ABSTRACT
Background
The study aimed to evaluate the agreement of prescribed drug dosages with renal dosing recommendations and describe adverse drug events (ADEs) contributing to hospital admissions of patients with chronic kidney disease (CKD).
Methods
This cross-sectional study focused on CKD patients admitted to University Hospital Hradec Králové, with an estimated glomerular filtration rate below 60 ml/min. The necessity for renal dosage adjustments was determined using the Summary of Product Characteristics (SmPC). For medications requiring renal dosage adjustment according to SmPC, agreement between the prescribed and recommended renal dosage was assessed. ADEs were adjudicated using the OPERAM drug-related hospital admissions adjudication guide.
Results
Of 375 CKD patients, 112 (30%, 95% CI 25–34) were prescribed drug dosages in disagreement with SmPC renal dosage recommendations. Perindopril, metformin, and ramipril were most frequently dosed in disagreement with SmPC. ADE-related hospital admissions occurred in 20% (95% CI 16–24) of CKD patients.
Conclusion
CKD patients are often prescribed medication dosages in disagreement with SmPC renal dosing recommendations. Besides explicit factors, treatment goals, feasibility of monitoring and alternative treatment must be weighed when assessing drug and dosage appropriateness. Gastrointestinal bleeding was the most frequent ADE that contributed to hospital admissions of CKD patients.
1. Introduction
Chronic kidney disease (CKD) affects around 11% to 13% of the population, with a higher prevalence among the older age groups [Citation1]. Polypharmacy and hyperpolypharmacy are common in patients with CKD, with a high use of cardiovascular medications due to a higher risk of cardiovascular events and a greater prevalence of cardiovascular comorbidities in this population [Citation2,Citation3]. Individuals with CKD are vulnerable to adverse drug events (ADEs), as many medications or their metabolites are excreted by the kidneys. Consequently, several medications require dosage adjustment, avoidance, or cautious use in patients with CKD. In addition, various medications can cause deterioration of renal function or increase the risk of other ADEs (e.g. hyperkalemia, hypoglycemia) and should be avoided or prescribed with caution in patients with CKD. ADEs related to impaired renal function have been reported to contribute to 10% −32% of hospital admissions [Citation4,Citation5].
Although inappropriate prescribing in patients with CKD, particularly the lack of dose adjustment with respect to renal function, is a well-documented issue in the literature [Citation6–10], there is a paucity of recent studies in this crucial field [Citation11]. Studies conducted earlier have emphasized the inappropriate prescribing of older medications such as ranitidine [Citation12–19], sulfonylureas [Citation12,Citation13,Citation16,Citation18,Citation20–22], enalapril [Citation6,Citation15,Citation19,Citation22] or atenolol [Citation19,Citation22–25].
However, clinical practice has evolved since then, and several newer medications (DOACs, SGLT2 inhibitors, DPP-4 inhibitors, and gabapentinoids) have entered clinical practice. Surprisingly, there is a lack of recent studies reporting which medications are prescribed inappropriately the most frequently in patients with CKD. Furthermore, previous studies were often limited to a specific group of medications, such as antibiotics, or limited to particular patient populations (such as those admitted to a single hospital ward).
The main objectives of this study were to evaluate the necessity of renal drug dosage adjustments or avoidance according to SmPC, the agreement between prescribed drug dosages and the renal drug dosage adjustments recommended by the SmPC and the prescription of contraindicated medications in patients with CKD admitted to the hospital. The study also aimed to describe ADE-related hospital admissions in patients with CKD and to identify those ADE-related hospital admissions that could be attributed to inappropriate renal drug dosages or inappropriate drug use in CKD patients.
2. Patients and methods
2.1. Design, data source and setting
This study represents a sub-study of our previous study focused on drug-related hospital admissions, which has been described earlier [Citation26]. This was a retrospective observational cross-sectional study that used electronic medical records as a data source. The setting of the study was University Hospital Hradec Králové (a teaching hospital in the Czech Republic that admits approximately 450 patients per month).
2.2. Eligibility criteria
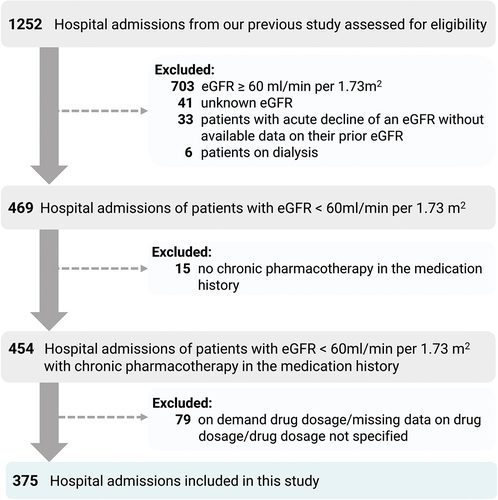
We have included patients admitted to the hospital through the emergency department in August-November 2018 with an estimated glomerular filtration rate (eGFR) below 60 ml/min per 1.73 m2. We have excluded patients with acute conditions that could potentially affect glomerular filtration rate without information on previous eGFR, patients on dialysis, patients without any chronic medications listed in the medication history and patients without information on medication dosages. In this study, a total of 375 hospital admissions were included. In the article, hospital admissions are referred to as patients for better readability. The flow chart can be found in . The information on the eGFR values was extracted from the hospital information system that used the CKD-EPI creatinine equation.
2.3. Evaluation
In the first step, the medication history of patients was screened in order to identify the necessity for renal drug dosage adjustments or avoidance in CKD patients, according to SmPC. We labeled these medications as renal risk medications (medications for which renal dosage adjustment or avoidance is recommended). Medications that required cautious use were not included due to ambiguous recommendations in the SmPC.
Renal risk medications were categorized into medications for which renal dosage adjustment is recommended and medications that are contraindicated under certain eGFR values.
