy (alone or in association with radiotherapy) is associated with a higher risk of developing CRF [Citation4,Citation5]; while use of taxanes increases fatigue while on treatment [Citation6], it does not seem to influence neither prevalence nor severity of long-term fatigue [Citation5]. Endocrine treatment, which is often given after chemotherapy in early breast cancer patients, can also contribute to the severity of this symptom.
3.1. Management
3.1.1. Screening and prevention
Cancer patients should be screened and assessed during and after chemotherapy treatment to enable timely management strategies. Fatigue can be self-reported by a Numeric Rating Scale (NRS) tool [Citation7], although in clinical practice, it is often ranked by physicians according to the Common Terminology Criteria for Adverse Events (CTCAE) scale. Several multi-dimensional tools have been validated, but there is currently no clear evidence about the most appropriate one [Citation8]. Recently, a predictive tool for long-term severe fatigue was validated based on the results of the CANTO trial [Citation9]; in this trial, the authors identified clinic-behavioral risk factors and generated a risk model for severe fatigue 2 years after diagnosis of breast cancer. Pretreatment fatigue, younger age, and symptoms at the time of diagnosis (such as pain, insomnia, depression, and anxiety) represented the most relevant predictors of long-term fatigue.
There is no evidence about the role of prevention strategies in this setting. Patients who report a high score in the aforementioned tools should be investigated for concomitant physical causes requiring treatment (such as anemia; active infection; malnutrition; and thyroid, renal, cardiovascular, and pulmonary disease) [Citation10–12]; moreover, a psychological evaluation to identify an underlying depressive disorder should be performed.
A recent study from Antonuzzo et al. demonstrated that providing information leaflets, associated to a phone-based nurse monitoring assessment and intervention, significantly reduced the time spent with both grade ≥3 fatigue (14.5% vs. 17.2%, p = 0.015) and grade ½ fatigue (60.3% vs 63.3%, p ≤ 0.05) [Citation13].
3.1.2. Non-pharmacological interventions
Although there is a plethora of data regarding the benefits of moderate intensity exercise in cancer patients, there is no specific exercise prescription for cancer-related fatigue. Two different meta-analyses concluded that moderate-intensity exercise, combining aerobic with strength and resistance training, reduced fatigue both during and after cancer therapy [Citation14,Citation15]. Walking at a moderate intensity has also been proven to reduce fatigue, sleep disruption and depression [Citation16]. ASCO guidelines on exercise report significant improvements in fatigue as a result of aerobic and combined resistance and aerobic exercise interventions [Citation17].
Psychoeducation interventions and cognitive behavior therapy have been proven clinically effective in reducing fatigue in cancer survivors [Citation18]. Some evidence suggests that mindfulness-based stress reduction (MBSR), a combined treatment of meditation exercises and psychoeducational elements, cognitive-behavioral interventions, and movement exercises may be useful in this setting [Citation19].
In two systematic reviews, yoga was found to be effective in decreasing CRF [Citation20,Citation21].
3.1.3. Complementary medicine
No nutraceutical intervention has shown efficacy in the management of CRF in breast cancer survivors. A double-blind, randomized, placebo-controlled phase 3 trial showed a benefit for use of Wisconsin ginseng on a heterogeneous population with different neoplasms and different stages of disease [Citation22]. There are conflicting data regarding use of guarana, while a placebo-controlled trial of mistletoe in BC patients undergoing treatment with CMF (cyclophosphamide, methotrexate, fluorouracil) showed an improvement in fatigue while on chemotherapy; however, there is no reported long-term outcome [Citation23]. Acupuncture appears to be a valid approach for treating fatigue in breast cancer patients, as evidenced by the results of a meta-analysis of 10 randomized trials [Citation24].
3.1.4. Pharmacological interventions
Several psychostimulant drugs have been investigated in the management of cancer-related fatigue, such as methylphenidate, dexmethylphenidate, long-acting methylphenidate, dexamphetamine, modafinil, and armodafinil. Most studies showed no advantage of psychostimulant drugs over placebo, while three studies with methylphenidate (5–30/40 mg day) and one with dexmethylphenidate (10–50 mg/day) reported an improvement in CRF [Citation25–28]. A subgroup analysis of two different studies suggested a benefit of long-acting methylphenidate and modafinil [Citation29,Citation30]. Considering the mostly negative results of these studies, ESMO guidelines do not advise routine use of psychostimulant drugs, with potential exceptions, such as use of methylphenidate, dexmethylphenidate, long-acting methylphenidate, and dexamphetamine in thoroughly selected patients [Citation8]. Paroxetine, donepezil, eszopiclone, megestrol acetate, and melatonin have not been proven to be effective [Citation31–36].
There is currently lack of evidence about use of steroids for long-term fatigue in early breast cancer, which, overall, is not advisable due to their mid- and long-term side effects [Citation8]. A double-blind, placebo-controlled trial is currently ongoing, testing the effectiveness in this setting of a 13-week treatment with bupropion [Citation37].
4. Insomnia
Insomnia, fatigue, and depression are often related and act synergistically to worsen each other and to lower QoL [Citation38–41]. Sleep disruption is prevalent during and after chemotherapy [Citation42,Citation43]. While treatment anxiety may contribute to the onset of insomnia, physical symptoms (such as pain and vasomotor syndrome) can cause sleep disruption during chemotherapy and may persist even after completion of treatment [Citation44]. Circadian rhythm disruption has been associated to a shorter overall survival (OS) in BC patients [Citation45], and to a higher incidence of cognitive impairment and metabolic disruption [Citation46,Citation47].
4.1. Management
4.1.1. Screening and prevention
Three groups of etiological factors have been recognized in cancer-related insomnia [Citation48]:
- Predisposing factors (pre-existing and non-modifiable), such as gender, younger age, and a history of psychiatric disorders.
- Precipitating factors: psychological and physical effects related to cancer diagnosis and treatment (i.e. stress, pain, use of corticosteroids during chemotherapy).
- Perpetuating factors: sleep behaviors which, due to a maladaptive mechanism, contribute in maintaining a state of sleep disturbance (i.e. sleeping in the afternoon rather than overnight).
Development of sleep disturbances seems to be related to a disruption of the circadian rhythm [Citation49], possibly due to a flattened curve of cortisol secretion. Overweight also can furtherance the propagation of a low-grade chronic inflammatory status, thus contributing to perpetuating the sleep disruption through an increased release of cytokines [Citation50–52]; an association between obesity, sleep disruption, and fatigue has indeed been reported [Citation53–56].
There is a dearth of evidence about the association between cancer-related post-traumatic stress disorder (PTSD) and insomnia, although PTSD and insomnia have been associated in non-cancer research [Citation57].
No prevention strategy has been recognized.
4.1.2. Non-pharmacological interventions
Moderate intensity exercise improves both sleep quality and circadian resynchronization [Citation58,Citation59]. However, there is a scarcity of evidence about physical intervention in cancer patients [Citation60,Citation61]; available data show the potential usefulness of Yoga [Citation62,Citation63].
In BC survivors, cognitive behavioral therapy for insomnia (CBT-I) improves the quality of sleep up to 12 months [Citation64–66]. Yet, CBT-I is a difficult treatment to implement routinely, as it requires mental health professionals formally trained in this approach.
Other behavioral interventions, such as sleep hygiene education and sleep restriction treatment, have been tested with mixed results [Citation67–70].
Controlled bright light exposure could be useful in improving sleep disruption and fatigue in breast cancer patients, by targeting the resynchronization of the biological clock [Citation71].
