ABSTRACT
Background
Four CGRP Monoclonal Antibodies (mAbs) have been approved for migraine prophylaxis by the Food and Drug Administration (FDA) since 2018. However, there are concerns about the safety of these four drugs for real-world use.
Objective
To compare the adverse event profiles of four CGRP-mAbs with FAERS data.
Methods
The study was based on records from the FAERS database. Only reports containing one of the active ingredients with CGRP-mAbs were included in this study. Disproportionality analyses including but not limited to reporting odds ratio (ROR) and information components (IC) were conducted to identify drug-AE associations.
Results
In total, 58110 reports were identified for CGRP-mAbs. 80 overlapping signals were disproportionately reported. They affected a range of organs and systems, including the gastrointestinal and cardiovascular systems, skin, and hair. Additionally, the rare cardiovascular adverse events were significantly different among the four CGRP-mAbs.
Conclusion
We identified numerous shared underlying signals (overlapping signals) for CGRP-mAbs as suspected drugs in multiple systems and organs. The unlabeled common signals may indicate potential safety issues. In addition, the underlying safety signals varied among the four CGRP-mAbs, particularly in the cardiovascular system, and further studies are needed to confirm these associations and the potential clinical implications.
1. Introduction
Migraine is a prevalent neurobiological headache disorder characterized by excessive excitability of the central nervous system (CNS) [Citation1]. It ranks among the most incapacitating diseases in the world [Citation2]. Preventive treatment reduces the frequency of migraines and enhances quality of life for patient [Citation1]. Since the development of monoclonal antibodies (mAbs) targeting calcitonin-generated peptide (CGRP) or the CGRP receptor (collectively referred to as CGRP mAbs), the landscape of migraine prevention has changed significantly. This new medicine has been recommended as a first-line agent for migraine prevention by European Headache Federation (EHF) guideline [Citation3]. There are four CGRP-mAbs currently approved by the Food and Drug Administration (FDA) for the prevention of migraine. The only CGRP receptor monoclonal antibody is erenumab, which is a fully human IgG2 monoclonal antibody, while the other three are humanized [Citation4]. Eptinezumab, fremanezumab, and galcanezumab are all CGRP ligand monoclonal antibodies. Except for eptinezumab, which is given intravenously, the other two are the same as erenumab, and are given by subcutaneous injection [Citation4].
CGRP-mAbs have been shown to be well tolerated, effective and safe in almost all clinical trials [Citation5,Citation6]. As the results of the main randomized controlled trials show that CGRP-mAbs have an excellent safety profile, the question that needs to be answered is whether adverse events (AEs) with CGRP-mAbs manifest differently in real-world use. For example, a recent study found that constipation emerged as a significant AE of monoclonal antibodies targeting the CGRP system in real-world post-approval surveys, and the impact of this AE may have been underestimated [Citation7]. This is in contrast to the results of clinical trials, which showed no potential for adverse gastrointestinal effects. With regard to the much-discussed cardiovascular safety, there have also been case reports of Raynaud’s phenomenon and other serious cardiovascular events [Citation8–11]. Although we have not been able to establish a causal relationship between medication use and cardiovascular events, current studies suggest that we should be vigilant for potential cardiovascular AEs.
A recent network meta-analysis provided valuable insights into the safety profiles of four different medicines, as evaluated through randomized controlled trials (RCTs) [Citation12]. Surprisingly, the analysis revealed no significant differences among these medications in terms of safety. To gain a more comprehensive understanding of potential variations in real-world safety, a thorough review was conducted. This comprehensive review encompassed an analysis of common AEs observed in real-world trials involving CGRP-mAb, as well as an examination of rare AE case reports [Citation13]. Furthermore, a notable real-life multicenter study emerged, shedding light on the comparative safety of fremanezumab. This retrospective study focused on a cohort of 162 patients who received erenumab, galcanezumab, and fremanezumab [Citation14].
To further investigate and compare the real-world safety profiles of these drugs, our study meticulously analyzed the AEs associated with the four CGRP-mAbs in actual clinical practice. One of the strengths of this investigation lies in its utilization of the extensive sample size provided by the Food and Drug Administration Adverse Event Reporting System (FAERS) database, reinforcing the robustness of the findings with real-world data.