For the medications for which the SmPC recommends renal dosage adjustment, we checked the agreement between the prescribed drug dosage and the renal dosing adjustment recommendation of the SmPC. The prescribed drug dosage was in agreement or disagreement with the SmPC renal dosing recommendation with respect to the patient’s renal function. The identified drug dosages in disagreement with the renal dosing recommendation of the SmPC were compared with the renal dosing adjustments recommended by Lexicomp [Citation27], Micromedex [Citation28], British National Formulary 84 [Citation29] and the Renal Drug Handbook [Citation30].
ADEs that contributed to hospital admissions were adjudicated using the OPERAM drug-related admission adjudication guide [Citation31]. The term ADE was defined as harm due to an adverse drug reaction or a medication error related to overuse, underuse, or misuse of prescription and non-prescription medications. The ADE adjudication was performed by three board-certified clinical pharmacists. Causality of ADEs was evaluated using WHO-UMC criteria [Citation31,Citation32] and the contribution of ADEs to hospital admission was evaluated using Hallas criteria [Citation33]. The methodology is described in our previous study on drug-related hospital admissions [Citation26]. In this study, only ADEs related to treatment safety were included. Hospital admissions due to medication nonadherence or omission of indicated medications were not considered ADE-related in this study.
2.4. Outcomes
The prevalence of patients with renal risk medication was calculated as the number of patients prescribed at least one renal risk medication () divided by the total number of patients.
Table 1. Categories of renal risk medications.
The prevalence of patients with medication for which renal dosage adjustment is necessary was calculated as the number of patients prescribed at least one medication for which renal dosage adjustment is necessary according to SmPC divided by the total number of patients.
The prevalence of patients with medication dosed in disagreement with SmPC renal dosage recommendation was calculated as the number of patients prescribed at least one medication dosed in disagreement with SmPC renal dosage recommendation divided by the total number of patients.
The prevalence of patients with contraindicated medication was calculated as the number of patients prescribed at least one contraindicated medication with respect to eGFR according to SmPC divided by the total number of patients.
The prevalence of patients with ADE-related hospital admission was calculated as the number of patients with ADE as the main or contributory reason for hospital admission divided by the total number of patients.
We reported the number of renal risk medications (and each subcategory) and the most frequent medications that represented renal risk medications (and each subcategory). Medications were classified by Anatomical Therapeutic Chemical (ATC) classification.
We have also provided the list of ADEs that were the main or contributory reason for hospital admissions of CKD patients. ADEs were classified according to MedDRA terminology.
2.5. Statistical analysis
The characteristics of the study sample were summarized as both numbers and percentages for categorical variables and the median and interquartile range (IQR) for continuous variables. The prevalences of main outcomes were reported as percentages with 95% confidence interval (CI).
To explore the associations between ADE-related hospital admission and patient characteristics, logistic regression analysis was employed. Initially, univariable logistic regression was used to screen for variables for multivariable logistic regression. The results of the univariable logistic regression were presented as crude odds ratio (OR) and corresponding p-value. Subsequently, multivariable logistic regression was conducted to eliminate potential confounding factors. The results of the multivariable logistic regression were presented as adjusted OR with 95% CI and corresponding p-value. P-values lower than 0.05 were considered statistically significant.
Statistical analyses were performed in Microsoft Excel and IBM SPSS Statistics for Windows, Version 27.0 (Armonk, NY: IBM Corp)
3. Results
3.1. Sample characteristics
The demographic and clinical characteristics of the study sample (n = 375) can be found in . The mean age of the patients was 79 (SD = 11), the mean Charlson comorbidity index was six (SD = 2), the mean number of medications listed in the medication history was eight (SD = 4) and the mean eGFR was 41 ml/min (SD = 12).
Table 2. Demographic and clinical characteristics of included patients.
3.2. Renal risk medications
Of the 3007 medications analyzed, 436 (14.5%) represented renal risk medications. The prevalence of patients with at least one renal risk medication was 63% (95% CI 58–68). For the flow chart showing the number of patients with renal risk medications, see .
Figure 2. The number of patients with renal risk medications.
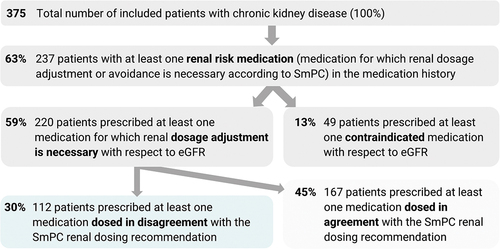
The prevalence of patients prescribed at least one medication for which dosage adjustment is necessary was 59% (95% CI 54–64), and the prevalence of patients prescribed at least one contraindicated medication was 13% (95% CI 10–16).
A total of 367 medications for which dosage adjustments are necessary, according to SmPC, were prescribed in 220 patients with CKD. The most frequently prescribed medications with the necessity for renal dosage adjustments included perindopril, ramipril, metformin, amiloride, spironolactone, pregabalin, gabapentin, tiapride, fenofibrate, rosuvastatin, digoxin, dabigatran etexilate, rivaroxaban, allopurinol. Regarding ATC medication groups, these medications represented agents acting on the renin-angiotensin system (C09, 30%), diuretics (C03, 15%), drugs used in diabetes (A10, 12%), antiepileptics (N03, 7%) and lipid modifying agents (C10, 6%) and antithrombotic agents (B01, 6%)
Overall, 112 (30%, 95% CI 25–34) patients were prescribed at least one medication dosed in disagreement with the SmPC renal dosage recommendation. All medications with disagreement between the prescribed dosage and SmPC renal dosing recommendations are shown in . Regarding ATC medication groups, these medications most frequently represented agents acting on the renin-angiotensin system (C09, 49%), drugs used in diabetes (A10, 10%) and lipid modifying agents (C10, 9%).
Table 3. The most common medications dosed in disagreement with the SmPC renal dosage recommendations.
