1. Introduction
Sodium nitroprusside (SNP) is a short-acting, light-sensitive, intravenous vasodilator consisting of a complex dianion [Fe(CN)5NO]2– in which an octahedral iron center is surrounded by five cyanide ligands and one linear nitric oxide ligand [Citation1]. Its primary mode of action entails the liberation of nitric oxide (NO), which is concurrent with the release of cyanide ions. NO binds to and directly activates soluble guanylate cyclase (sGC) in vascular smooth muscle cells, fueling the production of intracellular cyclic guanosine monophosphate (cGMP) which subsequently activates cGMP-dependent protein kinase G (PKG). Activated PKG then phosphorylates and activates myosin light-chain phosphatase, thus silencing the myosin complex and inducing relaxation of the vascular smooth muscle cells [Citation2]. Also, NO may reduce vascular smooth muscle contraction triggered by Bay K8644, an agonist of calcium channels located in the cellular membrane, and mastoparan-7, a direct G-protein activator [Citation3,Citation4]. This NO-initiated signaling cascade ultimately induces vasodilation, which is most prominent on the arteriolar vessels. Still, HF may exhibit decreased NO bioavailability as a consequence of oxidative stress; sGC stimulators, directly activating the NO-sGC-cGMP pathway independently of NO, have emerged as promising candidates for HF treatment in clinical trials [Citation5].
2. Historical background
SNP found its first applications in human medicine in the 1920s, with safety and efficacy data being published in 1955 [Citation6]. Originally adopted for the treatment of hypertensive crises, its use progressively expanded, embracing the acute heart failure (HF) setting [Citation7]. However, after the publication of a seminal work by Gheorghiade et al. in 2006 showing that low blood pressure at admission is an independent predictor of morbidity and mortality in patients hospitalized for acute HF [Citation8], the use of vasodilators was restrained to patients with normal-to-high blood pressure, as the potential hypotensive effect of these medications was feared to worsen outcomes in acute HF. However, the hemodynamic and clinical responses to SNP, and ensuing safety considerations, may strongly diverge based on the individual cardiovascular physiology. Accordingly, a description of different HF phenotypes is needed to fully grasp the potential efficacy and safety profiles of SNP in HF. No contemporary trials have tested SNP in terms of clinical outcomes in HF yet, and most clinical data reported throughout this manuscript are observational only.
3. Pathophysiological hemodynamic basis
Chronic congestive HF is characterized by progressive left ventricular (LV) dilation and dysfunction, with cardiac output still preserved at the cost of augmented LV end-diastolic pressure and volume [Citation9]; as chronic congestive HF progresses, significant mitral regurgitation (MR) often ensues, posing further hemodynamic overload on the failing heart. When systemic tissue perfusion decreases, the renin-angiotensin-aldosterone system and the sympathetic nervous system are over-activated in an attempt to increase intravascular volume and arterial load and prevent systemic hypoperfusion [Citation10]. These hemodynamic alterations, albeit adaptive in the chronic setting at the cost of increased cardiac workload, may become ominous in acute decompensation of advanced HF (adv-HF), where the failing heart may not be able to cope with such relatively elevated afterload. This can be best appreciated at invasive pressure-volume loop analysis of adv-HF, where arterial afterload (i.e. arterial elastance [Ea]) is disproportionately higher than LV contractility (i.e. end-systolic elastance [Ees]) in a condition of deleterious ventriculo-arterial uncoupling termed ‘afterload mismatch’ (). In this setting, SNP may restore ventriculo-arterial coupling by reducing Ea and improving LV stroke volume, as evidenced by Capomolla and collaborators [Citation11], who observed that SNP infusion significantly increased cardiac index (2.1 ± 0.5 vs 2.6 ± 0.5 l/min/m2, p < 0.004), reduced pulmonary artery wedge pressure (25 ± 6 vs 14 ± 4 mmHg, p < 0.0001) and diminished mitral regurgitation severity in 40 consecutive adv-HF patients undergoing right heart catheterization. Also, a prior study from our group including 200 patients with acute HF (88% adv-HF) treated with SNP reported a significant positive association between baseline LV end-diastolic diameter and SNP response, suggesting that the beneficial effects of SNP are amplified at higher preload levels as it simultaneously minimizes preload and afterload to enhance overall cardiac function [Citation12].
Figure 1. Efficacy of sodium nitroprusside according to different cardiac phenotypes and its effects on pressure-volume loops. In the upper panels the gray loop depicts basal conditions, and the black loop represents the alterations induced by sodium nitroprusside. In the lower part of the figure the gray curve indicates left ventricular end-diastolic diameter, and the black curve represents left ventricular ejection fraction.Adv: advanced; ea: arterial elastance; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; P: pressure; SNP: sodium nitroprusside; SV: stroke volume; VA: ventriculo-arterial.
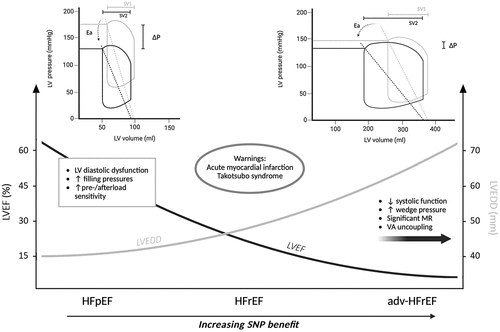
Non-dilated left ventricles typical of some conditions of de novo acute HF with reduced ejection fraction (HFrEF) and, most prominently, HF with preserved ejection fraction (HFpEF) express a different pathological substrate. Non-dilated failing hearts show increased LV filling pressures, but higher vulnerability to the venodilator effect of SNP, which leads to excessive preload reduction and enhanced risk of a drop in LV stroke volume and blood pressure [Citation13]. Again, this finding may be explained by pressure-volume loop analysis showing that HFpEF subjects present a balanced increase in both EEs and Ea, with a ventricular-arterial system already operating at near-maximum efficiency; abrupt SNP-induced preload and afterload reduction may not be adequately compensated and thus lead to hypotension without significant increase in stroke volume.