2. Methods
2.1. Database source
In this retrospective pharmacovigilance study, FAERS database was utilized for assessing AE reports of CGRP-mAbs. FAERS (or FDA AERS) is designed for post-marketing safety surveillance. Specifically, FAERS is a computerized database for the spontaneous reporting of AEs and medication errors involving human drugs and therapeutic biological products [Citation15]. FAERS receives millions of AE reports from consumers, healthcare professionals and product manufacturers around the world, with the majority of reports coming from the United States [Citation15]. This database contains key AE information based on the Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT). PT is a distinct descriptor (single medical concept) for a symptom, sign, disease diagnosis, therapeutic indication, investigation, surgical or medical procedure, and medical social or family history characteristic [Citation16]. A report may contain several PTs that are independent of each other. According to the MedDRA dictionary, each AE is automatically classified into an AE category in the FAERS database. A report is considered serious if the patient has one or more of the following outcomes: death, life-threatening illness, hospitalization or prolongation of existing hospitalization, permanent disability, or birth defect [Citation17].
2.2. Study procedure
We queried FAERS data using a highly interactive web-based tool called the FAERS public dashboard [Citation15]. We identified AE reports related to CGRP-mAbs with four active ingredients, specifically Erenumab-Aooe, Fremanezumab-Vfrm, Galcanezumab-Gnlm, and Eptinezumab-Jjmr. All eligible reports in the FAERS database from 2018 quarter 1 to 2022 quarter 3 were exported. A filter was used to include only reports in which the ‘suspect product active ingredients’ causing the AE was exclusively Erenumab-Aooe, Fremanezumab-Vfrm, Galcanezumab-Gnlm or Eptinezumab-Jjmr. That is, reports were excluded if the ‘suspect product active ingredients’ contained two or more agents. AEs categorized as product issues and AEs related to off-label use were excluded from the analysis.
3. Statistical analysis
3.1. Descriptive analyses
In order to capture the clinical and demographic traits of the study population, we categorized all FAERS reports that only involved CGRP-mAbs as the suspected drug, grouping them by specific drugs. We also examined the year distribution and adverse event frequency distribution of reports from the Eudravigilance database to assess the representativeness of the FAERS data.
3.2. Data mining (disproportionality analyses)
We used disproportionality analysis to determine the relationship between the drug of interest and AEs. If the reporting frequency of CGRP-mAbs is high compared to the average reporting frequency for other medications in the FAERS database, this indicates that there is a statistically significant association between the drug and the AE [Citation18]. Taking into account both sensitivity and specificity, two established pharmacovigilance indices were used to measure the disproportionality in AE reporting for CGRP-mAbs in this study [Citation19]. These two indices are reporting odds ratio (ROR) and information components (IC), which belong to frequentist and Bayesian statistical approaches, respectively. In addition, we applied statistical shrinkage (shrinkage parameter = 0.5) to calculate IC [Citation20]. The analysis framework is based on a contingency table of 2 × 2, which can be found in Supplementary Table S1. The formulas for the disproportionality measures are given in Supplementary Table S2. A potential signal occurred when the lower limits of the 95% confidence intervals of the ROR (ROR025) were greater than 1 and the lower limits of the 95% confidence intervals of the IC (IC025) were greater than 0 in at least three records [Citation21]. All analyses were performed using R software (version 4.1.2) and Microsoft EXCEL 2019.
4. Results
4.1. Descriptive results
After the treatment of CGRP-mAbs, we discovered 58,110 reports in total, the features of which are shown in . Erenumab received the highest number of prescriptions (n = 34328; 59.07%), followed by galcanezumab (n = 17614; 30.31%). Among all records for which this demographic information was available, the mean patient age was 48.98 years, and there were more female than male patients (85.98% vs. 14.02%). The majority of reports came from the United States. Serious reports accounted for 12.43% of all associated records and the proportion of deaths was 0.53%. Fremanezumab was associated with the highest percentage of hospitalizations or required interventions. Four different CGRP mAbs had approximately the same proportion of deaths.
Table 1. Characteristics of reports submitted to FAERS for CGRP-mAbs from January 2018 to September 2022.
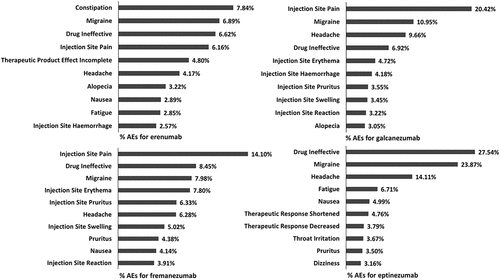
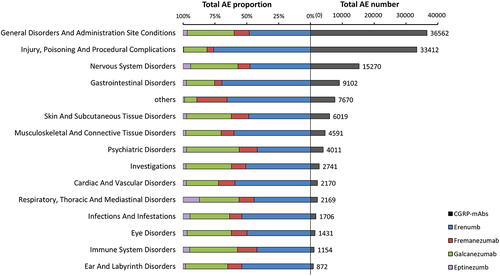
For all CGRP-mAbs, 128880 AEs from 58,110 reports were considered. The most frequently reported AE categories were ‘General disorders and administration site conditions’ (n = 36562; 28.37%), ‘injury, poisoning, and procedural complications’ (n = 33412; 25.92%), and ‘nervous system disorders’ (n = 15270; 11.85%). Erenumab-related AEs had the highest percentage of all the categories. For eptinezumab AEs, the category ‘Respiratory, Thoracic and Mediastinal Disorders’ had the highest percentage (). At the level of adverse events, constipation for erenumab, injection site pain for fremanezumab and galcanezumab, and drug ineffectiveness for eptinezumab were the most frequently reported AEs for individual CGRP-mAbs (). We found that injection/infusion site reactions (such as injection site pain, injection site erythema) were frequently reported for all four CGRP-mAbs. In addition, the gastrointestinal signal of nausea was ranked in the top 10 of AEs for three drugs. The hair signals, alopecia, ranked in the top 10 for two drugs.
