 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Bruton’s tyrosine kinase inhibitors (BTKis) are targeted treatments for B-cell tumors but have significant side effects. This study assesses and contrasts the side effects of BTKis alone and its four combination therapies.
Research design and methods
The reporting odds ratio (ROR) was used to analyze the data on three BTKis monotherapies and combinations of ibrutinib with rituximab, obinutuzumab, venetoclax, and lenalidomide in the FDA Adverse Event Reporting System (FAERS) database up to December 2022.
Results
We analyzed the top 20 PTs for each treatment regimen. In monotherapies, atrial fibrillation (ROR (95% CI): 9.88 (9.47–10.32)) in zanubrutinib and rash (6.97 (5.42–8.98)) in acalabrutinib had higher associations. In combinations, infection (6.86 (6.11–7.70)), atrial fibrillation (27.96 (22.61–34.58)) and myelosuppression (10.09 (8.89–11.46)) were vital signals when ibrutinib was combined with obinutuzumab, and pyrexia (4.22 (2.57–6.93)) had a high signal value when combined with lenalidomide. Hemorrhage had a lower signal value when combined with venetoclax compared to ibrutinib alone (2.50 (2.18–2.87) vs 3.60 (3.52–3.68)).
Conclusions
The ibrutinib-obinutuzumab combo has the highest risk of infection, atrial fibrillation, and myelosuppression, and the ibrutinib-lenalidomide combo has the highest risk of pyrexia. However, the ibrutinib-venetoclax combo has a lower risk of hemorrhage than monotherapy.
1. Introduction
B cell receptor (BCR) signaling is necessary for the proliferation and survival of B cell malignancies, and Bruton’s tyrosine kinase (BTK) plays a critical role in it [Citation1]. Numerous covalent BTK inhibitors (BTKis) have been developed [Citation2–4] and were widely used in B-cell malignancies such as chronic lymphocytic leukemia(CLL), small lymphocytic lymphoma (SLL), Waldenstrom macroglobulinemia (WM), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL) and diffuse large B-cell lymphoma (DLBCL) [Citation5]. Ibrutinib was the first in class approved by the US Food and Drug Administration (FDA) for mantle cell lymphoma (MCL) in 2013, acalabrutinib, approved in 2017, followed by zanubrutinib in 2019 [Citation6]. It is understood that BTKis attaches to the cytosine-481 (C481) of BTK covalently and irreversibly, preventing downstream signaling by preventing the phosphorylation of its substrates. It is generally recognized that BTKis has off-target effects such as arrhythmia, infection, and hemorrhage, some of which might harm patients irreversible [Citation7]. The next-generation drugs, zanubrutinib and acalabrutinib, were developed to minimize the off-target effects [Citation8]. BTKis is often used in combination with other drugs (such as rituximab, obinutuzumab, venetoclax, and lenalidomide) to uniform a chemo-free regimen to improve outcomes. We also wonder if the side effects of combination regimens are different from those of BTKis alone?
We aimed to evaluate BTKis alone or combined with other agents, based on a real-world analysis of adverse events (AEs) in the FDA’s Adverse Event Reporting System (FAERS). We hope to provide a rational choice of drugs for clinicians.
2. Materials and methods
2.1. Study design and data sources
This real-world drug safety study is based on a disproportionality analysis of the FDA’s FAERS database. All data from the time of drug marketing until December 2022 is included. The structure of the reporting information is based on the International Safety Reporting Guidelines (ICH E2B) published by ICH, and the adverse event and medication error terms in the report are coded according to the ICH International Medical Terminology Dictionary (MedDRA).