4.1.3. Pharmacological interventions
Lorazepam and zolpidem are commonly prescribed to treat insomnia [Citation72]; however, there is no evidence on prolonged use to address long-term sleep disruption following treatment for breast cancer.
A small randomized trial enrolled 95 BC survivors to receive either melatonin 6 mg or placebo after treatment, with promising results [Citation73].
5. Peripheral neuropathy
Many standard chemotherapy regimens in EBC, including platinum agents and taxanes, can induce chemotherapy-induced peripheral neuropathy (CIPN), which may persist even years after completion of treatment [Citation74–76]. In a systematic review, the estimated frequency of persistent CIPN 1 or more years post-treatment ranged from 11.0% to >80% [Citation77].
Incidence, severity, and clinical pattern of CIPN depend on the individual antineoplastic drug [Citation75,Citation78,Citation79]; it results in a predominant sensory axonal involvement (i.e. paresthesias, pain, muscle weakness, and ototoxicity), but sometimes it can occur as motor (i.e. reduction/absence of deep tendon reflexes, distal weakness and muscular atrophy, tremor, cramps) and autonomic dysfunction (i.e. abdominal pain, constipation, delayed gastric emptying, postural hypotension, reduced variability of heart rate and bladder disturbances) [Citation74,Citation75]. More distinctive symptoms are taxane-induced myalgias and cranial nerve palsy following the administration of vinca alkaloids [Citation74]. All platinum salts are characterized by the phenomenon of “coasting,” which consists in a worsening of symptoms in the months following the completion of the treatment [Citation74].
A summary of the clinical presentation of the main neuropathic drugs is presented in .
Table 2. Summary of clinical presentation of the main chemotherapy drugs for breast cancer inducing CIPN.
5.1. Management
5.1.1. Screening and prevention
Screening relies on identification of individual risk factors, which could affect the choice of chemotherapy; individual risk factors include age (≥75 years), gender, previous treatments, pre-existing peripheral neuropathy and diseases/deficiencies predisposing to neuropathy (i.e. alcohol abuse, diabetes, renal insufficiency, hypothyroidism, vitamin deficiency, infections, autoimmune rheumatologic conditions) [Citation74,Citation75]. In patients with advanced BC aged ≥65 years, the EFFECT phase II trial demonstrated that, with the same schedule, nab-paclitaxel 100 mg/m2 was associated with fewer neurotoxicity-related events than nab-paclitaxel 125 mg/m2 [Citation80]
Early detection of CIPN can be achieved by performing baseline and ongoing clinical evaluations of physical function, followed, if needed, by complementary neurophysiological investigations [Citation75,Citation81]. Sleep disturbance, anxiety, depression, and central sensitization of pain may aggravate neuropathic pain [Citation75].
Till date, there is no effective pharmacological agent to prevent CIPN [Citation75]. Among non-pharmacological prevention, only exercise and functional training can be recommended [Citation75,Citation82]. Acupuncture is discouraged, while cryotherapy is still debated: in the largest randomized phase III trial evaluating frozen gloves, no difference in CIPN subscales was demonstrated between the two arms. Besides, about one-third of the frozen gloves group discontinued the treatment [Citation83].
5.1.2. Non-pharmacological interventions
Physical exercise and functional training (i.e. vibration training) could be considered [Citation82,Citation84,Citation85]. For patients receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy, Kleckner et al. reported a statistically significant reduction of CIPN symptoms with exercise compared to the control group, especially for older patients, male, or BC survivors [Citation84,Citation85].
In a recent small, randomized pilot trial of 40 BC survivors, an 8-week acupuncture intervention demonstrated an improvement in subjective sensory symptoms [Citation86]. Implementing falls prevention strategies in patients with an increased risk of falling due to lower leg paresthesia is also essential [Citation87]. Bao et al. demonstrated that severity of CIPN was directly associated with a higher rate of falls [Citation76]. A recent systematic review demonstrated a good level of evidence for physical exercise, balance, and sensorimotor training, but only inconsistent evidence in use of cryotherapy, compression therapy, massages and related activities, and electrical stimulation [Citation88]. There is also some scant evidence regarding the use of photobiomodulation [Citation89].
5.1.3. Pharmacological interventions
Relief of neuropathic pain is the main objective of treatment. According to ESMO guidelines, duloxetine (30 mg/day for 1 week, then 60 mg/day for 4 weeks) is the only drug recommended for treatment of neuropathic pain [Citation90], while venlafaxine (37.5 mg b.i.d.), and amitriptyline (10 –50 mg/day) could be considered [Citation91,Citation92]; tramadol or strong opioids could represent a salvage strategy in the management of neuropathic pain [Citation75]. Antioxidants have shown their efficacy in treating CIPN in several clinical trials [Citation93]. Although almost all of these trials did not focus on a specific subtype of cancer, BC patients represented a significant percentage of the patients enrolled; the exception is the EFFOX trial [Citation91], where the majority of patients were affected by a gastrointestinal cancer; the advice for the use of venlafaxine, therefore, should be considered with attention, as it is extrapolated by a trial non directly regarding breast cancer survivors. A dedicated study should be performed, to assess the efficacy of this intervention in this subset of patients.
5.1.4. Complementary medicine
Dietary supplements represent a growing research field, such as vitamins (group B and E), acetyl-L-carnitine, unsaturated fatty acids, extracts of medical plants (goshajinkigan, curcumin, etc.) [Citation89,Citation94]. However, evidence is still scant and needs to be implemented to confirm a potential action against CIPN.
6. Cognitive impairment
Cognitive impairment affects up to 70% of non-CNS (central nervous system) cancer patients during or after chemotherapy [Citation95]; this phenomenon is commonly known as ‘chemobrain’ or ‘chemofog.’ In breast cancer, it is estimated that chemobrain can affect from 17% to 75% of cancer survivors [Citation96].
The term ‘chemobrain’ refers to a constellation of disorders that mainly concern the sphere of short-term memory, attention, learning, verbal ability, executive functions, and motor activities [Citation97–99]. The natural history of chemobrain comprises an onset phase, an acute phase, and finally a partial or complete recovery phase [Citation100].
Moreover, hormonal deprivation, secondary either to chemotherapy-induced menopause or to anti-hormonal treatments, could worsen patients’ cognitive functions. However, the scarce scientific evidence available is conflicting [Citation101–103]
Results from a randomized clinical trial comparing cognitive function in three different groups of BC patients treated with different types of chemotherapy showed an independent, direct dose-related effect on cognitive function [Citation104]
6.1. Management
6.1.1. Screening and prevention
Patients often do not report chemobrain to their oncologists, probably due to lack of awareness. At the same time, routine assessment of cognitive functions is not routinely performed, except within clinical trials. Several assessment tools have been proposed; these include the Wechsler Memory Scale (WMS), which is a verbal memory test; the Wechsler Adult Intelligence Scale (WAIS), which can identify even small changes in cognitive functions; the Mini Mental State Examination (MMSE), and some neuropsychological methods, such as the High Sensitivity Cognitive Screen (HSCS) [Citation105]. However, none of these has been established as the reference tool. Functional magnetic resonance imaging (fMRI) identified structural cerebral alterations – decrease in both the gray matter in the neocortex and cortex and in the white matter at the subcortical level – in patients with changes in neuropsychological tests [Citation106].
Increasing the awareness of this phenomenon both in doctors and in patients would allow early identification of signs and symptoms, to monitor their evolution over time and to facilitate the implementation of the aforementioned strategies.
To date, there are no known preventive strategies nor approved therapeutic interventions. Yet, a treatment strategy combining behavioral with psychopharmacological approaches could be considered.