Figure 1. The distribution of AE amounts in CGRP-mAbs among several AE categories is shown on the right side of the figure, and the proportion of four medications falling under each of these AE categories is shown on the left side of the figure. Others include the following reactions groups (based on MedDRA): metabolism and nutrition disorders; reproductive system and breast disorders; surgical and medical procedures; renal and urinary disorders; social circumstances; neoplasms benign, malignant and unspecified; blood and lymphatic system disorders; pregnancy, puerperium and perinatal conditions; endocrine disorders; hepatobiliary disorders; congenital, familial and genetic disorders.
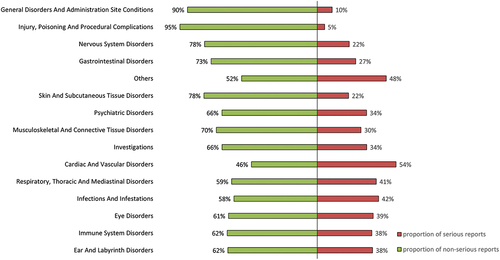
The results for severity of reports were summarized in . Most categories had less than 50% of serious reports, with only ‘Cardiac and Vascular disorders’ having 54% of serious reports.
Compared to the Eudravigilance database, we observed similar year-wise distribution of adverse event (AE) reports for the four drugs in both databases. Specifically, Erenumab showed notable similarities (Supplementary Figure S4). Additionally, the frequency distribution of AE reports across different systems in both databases displayed remarkable similarity (Supplementary Figure S5). Despite variations in report counts, AE distribution within different systems exhibited significant similarity.
4.2. Disproportionality Analyses Results
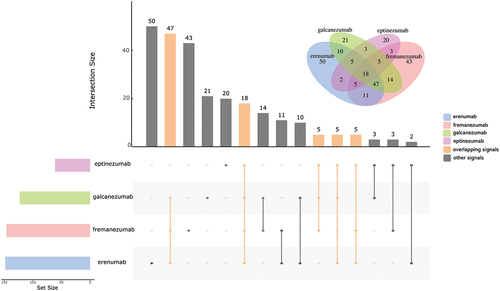
AEs associated with CGRP-mAbs were investigated for each medication. In this research, signals shared by three or more CGRP-mAbs were referred to be overlapping signals. 80 overlapping signals were owned by CGRP-mAbs (). summarizes the results of the disproportionality analysis about these overlapping signals. We discovered that there were a variety of overlapping signals across 12 different categories, with ‘General disorders and administration site conditions’ appearing the most frequently (due to the high number of injection site reactions). Following were ‘Gastrointestinal Disorders,’ ‘Nervous system disorders,’ ‘Psychiatric disorders’ and ‘Skin and Subcutaneous Tissue Disorders.’ Constipation, nausea, and diarrhea were the three AEs reported disproportionately for all four CGRP-mAbs under ‘Gastrointestinal Disorders.’ Erenumab’s signal for constipation (ROR0.25/IC0.25 = 11.48/3.47) was the strongest among the CGRP mAbs. Among the AEs listed under ‘Skin and Subcutaneous Tissue Disorders,’ pruritus, rash pruritic, and urticaria were associated with allergy symptoms, while alopecia was the sole AE that was reported disproportionately by all CGRP-mAbs. Significantly, the two signals of hypersensitivity and anaphylactic reaction were labeled as warnings under ‘Immune System Disorders,’ with 100% of serious reports for anaphylactic reaction but only 26% for hypersensitivity (Supplementary Figure S3). In the category of ‘Cardiac and Vascular disorders,’ two signals were found in erenumab, fremanezumab and galcanezumab. Raynaud's phenomenon, one of the two signals, was very strong for the three medications.
Table 2. CGRP-mAbs overlapping signals.
