ABSTRACT
Background
There are no local or international guidelines or consensus on the use of mAbs against the rabies virus.
Research design and methods
An expert group in the field of rabies prevention and control formulated the consensus presented in this paper.
Results
Class III exposed persons to rabies for the first time; Identify type II exposed persons with immune deficiency; those who are first exposed to Class II and re-exposed to Class III within 7 days. They can use ormutivimab injection after completing the PEP wound treatment. In the case of injection restrictions or a wound that is difficult to detect, it is recommended that the entire Ormutivimab dose be infiltrated close to the wound. For severe multi-wound bites, the recommended dosage of ormutivimab is 20 IU/kg. If the recommended dose cannot meet all of the wound infiltration requirements, appropriate dilution can be conducted at a dilution ratio of 3 ~ 5 times. If the requirements for infiltration cannot be met after dilution, it is recommended that the dosage be increased with caution (maximum dosage, 40 IU/kg). The use of Ormutivimab is safe and effective without any contraindications by all age groups.
Conclusions
This consensus standardizes clinical use of Ormutivimab, improves post-exposure prophylaxis of rabies in China, reduces infection rate.
1. Introduction
Approximately 29 million people worldwide undergo post-exposure prophylaxis (PEP) annually. Of these, 60% have class III exposure, but only 1%–10% of these patients receive rabies passive immunization preparations [Citation1]. The use of rabies passive immunization preparations is key to the successful implementation of PEP and prevention of rabies [Citation2]. Furthermore, vaccine-induced active immunity in patients with severely compromised immunity may fail [Citation3]. In addition to thorough wound cleaning, the application of passive immunization preparations is the most effective critical measure for preventing rabies [Citation4]. Currently, two rabies immunoglobulin (RIG) types are commonly used, human RIG (HRIG) and equine RIG (ERIG) (currently, only the horse-source rabies F(ab’)2 fragment is commercially available). Rabies immunoglobulin is a type of immunoglobulin G (IgG) that is isolated and purified from the serum of humans or other animals (typically horses) that have been vaccinated against rabies PEP. The advantages of RIG include its high level of efficiency, extensive viral neutralizing activity and ability to interact with multiple epitopes (polyspecific); however, its use involves ethical issues, such as donor use and multiple immunization rabies vaccines, as well as theoretical drawbacks, such as the potential risk of spreading known and unknown pathogens [Citation4,Citation5].
Since the 1970s and 1980s, with the development of recombinant monoclonal antibodies (mAbs) and their broad application in oncology, mAbs have presented a feasible and promising option for addressing the limitations of blood-derived RIG. Since the 1980s, the World Health Organization (WHO) has been vigorously promoting the development of rabies mAbs for PEP [Citation6,Citation7]. In 1990, a WHO thematic consultation meeting specifically proposed the development of mAbs in relation to rabies for PEP. In 2002, the WHO organized a further consultation on ‘monoclonal antibodies for rabies treatment after rabies exposure,’ with an action plan for the screening, evaluation and technology transfer of rabies mAbs for development into a PEP [Citation8]. The WHO Immunization Strategy Advisory Expert Group (Strategic Advisory Group of Experts) reviewed its recommendations on rabies vaccines and immunoglobulin, presented scientific evidence and protocols, and considered and discussed the inclusion of rabies mAbs [Citation8]. The WHO strongly recommended the transition from RIG to mAb-based PEP based on RIG in the latest rabies vaccine position paper and expert consultation documented in 2018 to achieve an adequate supply, reduce production costs, reduce the risk of adverse reactions and increase the availability of continuously effective vaccine batches [Citation9–11].
Monoclonal antibodies are presented in the form of concentrated products; therefore, infiltrating IgG molecules through an infiltration injection around the wound increases the abundance of molecules and makes mAbs more effective than RIG, which is in line with the WHO recommendation for developing passive immunity [Citation12]. Monoclonal antibodies have the following advantages: high specific activity (1,000 IU/mg), high local concentration, higher levels of neutralization with fewer injections, the ability to reduce the incidence of local damage and adverse reactions caused by a high injection dosage, and the strict homogeneity of the mAb preparations of different batches using standardized cell production processes. Furthermore, a single mAb targets only one or a few specific epitopes and is highly specific, and it has a reduced effect on active immunity when used together with the human rabies vaccine [Citation13].
1.1. Mechanism of action of rabies passive immunization preparations
The role of rabies passive immunization preparations is to provide neutralizing antibodies at the exposed site before the production of autoantibodies resulting from active immunity after vaccination. presents a schematic of rabies virus pathogenesis with standard PEP and without [Citation14].