6.1.2. Non-pharmacological interventions
Behavioral interventions include
Relaxation and mindfulness training [Citation107,Citation108]
Physical exercise to improve processing speed in BCS and enhanced neurogenesis [Citation100,Citation109]
Occupational therapy to recover or maintain daily and working life skills through activity
Brain-training computer-based programs to increase memory and processing speed [Citation110]
Electroencephalography (EEG) biofeedback to improve cognitive indexes, fatigue, sleep, and psychological scales [Citation111]
Lifestyle changes: using a detailed planner; getting enough rest; accepting support from relatives and friends; avoiding alcohol or other substances that could alter the mental state.
6.1.3. Pharmacological interventions
Despite the lack of data from randomized clinical trials, drugs that could be considered are
Central nervous system (CNS) stimulants: modafinil (200 mg day for 4 weeks) has shown potential in improving cognitive functions [Citation112]
Donepezil: a reversible acetylcholinesterase inhibitor used in patients with Alzheimer disease, which improves memory and attention in patients affected by brain cancer (5 mg/day for 6 weeks, then 10 mg/day for 18 weeks) [Citation113]
Antidepressive drugs, such as fluoxetine, or mood stabilizer drugs, such as lithium, appear to increase neurogenesis, especially in the hippocampus [Citation100]
7. Estrogen deprivation side-effects – fertility and bone health
Modern chemotherapy regimens hinder the normal ovarian function; complete ovarian failure occurs in more than half of BC patients who undergo chemotherapy in their forties and in 15–30% of patients younger than 35 years [Citation114,Citation115]. Use of alkylating agents, such as cyclophosphamide, is associated with a higher risk of ovarian failure, while anthracyclines and taxanes have an intermediate risk [Citation116].
Chemotherapy-induced premature ovarian failure often elicits the side effects of estrogen deprivation, which can be magnified by a concurrent endocrine therapy. Psychological and physical side effects comprise vasomotor symptoms, musculoskeletal pain, sexual dysfunctions, sleep deprivation, and depression, often more intense of those occurring during physiological menopause. Early iatrogenic menopause also influences bone health, disrupting the balance between bone formation and reabsorption.
7.1. Management
7.1.1. Prevention
Patients who are interested in preserving their fertility can undergo embryos, oocytes, or ovarian tissue cryopreservation prior to commencement of chemotherapy; however, these procedures are not always feasible due to time requirements, which are not always appropriate while commencing a primary treatment for a rapid growth type of cancer. Besides, embryos and oocytes preservation is not a treatment for prevention of premature ovarian insufficiency. In contrast, there is some limited evidence that ovarian tissue cryopreservation can allow ovarian function recovery [Citation117]. Another opportunity is represented by concomitant administration of gonadotropin-releasing hormone agonist (GnRHa) along chemotherapy, which protects ovarian tissue through different mechanisms, and it is not contraindicated in women who undergo cryopreservation procedures. GnRHa induces, however, a temporary suppression in ovarian function, thus eliciting menopausal symptoms [Citation117,Citation118]
7.1.2. Non-pharmacological interventions
Sexual disorders secondary to iatrogenic menopause can be managed with behavioral or non-hormonal intervention. Many trials suggested the benefit of cognitive behavioral therapy in breast cancer survivors, especially in those who experiment sexual dysfunction, intensified by adjuvant endocrine therapy. SHARE-OS trial highlighted an improvement in sexual health in women who received ovarian suppression by undertaking body awareness exercises and mindfulness-based therapy. In addition, given their availability and low-cost, non-hormonal vaginal lubricants, such as topic lidocaine, or vaginal dilators associated with pelvic floor exercises, should be advised to handle dyspareunia and vaginal dryness [Citation119,Citation120]. Vaginal laser therapy (CO2 or erbium) showed positive effects on genitourinary symptoms, but its use is controversial due to the lack of randomized trials with a long-term safety follow-up and to the high costs of this treatment [Citation121–123].
Vasomotor symptoms, typical of chemotherapy-induced menopause, can be managed with non-pharmacological strategies, such as weight maintenance, regular physical exercise, yoga, relaxation exercises, and acupuncture [Citation124,Citation125].
7.1.3. Pharmacological interventions
There is uncertainty about the safety of local estrogen or testosterone-based therapies; several studies showed an increase in serum estradiol levels, despite the lack of evidence of increased risk of recurrence [Citation126]. A more recent trial, however, assessed the safety and effectiveness of ultralow 0.005% estriol vaginal gel in a small group of 61 women with BC having treatment with nonsteroidal aromatase inhibitors; no significant difference in FSH, LH, and estradiol were observed; a transient and minimal absorption of estriol was observed at the beginning of treatment [Citation127].
Hormone-replacement therapy is the most effective treatment for estrogen-deficiency symptoms, but it is contraindicated because of the risk of breast cancer recurrence, as shown in the HABITS, Stockholm, and LIBERATE trials [Citation128–130]. Furthermore, some preclinical studies showed that ospemifene, an oral selective estrogen receptor modulator effective for the management of vaginal dryness, has an anti-estrogen activity on breast cancer cells, but due to the lack of safety data, its use in this setting is to date not recommended [Citation131].
Most severe cases of vasomotor symptoms require use of SSRI/SNRI antidepressant or anticonvulsant drugs such as gabapentin, pregabalin, or clonidine [Citation132,Citation133]. Venlafaxine (37.5 –75 mg/day) is the most studied agent for preventing hot flashes, but higher doses can cause loss of appetite, mouth dryness, nausea, and constipation [Citation134]. Similar efficacy, with a slightly different profile of side effects, is given by gabapentin (900–2,400 mg/day) and pregabalin. On the other hand, clonidine, an α-adrenergic agonist, has been shown to be less effective than venlafaxine [Citation135]. Oxybutynin, an anticholinergic drug, improves hot flashes in women with or without breast cancer [Citation136].
As for bone health, many trials demonstrated that both bisphosphonates (zoledronic acid, alendronate) and denosumab (a monoclonal antibody directed against RANKL) improve bone mineral density in women with osteoporosis or subjected to treatment with drugs capable of undermining bone health [Citation137]. The duration of anti-resorptive treatment is controversial and is usually related to the persistence of a high fracture risk. There is also evidence that the use of bisphosphonates is associated to a reduction of bone recurrence, in particular in postmenopausal women [Citation138]. Women should also be advised to adopt a lifestyle that promotes bone health, undergoing regular physical exercise to prevent bone loss; according to ESMO and NCCN guidelines, calcium and vitamin D supplementation is warmly recommended [Citation139,Citation140].
8. Cardiotoxicity
The term cardiotoxicity comprises a large spectrum of clinical entities, including cardiac dysfunction, ischaemia, vascular disorders, endothelial damage, and arrhythmias. Chemotherapy toxicity is rarely dependent on a single mechanism. Besides, long-term toxicities and cardiovascular (CV) comorbidities, pre-existing or related to aging, may overlap, and consequently increase patients’ morbidity [Citation141].
In breast cancer patients, we broadly differentiate three categories of cardiovascular impairment, according to the related drug: anthracycline-induced cardiotoxicity, non-anthracycline agent-induced cardiotoxicity, and anti-HER2 therapy-related cardiotoxicity.
8.1. Anthracycline-induced cardiotoxicity
Anthracyclines (AC) are a mainstay of treatment for BC [Citation142–145]. AC detrimental effect on cardiac function is well recognized and represent the dose-limiting adverse event of this therapy. AC-induced cardiotoxicity essentially entails a decreased left ventricular ejection fraction (LVEF) with a rising risk of congestive heart failure (CHF).