2.2. FAERS procedures
Currently, there are seven BTKis approved, including covalent inhibitors ibrutinib (2013), acalabrutinib (2017), zanubrutinib (2019), tirabrutinib (2020) and orelabrutinib (2020), and non-covalent inhibitors pirtobrutinib and edralbrutinib approved in 2023. Due to the lack of data support for the remaining four drugs, this study only focuses on ibrutinib, acalabrutinib, and zanubrutinib. In addition, we use ibrutinib to analyze its combination with rituximab, ocrelizumab, venetoclax, and lenalidomide for the same reason. We also removed duplicates from the extracted data to ensure that the records were unique. In the FAERS database, AEs are coded using preferred terms (PTs) according to the MedDRA. PTs are ranked by their frequency of occurrence, and the top 20 PTs are focused.
2.3. Statistical analysis
The reporting odds ratio (ROR) method, which is one of the most common algorithms used in drug vigilance analysis, is also known as ‘disproportionality analysis’ [Citation9]; It compares the reporting frequency of the target adverse event (AE) associated with the target drug against the reporting frequency of all other drugs. If the observed value surpasses the expected value, it signifies that attention should be paid to the AE. The calculation of ROR is based on a 2 × 2 table, where a represents the number of reports that include both the suspect drug and the suspect adverse event, b represents the number of reports that include suspect AEs associated with other drugs (excluding the target drug), c represents the number of reports that include other AEs associated with the target drug (excluding the target adverse event), d represents the number of reports that include other drugs and other AEs, and N represents the number of times they coincide. The calculation formula is as follows:
A positive signal is detected when the 95% CI>1 and N ≥ 3, indicating an objective association between the target drug and the target ADR. The higher the ROR value, the stronger the association. All analyses were performed using SAS 9.4.
3. Results
3.1. Demographics description of BTKis as monotherapy
There were 36,056,929 AE reports totally, including 46,272 for ibrutinib, 2,332 for acalabrutinib, and 512 for zanubrutinib. lists the demographic characteristics of AEs associated with BTKis as monotherapy. According to the results, the population reporting AEs in ibrutinib and acalabrutinib groups were predominantly male (ibrutinib: 58.3%; acalabrutinib: 54.8%), with the majority being older than 65 years (ibrutinib: 39.6%; acalabrutinib: 38.1%;), but there are less valid data for zanubrutinib. The country with the most reports was the United States for all groups. Ibrutinib and acalabrutinib are mainly reported by non-health professionals, while zanubrutinib is primarily reported by health professionals. In terms of the time of reporting, the number of cases reported for ibrutinib and acalabrutinib increased year by year before 2021 but showed a decline in 2022, while zanubrutinib showed a continuous increase, with the most cases reported in 2022. This phenomenon could potentially be attributed to the recent introduction of new BTK inhibitors. It is worth noting that when comparing the outcomes of AEs for the three drugs, acalabrutinib had the highest number of deaths caused by AEs (acalabrutinib 35.4% vs ibrutinib 17.8% and zanubrutinib 10.2%).
Table 1. Characteristics of patients at risk of AEs using BTK inhibitors based on the FAERS database.
3.2. Disproportionality analysis of BTKis as monotherapy
We extracted 4,936, 1,046, and 524 AEs associated with ibrutinib, acalabrutinib, and zanubrutinib, respectively. lists the ROR signal values and 95% confidence intervals (95% CI) for the top 20 AEs ranked by frequency of occurrence for three drugs. Overall, the frequency of occurrence of infection (9311 cases for ibrutinib, 302 cases for acalabrutinib, and 116 cases for zanubrutinib), hemorrhage (8462 cases for ibrutinib, 157 cases for acalabrutinib, and 101 cases for zanubrutinib), and myelosuppression (5199 cases for ibrutinib, 203 cases for acalabrutinib, and 74 cases for zanubrutinib) ranked in the top three for each, and the ROR signals were all associated. In ibrutinib and zanubrutinib, the top three were infection, hemorrhage, and myelosuppression in that order, but in acalabrutinib, the frequency of occurrence of myelosuppression was higher than that of hemorrhage. To conduct a parallel comparison of the AE signals, we counted the top 20 AEs ranked by frequency of occurrence reported simultaneously in three drugs () and then compared them (). As shown in , infection, hemorrhage, myelosuppression, arrhythmia, fatigue, and rash, all have significant signals in them. Among them, arrhythmia (ROR (95% CI) = 17.68 (13.10–23.86)) in zanubrutinib and rash (ROR (95% CI) = 6.97 (5.42–8.98)) in acalabrutinib have significantly higher associations than the others. Interestingly, when atrial fibrillation is singled out from arrhythmias (), the signal in the ibrutinib group is significantly higher than the other two groups (ibrutinib 9.88 (9.47–10.32) vs acalabrutinib 4.12 (2.96–5.75) vs zanubrutinib 4.48 (2.48–8.11)).