Figure 1. A schematic diagram of the pathogenesis of rabies virus under post-exposure prophylaxis (PEP).
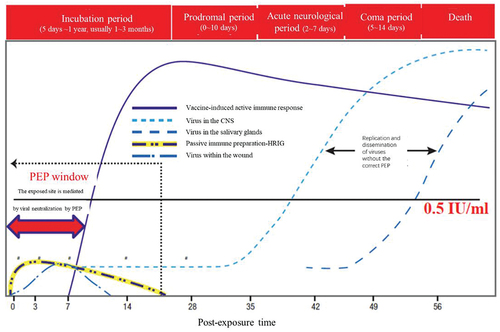
Rabies can be divided into five stages from infection to death as follows: infection, incubation period (5 days–1 year, typically 1–3 months), prodromal period (0–10 days), acute neurological period (2–7 days), and coma and death (5–14 days).
Vaccine-induced active immunity produces neutralizing antibodies between approximately 7 and 10 days, reaching the highest level 1–2 weeks after full vaccination. The presence of the virus in the wound area can be neutralized through passive immunization preparations against rabies, which can provide passive protection immediately before the vaccine is able to induce sufficient neutralizing antibodies (>0.5 IU/mL) (within 7 days).
If PEP is not performed according to the set standard, the virus enters the nerve and gradually moves to the central nervous system, replicating and spreading to the brain, and the patient will eventually die.
During PEP, after local treatment of the wound, blocking the residual viral infection of the body is achieved through the initial local infiltration of a rabies passive immunization preparation injection and neutralization of the antibodies produced by the subsequent vaccine-induced active immunity [Citation14]. The role of passive immunization preparations is to provide passive protection at the exposed site immediately before the vaccine typically induces the body to actively produce the necessary antibodies (7 days). However, the effect of the passive immunization preparation is not maintained for an extended period. After several weeks, it degrades and is cleared from the body; thus, rabies passive immunization preparations have a rapid but short-term effect. Vaccines can induce specific neutralizing antibody responses against the rabies G protein, but they take time to develop active immunity (typically 7–14 days). However, once antibodies have begun to be produced, they are continuously created and maintained for a longer period, thus having an immunological memory effect [Citation15]. Therefore, in addition to the complete clearing/washing of the wound, the complete PEP measure for rabies must be combined with a passive immunization preparation and a vaccine. The synergistic effect of these two measures can provide the entire protection effect process [Citation16]. Neutralizing antibodies involves several different mechanisms of action that act directly alongside the rabies virus, including adsorption onto viral particles, interference with the spatial binding of the virus to the receptor, inhibition of the endosomal acid-catalyzed fusion step required for virus uncoating, and the initiation of complement-dependent lysis of rabies-infected cells and antibody-dependent cell-mediated cytotoxic effects [Citation17].
1.2. Classification of rabies passive immunization preparations
For patients with class III rabies virus exposure, the wound should be thoroughly cleaned and injected with passive immunization preparations in the periphery along with vaccination to prevent the virus from entering the neural tissue and achieve a rapid protective effect [Citation18]. Rabies passive immunization preparation approaches can be divided into three categories:
Using the F(ab’)2 fragment of equine rabies
Using HRIG
Genetically engineered antibodies (mAb)
The characteristics of the three classes of passive immunization preparations are summarized in .
Table 1. Types and characteristics of rabies passive immune preparations.
The dosage of Chinese Ormutivimab injection (active ingredient, Ormutivimab, recombinant human mAb), 200 IU/1 mL; India Rabishield-100®, 100 IU/2.5 mL; Twinrab®, 3,000 IU/10 mL, 3,000 IU/5 mL, 1,500 IU/2.5 mL and 600 IU/1 mL.
Equine RIG is derived from already immunized healthy horse serum, and although products are very different in quality, they are also heterogeneous (). It is commonly known as an antiserum used before improving the production process (the product is a complete IgG molecule, and the purification process is simple). The incidence of serum diseases with ERIG (antiserum) is approximately 16%–46% and prone to lead to adverse reactions (F(ab’)2 fragment after the digestion process, with IgG molecules remaining intact) [Citation4,Citation5]. Equine RIG has been improved in terms of valence, purity and safety through the development of a new technique [Citation19], but it may still cause serious adverse reactions, including allergic responses (the incidence has decreased to 1/45,000). Only medical institutions with the correct facilities and medical personnel trained to deal with adverse reactions can use ERIG safely [Citation20]. In addition to the potential adverse reaction risk derived from heterogeneity, issues of cultural norms, religious beliefs and animal welfare limit the broader use of ERIG [Citation21].