Further disproportionality findings for each anti-CGRP mAb, excluding the overlapping signals mentioned above, were reported in Supplementary Table S3–6. According to the results, erenumab involved a total of 73 signals, fremanezumab 71, galcanezumab 48, and eptinezumab 28. We found that, compared to the other three CGRP-mAbs, the erenumab had more unique signals in ‘Gastrointestinal disorders,’ and eptinezumab had more unique signals in ‘Respiratory, Thoracic and Mediastinal Disorders’ (Supplementary Figure S1-2). We noticed that the unique signals of eptinezumab under ‘Respiratory, Thoracic and Mediastinal Disorders’ were related to symptoms of upper respiratory tract infection. Furthermore, these signals were strong, as shown by the following: nasal congestion (ROR025/IC025 = 10.67/3.11), oropharyngeal pain (ROR025/IC025 = 4.30/1.88), rhinorrhea (ROR025/IC025 = 4.62/1.94), sneezing (ROR025/IC025 = 9.10/2.57), throat irritation (ROR025/IC025 = 23.35/4.15). Moreover, we also found rare signals of ‘Cardiac and Vascular disorders,’ including coronary artery dissection(n = 5) and postural orthostatic tachycardia syndrome(n = 13) for erenumab; ventricular extrasystoles (n = 5), supraventricular tachycardia (n = 5), and peripheral coldness (n = 6) for fremanezumab. The percentage of severe reports for the both adverse events, palpitations, and Raynaud’s phenomenon, were less than 50%, but for all other cardiovascular unusual signals – with the exception of postural orthostatic tachycardia syndrome (53%), this rate soared to almost 100%.
We noticed that overlapping signals covered most of the labeled adverse drug reactions, as below: Constipation, muscle twitching and alopecia for erenumab; urticaria and pruritus for fremanezumab and galcanezumab; anaphylactic reaction and hypersensitivity for fremanezumab, galcanezumab, and eptinezumab; injection reaction (e.g. injection site erythema) for erenumab, fremanezumab and galcanezumab. Furthermore, the labeled AE hypertension, rash and nasopharyngitis in unique signals were reported disproportionately. Angioedema did not meet the criteria for signal detection, despite being listed on the drug label. Although we found a sizable number of unexpected AEs (not listed in the US package insert) for CGRP-mAbs, almost all of them were infrequent.
5. Discussion
In this extensive real-world study, we conducted a comprehensive analysis utilizing FAERS data to examine four FDA-approved CGRP-mAbs. Additionally, comparative analysis with the Eudravigilance database supported the strong representativeness of the FAERS data. Our aim was to identify similarities and differences in the risk of adverse events associated with these medications. While previous retrospective analyses have compared these four CGRP-mAbs, our study offers a more comprehensive and representative perspective.
Overall, three key conclusions were revealed: 1) The numerous overlapping signals indicate a common characteristic of adverse reactions in CGRP-mAbs, which affect a variety of organs and systems, including the gastrointestinal system, cardiovascular system, nervous system, respiratory System, immune system, skin, muscle and Hair. 2) Rare cardiovascular adverse events varied significantly among the four CGRP-mAbs; furthermore, eptinezumab had the narrowest toxicity profile with several unique respiratory signals, while erenumab had the most gastrointestinal events. 3) We detected numerous unlabeled adverse events, but the majority of these signals were reported at low frequencies.
Although they differ in terms of their target (CGRP or CGRP receptor), route of administration (intravenous or subcutaneous), and degree of humanization, the four CGRP-mAbs have similar pharmacological processes [Citation22].
5.1. Gastrointestinal disorders
We already know that CGRP plays a role in a number of physiological gastrointestinal processes, including motility, secretion and immune function [Citation23]. Therefore, it makes sense that abnormal CGRP activity could lead to impaired motility and disruption of the mucosal barrier [Citation24]. Gastrointestinal reactions are considered to be one of the most common side effects of CGRP mAbs [Citation7]. We found that three gastrointestinal signals – constipation, diarrhea and nausea – were shared by four CGRP mAbs. Constipation was most commonly reported with erenumab, while nausea was most commonly reported with the others. These gastrointestinal AEs have been widely reported in post-marketing studies [Citation25,Citation26]. Although gastrointestinal reactions are not labeled with the other three CGRP mAbs except erenumab, we believe they should not be ignored. In addition, the highest constipation signal was seen with erenumab, and this study also showed numerous unique gastrointestinal AEs to erenumab, some of which were related to gastrointestinal motility disorders, such as gastrointestinal motility disorder, impaired gastric emptying, gastrointestinal obstruction. This seems to indicate that gastrointestinal reactions are more severe when erenumab is used.