Figure 1. Overlapping common preferred terms (PTs) for the top 20 adverse events (AEs) associated with ibrutinib, acalabrutinib and zanubrutinib.
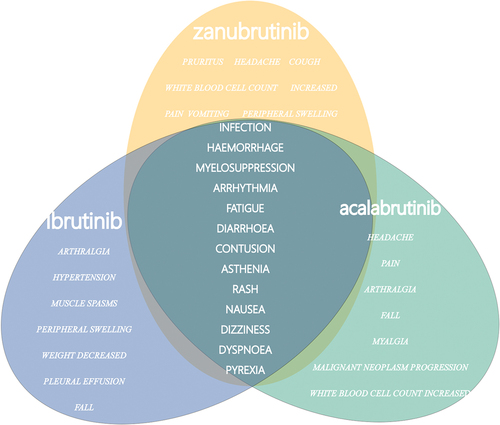
Figure 2. Forest plot of the overlapping common preferred terms (PTs) for the top 20 adverse events (AEs) associated with ibrutinib, acalabrutinib and zanubrutinib.
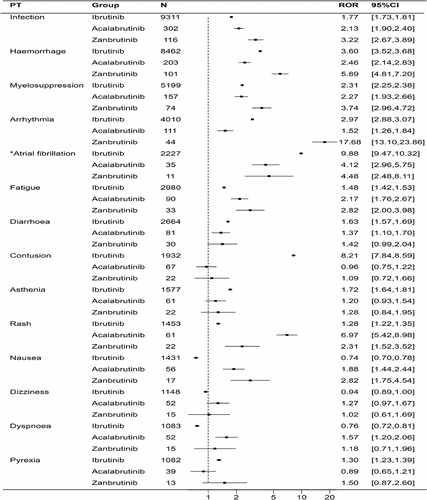
Table 2. Reporting odds ratios (RORs) of the top 20 AEs associated with ibrutinib, acalabrutinib, and zanubrutinib.
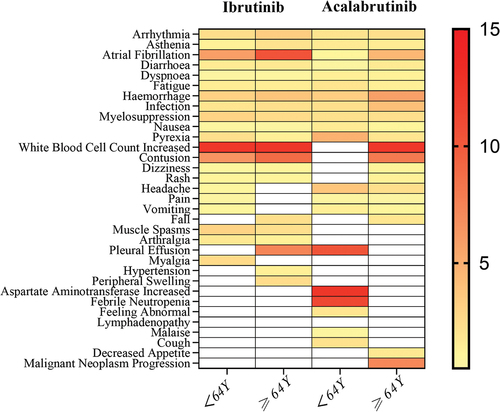
Additionally, we analyzed the influence of age on the AEs of ibrutinib and acalabrutinib (). For both ibrutinib and acalabrutinib, the signal values for bleeding, infection, arrhythmia, and bone marrow suppression were higher in the ≥64 age group compared to the <64 age group. Notably, when atrial fibrillation was singled out, the signal value in the ≥64 age group significantly increased (ibrutinib: ≥64Y 10.43 (9.83–11.05) vs <64Y 5.92 (5.15–6.81); acalabrutinib: ≥64Y 4.53 (2.73–7.53) vs <64Y 1.04 (0.15–7.36)).