Compared with ERIG, HRIG has good safety and tolerability without immunogenicity and relatively rarely causes allergic reactions or serum disease. Human RIG is derived from plasma from healthy volunteers who have been immunized with the rabies vaccine. Although healthy volunteers must meet strict screening criteria, including screening for known blood-borne pathogens (including viral hepatitis, human immunodeficiency virus (HIV) type 1 and syphilis) and markers of potential new pathogens, possible risks cannot be entirely ruled out (). In addition, to meet HRIG preparation requirements, volunteers are made hyperimmune (with multiple vaccinations) using a licensed cell-cultured rabies vaccine (e.g. HDCV, PCEC or PVRV) to produce high concentrations of neutralizing antibodies [Citation22]. Human RIG is, however, expensive and in short supply for meeting the needs of all patients who may require it [Citation23].
The WHO recommends that serious adverse reactions or any quality differences arising from RIG be monitored and reported. Monoclonal antibodies are prepared using genetic recombination technology, which enables their mass production as well as quality standardization with fewer obstacles concerning accessibility, safety and purity (). The supply of passive immunization preparations for rabies is inadequate worldwide. The WHO clearly states that mAbs are not produced using animals, can reduce the risk of adverse reactions, such as blood-borne infectious diseases, and are more effective compared with RIG. The replacement of RIG with mAb products is encouraged when conditions permit [Citation9,Citation10]. The anti-rabies virus mAb, Rabishield, listed in India at the end of 2016, can effectively neutralize the global rabies virus and is safe and effective for patients aged 1–85 years who have been exposed to the rabies virus [Citation9,Citation24,Citation25].
1.3. Clinical application of ormutivimab
Ormutivimab is a category 1 therapeutic biological product that has been independently developed in China [Citation26], and it is the third mAb approved for use against the rabies virus; SII RMab (Indian Serum Institute) was listed in 2016, RABIMABS (Zydus Research Center, India) was listed in 2019 and ormutivimab (North China Pharmaceutical Group New Drug Research and Development) was listed in 2022. Ormutivimab targets the highly conserved antigen site I of the rabies virus G protein [Citation27–29] and is a recombinant full human anti-rabies virus mAb expressed in Chinese hamster ovary cells. The ormutivimab antibody gene is derived from healthy volunteers as a full human mAb that is prepared using genetic recombination techniques [Citation27,Citation30–33]. Compared with murine mAb [Citation34,Citation35], human/mouse chimeric mAb [Citation36,Citation37] and humanized mAb [Citation38] produced using artificial modification technology, full human mAb does not include the murine IgG gene and has no heterogeneity; accordingly, the incidence of adverse reactions is greatly reduced, and the potency, efficacy and immunization persistency are higher [Citation24,Citation39,Citation40].
This consensus was formed by the expert group organized by the China Vaccine Industry Association, which was composed of the director of the dog injury clinics in China’s tertiary hospitals and the director of the Vaccine Institute of the Provincial Center for Disease Control and Prevention.
(1) Ormutivimab treatment population
The following patients represent suitable populations for treatment with ormutivimab:
Patients with class III exposure following initial exposure ( for details) [Citation18,Citation41];
Table 2. Recommended post-exposure immunization precautions according to the type of exposure.
Patients with class II exposure with long-term and heavy use of immunosuppressants and severely impaired immune function (e.g. HIV infection without ART or who are currently receiving ART, children <5 years with CD4 cell levels ≤ 25% or ≥5 years with a CD4 cell level < 200/mm3);
Patients with a first exposure rated as class II, but class III exposure occurred again within 7 days of PEP.
Re-exposure: Patients with class III exposure who had not been vaccinated with the human rabies vaccine (cell-culture vaccine).
Recommendation 1: Ormutivimab is recommended for those meeting the first rabies III degree exposure or with the immune deficiency or III degree re-exposure within 7 days and III degree re-exposure without full vaccine immunization
(2) Using ormutivimab
The ormutivimab dosage is 200 IU (1 mL)/bottle, with a recommended dosage of 20 IU/kg. A sufficient amount of this product should be used once following exposure to rabies and as soon as possible. As long as the anatomy of the injection site is appropriate (and avoids possible bone compartment syndrome), the entire ormutivimab infiltration should be injected near the wound at the calculated dose. All wounds should be injected with infiltration, regardless of size.
The immediate management of wounds from all bites and scratches is an important step in prophylactic treatment following rabies exposure. Wound management primarily consists of thorough washing and disinfection as well as comprehensive surgical management. These steps are described as follows:
Thorough rinsing: immediately rinse the inside of each wound meticulously for 15 min alternately with soap or a weak alkaline detergent and plenty of water.