5.2. Cardiovascular disorders
CGRP is found in nerve fibers innervating the heart and blood vessels [Citation27]and plays a role in blood pressure regulation [Citation28,Citation29]. It has also been implicated in tissue remodeling in pulmonary hypertension [Citation30]and in maintaining (cardio)vascular homeostasis during ischemic episodes [Citation31]. Therefore, the potential adverse cardiovascular effects of CGRP-mAbs have always been of great concern. In this study, we found one labeled disproportionate signal (hypertension), two overlapping signals (palpitations and Raynaud’s phenomenon), and several signals of rare AEs. Regarding Raynaud’s phenomenon, several case reports have been published [Citation10]. The AE of peripheral coldness has been implicated in Raynaud’s phenomenon [Citation32]. Reduced CGRP activity has been shown to contribute to the pathophysiology of Raynaud’s phenomenon, and CGRP infusions have been used to treat the condition [Citation33]. Our findings on Raynaud’s phenomenon are consistent with a published pharmacovigilance study [Citation32]. The European Headache Federation (EHF) guideline provides cautionary dosing tips for patients with a history of Raynaud’s phenomenon [Citation3]. In addition, palpitations have been documented in some post-marketing studies [Citation34]. However, the mechanism responsible for the palpitations is still unclear.
In this study, eptinezumab had essentially no cardiovascular signals, but galcanezumab had only two overlapping signals, and erenumab and fremanezumab each had some signals of rare AEs. Although there are fewer reports of cardiovascular adverse events and disproportionate signals with eptinezumab, this may be due to fewer prescriptions being written. We found that ventricular extrasystoles (n = 5), supraventricular tachycardia (n = 5), and peripheral coolness (n = 6) were the rare AEs for fremanezumab. Except for peripheral coolness, ventricular extrasystoles and supraventricular tachycardia are the rare AEs associated with cardiac function. A recent review article on fremanezumab lists the cardiovascular events that occurred in phase II-III clinical trials, including one case of new-onset atrial fibrillation [Citation35]. Moreover, the FOCUS study also published AEs of atrial fibrillation (n = 1), tachycardia (n = 2), and bradycardia (n = 1) in clinical application with fremanezumab [Citation34]. We also found that the AEs of coronary artery dissection(n = 5) and postural orthostatic tachycardia syndrome(n = 13) were reported disproportionately for erenumab. As far as we know, there have been no reports of either of these AEs in studies of erenumab. Despite there have been case reports of major cardiovascular events (such as myocardial infarction and stroke) in the past [Citation8,Citation11], we did not find any disproportionate reports of these events in our study. Our results suggest that although cardiovascular events are rare with the use of CGRP-mAbs, they should still be a priority safety issue, especially in patients at high cardiovascular risk.
5.3. Immune system disorders and associated signals
CGRP mAbs are designed to bind to either the CGRP peptide or receptor with high specificity and thus have minimal interaction with the immune system [Citation36]. his is consistent with what we observed in our study. The number of immune system AEs was 1154, which was only 0.9% of the total number of AEs. However, as with all therapeutic biologics, mAbs may be recognized as foreign, resulting in an immune response and the formation of anti-drug antibodies (ADAs), the latter of which may cause adverse events such as administration reactions (i.e. infusion or injection site reactions) and hypersensitivity reactions [Citation37]. We identified two overlapping signals, anaphylactic reaction and hypersensitivity, as well as a large number of injection/infusion site reactions that may be associated with ADAs. Anaphylactic reaction and hypersensitivity are labeled as boxed warnings. The mechanisms of adverse events such as pruritus, urticaria are similar to the injection site reactions described above and these events have been identified as disproportionate signals under ‘Skin and Subcutaneous Tissue Disorders.’ Pruritus, urticaria, and injection site reactions are common adverse events reported in postmarketing studies [Citation13]and described in the US package insert.
5.4. Psychiatric and neurological signals
Abnormal dreams, anxiety, depression, insomnia, and panic attack were five disproportionate signals for CGRP-mAbs under psychiatric disorders observed in our study. CGRP and its receptor are expressed in the anterior pituitary outside the blood-brain barrier, which is an area accessible to CGRP-mAbs [Citation38]. This suggests a possible involvement of CGRP in the regulation of hypothalamic-pituitary function [Citation39]. Alteration of hypothalamic-pituitary function has been implicated in the pathophysiological mechanisms of major depressive disorder [Citation40]. However, it should be emphasized that psychiatric comorbidity in migraine cannot be excluded as a confounding factor.