A total of 49 (13%) patients were prescribed at least one contraindicated medication, according to SmPC. Prescribed medications that should have been avoided with respect to patient renal function included low-dose acetylsalicylic acid, hydrochlorothiazide, rosuvastatin, fenofibrate, metformin, melperone, chlorthalidone, glimepiride, dabigatran etexilate, gliclazide, ibandronic acid, nitrofurantoin, alendronic acid, alfuzosin, eplerenone, naproxen and nimesulide.
Out of 367 medications for which renal dosage adjustment is necessary, 37% of the medication dosages were found to be in disagreement with the SmPC renal dosage recommendations, while 63% were in agreement with the SmPC renal dosage recommendations. For the flow chart, see .
Figure 3. The number of renal risk medications and various subgroups.
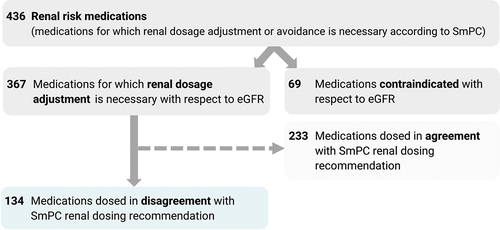
For the medications used more than once, the relatively highest disagreement with the SmPC renal dosing recommendations was found in levocetirizine and cetirizine (the dosages of these medications were never in agreement with the SmPC renal dosing recommendations), followed by perindopril (92%), fenofibrate (91%), trimetazidine (83%), sitagliptin (75%), solifenacin (67%), and trospium (67%). The lowest rate of disagreement with SmPC was observed for pregabalin, gabapentin, tiapride, ramipril and apixaban.
The subgroup analysis can be found in Supplementary Table S1, Supplementary Table S2, Supplementary Figure S1, Supplementary Table S3 and Supplementary Figure S2. The lowest prevalence of medications for which renal dosage adjustment is necessary, according to SmPC, was found among patients with CKD stage G4. In contrast, 97% of the contraindicated medications were contraindicated in patients with CKD stage G4, with only two cases of desmopressin contraindicated in CKD stage G3B.
3.3. Adverse drug events
Supplementary Table S4 shows the overview of ADEs that were the main reason for or contributed to hospital admissions of CKD patients. These ADEs most frequently concerned Gastrointestinal disorders (Gastroduodenal hemorrhage), Metabolism and nutrition disorders (Hypoglycemia, Hyperkalemia, Hyponatremia, Dehydration) and Blood and lymphatic system disorders (Microcytic anemia, Bone marrow toxicity). The prevalence of ADE-related admissions was 20% (95% CI 16–24). The medication classes most frequently involved in ADEs included Antithrombotic agents (B01). Antithrombotic agents (antiplatelets, anticoagulants) and nonsteroidal anti-inflammatory drugs (NSAIDS) were involved in Gastroduodenal hemorrhage, Intestinal hemorrhage and Microcytic anemia.
Out of 75 ADE-related hospital admissions, only four were associated with inappropriate renal dosages. Supplementary Table S5 shows the medications involved in ADE-related hospital admissions associated with inappropriate renal dosages included dabigatran etexilate, naproxen, perindopril and tiapride.
3.4. Risk factors of adverse drug events
Logistic regression analysis showed no association between the prescription of medications dosed in disagreement with the renal dosing recommendation of the SmPC and ADE-related hospital admission. This finding aligns with our observation that the majority of ADE-related hospital admissions in CKD patients were not attributed to inappropriate renal dosing of medications. The results of the univariable logistic regression and multivariable logistic regression can be found in Supplementary Table S6.
3.5. Comparison with other drug information sources
shows the comparison with the recommendations of other drug information sources regarding eGFR thresholds that require drug dosage adjustments.
Table 4. Comparison of drug information sources – eGFR thresholds that require dosage adjustment.
Specific renal dosing recommendations from other drug information sources can be found in Supplementary Table S7. Supplementary Table S8 provides an overview of the medications listed in explicit criteria of potentially inappropriate medications (PIMs) in older patients – American Geriatrics Society Beers Criteria ®2023 [Citation34], STOPP 2023 [Citation35], GheOP3S [Citation36] and EU(7)PIM list [Citation37].
For perindopril, other drug information sources suggested mostly only initial dosage adjustments. Based on the consensus of SmPC with other drug information sources, the prevalence of inappropriate dosage adjustment would be 18% (excluding patients with renal dosage disagreement only for perindopril).
4. Discussion
4.1. Comparison with the literature
In our study, 63% of the included CKD patients were prescribed at least one medication for which renal dosage adjustment or avoidance is necessary, and 30% were prescribed at least one medication dosed in disagreement with the SmPC renal dosing recommendation. The prevalence of patients with at least one medication dosed in disagreement with renal dosing recommendations varies widely in the literature, ranging from 13% [Citation21] to 84.3% [Citation38]. Several factors can influence the prevalence, such as the subset of medications analyzed, the drug information source used to guide renal dosing, the equation used to estimate glomerular filtration rate (GFR) and the characteristics of the included patients (e.g. hospital ward, threshold of eGFR value, their age, comorbidities or medications used).
Apart from the drug information sources used in our study, some studies used national guidelines [Citation7,Citation23] or Beers criteria [Citation39]. The SmPC was our primary focus as it is the most readily available source of drug information for drug dosing guidance. The formula most commonly used to estimate GFR in previous studies was the Cockcroft-Gault [Citation13,Citation14,Citation19–21,Citation23,Citation38,Citation40–46], followed by MDRD [Citation7,Citation25,Citation47,Citation48]. Only a few studies used CKD-EPI or multiple formulas [Citation8,Citation9,Citation21,Citation39,Citation49]. The proportions of patients with at least one inappropriate dosage might vary depending on the equation used to estimate renal function, with the CKD-EPI equation yielding higher proportions.