Disinfection: use a 0.05% povidone – iodine (iodine – iodine) solution or another skin and mucosa disinfectant with a virus inactivation effect to wipe or disinfect the wound internally. Thereafter, a thorough debridement of all wounds should be performed according to the principles of surgical debridement, after which ormutivimab should be injected. If the pain from a serious bite wound is severe, local wound anesthesia infiltration, nerve block anesthesia or even extraspinal anesthesia/general anesthesia can be selected before dealing with the wound.
Recommendation 2: A thorough and standardized surgical treatment of the wound as soon as possible is the first step in rabies PEP and one of the key stages for preventing the disease after exposure. Ormutivimab can subsequently be used.
After completing surgical treatment, ormutivimab drops are first applied according to the wound size to directly neutralize any possible presence of the virus that may have remained on the wound surface after debridement. The injection needle is then inserted approximately 0.5–1 cm from the wound’s edge for an infiltration injection. The injection depth should exceed the depth of the wound and is delivered first to the base of the wound. No blood is drawn back, and there is no reinjection. Draw back the needle while injecting the infiltration to avoid injecting ormutivimab into the vessel until a small amount of the ormutivimab injection gradually seeps from the wound. Avoid direct injection into the wound or through the wound to the contralateral side, which may allow the virus to pass into deep tissue. The number and angle of punctures depends on the nature of the wound. The number of punctures should be minimized as much as possible, but infiltration must be ensured for the entire wound area.
Infiltration injection in injection-restricted sites (e.g. fingers, toes, nose tip, auricle or male external genitalia) or where wounds are not easily detectable should consider the local anatomy to avoid excessive injection, blocking peripheral blood circulation or causing bone compartment syndrome. It is recommended that ormutivimab be injected according to the prescribed dosage near the wound. Some ormutivimab may remain after infiltration injection for all wounds; however, there is no authoritative guidance on how to use remaining passive immune preparations. China still implements the distant intramuscular injection of remaining passive immunization preparations. Recent literature revealed that the injection of these preparations into muscle far from the wound had no or only minimal additional protective effects against the occurrence of rabies compared with performing infiltration near the wound only [Citation42]. If the remaining passive immunization preparation matches a calculated dose, the WHO no longer recommends intramuscular injection far from the wound; rather, the remaining preparation should be divided into multiple small syringes for treating other patients, which is particularly useful when the passive immunization preparation is in short supply.
Recommendation 3: To include injection-restricted areas or wounds that are not easy to detect or inspect in treatment, if anatomically permitted (and to avoid bone compartment syndrome), ormutivimab injection is recommended for infiltration injection around all observable wounds. The remaining ormutivimab should be injected intramuscularly as close as possible to the putative wound.
If there are multiple wounds and ormutivimab is insufficient based on the recommended dose for the infiltration injection of all wounds, it can be appropriately diluted with normal saline to ensure that all wounds can be fully injected. With high specific activity and high local concentration, mAbs should have higher protective efficacy at the same dilution ratio. Current WHO guidelines and the latest literature recommend a threefold to fivefold dilution of RIG [Citation43–47] but no more than fivefold. The results of phases Ia, Ib, IIa and IIb of ormutivimab trials revealed that a dosage of 40 IU/kg did not increase the inhibitory effect on the active immunization of the vaccine. Therefore, in extreme cases, such as where multiple wounds and severe bites are present, when adequate dilution still fails to meet the satisfactory infiltration requirements for all wounds, an increased dosage of ormutivimab is recommended, but with caution (no higher than 40 IU/kg).
Recommendation 4: The recommended dose of ormutivimab is 20 IU/kg. If the recommended dose cannot meet the infiltration requirements for all wounds, appropriate dilution can be performed (the recommended a threefold to fivefold dilution). If the infiltration requirements still cannot be met after dilution, increasing the dose is recommended but with caution (maximum dose of 40 IU/kg).
If exposure is not from bites or exposure to bats, an intramuscular injection of ormutivimab as near as possible to the putative wound is recommended.
Recommendation 5: For mucosal exposure, a local or peripheral infiltration injection of ormutivimab or an intramuscular injection as close to the assumed exposed site as possible is recommended if anatomically permitted.
Recommendation 6: A trapezius intramuscular injection of ormutivimab is recommended for exposure to suspected rabies aerosols or strongly suspected high-risk animals without the presence of wounds (such as bats or animals with confirmed rabies).
Ormutivimab has completed a pre-marketing phase I – III registry clinical trial. Key indicators of safety, antibody neutralization activity, immunogenicity and the tolerability of ormutivimab on its own and combined with a human rabies vaccine and/or HRIG have been studied and verified. The results revealed that ormutivimab combined with a human rabies vaccine is safe and effective for rabies PEP as well as for pregnant and lactating women, the elderly, children and infants.
