Glucagon-like peptide-1 (GLP-1) receptor agonists have been increasingly utilized over the last years for the treatment of type 2 diabetes mellitus (T2DM) [Citation1], while, according to the most recent guidelines by the American Diabetes Association and the European Association for the Study of Diabetes, their use should be prioritized for subjects with concomitant atherosclerotic cardiovascular disease (CVD), or multiple cardiovascular risk factors (such as age ≥55 years, obesity, hypertension, smoking, dyslipidaemia or albuminuria) without established CVD, and for subjects with underlying non-dialysis dependent chronic kidney disease (CKD), not eligible for a sodium-glucose co-transporter-2 (SGLT-2) inhibitor [Citation2].
GLP-1 receptor agonists provide substantial benefits in glycemic control and body weight loss while they improve health-related quality of life among individuals with T2DM; in addition, GLP-1 receptor agonists have been shown to significantly decrease the risk for all-cause and cardiovascular death in T2DM, also producing a significant reduction in the risk for non-fatal myocardial infarction and non-fatal stroke [Citation3]. However, they do not affect heart failure-related outcomes [Citation3]. In addition, their administration might lead to mild gastrointestinal adverse events, mainly nausea, vomiting and diarrhea, which are mostly mild and transient [Citation4]. No association of their use with severe hypoglycemia, diabetic ketoacidosis, pancreatitis, pancreatic cancer, or medullary thyroid cancer has been found [Citation4]. Of note, initial concerns regarding their association with increased risk for cholelithiasis have been recently confirmed since their use was, indeed, linked to a significant increase in the risk for gallbladder and biliary diseases, especially when GLP-1 receptor agonists are administered at higher doses and for longer duration [Citation5].
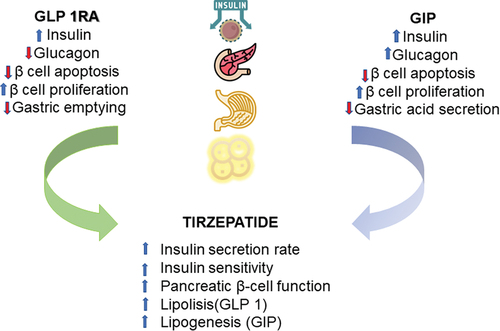
Tirzepatide (LY3298176) is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist, recently developed and finally approved in May 2022 by the Food and Drug Administration for the treatment of T2DM as an adjunct to diet and exercise [Citation6]. In subjects with T2DM, dual GIP and GLP-1 receptor agonism with tirzepatide, compared to single GLP-1 receptor agonism with semaglutide, produced significantly more significant improvements in total insulin secretion rate and insulin sensitivity, reflecting a significant improvement in pancreatic β-cell function [Citation7]. Similar effects were also documented in another mechanistic trial comparing tirzepatide with dulaglutide, another GLP-1 receptor agonist, suggesting that dual receptor agonism might indeed be responsible for improving insulin sensitivity, especially since the observed effect was shown to be only partially attributable to weight loss [Citation8]. Furthermore, the effect of tirzepatide on delayed gastric emptying is comparable to that induced by single GLP-1 receptor agonism, according to a recent experimental study in subjects with T2DM [Citation9]. Recent evidence further suggests a potential role of dual GIP/GLP1 RA in the treatment of several components of the metabolic syndrome, including obesity, diabetes as well as nonalcoholic fatty liver disease [Citation10]. The combined effect of GLP1 RA and GIP is shown in .
The question that inevitably arises is whether tirzepatide is more efficacious and, of course, if it is equally safe to GLP-1 receptor agonists. Based on the actions mentioned above, tirzepatide has greater glycemic efficacy than GLP-1 receptor agonists and might produce more significant weight loss. However, at the same time, gastrointestinal side effects rates should be similar to that observed with GLP-1 receptor agonists. According to a formerly published, comprehensive meta-analysis of seven trials with tirzepatide in a total of 6609 subjects with T2DM [Citation11], tirzepatide at all doses (5 mg, 10 mg and 15 mg) has significantly increased the odds for nausea and vomiting compared with placebo; at the same time, tirzepatide 15 mg also significantly increased the odds of diarrhea compared with a placebo. However, when tirzepatide was compared with GLP-1 receptor agonists, it was not associated with a significant increase in the odds of nausea, vomiting or diarrhea, except for tirzepatide 10 mg, which correlated with a significant increase of 51% in the odds for diarrhea, compared to GLP-1 receptor agonist treatment [Citation11].
In addition, the same researchers found that tirzepatide use was associated with a non-significant effect on the odds of hypoglycemia compared with placebo and a significant decrease in the odds of hypoglycemia compared with basal insulin [Citation11]. Of note, the use of tirzepatide in subjects with T2DM did not significantly impact the incidence of any serious adverse event compared with placebo, basal insulin or GLP-1 receptor agonists; moreover, only the highest dose of tirzepatide at 15 mg was associated with a significant increase in the odds of treatment discontinuation compared to the abovementioned drugs [Citation11]. Therefore, tirzepatide is equally safe with GLP-1 receptor agonists and possibly more efficacious regarding glycemic control and body weight loss.