Vertigo and dizziness, as two of the overlapping signals in our study, have been previously described and pathophysiological mechanisms have been provided elsewhere [Citation41–43]. We observed that four disproportionality signals for CGRP-mAbs were related to feeling abnormal, separately, they are feeling abnormal, paresthesia, paresthesia oral and hypoaesthesia. migraine is associated with temporomandibular disorders, the possible pathophysiological mechanisms of which are hyperexcitability with the involvement of CGRP in the trigeminal distribution [Citation44]. It is reasonable to speculate that antagonism of CGRP biological effects can cause feeling abnormal.
5.5. Respiratory system and infection signals
Current physiological studies of CGRP indicate that CGRP and its receptors are found throughout the respiratory tract [Citation45–47]. Although the role of CGRP in the respiratory system is unclear, there is strong evidence that elevated CGRP plays a protective role in the lung [Citation48–50]. Therefore, the use of CGRP mAbs may have a potential adverse effect on the respiratory system. In a recent review of real-world studies of CGRP-mAbs, the AE of flu-like symptoms was found to be one of the most common unlabeled AEs [Citation13]. Flu-like symptoms, pharyngeal swelling, and Throat Tightness were identified as overlapping signals in this study. We also identified respiratory signals unique to eptinezumab such as nasal congestion, oropharyngeal pain, rhinorrhea, sneezing and throat irritation. These signals were associated with upper respiratory tract infections.
5.6. Musculoskeletal and hair signals
A known adverse event of erenumab is muscle spasms [Citation51], which may explain several relevant signals found in the study. These overlapping signals were blepharospasm, muscle twitching, arthralgia, myalgia, neck pain.
We found two overlapping signals related to hair, one is alopecia, which is a labeled AE for erenumab, another is an unlabeled AE trichorrhexis. Both can be explained by previous studies showing that in alopecia areata, CGRP is reduced in the vicinity of nerve fibers, multiple layers of the epidermis and hair follicles [Citation39]. Furthermore, these patients were found to have lower serum plasma concentrations of CGRP [Citation40]. Consistent with our findings, alopecia was reported as a safety signal in a recent pharmacovigilance study [Citation52].
6. Strengths and limitations
The inclusion of a large number of CGRP-mAb reports was a major strength of our study, providing the first comprehensive view of postmarketing AEs to our knowledge. However, our study also has limitations. On the one hand, shortcomings inherent in a spontaneous reporting system must be considered, such as reporting bias (over- or underreporting), incomplete reporting, variable reporting quality, and lack of denominator data. On the other hand, limitations of a disproportionality analysis that cannot be ignored include limited detailed demographic data, exposure duration, risk factors, and comorbidities. Therefore, disproportionality analyses do not infer a causal relationship between reported AEs and the use of CGRP mAbs, but they do reveal potential signals that may require further investigation.
7. Conclusion
We identified numerous shared underlying signals (overlapping signals) for CGRP-mAbs as a suspected drug in multiple systems and organs. The unlabeled common signals may indicate potential safety issues. In addition, the underlying safety signals varied among the four CGRP-mAbs, particularly in the cardiovascular system, and further studies are needed to confirm these associations and the potential clinical implications.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer is a PI for RCT and observational studies on erenumab, galcanezumab, fremanezumab and eptinezumab. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
supplemental materials.docx
Download MS Word (1.1 MB)supplemental study.docx
Download MS Word (16.9 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2023.2250720
Additional information
Funding
References
- Silberstein SD. Migraine. Lancet. 2004;363(9406):381–391. doi: 10.1016/S0140-6736(04)15440-8
- Steiner TJ, Stovner LJ, Jensen R, et al. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi: 10.1186/s10194-020-01208-0
- Sacco S, Amin FM, Ashina M, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention – 2022 update. J Headache Pain. 2022;23(1):67. doi: 10.1186/s10194-022-01431-x