Recommendation 7: Ormutivimab can be safely and effectively used by all age groups, and pregnant and lactating women can also be treated in accordance with the regulations.
(3) Notes for the use of ormutivimab
Injection of ormutivimab and a human rabies vaccine are prohibited at the same site;
Ormutivimab should not be used as an intravenous or drip injection. Accidental injection into the blood vessels during local infiltration injection should be avoided;
Ormutivimab and a human rabies vaccine should never be injected using the same syringe;
No allergy test is needed prior to administering ormutivimab;
Ormutivimab should be stored and transported at 2°C–8°C in a dark environment.
(4) Contraindications for using ormutivimab
Rabies is a fatal disease. In principle, there is no contraindication for using ormutivimab for those who meet the conditions for receiving passive immunization preparations after rabies exposure; however, detailed medical information should be provided by the patient (e.g. history of serious allergies, other serious diseases) before being injected. If the patient has an allergy history linked to active ormutivimab ingredients or excipients (glycine, histidine, mannitol, polysorbate 80, edetate disodium), other passive immunization preparations without these can be used. If the patient is not suitable for injection and ormutivimab injection is required, it should be used under close monitoring. In case of an emergency or accident, timely and standardized medical treatment should be provided.
Recommendation 8: There is no contraindication to the clinical use of ormutivimab, but allergies to the active components and excipients of the drug should be excluded.
(5) Adverse effects of ormutivimab
Ormutivimab is a recombinant whole-human anti-rabies virus mAb and is not heterologous. In phase III clinical trials, class III adverse reactions mainly included swelling at the injection site (17.22%), erythema at the injection site (3.33%), pain at the injection site (0.28%) and rare systemic urticaria (0.28%), most of which were mild or moderate and could be relieved spontaneously after the injection. If the influence of the underlying wound status is considered (excluding local swelling and erythema caused by the wound itself and/or debridement before injection), the incidence of class III swelling and erythema at the ormutivimab injection site is 3.06% and 0.56%, respectively [Citation48].
2. Conclusion
The fatality rate of rabies is almost 100%, but rabies is an almost 100% preventable zoonotic infectious disease [Citation9,Citation10,Citation49]. The key to prevention is to conduct PEP in a timely and standardized manner, and one of the important aspects of standardizing PEP is using rabies passive immunization agents [Citation2]. The traditional passive immunization agents for rabies are blood products, which are employed to immunize animals and people. These carry risks in the form of potential adverse reactions related to, for example, the source of blood, limited production capacity and ethical disputes [Citation4,Citation50,Citation51]. At present, the supply of passive immunization preparations against rabies remains seriously insufficient worldwide. Monoclonal antibodies, prepared using gene recombination technology, do not use animals in the production process, do not carry the risk of adverse blood reactions, can be produced on a large scale and with standardized quality, exclude ethical obstacles and have the same function as HRIG [Citation9,Citation10]. Research has demonstrated that anti-rabies virus mAbs can effectively neutralize the North American bat mutant strain of the virus, whereas HRIG is ineffective in this regard [Citation52]. Anti-rabies virus mAbs can be effectively applied for the prevention of rabies after exposure through passive immunization agents, a new passive immunization agent that has been strongly recommended and supported by the WHO for rabies PEP in recent years [Citation9,Citation10,Citation53,Citation54].
At present, there is no clinical application guide or consensus for a new type of passive immunization agent for rabies or anti-rabies virus mAb in either China or abroad. This consensus refers to the latest strategy for the prevention and treatment of rabies after exposure (locally and abroad) and discusses the controversial dilution multiple, whether to recommend distal muscle injection in cases where a residual dose is available and the qualifying population in the current clinical use of passive immunization agents for rabies. To better guide the clinical use of the first new rabies passive immunization agent in China, ormutivimab monoclonal injection, the authors convened domestic experts in this field to formulate the following consensus.