Frequently prescribed medications for which renal dosage adjustment is necessary according to SmPC included cardiovascular medications (ACE inhibitors), antidiabetics (metformin), lipid modifying agents (fenofibrate, rosuvastatin) and anticoagulants (dabigatran, rivaroxaban). The indications of these medications correspond to the most frequent comorbidities in our studied sample (arterial hypertension, dyslipidemia, diabetes mellitus, coronary artery disease and atrial fibrillation)
Disagreement with the SmPC’s renal dosage recommendations was present in 37% of the medication dosages for which renal dosage adjustment is recommended by SmPC. Several medications that were most frequently dosed in disagreement with the SmPC renal dosing recommendations in our study were consistent with those identified in previous studies – perindopril [Citation8,Citation20,Citation40,Citation48,Citation50,Citation51], fenofibrate [Citation20,Citation22,Citation38,Citation48,Citation51–53], metformin [Citation6,Citation7,Citation13,Citation22,Citation23,Citation42,Citation48,Citation52,Citation54–59], ramipril [Citation8,Citation22,Citation40,Citation47,Citation48,Citation60] and spironolactone [Citation38,Citation42,Citation43,Citation47,Citation48,Citation51,Citation54,Citation61].
Our findings are consistent with a systematic review [Citation62] that reported that cardiovascular-acting medications (mainly ACE inhibitors) and antidiabetics are frequently prescribed in disagreement with renal dosing guidelines. The overview of medications dosed in disagreement with renal dosing guidelines in other studies can be found in Supplementary Table S9. The overview of contraindicated medications identified in other studies can be found in Supplementary Table S10. Unlike our study, previous studies frequently identified ranitidine [Citation12–19,Citation63], atenolol [Citation19,Citation22–25,Citation42] and enalapril [Citation6,Citation13,Citation15,Citation19,Citation22]. However, these older medications were not prescribed so frequently in our sample. Previous studies often reported that allopurinol [Citation6–8,Citation13,Citation15,Citation18,Citation21,Citation22,Citation38,Citation40,Citation43,Citation50,Citation53,Citation54,Citation64] was dosed in disagreement with renal dosing recommendations, which was not the case in our study. In our sample, most patients were prescribed allopurinol at a dose of 100 mg daily. In addition, in some of the previous studies [Citation13,Citation21,Citation53] different dosing recommendations were used to assess the appropriateness of drug dosing.
Levocetirizine and cetirizine were always dosed in disagreement with the renal dosage recommendations of SmPC, consistent with the findings of a study from the Netherlands [Citation7]. Agreement with the renal dosage recommendations was observed more frequently for medications with narrow therapeutic windows, such as digoxin and DOACs, and for medications with potentially serious adverse effects, such as lactic acidosis (metformin). In contrast, medications with wide therapeutic windows, such as levocetirizine and cetirizine, were not adjusted as frequently.
The most clinically relevant medications with inappropriate drug dosages would be DOACs, metformin, and digoxin. Apart from the therapeutic index, the proportion of non-renal elimination (apixaban > rivaroxaban > dabigatran etexilate) influences the clinical relevance of inappropriate renal dosage adjustments.
Furthermore, the necessity for dosage adjustment will depend on the feasibility of the monitoring. The potential adverse effects of perindopril, the medication prescribed most frequently in disagreement with the SmPC renal dosing guideline, could be monitored through parameters such as blood pressure, potassium levels, and renal function.
In CKD patients, there is an increased risk of various ADEs (such as hyperkalemia, hypoglycemia, acute kidney injury, and bleeding). Therefore, apart from dose adjustments for medications with renal excretion, it is important to exercise caution or avoid medications that increase the risks of these complications. Within the group of contraindicated medications, dabigatran etexilate, metformin, sulfonylureas, and NSAIDs have the greatest clinical significance.
The contraindicated medications we identified in our study align with those found in earlier studies. These medications include metformin [Citation7,Citation16,Citation21,Citation22,Citation24,Citation40–42,Citation51–53,Citation59,Citation65,Citation66], alendronic acid [Citation6,Citation7,Citation16,Citation20–22,Citation51,Citation52], hydrochlorothiazide [Citation6,Citation7,Citation13,Citation21,Citation40,Citation60], nitrofurantoin [Citation6,Citation7,Citation12,Citation21,Citation22,Citation53], rosuvastatin [Citation22,Citation41], fenofibrate [Citation22], low-dose acetylsalicylic acid [Citation60,Citation67], glimepiride [Citation22], eplerenone [Citation7], naproxen [Citation22] and dabigatran etexilate [Citation52] Unlike previous studies [Citation12,Citation13,Citation16,Citation18,Citation20–22], we have not observed any contraindicated use of glibenclamide, as it was never prescribed in our sample. On the contrary, we have identified the contraindicated use of nimesulide, which has been withdrawn from the market in several other countries. Interestingly, we did not encounter any ADEs linked to the use of contraindicated medication with respect to eGFR. However, our scope of identification was limited to recognizing only severe ADEs that were present at the hospital admissions of patients with CKD.
Understanding the rationale behind the contraindication is crucial. For metformin and dabigatran, avoidance stems from an elevated risk of ADEs, while for hydrochlorothiazide and chlorthalidone, reduced efficacy is the primary concern. Upon consulting other sources of information, it becomes evident that their recommendations are not uniform. Although the SmPC contraindicates the use of hydrochlorothiazide and chlorthalidone in patients with severe renal impairment (creatinine clearance <30 ml/min), Lexicomp [Citation27] states that although the diuretic effect decreases, the antihypertensive effect may be preserved. Likewise, SmPC contraindicates the use of rosuvastatin in patients with severe renal impairment (creatinine clearance <30 ml/min), which conflicts with other drug information sources [Citation27,Citation28,Citation30,Citation68], which recommend only adjusting the dosage. Similarly, while SmPC contraindicates the use of low-dose acetylsalicylic acid in patients with severe renal impairment, Lexicomp [Citation27] suggests that the benefits of low-dose acetylsalicylic acid outweigh the risks, making it a suitable option as an antiplatelet agent despite severe kidney impairment.