- Szkutnik-Fiedler D. Pharmacokinetics, pharmacodynamics and drug–drug interactions of new anti-migraine drugs—lasmiditan, gepants, and calcitonin-gene-related peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics. 2020;12(12):1180. doi: 10.3390/pharmaceutics12121180
- González-Hernández A, Condés-Lara M, García-Boll E, et al. An outlook on the trigeminovascular mechanisms of action and side effects concerns of some potential neuropeptidergic antimigraine therapies. Expert Opin Drug Metab Toxicol. 2021;17(2):179–199. doi: 10.1080/17425255.2021.1856366
- Messina R, Huessler EM, Puledda F, et al. Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: a systematic review and network meta-analysis. Cephalalgia. 2023;43(3):3331024231152169. doi: 10.1177/03331024231152169
- Holzer P, Holzer-Petsche U. Constipation caused by anti-calcitonin gene-related peptide migraine therapeutics explained by antagonism of calcitonin gene-related peptide’s motor-stimulating and prosecretory function in the intestine. Front Physiol. 2021;12:820006. doi: 10.3389/fphys.2021.820006
- Aradi S, Kaiser E, Cucchiara B. Ischemic stroke associated with calcitonin gene-related peptide inhibitor therapy for migraine: a case report. J Stroke Cerebrovasc Dis. 2019;28(10):104286. doi: 10.1016/j.jstrokecerebrovasdis.2019.07.002
- Evans RW. Raynaud’s phenomenon associated with calcitonin gene-related peptide monoclonal antibody antagonists. Headache. 2019;59(8):1360–1364. doi: 10.1111/head.13596
- Manickam AH, Buture A, Tomkins E, et al. Raynaud’s phenomenon secondary to erenumab in a patient with chronic migraine. Clin Case Rep. 2021;9(8):e04625. doi: 10.1002/ccr3.4625
- Perino J, Corand V, Laurent E, et al. Myocardial infarction associated with erenumab: a case report. Pharmacotherapy. 2022;42(7):585–589. doi: 10.1002/phar.2706
- Wang X, Wen D, He Q, et al. Efficacy and safety of monoclonal antibody against calcitonin gene-related peptide or its receptor for migraine patients with prior preventive treatment failure: a network meta-analysis. J Headache Pain. 2022;23(1):105. doi: 10.1186/s10194-022-01472-2
- Pavelic AR, Wöber C, Riederer F, et al. Monoclonal antibodies against calcitonin gene-related peptide for migraine prophylaxis: a systematic review of real-world data. Cells. 2022;12(1):143. doi: 10.3390/cells12010143
- Muñoz-Vendrell A, Campoy S, Caronna E, et al. Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: a real-life multicentre analysis of 162 patients. J Headache Pain. 2023;24(1):63. doi: 10.1186/s10194-023-01585-2
- Questions and Answers on FDA’s Adverse Event Reporting System (FAERS). Available from: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers.
- MedDRA Hierarchy. Available from: https://www.meddra.org/how-to-use/basics/hierarchy.
- Dores GM, Bryant-Genevier M, Perez-Vilar S. Adverse events associated with the use of Sipuleucel-T reported to the US Food and drug administration’s adverse event reporting system, 2010-2017. JAMA Netw Open. 2019;2(8):e199249. doi: 10.1001/jamanetworkopen.2019.9249
- Noguchi Y, Tachi T, Teramachi H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform. 2021;22(6). doi: 10.1093/bib/bbab347
- Hauben M, Patadia VK, Goldsmith D. What counts in data mining? Drug Saf. 2006;29(10):827–832. doi: 10.2165/00002018-200629100-00001
- Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69. doi: 10.1177/0962280211403604
- van Puijenbroek EP, Bate A, Leufkens HGM, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10. doi: 10.1002/pds.668
- de Vries T, Villalón CM, MaassenVandenbrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther. 2020;211:107528. doi: 10.1016/j.pharmthera.2020.107528
- Ma WJ, Yin Y-C, Zhang B-K, et al. Calcitonin gene‑related peptide‑mediated pharmacological effects in cardiovascular and gastrointestinal diseases (review). Mol Med Rep. 2021;23(1):1–1. doi: 10.3892/mmr.2020.11665
- Evangelista S. Role of calcitonin gene-related peptide in gastric mucosal defence and healing. Curr Pharm Des. 2009;15(30):3571–3576. doi: 10.2174/138161209789207024
- Sakai F, Suzuki N, Kim BK, et al. Efficacy and safety of fremanezumab for episodic migraine prevention: multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache. 2021;61(7):1102–1111. doi: 10.1111/head.14178
- Silberstein S, Diamond M, Hindiyeh NA, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (prevention of migraine via intravenous ALD403 safety and efficacy–2) study. J Headache Pain. 2020;21(1):120. doi: 10.1186/s10194-020-01186-3
- Uddman R, Edvinsson L, Ekblad E, et al. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. 1986;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6