The following patients represent suitable populations for treatment with ormutivimab: patients with first time exposure to class III rabies; patients with immune deficiency with class II exposure; patients presenting initially with class II exposure and then re-exposed to class III exposure within 7 days. Patients with class III re-exposure who have not completed the entire course of vaccine immunization [Citation18,Citation41] can receive the ormutivimab mAb injection after completing the PEP wound treatment if one of the above conditions is met. In the case of injection restrictions or a wound that is difficult to detect, if anatomically possible (to avoid osteofascial compartment syndrome caused by excessive injection volume), it is recommended that the entire ormutivimab dose be infiltrated close to the wound. If a residual dose remains after infiltration injection, the WHO no longer recommends a deep intramuscular injection of the remaining mAb in areas distant from the wound [Citation9,Citation10]. For severe multi-wound bites, the recommended dosage of ormutivimab mAb is 20 IU/kg. If the recommended dose cannot meet all of the wound infiltration requirements, threefold to fivefold dilution can be conducted. If the requirements for infiltration cannot be met after dilution, it is recommended that the dosage be increased with caution (maximum dosage, 40 IU/kg). For mucous membrane exposure, viral aerosols or bats and other animals that are strongly suspected of carrying the virus, it is recommended that ormutivimab be injected intramuscularly as close as possible to the presumed exposure wound. For special populations exposed to rabies, such as pregnant and lactating women, the elderly, children and infants [Citation50,Citation51,Citation55], the use of ormutivimab is safe and effective without any contraindications.
In recent years, the number of reported rabies cases in China has decreased annually, which could not have been achieved without standardized post-exposure prevention and treatment guidelines. The marketing of a new type of passive immunization agent for rabies, ormutivimab mAb injection, has filled the gap in the field of rabies prevention and control by addressing the current situation reflecting the insufficient application of passive immunization agents against rabies and helping to achieve the goal of eliminating the transmission of rabies from dogs to humans by 2030.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution statement
Study conception and design: Ruifeng Chen; Hu Li; Wuyang Zhu; Data collection: Hongbin Cheng; Yu Li; Xiaomei Li; Data analysis and interpretation: Faliang Li; Xiaoqiang Liu; Shixiong Hu; Drafting of the article: Baigang Yan; Yishan Zheng; Yongbo Zuo; Critical revision of the article: Guanmu Dong; Xiangming Li.
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Additional information
Funding
References
- Sparrow E, Torvaldsen S, Newall AT, et al. Recent advances in the development of monoclonal antibodies for rabies post exposure prophylaxis: A review of the current status of the clinical development pipeline. Vaccine. 2019;37(Suppl 1):A132–A139. doi: 10.1016/j.vaccine.2018.11.004
- Both L, Banyard AC, van Dolleweerd C, et al. Passive immunity in the prevention of rabies. Lancet Infect Dis. 2012 May;12(5):397–407.
- Mohindra R, Suri V, Chatterjee D, et al. Measuring antibody titres following rabies postexposure prophylaxis in immunosuppressed patients: a norm rather than the exception. BMJ Case Rep. 2021 Nov 11;14(11):e245171. doi: 10.1136/bcr-2021-245171
- Rupprecht CE, Yager ML, Newhouse RH. Passive Immunity in Rabies Prophylaxis[M]//ERTL H C J. Rabies and Rabies Vaccines Cham: Springer International Publishing; 2020. p. 117–139.
- Bourhy H, Dacheux L, Ribadeau-Dumas F. The use of passive rabies immunotherapy: from the past to the future. Biol Aujourdhui. 2010;204(1):71–80. doi: 10.1051/jbio/2009049
- Diagnosis WHO Comair, Research, World Health Organization, Veterinary Public Health U. Report of the Sixth WHO consultation on monoclonal antibodies in rabies[M]. The Wistar Institute, 1990 April, Philadelphia, Pennsylvania, USA. Geneva: World Health Organization; 1990. p. 2–3.
- Gongal G, Sampath G. Monoclonal antibodies for rabies post-exposure prophylaxis: A paradigm shift in passive immunization. Arch Prev Med. 2020;5(1):35–38.
- World Health Organization. Consultation on a rabies monoclonal antibody cocktail for rabies post exposure treatment. Geneva: WHO; 2002Vol. 5p. 23–24.
- World Health Organization. Rabies vaccines: WHO position paper-April 2018. Weekly Epidemiological Rec. 2018;93(16):201–220.
- World Health Organization. WHO expert consultation on rabies: third report [M]. Geneva: World Health Organization, 2018.
- O’Brien KL, Nolan T, SAGE WG on Rabies. The WHO position on rabies immunization-2018 updates. Vaccine. 2019;37(Suppl 1):A85–87. doi: 10.1016/j.vaccine.2018.10.014
- World Health Organization. Driving progress towards rabies elimination: new WHO recommendations on human rabies immunization and results of Gavi’s learning agenda on rabies and 2nd international meeting of the Pan-African Rabies Control Network (PARACON): meeting report. Johannesburg, South Africa; 2018 Sep 12–14.
- Ilina EN, Larina MV, Aliev TK, et al. Recombinant monoclonal antibodies for rabies post-exposure prophylaxis. Biochemistry (Mosc). 2018 Jan;83(1):1–12.
- Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2010;59(RR–2):1–9.