8.1.1. Screening
The main risk factor is represented by cumulative dose (over 450–550 mg/m2 of doxorubicin and 900 mg/m2 of epirubicin) [Citation146,Citation147], but pre-existing cardiovascular diseases, obesity, diabetes mellitus, hypertension, smoking habit, age greater than 65 years, anti-HER-2 agents and chest irradiation increase the susceptibility to cardiac damage [Citation148]. AC-induced cardiotoxicity should be suspected in the presence of CHF symptoms, such as declivous edema, dyspnea, and syncope, but requires echocardiographic evidence of decreased LVEF (<10% than basal, absolute value <53%) [Citation149]. Testing circulating biomarkers, such as troponin, could reveal a preclinical myocytic damage and predict an HF diagnosis [Citation150].
Several studies have demonstrated that early detection and treatment allow a functional recovery; conversely, a delayed diagnosis leads to a lack of response to treatment [Citation151,Citation152].
Every patient should undergo a baseline cardiological evaluation, comprising ECG and echocardiography (ECHO) before starting AC-treatment. If chemotherapy with doxorubicin doses <200 mg/m2 is planned, patients should be reassessed at the end of treatment and every 6 months for the first year. Additional follow-up should be modulated on basal risk [Citation153].
8.1.1. Prevention
Primary prevention, such as correction of CV basal risk factors (i.e. smoking habits, hypertension, and dyslipidaemia), is fundamental. In addition, liposomal formulations and continuous infusion instead of bolus dosing reduce direct AC cardiotoxic effect [Citation154–158]. The most investigated cardioprotective drug is dexrazoxane, which showed benefits both in reduction of HF risk and subclinical cardiotoxicity [Citation159,Citation160]. Although it was initially suspected of reducing AC efficacy and inducing secondary malignancies, recent literature has assessed its safety and dexrazoxane is approved by regulatory agencies for patients exposed to previous cumulative doses of doxorubicin >300 mg/m2 or epirubicin >540 mg/m2 [Citation158,Citation161,Citation162]. Therefore, dexrazoxane (1 mg for each 10 mg of doxorubicin, given at least 30 minutes prior to each doxorubicin infusion) should be considered in patients with either a high cardiovascular risk, or about to receive a high total cumulative anthracycline dose for curative treatment. The role of neurohormonal antagonists, such as angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockade (ARB), and aldosterone antagonists, is not well established for patients with a low CV risk [Citation141,Citation153]. Two meta-analyses reported a benefit in preventing LVEF reduction with the use of cardioprotective drugs (renin–angiotensin–aldosterone system blockers, beta-blockers, aldosterone antagonists, ACE inhibitors) [Citation163,Citation164]; however, there was no statistically significant benefit in the incidence of heart failure and adverse clinical effects. A different meta-analysis confirmed the benefit in protecting the LV systolic function for a combination of candesartan and carvedilol, spironolactone, enalapril, and statins [Citation165]. A trial showed the efficacy of enalapril (2.5 –20 mg daily) in preventing left ventricular dysfunction in patients with an elevation in troponin value during or after chemotherapy [Citation166]. Authors in the PRADA trial proved the role of candesartan (32 mg q.d.) in reducing the decline in LVEF [Citation167]. Globally, neuro-hormonal therapies seem to reduce LVEF dysfunction with a trend of statistical significance [Citation161]. Finally, third-generation beta-blockers (i.e. carvedilol and nebivolol) showed cardioprotective properties preventing a decrease in LVEF and left ventricular enlargement [Citation168–171]; a meta-analysis showed a reduction in clinically detectable cardiotoxicity with the prophylactic use of beta-blockers, although there was no improvement in the incidence of early asymptomatic LVEF decrease [Citation172]. The 2022 ESC guidelines on cardio-oncology suggest considering ACE-I, ARB, beta-blockers, and statins for primary prevention in high- and very high-risk patients receiving cancer therapies that may cause heart failure [Citation158]. Patients who presented with signs or symptoms of cardiac dysfunction during or after chemotherapy should be treated according to guidelines for HF [Citation141].
8.2. Non-anthracycline agents
Cardiotoxicity is associated with other antineoplastic agents commonly used in early BC, such as cyclophosphamide, 5-fluorouracil, platinum, and taxanes, is less frequent, typically not dose-related, and characterized by an early onset. Due to the intent of this review, acute cardiotoxicity will not be analyzed extensively. In summary, the main toxicities reported are cardiac ischemia, rhythm disturbances (QTc prolongation, arrhythmia, sinus bradycardia), coronary vasospasm, and vasospastic angina [Citation173,Citation174].
Considering the low incidence, no cardiac monitoring or prophylactic therapy are routinely required [Citation175].
8.3. Anti-HER2 therapy-related cardiotoxicity
HER2 pharmacological blockade may induce cardiac alterations, resulting in structural and functional changes which manifest clinically with a decline in LVEF and CHF [Citation176]. Trastuzumab therapy in the adjuvant setting had a relative risk (RR) of 5,1 for impaired LVEF and 1,8 for CHF [Citation177]. Seidman and colleagues found a higher rate of cardiotoxicity in those patients who underwent AC-based chemotherapy concomitantly (27% for combination therapy, 8% for CT alone) rather than sequentially [Citation178–180].
Previous history of exposure to AC, especially if recent, BMI >25 kg/sqm, low basal LVEF, older age, and duration of treatment >1 year are known risk factors for trastuzumab-induced cardiotoxicity [Citation141,Citation175–177,Citation181–184]. Recognition of risk factors, careful selection, and adequate and periodic cardiac follow-up allowed to reduce the incidence of cardiotoxicity [Citation176].
Treatment suspension represents the main intervention to allow functional cardiac recovery, whereas the role of HF drugs is still controversial [Citation141,Citation185].
In adjuvant setting, CV assessment consists of ECHO every 3 months or more frequently if clinically indicated. Throughout the treatment, a value of LVEF between 40% and 49% requires a cardiologist evaluation and initiation of a cardioprotective therapy (i.e, ACEi or ARB). A decrease in LVEF > 15% should prompt discontinuation of trastuzumab; cardiac function should be reassessed every 4–6 weeks until an LVEF >50%.
Anti-HER2-associated toxicity has often an early onset and is mostly reversible; thus, a specific CV follow-up is not required; an elevation of troponin could be a predictor of LVEF reduction and poor cardiac outcome, identifying a group of patients less likely to recover from cardiotoxicity [Citation186].
Other anti-HER2 drugs, such as pertuzumab, trastuzumab-metansine (T-DM1), and lapatinib showed a lower CV toxicity profile. In particular, the addition of pertuzumab to trastuzumab is not associated with higher toxicity [Citation187–190].
9. Second cancers
The association between chemotherapy and acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) is well known [Citation191–194]. In general, topoisomerase II inhibitors, cyclophosphamide, and platinum compounds possess a leukemogenic potential [Citation194–197]. The risk of AML and MDS appears to peak 2–5 years after chemotherapy, with a decline after 10 years [Citation191]. Pancytopenia, marrow failure, or myelodysplasia are often the first signs recognized. Chemotherapy-related AML/MDS are often characterized by a poor prognosis, due to an intrinsic drug resistance [Citation191].
Alkylating drugs have been also linked to an increased risk of a wide range of solid malignancies, especially lung cancers, gastrointestinal cancers, sarcoma, and bladder cancers, with a dose–response relationship [Citation191,Citation198,Citation199]. Similarly, topoisomerase II inhibitors may increase the risk of new breast cancers [Citation199]. The rate of development of chemotherapy-related solid cancers seems to persist even for decades [Citation199]. Nothing is known about the long-term carcinogenic potential of targeted therapies.