In addition, it is important to note that medical knowledge is constantly evolving, and new clinical trials influence the recommendations for adjusting renal drug dosages and implementing avoidance strategies. For instance, trials investigating the cardiorenal benefits of SGLT-2 inhibitors have broadened their inclusion criteria to encompass patients in more advanced CKD stages [Citation69]. This expansion has consequently resulted in a shift in the eGFR threshold that requires avoidance.
Few studies address ADEs due to inappropriate renal drug dosing in CKD patients. In a Swedish study [Citation5] involving 22 patients with adverse drug reactions leading to acute hospital admissions, 32% were linked to reduced renal function. Specifically, tramadol caused multiple adverse drug reactions due to the decline in renal function that affected its elimination [Citation5]. In a Dutch study [Citation4] involving 714 ADE-related admissions, 10% were related to a medication error and impaired renal function. Among 46 patients (6.4%), inappropriate drug dosage adjustment was assessed as related to the reason for admission. The most common medications with non-guideline dosing included ACE inhibitors, diuretics, metformin and digoxin. Our study found ADE-related admissions associated with inappropriate renal dosing for dabigatran etexilate, naproxen, perindopril and tiapride. These disparities emphasize the need for further research on ADEs arising from inappropriate renal dosage adjustments.
4.2. Strengths
This study has several strengths. Firstly, electronic medical records were used as a data source, enabling the evaluation of recommended dosages with respect to patient clinical details. This approach allowed consideration of factors such as indication, body weight, and age, which can influence dosage recommendations (e.g. for apixaban), which would not be possible when using administrative databases as a data source.
Secondly, although this was a single-center study, the patient population and the medications evaluated in this study were relatively representative. Unlike studies that evaluate only a subset of medications [Citation7,Citation12,Citation13,Citation18–20,Citation24,Citation52–54,Citation65,Citation66,Citation70] or focus on a single hospital ward, this study applied few explicit criteria with respect to patients and no exclusion criteria with respect to medications.
Thirdly, this study compared its findings with recommendations from other drug information sources, providing valuable insight into discrepancies in dosage recommendations between different sources.
Lastly, we have also identified ADEs that contributed to hospital admissions of CKD patients and ADEs that were associated with inappropriate renal dosages in patients with CKD.
4.3. Limitations
This study is subject to several limitations. Due to cross-sectional design, our study is subject to several biases – e.g. Neyman bias (prevalence-incidence bias). A late look at those exposed to inappropriate drug prescribing (during hospital admission) will miss fatal or mild cases of adverse drug events. A cohort study looking at the incidence of ADEs would minimize this type of bias.
The data collection was retrospective, which precluded the possibility of conducting patient or physician interviews. Furthermore, no medication reconciliation was performed, which means that information on over-the-counter medication use or medication adherence was not precise. It remains unknown whether inappropriate dosages were intentional or overlooked.
Furthermore, in the medical history of the included patients with an eGFR below 60 ml/min per 1.73 m2, chronic kidney disease was recorded only in 27% of the cases. As a result, we lack information on physicians’ awareness of the renal function in these patients. In a few cases, long-term eGFR values were not available in the electronic health records. Consequently, there is a possibility that we have overestimated the number of patients with CKD. Nevertheless, it is essential to note that we have excluded patients with acute conditions that could potentially affect glomerular filtration rate and for whom prior eGFR values were unavailable.
Secondly, the study lacked a gold-standard methodology, hampering direct comparisons with other research. A systematic review [Citation11] highlighted that the prevalence varies due to the variety of methodological approaches. Our findings rely on SmPC recommendations and are only secondarily compared with other drug information sources (Lexicomp, Micromedex, Renal Drug Handbook, British National Formulary). We opted for the SmPC as our primary reference due to its legally binding nature and the limited utilization of alternative drug information sources by prescribing physicians in the Czech Republic. Consequently, some medications without explicit renal dosage adjustment or avoidance stated in the SmPC might have been missed. For instance, while Micromedex [Citation28] and Lexicomp [Citation27] recommended a specific dose reduction of tramadol when eGFR drops below 30 ml/min, the SmPC for tramadol (instant-release tablet) recommended only considering prolongation of dosage intervals. Another potential variation in drug dosage recommendations may occur if there are differences in the SmPC of medicinal products either between countries or among different medicinal products.
Third limitation concerns the estimation of the GFR. Estimating the GFR based on serum creatinine may not be accurate in specific patient groups, such as those with cachexia. Reduced muscle mass in these patients can yield normal serum creatinine levels, potentially underestimating GFR. Even when estimating GFR using cystatin C, there are several nonGFR determinants of serum cystatin C, such as malignancy and thyroid status of the patients [Citation71,Citation72]. Additionally, for patients with substantial deviations from a body surface area (BSA) of 1.73 m2, eGFR automatically calculated using CKD-EPI should be adjusted for the BSA calculated from actual weight and height [Citation73]. Furthermore, several recommendations from SmPC for renal drug dosing were derived using the Cockcroft-Gault equation, which differs from the method used to estimate GFR in our study.
Lastly, we did not have all the necessary information to assess implicit inappropriateness (the frequency of monitoring, including monitoring of drug levels, electrolytes or self-monitoring) or the tolerance of alternative therapy.
5. Conclusion
Almost two-thirds of included CKD patients admitted to the hospital were prescribed medications for which renal dosage adjustment or avoidance is recommended by the SmPC. These included clinically relevant medications such as metformin, dabigatran etexilate, digoxin, rivaroxaban, apixaban, pregabalin, gabapentin, and baclofen.
Almost one-third of the included patients were prescribed at least one medication dosed in disagreement with the renal dosage adjustments recommended by SmPC. Common examples were perindopril, metformin, ramipril, and fenofibrate. It is important to note that assessing implicit inappropriateness of drug dosages should encompass considerations beyond explicit guidelines, such as treatment goals, feasibility of monitoring or alternative treatment.