- Malerczyk C, Freuling C, Gniel D, et al. Cross-neutralization of antibodies induced by vaccination with Purified Chick Embryo Cell Vaccine (PCECV) against different Lyssavirus species. Hum Vaccin Immunother. 2014;10(10):2799–2804. doi: 10.4161/21645515.2014.972741
- Kopel E, Oren G, Sidi Y, et al. Inadequate antibody response to rabies vaccine in immunocompromised patient. Emerg Infect Dis. 2012;18(9):1493–1495. doi: 10.3201/eid1809.111833
- Katz ISS, Guedes F, Fernandes ER, et al. Immunological aspects of rabies: a literature review. Arch Virol. 2017;162(11):3251–3268. doi: 10.1007/s00705-017-3484-0
- Zhou H, Li Y, Chen RF, et al. Chinese Center for Disease Control and Prevention. [Technical guideline for human rabies prevention and control (2016)]. Zhonghua Liu Xing Bing Xue Za Zhi. 2016Feb;37(2):139–163. Chinese. doi: 10.3760/cma.j.issn.0254-6450.2016.02.001
- Lang J, Attanath P, Quiambao B, et al. Evaluation of the safety, immunogenicity, and pharmacokinetic profile of a new, highly purified, heat-treated equine rabies immunoglobulin, administered either alone or in association with a purified, Vero-cell rabies vaccine. Acta Trop. 1998;70(3):317–333. doi: 10.1016/S0001-706X(98)00038-2
- Feige K, Ehrat FB, Kastner SB, et al. Automated plasmapheresis compared with other plasma collection methods in the horse. J Vet Med Physiol Pathol Clin Med. 2003;50(4):185–189. doi: 10.1046/j.1439-0442.2003.00528.x
- Gerber F, Tetchi M, Kallo V, et al. RABIES IMMUNOGLOBULIN: Brief history and recent experiences in Côte d’Ivoire. Acta Trop. 2020;211:105629. doi: 10.1016/j.actatropica.2020.105629
- Johnson N, Cunningham AF, Fooks AR. The immune response to rabies virus infection and vaccination. Vaccine. 2010;28(23):3896–3901. doi: 10.1016/j.vaccine.2010.03.039
- Tantawichien T, Rupprecht CE. Modern biologics for rabies prophylaxis and the elimination of human cases mediated by dogs. Expert Opin Biol Ther. 2020;20(11):1347–1359. doi: 10.1080/14712598.2020.1766021
- Gogtay NJ, Munshi R, Ashwath Narayana DH, et al. Comparison of a novel human rabies monoclonal antibody to human rabies immunoglobulin for postexposure prophylaxis: a phase 2/3, randomized, single-blind, noninferiority, controlled study. Clin Infect Dis. 2018 Jan 18;66(3):387–395. doi: 10.1093/cid/cix791
- Hobart-Porter N, Stein M, Toh N, et al. Safety and efficacy of rabies immunoglobulin in pediatric patients with suspected exposure. Hum Vaccin Immunother. 2021 Jul 3;17(7):2090–2096. doi: 10.1080/21645515.2020.1854000
- State drug administration. Biological products registration classification and application data requirements[M]. 2020.
- Dietzschold B, Gore M, Casali P, et al. Biological characterization of human monoclonal antibodies to rabies virus. J Virol. 1990 Jun;64(6):3087–3090.
- Prosniak M, Faber M, Hanlon CA, et al. Development of a cocktail of recombinant-expressed human rabies virus-neutralizing monoclonal antibodies for postexposure prophylaxis of rabies. J Infect Dis. 2003 Jul 1;188(1):53–56. doi: 10.1086/375247
- Marissen WE, Kramer RA, Rice A, et al. Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis. J Virol. 2005 Apr;79(8):4672–4678.
- Aman P, Ehlin-Henriksson B, Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984;159(1):208–220. doi: 10.1084/jem.159.1.208
- Traggiai E, Becker S, Subbarao K, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004 Aug;10(8):871–875.
- Bonsignori M, Hwang KK, Chen X, et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011 Oct;85(19):9998–10009.
- Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010 Aug 13;329(5993):856–861. doi: 10.1126/science.1187659
- Wiktor TJ, Koprowski H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: detection of antigenic variants. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3938–3942. doi: 10.1073/pnas.75.8.3938
- Müller T, Dietzschold B, Ertl H, et al. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl Trop Dis. 2009 Nov 3;3(11):e542. doi: 10.1371/journal.pntd.0000542
- Morrison SL, Johnson MJ, Herzenberg LA, et al. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6851–6855.