The genesis of a second cancer is unpredictable and up to date there are no effective prevention tools. Henceforth, especially in heavily pretreated patients, strategies to favor the adherence to national cancer screening programs should be implemented. A careful and timely clinical evaluation remains, to date, the cornerstone of early detection of second neoplasms.
10. Conclusion
In our review, we aim to provide a comprehensive summary of the most common long-term side effects and their management. We found that some symptoms are often unrecognized, and there is a dearth of prevention strategies and standardized screening systems. Although few data on pharmacological agents exist, there is evidence that lifestyle approaches are often the best management strategy.
11. Expert opinion
Long-term survivorship, in oncology, is a relatively new and scarcely explored, although substantial, field. Thanks to the screening programs and the advances in anticancer therapy, the number of cancer survivors is increasing overtime. However, such a population may present treatment-induced chronic ailments that generate a public health problem to be addressed in a consistent way.
Most treatments in oncology, when assessed for safety, are evaluated on the basis of acute side effects, rather than chronic issues that, although less life-threatening, can significantly affect the quality of life of the survivors for the years to come. Follow-up of oncology patients is often solely aimed to assess recurrence, rather than addressing other issues which are, sometimes, even unrecognized (like insomnia and cognitive impairment). With the increasing number of survivors, especially in breast cancer – the most common cancer in women – the need to address such symptoms in a more coherent way is starting to emerge.
This review focuses on the practical management of long-term toxicities. In the real world, often these toxicities are managed empirically, when addressed at all. The aim of our study is to provide an evidence-based, comprehensive, pragmatical basis for management of these symptoms, thus improving the real-world long-term quality of life outcomes of breast cancer survivors. Changes in current practice would require, in first instance, a shift of paradigm to focus not only on the mere survival of these patients but on their quality of life and the challenges issued in daily life by the residual toxicities of the treatments they underwent. We propose several measures that could be implemented; while some of them would require additional funding and availability of trained health-care professionals, others could be more easily adopted by most breast cancer centers.
The first measure to be implemented should be increased education for both patients and health-care operators. This would equally increase the capability of patients to recognize and, therefore, report significant symptoms and the ability of health-care providers to intervene in an appropriate way, being aware not only of the problem but also of the potential treatments available, which are not confined to pharmacological options.
Management of symptoms, in oncology, has often been delegated to palliative care; in our opinion, supportive care needs to be routinely implemented in breast cancer centers. Moreover, as the beneficial effects of exercise are recognized in many fields, dedicated facilities could be helpful in implementing a routine pattern of exercise in breast cancer survivorship.
A phone-based nurse-monitoring intervention has been proven effective in reducing the severity and the duration of different treatment side effects; in cases where a supportive care clinic cannot be implemented due to lack of resources (either financial or in terms of staff availability), a nurse-led phone-based follow-up clinic could be effective and easier to establish.
We recognize as key areas for improvement the standardization of assessments and treatments, patients and health-care providers education, and implementation of supportive care clinics associated with breast cancer centers. Most of the symptoms we discussed do not have any recognized prevention strategy; moreover, there is lack of standardization in their respective scoring systems, which are scarcely used in clinical practice. This is a poorly explored area for research; further studies would be needed to identify reliable and easy-to-use scoring systems, and subsequently effective prevention strategies.
We found that the most useful management strategies are often lifestyle and behavioral interventions; these should be routinely discussed with patients. However, these interventions often require the support of specifically trained health-care operators, which are few and not always available; ideally, all breast cancer centers should have a referral system to a specific supportive care clinic. Whenever lifestyle and behavioral interventions are not possible, a pharmacological approach could be considered, with the aim of minimizing the number of different medications taken and therefore choosing, whenever possible, medications that will have a beneficial effect on more adverse events at the same time (i.e. venlafaxine for peripheral neuropathy and vasomotor syndrome).
In our opinion, improvements in this field, in the next few years, should focus on two main aspects. First, as there is a dearth of prevention strategies for most of these symptoms, a research effort should be considered to establish clear and effective protocols to be empowered in the clinical setting. In second place, we believe that specific clinics for treatment of cancer treatments long-term side effects should be implemented and available to all breast cancer centers, or, alternatively, that specific education should be given to patients and health-care operators. As the survival rate of patients increases, so does the need for an evidence-based optimization of survivorship management; in the next few years, dedicated clinics should become increasingly common, and referral to dedicated training and exercise programs tailored on these patient-specific needs should become available in most breast cancer centers.
Article highlights
Long-term side effects secondary to chemotherapy for breast cancer are an increasing health concern, affecting quality of life of a growing number of breast cancer survivors
There is lack of standardization and a dearth of research about prevention strategies and optimal management
For many side effects, the optimal management is represented by lifestyle changes, such as regular exercise, yoga, and mindfulness courses, rather than a pharmacological approach.
Optimization of management could be implemented through dedicated supportive care clinics.
Optimal management of certain side effects, such as cardiotoxicity, requires strict collaboration between different specialists.
Author contribution statement
Conceptualization: P Di Nardo and F Puglisi; investigation: P Di Nardo, C Lisanti, M Garutti, S Buriolla, M Alberti, and R Mazzeo; writing: P Di Nardo, C Lisanti, M Garutti, S Buriolla, M Alberti, and R Mazzeo; supervision: F Puglisi. All authors have read and agreed to the published version of the manuscript.
Declaration of interests
M Garutti reports advisory board from Novartis, Eli Lilly, PierreFabre, Roche and travel fees from Daichii Sankyo all outside the submitted work. F Puglisi received a honorarium for advisory boards, activities as a speaker, travel grants, research grants: Amgen, AstraZeneca, Daichii Sankyo, Celgene, Eisai, Eli Lilly, Gilead, Ipsen, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, Viatris. Research funding: AstraZeneca, Eisai, Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (Concord-2). Lancet. 2015;385(9972):977–1010.
- Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/american Society of Clinical Oncology Breast Cancer survivorship care guideline. J Clin Oncol. 2016;34(6):611–635.
- Mock V, Atkinson A, Barsevick A, et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park). 2000;14(11A):151–161.
- Schmidt ME, Chang-Claude J, Vrieling A, et al. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. J Cancer Surviv. 2012;6(1):11–19.
- Goedendorp MM, Andrykowski MA, Donovan KA, et al. Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls. Cancer. 2012;118(15):3833–3841.
- Martín M, Ruiz A, Ruiz Borrego M, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: results from the GEICAM/2003-02 study. J Clin Oncol. 2013;31(20):2593–2599.
- Fisher MI, Davies C, Lacy H, et al. Oncology section EDGE task force on cancer: measures of cancer-related fatigue—a systematic review. Rehab Oncol. 2018;36(2):93–105.
- Fabi A, Bhargava R, Fatigoni S, et al. Cancer-related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723.
- Vaz-Luis I, Di Meglio A, Havas J, et al. Long-term longitudinal patterns of patient-reported fatigue after breast cancer: a group-based trajectory analysis. J Clin Oncol. 2022;40(19):2148–2162.
- Puglisi F, Deroma L, Russo S, et al. Effect of age on hemoglobin levels and quality of life following treatment with epoetin alfa in cancer patients. Crit Rev Oncol Hematol. 2009;69(2):175–182.
- Russo S, Cinausero M, Gerratana L, et al. Factors affecting patient’s perception of anticancer treatments side-effects: an observational study. Expert Opin Drug Saf. 2014;13(2):139–150.
- Sobrero A, Puglisi F, Guglielmi A. et alFatigue: a main component of anemia symptomatology. Semin Oncol. 2001;28(2, Suppl 8):15–18.