Discrepancies exist between manufacturer-provided dosage recommendations (SmPC) and other drug information sources (Lexicomp, Micromedex, Renal Drug Handbook, British National Formulary). Clinical pharmacists could help bridge the gap between drug dosing recommendations found in drug information sources and the practical implementation of medication regimens for CKD patients.
The prevalence of hospital admissions related to adverse drug events in included CKD patients was 20%, with only a small proportion attributed to inappropriate renal dosage adjustments.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
ZO conceived and designed the study. ZO created a Microsoft Access database for data analysis. JP and ZO evaluated the appropriateness of drug dosages and performed data analysis. ZO, MM, and JV were involved in the clinical adjudication of adverse drug events. ZO and JP performed a review of the literature. ZO, MM, and JV were involved in the interpretation of data. JV supervised the study and critically revised the manuscript for important intellectual content. ZO drafted the manuscript, and all coauthors contributed to and approved the final manuscript.
Ethics statement
THE study was approved by the Ethics Committee of the University Hospital Hradec Králové and the Ethics Committee of the Faculty of Pharmacy in Hradec Králové. Written informed consent for participation was not required for this study due to the retrospective design.
Supplementary Material - revised.docx
Download MS Word (282.3 KB)Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14740338.2023.2295980
Additional information
Funding
References
- Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi: 10.1371/journal.pone.0158765
- Hayward S, Hole B, Denholm R, et al. International prescribing patterns and polypharmacy in older people with advanced chronic kidney disease: results from the European quality study. Nephrol Dial Transplant. 2021 Feb 20;36(3):503–511. doi: 10.1093/ndt/gfaa064
- Offurum A, Wagner LA, Gooden T. Adverse safety events in patients with chronic kidney disease (CKD). Expert Opin Drug Saf. 2016 Dec;15(12):1597–1607. doi: 10.1080/14740338.2016.1236909
- Leendertse AJ, van Dijk EA, De Smet PA, et al. Contribution of renal impairment to potentially preventable medication-related hospital admissions. Ann Pharmacother. 2012 May;46(5):625–33.
- Helldén A, Bergman U, von Euler M, et al. Adverse drug reactions and impaired renal function in elderly patients admitted to the emergency department: a retrospective study. Drugs Aging. 2009;26(7):595–606. doi: 10.2165/11315790-000000000-00000
- van Dijk EA, Drabbe NR, Kruijtbosch M, et al. Drug dosage adjustments according to renal function at hospital discharge. Ann Pharmacother. 2006 Jul;40(7–8):1254–60. doi: 10.1345/aph.1G742
- Drenth-van Maanen AC, van Marum RJ, Jansen PA, et al. Adherence with dosing guideline in patients with impaired renal function at hospital discharge. PLoS One. 2015;10(6):e0128237. doi: 10.1371/journal.pone.0128237
- Laville SM, Metzger M, Stengel B, et al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort. Br J Clin Pharmacol. 2018 Dec;84(12):2811–2823.
- O’Shaughnessy M, Allen N, O’Regan J, et al. Agreement between renal prescribing references and determination of prescribing appropriateness in hospitalized patients with chronic kidney disease. QJM. 2017 Oct 1;110(10):623–628. doi: 10.1093/qjmed/hcx086
- Alruqayb WS, Price MJ, Paudyal V, et al. Drug-related problems in hospitalised patients with chronic kidney disease: a systematic review. Drug Saf. 2021 Oct;44(10):1041–1058. doi: 10.1007/s40264-021-01099-3
- Dörks M, Allers K, Schmiemann G, et al. Inappropriate medication in non-hospitalized patients with renal insufficiency: a systematic review. J Am Geriatr Soc. 2017 Apr;65(4):853–862.
- Hanlon JT, Wang X, Handler SM, et al. Potentially inappropriate prescribing of primarily renally cleared medications for older veterans affairs nursing home patients. J Am Med Dir Assoc. 2011 Jun;12(5):377–83.
- Papaioannou A, Clarke JA, Campbell G, et al. Assessment of adherence to renal dosing guidelines in long-term care facilities. J Am Geriatr Soc. 2000 Nov;48(11):1470–3. doi: 10.1111/j.1532-5415.2000.tb02639.x
- Sweileh WM, Janem SA, Sawalha AF, et al. Medication dosing errors in hospitalized patients with renal impairment: a study in Palestine. Pharmacoepidemiol Drug Saf. 2007 Aug;16(8):908–12.
- Steinman MA, Miao Y, Boscardin WJ, et al. Prescribing quality in older veterans: a multifocal approach. J Gen Intern Med. 2014 Oct;29(10):1379–86.
- Blix HS, Viktil KK, Moger TA, et al. Use of renal risk drugs in hospitalized patients with impaired renal function–an underestimated problem? Nephrol Dial Transplant. 2006 Nov;21(11):3164–71. doi: 10.1093/ndt/gfl399
- Alahdal AM, Elberry AA. Evaluation of applying drug dose adjustment by physicians in patients with renal impairment. Saudi Pharm J. 2012 Jul;20(3):217–20. doi: 10.1016/j.jsps.2011.12.005
- Chang F, O’Hare AM, Miao Y, et al. Use of renally inappropriate medications in older veterans: a National study. J Am Geriatr Soc. 2015 Nov;63(11):2290–7. doi: 10.1111/jgs.13790
- Yap C, Dunham D, Thompson J, et al. Medication dosing errors for patients with renal insufficiency in ambulatory care. Jt Comm J Qual Patient Saf. 2005 Sep;31(9):514–21. doi: 10.1016/S1553-7250(05)31066-X
- Khanal A, Peterson GM, Castelino RL, et al. Potentially inappropriate prescribing of renally cleared drugs in elderly patients in community and aged care settings. Drugs Aging. 2015 May;32(5):391–400. doi: 10.1007/s40266-015-0261-1
- Sönnerstam E, Sjölander M, Gustafsson M. Inappropriate prescription and renal function among older patients with cognitive impairment. Drugs Aging. 2016 Dec;33(12):889–899. doi: 10.1007/s40266-016-0408-8
- Schmidt-Mende K, Wettermark B, Andersen M, et al. Prevalence of renally inappropriate medicines in older people with renal impairment - a cross-sectional register-based study in a large primary care population. Basic Clin Pharmacol Toxicol. 2019 Mar;124(3):256–265.