- Both L, van Dolleweerd C, Wright E, et al. Production, characterization, and antigen specificity of recombinant 62-71-3, a candidate monoclonal antibody for rabies prophylaxis in humans. FASEB J. 2013 May;27(5):2055–2065.
- Chao TY, Ren S, Shen E, et al. SYN023, a novel humanized monoclonal antibody cocktail, for post-exposure prophylaxis of rabies. PLoS negl trop dis. 2017;11(12):e0006133. doi: 10.1371/journal.pntd.0006133
- Singh S, Kumar NK, Dwiwedi P, et al. Monoclonal antibodies: a review. Curr Clin Pharmacol. 2017;12(2):85–99. doi: 10.2174/1574884712666170809124728
- Lu RM, Hwang YC, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020 Jan 2;27(1):1. doi: 10.1186/s12929-019-0592-z
- Jun W. Practice for prevention and disposal of rabies exposure (2009 edition). China Working Dog. 2010;2:60–61.
- Bharti OK, Madhusudana SN, Wilde H. Injecting rabies immunoglobulin (RIG) into wounds only: a significant saving of lives and costly RIG. Hum Vaccin Immunother. 2017;13(4):762–765. doi: 10.1080/21645515.2016.1255834
- Bharti OK, Madhusudana SN, Gaunta PL, et al. Local infiltration of rabies immunoglobulins without systemic intramuscular administration: an alternative cost effective approach for passive immunization against rabies. Hum Vaccin Immunother. 2016;12(3):837–842. doi: 10.1080/21645515.2015.1085142
- Madhusudana SN, Ashwin BY, Sudarshan S. Feasibility of reducing rabies immunoglobulin dosage for passive immunization against rabies: results of in vitro and in vivo studies. Hum Vaccin Immunother. 2013;9(9):1914–1917. doi: 10.4161/hv.25431
- Saesow N, Chaiwatanarat T, Mitmoonpitak C, et al. Diffusion and fate of intramuscularly injected human rabies immune globulin. Acta Trop. 2000;76(3):289–292. doi: 10.1016/S0001-706X(00)00107-8
- Atanasiu P, Bahmanyar M, Baltazard M, et al. Rabies neutralizing antibody response to different schedules of serum and vaccine inoculations in non-exposed persons. II. Bull World Health Organ. 1957;17(6):911–932.
- Bookstaver PB, Akpunonu P, Nguyen HB, et al. Administration of rabies immunoglobulin: Improving evidence-based guidance for wound infiltration. Pharmacotherapy. 2021 Aug;41(8):644–648.
- Liu X, Li Y, Li J, et al. Comparing recombinant human rabies monoclonal antibody (ormutivimab) with human rabies immunoglobulin (HRIG) for postexposure prophylaxis: A phase III, randomized, double-blind, non-inferiority trial. Int J Infect Dis. 2019 May 19;134:53–62.
- de Melo GD, Hellert J, Gupta R, et al. Monoclonal antibodies against rabies: current uses in prophylaxis and in therapy. Curr Opin Virol. 2022 Apr;53:101204.
- Quiambao BP, Dy-Tioco HZ, Dizon RM, et al. Rabies post-exposure prophylaxis with purified equine rabies immunoglobulin: one-year follow-up of patients with laboratory-confirmed category III rabies exposure in the Philippines. Vaccine. 2009 Nov 27;27(51):7162–7166. doi: 10.1016/j.vaccine.2009.09.036
- Haradanhalli RS, Fotedar N, Kumari N, et al. Safety and clinical efficacy of human rabies immunoglobulin in post exposure prophylaxis for category III animal exposures. Human Vaccines & Immunotherapeutics. 2022 Jun;10(5):1–5. doi: 10.1080/21645515.2022.2081024
- Ejemel M, Smith TG, Greenberg L, et al. A cocktail of human monoclonal antibodies broadly neutralizes North American rabies virus variants as a promising candidate for rabies post-exposure prophylaxis. Sci Rep. 2022 Jun 7;12(1):9403. doi: 10.1038/s41598-022-13527-0
- World Health Organization. The Selection and Use of Essential Medicines (2021) -TRS 1035. WHO Technical Report Series. 2022 Jan;1035:499–506.
- Fan L, Zhang L, Li J, et al. Advances in the progress of monoclonal antibodies for rabies. Hum Vaccin Immunother. 2022 Dec 31;18(1):2026713. doi: 10.1080/21645515.2022.2026713
- Anwith HS, Ravish HS, Ashwathnarayana DH. Safety of new indigenous human Rabies Monoclonal Antibody (RMAb) for post exposure prophylaxis corresponding author citation article cycle. Indian J Community Health. 2018;30(3):196–201. doi: 10.47203/IJCH.2018.v30i03.004