- Antonuzzo A, Ripamonti CI, Roila F, et al. Effectiveness of a phone-based nurse monitoring assessment and intervention for chemotherapy-related toxicity: a randomized multicenter trial. Front Oncol. 2022;12:925366.
- Brown JC, Huedo-Medina TB, Pescatello LS, et al. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133.
- Tomlinson D, Diorio C, Beyene J, et al. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 2014;93(8):675–686.
- Mock V, Pickett M, Ropka ME, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract. 2001;9(3):119–127.
- Ligibel JA, Bohlke K, May AM, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40(22):2491–2507.
- Gielissen MFM, Verhagen S, Witjes F, et al. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol. 2006;24(30):4882–4887.
- Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(3):194–232.
- Buffart LM, van Uffelen JGZ, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12(1):559.
- Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979–1989.
- Barton DL, Liu H, Dakhil SR, et al. Wisconsin ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105(16):1230–1238.
- Semiglazov VF, Stepula VV, Dudov A, et al. Quality of life is improved in breast cancer patients by standardised mistletoe extract PS76A2 during chemotherapy and follow-up: a randomised, placebo-controlled, double-blind, multicentre clinical trial. Anticancer Res. 2006;26(2B):1519–1529.
- Zhang Y, Lin L, Li H, et al. Effects of acupuncture on cancer-related fatigue: a meta-analysis. supportive care in cancer. J Multinat Assoc Support Care Cancer. 2018;26(2):415–425.
- Roth AJ, Nelson C, Rosenfeld B, et al. Methylphenidate for fatigue in ambulatory men with prostate cancer. Cancer. 2010;116(21):5102–5110.
- Kerr CW, Drake J, Milch RA, et al. Effects of methylphenidate on fatigue and depression: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 2012;43(1):68–77.
- Richard PO, Fleshner NE, Bhatt JR, et al. Phase II,randomised, double-blind, placebo-controlled trial of methylphenidate for reduction of fatigue levels in patients with prostate cancer receiving LHRH-agonist therapy. BJU Int. 2015;116(5):744–752.
- Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38(5):650–662.
- Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28(23):3673–3679.
- Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a university of Rochester cancer center community clinical oncology program research base study. Cancer. 2010;116(14):3513–3520.
- Morrow GR, Jickok JT, Roscoe JA, et al. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21(24):4635–4641.
- Roscoe A, Morrow GR, Hickok JT, et al. Effect of paroxetine hydrochloride (Paxil) on fatigue and depression in breast cancer patients receiving chemotherapy. Breast Cancer Res Treat. 2005;89(3):243–39.
- Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25(23):3475–3481.
- Dimsdale JE, Ball ED, Carrier E, et al. Effect of eszopiclone on sleep, fatigue, and pain in patients with mucositis associated with hematologic malignancies. Support Care Cancer. 2011;19(12):2015–2020.
- Bruera E, Ernst S, Hagen N, et al. Effectiveness of megestrol acetate in patients with advanced cancer: a randomized, double-blind, crossover study. Cancer Prevent Cont. 1998;2(2):74–78.
- Lund Rasmussen C, Olsen MK, Johnsen AT, et al. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: a double-blind placebo-controlled crossover trial. Cancer. 2015;121(20):3727–3736.
- Jim HSL, Hoogland AI, Han HS, et al. A randomized placebo-controlled trial of bupropion for cancer-related fatigue: study design and procedures. Contemp Clin Trials. 2020;91:105976.
- Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292–298.
- Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl). 2001;10(4):245–255.
- Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biol Psychol. 2007;75(1):37–44.
- Leysen L, Lahousse A, Nijs J, et al. Prevalence and risk factors of sleep disturbances in breast cancer survivors: systematic review and meta-analyses. Support Care Cancer. 2019;27(12):4401–4433.
- Savard J, Ivers H. The initiation of chemotherapy, but not radiation therapy, coincides with increased insomnia. Psychooncology. 2012;21:1–130.
- Palesh O, Peppone L, Innominato F, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162.
- Sanford SD, Wagner LI, Beaumont JL, et al. Longitudinal prospective assessment of sleep quality: before, during, and after adjuvant chemotherapy for breast cancer. Support Care Cancer. 2013;21(4):959–967.
- Sephton SE, Sapolsky RM, Kraemer HC, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000.
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13(5):325–335.
- Garaulet M, Madrid J. A. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62(9–10):967–978.
- Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908.
- Berger AM, Wielgus K, Hertzog M, et al. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support Care Cancer. 2010;18(1):105–114.
- Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin cAncer Res Off J Am Assoc Cancer Res. 2005;11(5):1757–1764.
- Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9(5):355–364.
- Liu L, Mills PJ, Rissling M, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26(5):706–713.
- Panossian LA, Veasey SC. Daytime sleepiness in obesity: mechanisms beyond obstructive sleep apnea—a review. Sleep. 2012;35(5):601–615.
- Lucassen EA, Rother KI, Cizza G. Interacting epidemics? Sleep curtailment, insulin resistance, and obesity. Ann N Y Acad Sci. 2012;1264(1):110–134.
- Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–2269.
- Gerber LH, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer. 2011;19(10):1581–1591.
- Koffel E, Khawaja IS, Germain A. Sleep disturbances in posttraumatic stress disorder: updated review and implications for treatment. Psychiatr Ann. 2016;46(3):173–176.
- King AC, Oman RF, Brassington GS, et al. Moderate-intensity exercise and self-rated quality of sleep in older adults. a randomized controlled trial. JAMA. 1997;277(1):32–37.
- Tworoger SS, Yasui U, Vitiello MV, et al. Effects of a year long moderate-intensity exercise and a stretching intervention on sleep quality in postmenopausal women. Sleep. 2003;26(7):830–836.
- Humpel N, Iverson DC. Sleep quality, fatigue and physical activity following a cancer diagnosis. Eur J Cancer Care (Engl). 2010;19(6):761–768.
- Young-McCaughan S, Mays MZ, Arzola SM, et al. Research and commentary: change in exercise tolerance, activity and sleep patterns, and quality of life in patients with cancer participating in a structured exercise program. Oncol Nurs Forum. 2003;30(3):441–454.
- Mustian KM, Palesh O, Sprod L, et al. Effect of YOCAS yoga on sleep, fatigue, and quality of life: a URCC CCOP randomized, controlled clinical trial among 410 cancer survivors. J Clin Oncol. 2010;28(15_suppl):9013.
- Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–3241.
- Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. an American Academy of Sleep Medicine Report. Sleep. 2006;29(11):1415–1419.
- Savard J, Simard S, Ivers H, et al. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096.
- Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658.
- Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18(6):634–646.
- Berger AM, Kuhn BR, Farr LA, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. J Clin Oncol. 2009;27(35):6033–6040.
- Barsevick A, Beck SL, Dudley WN, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage. 2010;40(2):200–216.
- Chung K, Lee C, Yeung W, et al. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2018;35(4):365–375.
- Neikrug AB, Rissling M, Trofimenko V, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10(3):202–216.
- Costantini C, Ale-Ali A, Helsten T. Sleep aid prescribing practices during neoadjuvant or adjuvant chemotherapy for breast cancer. J Palliat Med. 2011;14(5):563–566.
- Chen WY, Giobbie-Hurder A, Gantman K, et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145(2):381–388.
- Cavaletti G, Marmiroli P, and Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–666.
- Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31(10):1306–1319.
- Bao T, Basal C, Seluzicki C, et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159(2):327–333.
- Rivera DR, Ganz PA, Weyrich MS, et al. Chemotherapy-associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J Natl Cancer Inst. 2018;110(2).