Figure 3. Heatmap of the reporting odds ratios (RORs) for the top 20 adverse events (AEs) associated with ibrutinib and acalabrutinib across different age groups.
3.3. Time-to-onset analysis and disproportionality analysis of ibrutinib and its combination
There have been 1213 reports of AEs associated with the usage of ibrutinib in combination with rituximab, 598 reports with obinutuzumab, 1123 reports with venetoclax and 294 reports with lenalidomide. As shown in , including ibrutinib monotherapy and four combination regimens mentioned above, the time of occurrence of AEs was highest in the 0–30 days for all groups (36.00% for ibrutinib alone, and 32.89%, 32.55%, 24.76%, and 39.81% for combination with rituximab, obinutuzumab, venetoclax and lenalidomide). However, it is a significant proportion of AEs occurred after more than 180 days in all groups. The top 20 AEs for the four combination regimens are listed in . Similar to the ibrutinib alone, the top four AEs were infection, hemorrhage, myelosuppression, and arrhythmia, and the ROR signals were statistically significant. However, the frequency of these four AEs varied among different regimens. To compare the ROR signals of different regimens, we separately listed the overlapping parts of the top 20 AEs in frequency for ibrutinib monotherapy and its four combinations and created a forest plot (). We found that in the four combinations, the signal values for infection, myelosuppression, arrhythmia, and pyrexia were all higher than those for the ibrutinib monotherapy group. Among them, the most vital signals were infection (ROR (95% CI) = 6.86 (6.11–7.70)) and myelosuppression (ROR (95% CI) = 10.09 (8.89–11.46)) when ibrutinib was combined with obinutuzumab, and for pyrexia (ROR (95% CI) = 4.22 (2.57–6.93)), when ibrutinib was combined with lenalidomide. It is particularly noteworthy that in the ibrutinib combined with lenalidomide, the signal value for arrhythmia (ROR (95% CI) = 19.26 (14.00–26.50)) is exceptionally high, indicating an influential association between this regimen and arrhythmia. However, when atrial fibrillation is singled out, the signal value of ibrutinib combined with obinutuzumab is the highest (ROR (95% CI) = 27.96 (22.61–34.58)). For hemorrhage, the signal value for ibrutinib combined with venetoclax is lower than that for the ibrutinib alone (ibrutinib + venetoclax 2.50 (2.18–2.87) vs ibrutinib 3.60 (3.52–3.68)). In contrast, the signal values for the other combination groups are higher than the monotherapy group, which suggests that the risk of bleeding is relatively lower with the ibrutinib in combination with venetoclax regimen compared to others. In addition, for fatigue and diarrhea, which have signals in the ibrutinib monotherapy group, combination with venetoclax group is comparable to monotherapy group. In contrast, no fatigue signal was detected in the other groups. For diarrhea, no signal was detected in combination with obinutuzumab group, and combination with rituximab group was slightly lower than monotherapy group. Other two combination groups were slightly higher than monotherapy group.
Figure 4. Summary of the time to onset of adverse reactions for ibrutinib and its four combination therapy regimens.
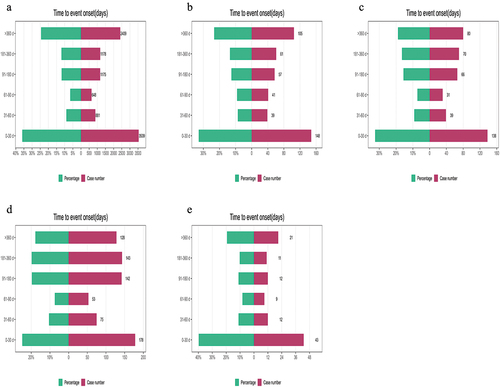
Figure 5. Forest plot of the overlapping common preferred terms (PTs) for the top 20 adverse events (AEs) associated with ibrutinib in combination with rituximab, obinutuzumab, lenalidomide and venetoclax.