- Pillans PI, Landsberg PG, Fleming AM, et al. Evaluation of dosage adjustment in patients with renal impairment. Intern Med J. 2003 Jan;33(1–2):10–3.
- Rothberg MB, Kehoe ED, Courtemanche AL, et al. Recognition and management of chronic kidney disease in an elderly ambulatory population. J Gen Intern Med. 2008 Aug;23(8):1125–30.
- Decloedt E, Leisegang R, Blockman M, et al. Dosage adjustment in medical patients with renal impairment at Groote Schuur hospital. S Afr Med J. 2010 May 4;100(5):304–6. doi: 10.7196/SAMJ.3955
- Očovská Z, Maříková M, Kočí J, et al. Drug-related hospital admissions via the department of emergency medicine: a cross-sectional study from the Czech Republic. Front Pharmacol. 2022;13:899151. doi: 10.3389/fphar.2022.899151
- Lexicomp database. [internet]. Waltham (MA): UpToDate, Inc. Available from: https://www.uptodate.com Subscription required to view.
- Micromedex database [internet]. Merative. Available from: https://www.micromedexsolutions.com Subscription required to view.
- Joint Formulary Committee. BNF 84: September 2022-March 2023. London: Pharmaceutical Press; 2022. p. 84.
- Ashley C, Dunleavy A. The renal drug Handbook: the ultimate prescribing guide for renal practitioners. 5th ed. Boca Raton, Florida: CRC Press; 2018.
- Thevelin S, Spinewine A, Beuscart JB, et al. Development of a standardized chart review method to identify drug-related hospital admissions in older people. Br J Clin Pharmacol. 2018 Nov;84(11):2600–2614.
- Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment: World Health Organisation-Uppsala Monitoring Centre; 2018. Available from: https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf
- Hallas J, Harvald B, Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990 Aug;228(2):83–90.
- American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023 May;71(7):2052–2081.
- O’Mahony D, Cherubini A, Guiteras AR, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3. Eur Geriatr Med. 2023 May 31;14(4):625–632. doi: 10.1007/s41999-023-00777-y
- Foubert K, Capiau A, Mehuys E, et al. Ghent older people’s prescriptions community pharmacy screening (GheOP(3)S)-tool version 2: update of a tool to detect drug-related problems in older people in primary care. Drugs Aging. 2021 Jun;38(6):523–533.
- Renom-Guiteras A, Meyer G, Thürmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol. 2015 Jul;71(7):861–75. doi: 10.1007/s00228-015-1860-9
- Saad R, Hallit S, Chahine B. Evaluation of renal drug dosing adjustment in chronic kidney disease patients at two university hospitals in Lebanon. Pharm Pract (Granada). 2019 Jan;17(1):1304. doi: 10.18549/PharmPract.2019.1.1304
- Jones SA, Bhandari S. The prevalence of potentially inappropriate medication prescribing in elderly patients with chronic kidney disease. Postgrad Med J. 2013 May;89(1051):247–50. doi: 10.1136/postgradmedj-2012-130889
- Hoffmann F, Boeschen D, Dörks M, et al. Renal insufficiency and medication in nursing home residents. A cross-sectional study (IMREN). Dtsch Arztebl Int. 2016 Feb 12;113(6):92–8. doi: 10.3238/arztebl.2016.0092
- Yang P, Chen N, Wang RR, et al. Inappropriateness of medication prescriptions about chronic kidney disease patients without dialysis therapy in a Chinese tertiary teaching hospital. Ther Clin Risk Manag. 2016;12:1517–1524. doi: 10.2147/TCRM.S116789
- Doody HK, Peterson GM, Watson D, et al. Retrospective evaluation of potentially inappropriate prescribing in hospitalized patients with renal impairment. Curr Med Res Opin. 2015 Mar;31(3):525–35. doi: 10.1185/03007995.2015.1010036
- Getachew H, Tadesse Y, Shibeshi W. Drug dosage adjustment in hospitalized patients with renal impairment at tikur anbessa specialized hospital, Addis Ababa, Ethiopia. BMC Nephrol. 2015 Oct 7;16:158. doi: 10.1186/s12882-015-0155-9
- Bilge U, Sahin G, Unluoglu I, et al. Inappropriate use of nonsteroidal anti-inflammatory drugs and other drugs in chronic kidney disease patients without renal replacement therapy. Ren Fail. 2013 Jul;35(6):906–10.
- Prajapati A, Ganguly B. Appropriateness of drug dose and frequency in patients with renal dysfunction in a tertiary care hospital: a cross-sectional study. J Pharm Bioallied Sci. 2013 Apr;5(2):136–40. doi: 10.4103/0975-7406.111829
- Salomon L, Deray G, Jaudon MC, et al. Medication misuse in hospitalized patients with renal impairment. Int J Qual Health Care. 2003 Aug;15(4):331–5.
- Nielsen AL, Henriksen DP, Marinakis C, et al. Drug dosing in patients with renal insufficiency in a hospital setting using electronic prescribing and automated reporting of estimated glomerular filtration rate. Basic Clin Pharmacol Toxicol. 2014 May;114(5):407–13.
- Altunbas G, Yazc M, Solak Y, et al. Renal drug dosage adjustment according to estimated creatinine clearance in hospitalized patients with heart failure. Am J Ther. 2016 Jul;23(4):e1004–8.