- Argyriou AA, Koltzenburg M, Polychronopoulos P, et al. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol. 2008;66(3):218–228.
- Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol. 2015;75(4):659–670.
- Biganzoli L, Cinieri S, Berardi R, et al. EFFECT: a randomized phase II study of efficacy and impact on function of two doses of nab-paclitaxel as first-line treatment in older women with advanced breast cancer. BCR. 2020;22(1):83.
- Tofthagen C, Visovsky CM, Hopgood R. Chemotherapy-induced peripheral neuropathy: an algorithm to guide nursing management. Clin J Oncol Nurs. 2013;17(2):138–144.
- Crevenna R, Ashbury FD. Physical interventions for patients suffering from chemotherapy-induced polyneuropathy. Support Care Cancer. 2018;26(4):1017–1018.
- Beijers AJM, Bonhof CS, Mols F, et al. Multicenter randomized controlled trial to evaluate the efficacy and tolerability of frozen gloves for the prevention of chemotherapy-induced peripheral neuropathy. Ann Oncol. 2020;31(1):131–136.
- Kleckner IR, Kamen C, Cole C, et al. Effects of exercise on inflammation in patients receiving chemotherapy: a nationwide NCORP randomized clinical trial. Support Cancer Ther. 2019;27(12):4615–4625.
- Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018;26(4):1019–1028.
- Lu W, Giobbie-Hurder A, Freedman RA, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. 2020;25(4):310–318.
- Kolb NA, Smith G, Singleton JR, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016;73(7):860–866.
- Tamburin S, Park SB, Schenone A, et al. Rehabilitation, exercise, and related non-pharmacological interventions for chemotherapy-induced peripheral neurotoxicity: systematic review and evidence-based recommendations. Crit Rev Oncol Hematol. 2022;171:103575.
- Li Y, Lustberg MB, Hu S. Emerging pharmacological and non-pharmacological therapeutics for prevention and treatment of chemotherapy-induced peripheral neuropathy. Cancers (Basel). 2021;13(4):766.
- Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359.
- Durand JP, Deplanque G, Montheil V, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase iii trial. Ann Oncol. 2012;23(1):200–205.
- Kautio A, Haanpää M, Saarto T, et al. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35(1):31–39.
- Desforges AD, Hebert CM, Spence AL, et al. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: an update. Biomed Pharmacother. 2022;147:112671.
- Szklener K, Szklener S, Michalski A, et al. Dietary supplements in chemotherapy-induced peripheral neuropathy: a new hope? Nutrients. 2022;14(3):625.
- Zandbergen N, de Rooij BH, Vos MC, et al. Changes in health-related quality of life among gynecologic cancer survivors during the two years after initial treatment: a longitudinal analysis. Acta Oncol. 2019;58(5):790–800.
- Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686.
- Chen VC, Lin T, Yeh D, et al. Predicting chemo-brain in breast cancer survivors using multiple MRI features and machine-learning. Magn Reson Med. 2019;81(5):3304–3313.
- Minisini A, Atalay G, Bottomley A, et al. What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol. 2004;5(5):273–282.
- Minisini AM, De Faccio S, Ermacora P, et al. Cognitive functions and elderly cancer patients receiving anticancer treatment: a prospective study. Crit Rev Oncol Hematol. 2008;67(1):71–79.
- Nguyen LD, Ehrlich BE. Cellular mechanisms and treatments for chemobrain: insight from aging and neurodegenerative diseases. EMBO Mol Med. 2020;12(6):e12075.
- Breuer B, Anderson R, and Anderson R. The relationship of tamoxifen with dementia, depression, and dependence in activities of daily living in elderly nursing home residents. Women Health. 2000;31(1):71–85.
- Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 2000;64(2):165–176.
- Phillips K, Regan MM, Ribi K, et al. Adjuvant ovarian function suppression and cognitive function in women with breast cancer. Br J Cancer. 2016;114(9):956–964.
- van Dam FS, Schagen SB, Muller MJ, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90(3):210–218.
- Marín AP, Sánchez AR, Arranz EE, et al. Adjuvant chemotherapy for breast cancer and cognitive impairment. South Med J. 2009;102(9):929–934.
- Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Neurol. 2003;8(4):201–216.
- Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? a systematic review of neuropsychological findings. Clin Psychol Rev. 2011;31(3):449–464.
- Duval A, Davis CG, Khoo EL, et al. Mindfulness-based stress reduction and cognitive function among breast cancer survivors: a randomized controlled trial. Cancer. 2022;128(13):2520–2528.
- Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124(1):192–202.
- Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135(3):799–809.
- Alvarez J, Meyer FL, Granoff DL, et al. The effect of EEG biofeedback on reducing postcancer cognitive impairment. Integr Cancer Ther. 2013;12(6):475–487.
- Kohli S, Fisher SG, Tra Y, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115(12):2605–2616.
- Shaw EG, Rosdhal R, D’Agostino RB, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–1420.
- Dinas KD. Impact of breast cancer treatment on fertility. Adv Exp Med Biol. 2020;1252:175–179.
- Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17(8):2365–2370.
- Goldfarb SB, Turan V, Bedoschi G, et al. Impact of adjuvant chemotherapy or tamoxifen-alone on the ovarian reserve of young women with breast cancer. Breast Cancer Res Treat. 2021;185(1):165–173.
- Arecco L, Ruelle T, Martelli V, et al. How to protect ovarian function before and during chemotherapy? J Clin Med. 2021;10(18):4192.
- Moore HCF, Unger JM, Phillips K, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372(10):923–932.
- Franzoi MA, Agostinetto E, Perachino M, et al. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021;22(7):e303–13.
- Bober SL, Fine E, Recklitis CJ. Sexual health and rehabilitation after ovarian suppression treatment (SHARE-OS): a clinical intervention for young breast cancer survivors. J Cancer Survivorship. 2020;14(1):26–30.
- Enemchukwu EA. CO2 laser treatment is effective for symptoms of vaginal atrophy: no. J Urol. 2017;198(6):1228–1229.
- Arêas F, Valadares Ana LR, Conde DM, et al. The effect of vaginal erbium laser treatment on sexual function and vaginal health in women with a history of breast cancer and symptoms of the genitourinary syndrome of menopause: a prospective study. Menopause. 2019;26(9):1052–1058.
- Becorpi A, Campisciano G, Zanotta N, et al. Fractional CO2 laser for genitourinary syndrome of menopause in breast cancer survivors: clinical, immunological, and microbiological aspects. Lasers Med Sci. 2018;33(5):1047–1054.
- Carson JW, Carson KM, Porter LS, et al. Yoga of awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17(10):1301–1309.
- Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor–positive breast cancer: a randomized controlled trial. J Clin Oncol. 2010;28(4):634–640.
- Moegele M, Buchholz S, Seitz S, et al. Vaginal estrogen therapy in postmenopausal breast cancer patients treated with aromatase inhibitors. Arch Gynecol Obstet. 2012;285(5):1397–1402.
- Sanchez-Rovira P, Hirschberg AL, Gil-Gil M, et al. A phase II prospective, randomized, double-blind placebo-controlled and multicenter clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitor in the adjuvant setting. Oncologist. 2020;25(12):e1846–1854.
- Holmberg L, Anderson H. HABITS (Hormonal Replacement Therapy after Breast Cancer—Is it safe?), A randomised comparison: trial stopped. Lancet. 2004;363(9407):453–455.
- Fahlén M, Fornander T, Johansson H, et al. Hormone replacement therapy after breast cancer: 10 year follow up of the Stockholm randomised trial. Eur J Cancer. 2013;49(1):52–59.