Over the last decade, a number of novel antidiabetic drug classes, mainly incretin-based drugs, SGLT-2 inhibitors and nonsteroidal mineralocorticoid receptor agonists, have been shown to exert significant cardiovascular benefits for subjects with T2DM [Citation12–14]. Based on its mechanisms of action and its clinical efficacy, compared with other treatment options, especially GLP-1 receptor agonists, it appears reasonable that tirzepatide will be similarly efficacious with this drug class in terms of cardio-protection. In their pre-specified cardiovascular event risk assessment meta-analysis, Sattar and colleagues [Citation15] pooled data from seven trials in a total of 7215 subjects with T2DM, randomized either to tirzepatide or placebo or an active comparator, showing that tirzepatide use was safe, at least at cardiovascular level. Indeed, tirzepatide use was associated with a non-significant decrease in the risk for four-component major adverse cardiovascular events (MACE-4; cardiovascular death, myocardial infarction, stroke and hospitalized unstable angina) and all-cause death [Citation15]. In addition, the effect of tirzepatide on the risk for MACE-4 was not amended by baseline characteristics of interest, namely sex, age category, baseline glycated hemoglobin levels, race, country of origin and SGLT-2 inhibitor baseline use [Citation15]. The ongoing cardiovascular outcome trial SURPASS-CVOT (NCT04255433) is eagerly awaited in order to answer whether tirzepatide also displays cardiovascular efficacy, besides cardiovascular safety.
1. Expert opinion
Tirzepatide is a very promising agent, since it exerts a number of favorable effects on most components of the ‘cardiometabolic continuum,’ and thus, it is likely to play a crucial role in the treatment of T2DM and related co-morbidities within the following years, in the era of evidence-based medicine with a patient-centered approach. Current evidence suggests that tirzepatide might be more efficacious than GLP-1 receptor agonists in terms of improvement in glycemia, body weight control, amelioration of insulin sensitivity and improvement of β-cell function. In addition, it seems that tirzepatide is at least equally safe as GLP-1 receptor agonists, not increasing the odds for serious adverse events. Whether tirzepatide exerts cardio-protective effects similar to that observed with GLP-1 receptor agonists, it remains to be answered by the ongoing CVOT, whose results will possibly answer the dilemma ‘Which one to choose? Tirzepatide or GLP-1 receptor agonist?.’ Of course, cost-effectiveness analyses are required, as well, in order to directly place tirzepatide in the treatment algorithms of T2DM and related co-morbidities. Yet, it has to be highlighted that the global SURPASS-CVOT is a study where tirzepatide is compared to dulaglutide on major cardiovascular events in patients with T2D and increased CV risk, and therefore assessing the noninferiority and superiority of tirzepatide against an agent within the class of GLP-1 receptor agonists. Since dulaglutide has a confirmed cardio-protective effect, this head-to-head study will be particularly informative and useful for clinicians dealing with T2DM. In conclusion, it appears that tirzepatide represents an additional excellent and safe agent in the treatment against the growing T2DM pandemic.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution statement
The authors contributed equally to the writing and revision of the present editorial.
Additional information
Funding
References
- Nauck MA, Quast DR, Wefers J, et al. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102.
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetologia. 2022;65(12):1925–1966. doi: 10.1007/s00125-022-05787-2
- Rizzo M, Nauck MA, Mantzoros CS. Incretin-based therapies in 2021 - Current status and perspectives for the future. Metabolism. 2021;122:154843. doi: 10.1016/j.metabol.2021.154843
- Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diab Obes Metab. 2017;19(9):1233–1241. doi: 10.1111/dom.12926
- He L, Wang J, Ping F, et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2022;182(5):513–519. doi: 10.1001/jamainternmed.2022.0338
- Rizvi AA, Rizzo M. The emerging role of dual GLP-1 and GIP receptor agonists in glycemic Management and cardiovascular risk reduction. Diabetes Metab Syndr Obes. 2022;15:1023–1030. doi: 10.2147/DMSO.S351982
- Heise T, Mari A, DeVries JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomized, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10(6):418–429. doi: 10.1016/S2213-8587(22)00085-7
- Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(2):388–396. doi: 10.1210/clinem/dgaa863
- Urva S, Coskun T, Loghin C, et al. The novel dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long-acting GLP-1 receptor agonists. Diab Obes Metab. 2020;22(10):1886–1891. doi: 10.1111/dom.14110
- Muzurović EM, Volčanšek Š, Tomšić KZ, et al. Glucagon-like peptide-1 receptor agonists and dual glucose-dependent insulinotropic polypeptide/Glucagon-like peptide-1 receptor agonists in the treatment of obesity/metabolic syndrome, prediabetes/diabetes and non-alcoholic fatty liver disease-current evidence. J Cardiovasc Pharmacol Ther. 2022;27:10742484221146371. doi: 10.1177/10742484221146371
- Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia. 2022;65(8):1251–1261. doi: 10.1007/s00125-022-05715-4
- Rizvi AA, Linhart A, Vrablik M, et al. Safety and benefit of incretin-based therapies in patients with type 2 diabetes: learnings and reflections. Expert Opin Drug Saf. 2022;21(3):291–293. doi: 10.1080/14740338.2022.2043848
- Patoulias D, Fragakis N, Rizzo M. The therapeutic role of SGLT-2 inhibitors in acute heart failure: from pathophysiologic mechanisms to clinical evidence with pooled analysis of relevant studies across safety and efficacy endpoints of interest. Life. 2022;12(12):2062. doi: 10.3390/life12122062
- Marzolla V, Infante M, Armani A, et al. Efficacy and safety of finerenone for treatment of diabetic kidney disease: current knowledge and future perspective. Expert Opin Drug Saf. 2022;21(9):1161–1170. doi: 10.1080/14740338.2022.2130889
- Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28(3):591–598. doi: 10.1038/s41591-022-01707-4