- Bertsche T, Fleischer M, Pfaff J, et al. Pro-active provision of drug information as a technique to address overdosing in intensive-care patients with renal insufficiency. Eur J Clin Pharmacol. 2009 Aug;65(8):823–9.
- Desmedt S, Spinewine A, Jadoul M, et al. Impact of a clinical decision support system for drug dosage in patients with renal failure. Int J Clin Pharm. 2018 Oct;40(5):1225–1233.
- Gheewala PA, Peterson GM, Curtain CM, et al. Impact of the pharmacist medication review services on drug-related problems and potentially inappropriate prescribing of renally cleared medications in residents of aged care facilities. Drugs Aging. 2014 Nov;31(11):825–35.
- Castelino RL, Saunder T, Kitsos A, et al. Quality use of medicines in patients with chronic kidney disease. BMC Nephrol. 2020 Jun 5;21(1):216. doi: 10.1186/s12882-020-01862-1
- Marquito AB, Pinheiro HS, Fernandes N, et al. Pharmacotherapy assessment in chronic kidney disease: validation of the PAIR instrument for use in Brazil. J Bras Nefrol. 2020 Oct;42(4):400–412. doi: 10.1590/2175-8239-jbn-2019-0205
- Van Pottelbergh G, Mertens A, Azermai M, et al. Drug prescriptions unadapted to the renal function in patients aged 80 years and older. Eur J Gen Pract. 2014 Sep;20(3):190–5.
- Hayat M, Ahmad N, Khan SLA, et al. Pattern, frequency and factors associated with inappropriate high dosing in chronic kidney disease patients at a tertiary care hospital in Pakistan. BMC Nephrol. 2023 May 1;24(1):118. doi: 10.1186/s12882-023-03167-5
- Guirguis-Blake J, Keppel GA, Holmes J, et al. Prescription of high-risk medications among patients with chronic kidney disease: a cross-sectional study from the Washington, Wyoming, Alaska, Montana and Idaho region practice and research network. Fam Pract. 2018 Sep 18;35(5):589–594. doi: 10.1093/fampra/cmy001
- Markota NP, Markota I, Tomic M, et al. Inappropriate drug dosage adjustments in patients with renal impairment. J Nephrol. 2009 Jul;22(4):497–501.
- Holm H, Bjerke K, Holst L, et al. Use of renal risk drugs in patients with renal impairment. Int J Clin Pharm. 2015 Dec;37(6):1136–42. doi: 10.1007/s11096-015-0175-3
- Kassa Birarra M, Mekonnen GB, Gelayee DA, et al. Drug dose adjustment in patients with renal impairment attending a specialized referral hospital, Northwest Ethiopia. Metabol Open. 2022 Dec;16:100211.
- MacRae C, Mercer S, Guthrie B. Potentially inappropriate primary care prescribing in people with chronic kidney disease: a cross-sectional analysis of a large population cohort. Br J Gen Pract. 2021 Jul;71(708):e483–e490. doi: 10.3399/BJGP.2020.0871
- Saleem A, Masood I. Pattern and predictors of medication dosing errors in chronic kidney disease patients in Pakistan: a single center retrospective analysis. PLoS One. 2016;11(7):e0158677. doi: 10.1371/journal.pone.0158677
- Tesfaye WH, Castelino RL, Wimmer BC, et al. Inappropriate prescribing in chronic kidney disease: a systematic review of prevalence, associated clinical outcomes and impact of interventions. Int J Clin Pract. 2017 Jul;71(7). doi: 10.1111/ijcp.12960.
- Hassan Z, Ali I, Ullah AR, et al. Assessment of medication dosage adjustment in hospitalized patients with chronic kidney disease. Cureus. 2021 Feb 20;13(2):e13449. doi: 10.7759/cureus.13449
- Erler A, Beyer M, Petersen JJ, et al. How to improve drug dosing for patients with renal impairment in primary care - a cluster-randomized controlled trial. BMC Fam Pract. 2012 Sep 6;13:91. doi: 10.1186/1471-2296-13-91
- Deskur-Śmielecka E, Chudek J, Neumann-Podczaska A, et al. Use of renal risk drugs in a nation-wide Polish older adult population: an analysis of PolSenior database. BMC Geriatr. 2019 Mar 5;19(1):70. doi: 10.1186/s12877-019-1075-5
- Bezabhe WM, Kitsos A, Saunder T, et al. Medication prescribing quality in Australian primary care patients with chronic kidney disease. J Clin Med. 2020 Mar 13;9(3):783. doi: 10.3390/jcm9030783
- Barbieri MA, Rottura M, Cicala G, et al. Chronic kidney disease management in general practice: a focus on inappropriate drugs prescriptions. J Clin Med. 2020 May 4;9(5):1346. doi: 10.3390/jcm9051346
- Golightly IT LK, Kiser TH, Levin DA, editors . Renal pharmacotherapy: dosage adjustment of medications eliminated by the kidneys. 2nd ed. New york (NY): Springer; 2021.
- Yau K, Dharia A, Alrowiyti I, et al. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022 Jul;7(7):1463–1476. doi: 10.1016/j.ekir.2022.04.094
- Sellier E, Colombet I, Sabatier B, et al. Effect of alerts for drug dosage adjustment in inpatients with renal insufficiency. J Am Med Inform Assoc. 2009 Mar;16(2):203–10.
- Jones M, Denieffe S, Griffin C, et al. Evaluation of cystatin C in malignancy and comparability of estimates of GFR in oncology patients. Pract Lab Med. 2017 Aug;8:95–104.
- Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005 Apr;28(4):346–9.
- Seiberth S, Bauer D, Schönermarck U, et al. Correct use of non-indexed eGFR for drug dosing and renal drug-related problems at hospital admission. Eur J Clin Pharmacol. 2020 Dec;76(12):1683–1693.