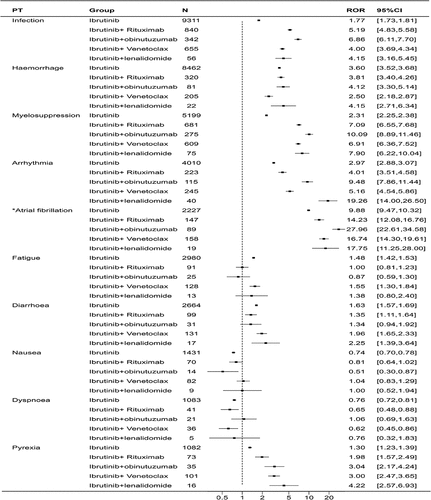
Table 3. Reporting odds ratios (RORs) of the top 20 adverse events (AEs) associated with ibrutinib in combination with rituximab, obinutuzumab, lenalidomide and venetoclax.
4. Discussion
Ibrutinib (PCI-32765) is one of the most well-known irreversible BTKis, which covalently binds to cysteine at position 481 in the kinase domain and thereby blocks kinase activity [Citation10]. The FDA has approved ibrutinib, acalabrutinib, and zanubrutinib for the treatment of hematological cancer, including both therapy-naive and relapsed/refractory settings [Citation2–4]. Except ibrutinib, many other drugs have been created with improved selectivity to lessen inhibition of ‘off-target’ (non-BTK) kinases. Due to the higher Bruton’s tyrosine kinase (BTK) selectivity and target specificity, acalabrutinib had less occurrence of off-target effects [Citation11,Citation12].
The onset of ibrutinib resistance and the dependency of B-cell malignancies on their microenvironment have arisen the innovation of combination therapies. Many combination regimens are now being applied to achieve a deeper remission and conquer drug resistance [Citation13–15]. The PFS and OS benefits were observed for ibrutinib plus rituximab over FCR in the E1912 trial [Citation10]. In iLLUMINATE study [Citation16], Moreno et al. evaluated the efficacy and safety of ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in treatment-naïve patients with CLL/SLL, after a median follow-up duration of 31.3 months, median progression-free survival was significantly longer in the ibrutinib plus obinutuzumab group (median not reached [95% CI 33.6 – non-estimable]) than in the chlorambucil plus obinutuzumab group (19.0 months [15.1–22.1]). In clinical studies, late or rare AE is easily overlooked due to limited sample size and follow-up time.
It is noteworthy that BTKis may also suppress other kinases, leading to adverse reactions. Interleukin-2-inducible T-cell kinase [ITK] and tyrosine kinase expressed in hepatocellular carcinoma [TEC]-family kinases are two examples of other kinases that ibrutinib irreversibly binds with varying affinities. This could affect normal T-cell, macrophage, and platelet function [Citation17]. These off-target effects may influence the AE profile associated with ibrutinib therapy. Ibrutinib’s effects on BTK and TEC are thought to be responsible for bleeding, while effects on the EGFR may be responsible for rashes and diarrhea. The C-terminal Src kinase (CSK) has also been identified as a molecular target for the development of atrial fibrillation [Citation18]. The right BTKis must be chosen based on a variety of factors, including cost, desired therapeutic results, side effect profiles, comorbidities, concurrent drugs, and potential drug–drug interactions, while the side effects of drugs are most concerned for clinicians. Hence, we analyzed the differences between BTKis monotherapy and combination regimens to provide a reference for clinicians.
In a comparison of the common adverse reactions among the three BTK inhibitor monotherapies, ‘infection,’ ‘hemorrhage,’ ‘myelosuppression,’ ‘arrhythmia,’ ‘fatigue,’ and ‘rash’ had a strong correlation signal. The ASPEN study reported that when comparing the treatment of Waldenström’s macroglobulinemia with ibrutinib and zanubrutinib, the incidence of atrial fibrillation, bruising, diarrhea, peripheral edema, bleeding, muscle spasms, and pneumonia was higher in the ibrutinib group, while the incidence of neutropenia was higher in the zanubrutinib group [Citation4]. Our results suggest that despite zanubrutinib’s high arrhythmia signal, ibrutinib shows the strongest link to atrial fibrillation when analyzed separately. It may be because we included atrial fibrillation, atrial flutter, sinus tachycardia/bradycardia, premature contractions, and atrioventricular block in the scope of arrhythmias, and asymptomatic sinus tachycardia/bradycardia, premature contractions, and atrioventricular block usually do not require treatment. Atrial fibrillation, which has a higher risk, is an adverse event (AE) of more concern to clinicians.