- Sismondi P, Kimmig R, Kubista E, et al. Effects of tibolone on climacteric symptoms and quality of life in breast cancer patients—data from LIBERATE trial. Maturitas. 2011;70(4):365–372.
- Wurz GT, Soe LH, DeGregorio MW. Ospemifene, vulvovaginal atrophy, and breast cancer. Maturitas. 2013;74(3):220–225.
- Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J Clin Oncol. 2009;27(17):2831–2837.
- Goldberg RM, Loprinzi CL, O’Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12(1):155–158.
- Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356(9247):2059–2063.
- Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2011;29(29):3862–3868.
- Leon-Ferre RA, Novotny PJ, Wolfe EG, et al. Oxybutynin vs placebo for hot flashes in women with or without breast cancer: a randomized, double-blind clinical trial (ACCRU SC-1603). JNCI Cancer Spectrum. 2020;4(1):kz088.
- Rachner TD, Coleman R, Hadji P, et al. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol. 2018;6(11):901–910.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361.
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220.
- Gralow JR, Biermann JS, Farooki A, et al. NCCN task force report: bone health in cancer care. J National Compr Cancer Network. 2013;11(suppl 3):S-1-S–50.
- Zamorano JL, Lancellotti P, Muñoz DR, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–2801.
- Moliterni A, Bonadonna G, Valagussa P, et al. Cyclophosphamide, methotrexate, and fluorouracil with and without doxorubicin in the adjuvant treatment of resectable breast cancer with one to three positive axillary nodes. J Clin Oncol. 1991;9(7):1124–1130.
- Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes: ten-year results. JAMA. 1995;273(7):542–547.
- Bonadonna G, Zambetti M, Bumma C, et al. Multimodal treatment with primary single-agent epirubicin in operable breast cancer: 5-year experience of the Michelangelo Cooperative Group. Ann Oncol. 2002;13(7):1049–1058.
- Earl HM, Hiller L, Dunn JA, et al. for the NEAT Investigators and the SCTBG. Adjuvant epirubicin followed by cyclophosphamide, methotrexate and fluorouracil (CMF) vs CMF in early breast cancer: results with over 7 years median follow-up from the randomised phase III NEAT/BR9601 trials. Br J Cancer. 2012;107(8):1257–1267.
- Von Hoff D. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710.
- Bonadonna G, Gianni L, Santoro A, et al. Drugs ten years later: epirubicin. Ann Oncol. 1993;4(5):359–369.
- Wojtukiewicz MZ, Omyla J, Kozlowski L, et al. Cardiotoxicity of anthracycline. Postepy Hig Med Dosw (Online). 2000;54(4):467–485.
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Euro Heart J Cardiovascular Imaging. 2014;15(10):1063–1093.
- Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988.
- Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2010;55(3):213–220.
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy–induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481.
- Raccomandazioni pratiche. CARDIO-ONCOLOGIA 2019. N.d. n.d.
- van Dalen EC, van der Pal HJH, Kremer LCM. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Edited by Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group. Cochrane Database Syst Rev. 2016.
- Rafiyath SM, Rasul M, Lee B, et al. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol. 2012;1(1):10.
- Yamaguchi N, Fujii T, Aoi S, et al. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: a Bayesian network meta-analysis. Eur J Cancer. 2015;51(16):2314–2320.
- O’Brien MER, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl(CAELYXTM/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449.
- Lyon AR, López-Fernández T, Couch LS, et al. ESC scientific document group, 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Euro Heart J Cardiovascular Imaging. 2022;23(10):e333–e465.
- van Dalen EC, Caron HN, Dickinson HO, et al. Cardioprotective interventions for cancer patients receiving anthracyclines. edited by Cochrane gynaecological, neuro-oncology and orphan cancer group. Cochrane Database Syst Rev. 2011;2016(9).
- Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10(1):337.
- Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group randomized trial Pediatric Oncology Group 9404. J Clin Oncol. 2016;34(8):854–862.
- Reichardt P, Tabone M, Mora J, et al. Risk–benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling. Future Oncol. 2018;14(25):2663–2676.
- Caspani F, Tralongo AC, Campiotti L, et al. Prevention of anthracycline-induced cardiotoxicity: a systematic review and meta-analysis. Intern Emerg Med. 2021;16(2):477–486.
- Vaduganathan M, Hirji S, Qamar A, et al. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. J Am Coll Cardiol CardioOnc. 2019;1(1):54–65.
- Li X, Li Y, Zhang T, et al. Role of cardioprotective agents on chemotherapy-induced heart failure: a systematic review and network meta-analysis of randomized controlled trials. Pharmacol Res. 2020;151:104577.
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin converting enzyme inhibition. Circulation. 2006;114(23):2474–2481.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671–1680.
- Vaduganathan M, Jirji SA, Qamar A, et al. Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. JACC: CardioOncology. 2019;1(1):54–65.
- Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. 2013;167(5):2306–2310.
- Shah P, Garris R, Abboud R, et al. Meta-analysis comparing usefulness of beta blockers to preserve left ventricular function during anthracycline therapy. Am J Cardiol. 2019;124(5):789–794.
- Ma Y, Bai F, Qin F, et al. Beta-blockers for the primary prevention of anthracycline-induced cardiotoxicity: a meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol. 2019;20(1):18.
- Huang S, Zhao Q, Yang ZG, et al. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity-a systematic review and meta-analysis of carvedilol. Heart Fail Rev. 2019;24(3):325–333.
- Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974–984.
- Rowinsky EK, McGuire WP, Guarnieri T, et al. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9(9):1704–1712.
- Martel S, Maurer C, Lambertini M, et al. Breast cancer treatment-induced cardiotoxicity. Expert Opin Drug Saf. 2017;16(9):1021–1038.
- Pondé NF, Lambertini M, de Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1(4):e000073.
- Moja L., Brambilla C., Compagnoni A., et al. Trastuzumab Containing Regimens for Early Breast Cancer. In Cochrane Database of Systematic Reviews, edited by The Cochrane Collaboration. 2006
- Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–1221.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792.
- Advani PP, Ballman KV, Dockter TJ, et al. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J Clin Oncol. 2016;34(6):581–587.
- Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol. 2015;12(9):547–558.
- Jawa Z, Perez RM, Garlie L, et al. Risk factors of trastuzumab-induced cardiotoxicity in breast cancer: a meta-analysis. Medicine (Baltimore). 2016;95(44):e5195.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205.
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–1028.
- Barish R, Gates E, Barac A. Trastuzumab-Induced Cardiomyopathy. Cardiol Clin. 2019 18;37(4):407–418.
- Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–3916.
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131.
- Gianni L, Pienkowski T, Im Y, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
- von Minckwitz G, Huang C, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628.
- Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with Trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640.
- Wood ME, Vogel V, Ng A, et al. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30(30):3734–3745.
- Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the national surgical adjuvant breast and bowel project experience. J Clin Oncol. 2003;21(7):1195–1204.
- Wolff AC, Blackford AL, Visvanathan K, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33(4):340–348.
- Leone G, Pagano L, Ben-Yehuda D, et al. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92(10):1389–1398.
- Azarova AM, Lyu YL, Lin C, et al. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc Natl Acad Sci U S A. 2007;104(26):11014–11019.
- Travis LB, Andersson M, Gospodarowicz M, et al. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92(14):1165–1171.
- Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340(5):351–357.
- Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol. 2008;26(8):1239–1246.
- Turcotte LM, Liu Q, Yasui Y, et al. Chemotherapy and risk of subsequent malignant neoplasms in the childhood cancer survivor study cohort. J Clin Oncol. 2019;37(34):3310–3319.