- Lindstedt IH, Edvinsson ML, Edvinsson L. Reduced responsiveness of cutaneous microcirculation in essential hypertension–a pilot study. Blood Press. 2006;15(5):275–280. doi: 10.1080/08037050600996586
- Russell FA, King R, Smillie S-J, et al. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–1142. doi: 10.1152/physrev.00034.2013
- Keith IM, Tjen-A-Looi S, Kraiczi H, et al. Three-week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2000;279(4):H1571–8. doi: 10.1152/ajpheart.2000.279.4.H1571
- MaassenVandenbrink A, Meijer J, Villalón CM, et al. Wiping Out CGRP: Potential cardiovascular Risks. Trends Pharmacol Sci. 2016;37(9):779–788. doi: 10.1016/j.tips.2016.06.002
- Gérard AO, Merino D, Van Obberghen EK, et al. Calcitonin gene-related peptide-targeting drugs and Raynaud’s phenomenon: a real-world potential safety signal from the WHO pharmacovigilance database. J Headache Pain. 2022;23(1):53. doi: 10.1186/s10194-022-01424-w
- Bunker CB, Reavley C, Dowd PM, et al. Calcitonin gene-related peptide in treatment of severe peripheral vascular insufficiency in Raynaud’s phenomenon. Lancet. 1993;342(8863):80–83. doi: 10.1016/0140-6736(93)91286-U
- Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–1040. doi: 10.1016/S0140-6736(19)31946-4
- Robblee J, VanderPluym J. Fremanezumab in the treatment of migraines: evidence to date. J Pain Res. 2019;12:2589–2595. doi: 10.2147/JPR.S166427
- Levin M, Silberstein SD, Gilbert R, et al. Basic Considerations for the use of monoclonal antibodies in migraine. Headache. 2018;58(10):1689–1696. doi: 10.1111/head.13439
- Silberstein S, Lenz R, Xu C. Therapeutic monoclonal antibodies: What Headache Specialists Need to know. Headache. 2015;55(8):1171–1182. doi: 10.1111/head.12642
- Eftekhari S, Salvatore CA, Johansson S, et al. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood–brain barrier. Brain Res. 2015;1600:93–109. doi: 10.1016/j.brainres.2014.11.031
- Wimalawansa SJ, el-Kholy AA. Comparative study of distribution and biochemical characterization of brain calcitonin gene-related peptide receptors in five different species. Neuroscience. 1993;54(2):513–519. doi: 10.1016/0306-4522(93)90270-P
- Kennis M, Gerritsen L, van Dalen M, et al. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25(2):321–338. doi: 10.1038/s41380-019-0585-z
- Hou M, Xing H, Cai Y, et al. The effect and safety of monoclonal antibodies to calcitonin gene-related peptide and its receptor on migraine: a systematic review and meta-analysis. J Headache Pain. 2017;18(1):42. doi: 10.1186/s10194-017-0750-1
- Kong WJ, Scholtz AW, Kammen-Jolly K, et al. Ultrastructural evaluation of calcitonin gene-related peptide immunoreactivity in the human cochlea and vestibular endorgans. Eur J Neurosci. 2002;15(3):487–497. doi: 10.1046/j.0953-816x.2001.01880.x
- Marco RA, Hoffman LF, Wackym PA, et al. Distribution of calcitonin gene-related peptide immunoreactivity in vestibular efferent neurons of the chinchilla. Hear Res. 1996;97(1–2):95–101. doi: 10.1016/S0378-5955(96)80011-6
- Cady RJ, Glenn JR, Smith KM, et al. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain. 2011;7:94. doi: 10.1186/1744-8069-7-94
- Springer J, Geppetti P, Fischer A, et al. Calcitonin gene-related peptide as inflammatory mediator. Pulm Pharmacol Ther. 2003;16(3):121–130. doi: 10.1016/S1094-5539(03)00049-X
- Sui P, Wiesner DL, Xu J, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360(6393). doi: 10.1126/science.aan8546
- Voisin T, Bouvier A, Chiu IM. Neuro-immune interactions in allergic diseases: novel targets for therapeutics. Int Immunol. 2017;29(6):247–261. doi: 10.1093/intimm/dxx040
- Champion HC, Bivalacqua TJ, Toyoda K, et al. In vivo gene Transfer of Prepro-calcitonin gene–related peptide to the Lung Attenuates chronic Hypoxia-Induced pulmonary hypertension in the Mouse. Circulation. 2000;101(8):923–930. doi: 10.1161/01.CIR.101.8.923
- Tjen ALS, Ekman R, Lippton H, et al. CGRP and somatostatin modulate chronic hypoxic pulmonary hypertension. Am J Physiol. 1992;263(3):H681–90. doi: 10.1152/ajpheart.1992.263.3.H681
- Xie W, Fisher JT, Lynch TJ, et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121(8):3144–3158. doi: 10.1172/JCI41857
- Breen ID, Mangold AR, VanderPluym JH. The evolving understanding of risk with calcitonin gene-related peptide monoclonal antibodies based on real-world data: a focus on hypertension and Raynaud phenomenon. Headache. 2021;61(8):1274–1276. doi: 10.1111/head.14198
- Woods RH. Alopecia signals associated with calcitonin gene-related peptide inhibitors in the treatment or prophylaxis of migraine: a pharmacovigilance study. Pharmacotherapy. 2022;42(10):758–767. doi: 10.1002/phar.2725