In the comparison of the four combination groups, the signal values of infection, myelosuppression, arrhythmia, and pyrexia are all higher than ibrutinib monotherapy group. In terms of infection, ibrutinib combined with obinutuzumab group has a maximal reporting odds ratio, indicating that patients with the therapy may have more chance of infections. Myelosuppression is another common AE, and in combination groups had higher incidence rates than ibrutinib monotherapy group. As to arrhythmia, it seems that ibrutinib plus lenalidomide group had the highest ROR rate of 19.26. In the ORIGIN trial, when comparing lenalidomide (LEN) with Chlorambucil (CHB) treatment, the number of patients in the LEN group who discontinued treatment due to cardiac disease was significantly higher than the control group (3% vs 1.7%) [Citation19]. This suggests that the high arrhythmia signal observed in the ibrutinib combined with lenalidomide group may be due to the additive cardiac toxicity of ibrutinib and lenalidomide. Interestingly, the strongest association with atrial fibrillation was found in the ibrutinib combined with obinutuzumab group, thus the cardiac toxicity of this group also warrants attention. At the same time, based on signal strength, the combination treatment regimen did not appear to differ significantly from ibrutinib monotherapy for the side effects of fatigue, nausea, and dyspnea aspect. These findings are not entirely consistent with existing clinical research data. GOY et al. [Citation20] evaluated the safety and tolerability of ibrutinib plus lenalidomide and rituximab in transplant ineligible patients with relapsed/refractory DLBCL. The most frequently reported TEAEs across cohorts were gastrointestinal events, myelosuppression, fatigue, hypokalemia, peripheral edema, and maculopapular rash, and five patients (11.1%) experienced new-onset atrial fibrillation (AF). In a phase 3 Trial of Ibrutinib plus Rituximab in Waldenström’s Macroglobulinemia [Citation3], the most common such AEs that occurred more frequently with ibrutinib – rituximab than with placebo – rituximab included hypertension (13% vs. 4%) and atrial fibrillation (12% vs. 1%), and the most common serious AEs included pneumonia (8%), atrial fibrillation (7%), and respiratory tract infection. This discrepancy may be attributed to the relatively limited sample size in clinical studies.
Because some BTKis have a short marketing time, our study only compared and examined the safety signals of ibrutinib, acalabrutinib, and zanubrutinib and the side effects of the combination regimen of ibrutinib. We found that, the reporting frequency of infection, hemorrhage, myelosuppression, and arrhythmia was high regardless of the medication regimen, which is consistent with reports from clinical trials [Citation16,Citation21]. However, hypertension, which is commonly reported in clinical trials, was not particularly high in our data. It only appeared in the top 20 pts in the ibrutinib combined with obinutuzumab group. Overall, our study based on the extensive data from the FAERS database analyzed AEs in the real world. Under some circumstances, the combination treatment regimen may be further aggravated by some drug side effects, which need to be paid more attention. Broader pharmacovigilance research will be done in future studies. The constraints of this study are that the data in the FAERS database are spontaneously reported and are geographically limited.
5. Conclusion
This study is the first to evaluate and compare the adverse reactions of BTKis monotherapy and combination therapy. In our analysis of the top 20 PTs, the ibrutinib–obinutuzumab combination showed the strongest signals for infection and myelosuppression, while the ibrutinib–lenalidomide combination had the highest signals for pyrexia and arrhythmia. However, when isolating atrial fibrillation, the ibrutinib–obinutuzumab combination has the highest signal value. However, the ibrutinib–venetoclax combination had lower hemorrhage signals compared to ibrutinib alone. These findings enable clinicians to customize treatment options by considering the patient’s risk of complications. It also facilitates early intervention of AEs during treatment.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer is a coauthor of studies leading to approval of ibrutinib, acalabrutinib, and zanubrutinib. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Author contribution statement
Hangping Ge, Yu Zhang and Jianping Shen: conception and design of research; Sichun Xianga, and Rongbin Shen: analyzed and interpreted the data; Sichun Xiang and Jingjing Xiang: drafted manuscript; Ni Zhu and Jianyou Gu: edited and revised manuscript. All authors agree to be accountable for all aspects of the work.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors. This study is based on anonymous data that can be downloaded from a publicly available source. Therefore, no ethical approval is required.
Additional information
Funding
References
- Burger JA, Wiestner A. Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer. 2018;18(3):148–167. doi: 10.1038/nrc.2017.121
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798. doi: 10.1038/s41375-019-0602-x
- Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020 Apr 18;395(10232):1278–1291. doi: 10.1016/s0140-6736(20)30262-2
- Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020 Oct 29;136(18):2038–2050. doi: 10.1182/blood.2020006844
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569
- Sutanto F, Konstantinidou M, Dömling A. Covalent inhibitors: a rational approach to drug discovery. RSC Med Chem. 2020 Aug 1;11(8):876–884. doi: 10.1039/d0md00154f
- O’Brien SM, Brown JR, Byrd JC, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Front Oncol. 2021;11:720704. doi: 10.3389/fonc.2021.720704
- Lewis KL, Cheah CY. Non-covalent BTK inhibitors—the new BTKids on the block for B-Cell malignancies. J Pers Med. 2021 Aug 3;11(8):764. doi: 10.3390/jpm11080764
- Hauben M, Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov Today. 2009;14(7–8):343–357. doi: 10.1016/j.drudis.2008.12.012
- Shanafelt TD, Wang XV, Hanson CA, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022 Jul 14;140(2):112–120. doi: 10.1182/blood.2021014960
- Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016 Jan 28;374(4):323–332. doi: 10.1056/NEJMoa1509981
- Wen T, Wang J, Shi Y, et al. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia. 2021 Feb;35(2):312–332. doi: 10.1038/s41375-020-01072-6
- Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019 May 30;380(22):2095–2103. doi: 10.1056/NEJMoa1900574
- Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018 Jun 21;378(25):2399–2410. doi: 10.1056/NEJMoa1802917
- Wang M, Ramchandren R, Chen R, et al. Concurrent ibrutinib plus venetoclax in relapsed/refractory mantle cell lymphoma: the safety run-in of the phase 3 SYMPATICO study. J Hematol Oncol. 2021 Oct 30;14(1):179. doi: 10.1186/s13045-021-01188-x
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019 Jan;20(1):43–56.
- Wu J, Liu C, Tsui ST, et al. Second-generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016 Sep 2;9(1):80. doi: 10.1186/s13045-016-0313-y
- Xiao L, Salem JE, Clauss S, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-Terminal Src Kinase. Circulation. 2020 Dec 22;142(25):2443–2455. doi: 10.1161/circulationaha.120.049210
- Chanan-Khan A, Egyed M, Robak T, et al. Randomized phase 3 study of lenalidomide versus chlorambucil as first-line therapy for older patients with chronic lymphocytic leukemia (the ORIGIN trial). Leukemia. 2017 May;31(5):1240–1243. doi: 10.1038/leu.2017.47
- Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019 Sep 26;134(13):1024–1036. doi: 10.1182/blood.2018891598
- Moreno C, Greil R, Demirkan F, et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: final analysis of the randomized, phase III iLLUMINATE trial. Haematologica. 2022 Sep 1;107(9):2108–2120. doi: 10.3324/haematol.2021.279012