However, the intricate relationships between cardiovascular pressures and volumes must be interpreted within individual clinical scenarios to better appreciate the safety and efficacy profiles of SNP, as described in the following paragraph.
4. Heart failure phenotypes
HF is a complex systemic syndrome with multiple phenotypic expression; three acute HF phenotypes will be hereby described, namely acutely decompensated adv-HF, de-novo HFrEF, and HFpEF.
Acutely decompensated adv-HF is the condition most likely to benefit from SNP. In an observational study on 175 patients with acutely decompensated adv-HF (LVEF ejection fraction: 15 ± 6%; LV end-diastolic diameter: 7 ± 1 cm; mean arterial pressure: 84 ± 11 mmHg; cardiac index: 1.6 ± 0.2 l/min/m2), Mullens et al. showed that individuals treated with SNP had lower all-cause mortality (OR: 0.48; p = 0.005) than non-SNP-treated patients; also, no significant reduction in mean arterial pressure was observed in SNP-treated individuals, corroborating the hypothesis that afterload reduction during SNP administration usually leads to a marked increase in cardiac output which prevents the development of significant hypotension [Citation14].
In de novo HFrEF, the sudden manifestation of cardiac failure due to variable clinical causes (acute coronary ischemia, Takotsubo syndrome, myocarditis, etc.) does not usually allow for progressive LV dilatation and adaptation of the renin-angiotensin-aldosterone and sympathetic nervous systems to take place. Precocious initiation of SNP during acute coronary ischemia may lead to a ‘coronary steal’ effect, which results in redistribution of blood flow away from an ischemic area with an increase in myocardial injury [Citation15]; a randomized double-blind placebo-controlled trial on patients with acute myocardial infarction and high LV filling pressures demonstrated that precocious (<9 h) administration of SNP had a deleterious effect with higher mortality rate compared to placebo, whereas SNP yielded a beneficial effect if begun later [Citation16]. On the other hand, mechanical complications of acute myocardial infarction (interventricular septum defects, rupture of papillary muscles or chordae tendineae, etc.) might benefit from LV unloading, be it achieved either pharmacologically (e.g. SNP) or mechanically (e.g. intra-aortic balloon pump). As for Takotsubo syndrome, excessive afterload lowering should be generally avoided to prevent significant LV outflow tract obstruction from hypercontractile ventricular basal segments and MR due to systolic anterior movement of the anterior mitral leaflet [Citation17]. Of note, de novo HFrEF may be the first abrupt clinical presentation of latent cardiomyopathies with features of severe LV dilation and afterload-dependency; this phenotype is generally characterized by a hemodynamic response to SNP that is similar to adv-HF and should be considered accordingly.
HFpEF describes a multispecialty disorder characterized by an LV ejection fraction >50%; patients with HFpEF are commonly older, with higher prevalence of arterial hypertension, obesity, atrial fibrillation, and chronic kidney disease compared to their HFrEF counterpart. Diastolic dysfunction with increased filling pressure is the main driver of HFpEF, while LV contractility is normal and ventriculo-arterial coupling is rather balanced with heightened preload and afterload sensitivity [Citation18]; accordingly, therapies that rapidly alter preload or afterload like SNP appear less beneficial or even harmful. Also, higher blood pressure may be needed to provide adequate end-organ perfusion in older patients with increased peripheral artery stiffness [Citation19].
5. Sodium nitroprusside toxicity
Cyanide radicals and NO, spontaneous breakdown products of SNP, undergo rapid non-enzymatic clearance by interacting with sulfhydryl groups on surrounding tissue and erythrocyte proteins [Citation20]. Some of the cyanide radicals released by SNP molecules immediately bind methemoglobin to form cyanomethemoglobin, which is in equilibrium with free cyanide radicals and is considered nontoxic; adverse effects from methemoglobinemia generated by SNP breakdown are rare. The remaining cyanide radicals enter the ‘cyanide pool’ and are converted to thiocyanate via hepatic transsulfuration. The rhodanese enzyme catalyzes this transformation, utilizing thiosulfate as a sulfur donor; theoretically, the reaction is reversible via thiocyanate oxidase in erythrocytes, but the thermodynamics favors thiocyanate production. Prolonged (above 48 h) SNP infusions exceeding 2 mcg/kg/min or depletion of sulfur donors and methemoglobin can lead to cyanide radicals accumulation and clinical cyanide toxicity. Free cyanide radicals bind and inactivate tissue cytochrome oxidase, disrupting oxidative phosphorylation; this can precipitate tissue anoxia, anaerobic metabolism, and lactic acidosis. Thiocyanate toxicity manifests with nonspecific symptoms, like fatigue, tinnitus, nausea, and vomiting, and neurological signs, including hyperreflexia, confusion, psychosis, and miosis [Citation21]. Hepatic or renal impairment may increase cyanide toxicity during SNP infusion. Cyanide toxicity associated with prolonged SNP administration is uncommon in current clinical practice; specifically, our study on 200 acute HF patients treated with SNP (median dose: 0.3 mcg/kg/min; median treatment duration: 5 days) reported no thiocyanate-related adverse events and rare episodes of acute kidney injury despite a relatively high creatinine level at baseline (median 1.4 mg/dL) [Citation12]. However, vigilance remains crucial, as the constellation of central nervous system dysfunction, hemodynamic instability, and progressive metabolic acidosis in an SNP-treated patient warrants prompt investigation for potential cyanide poisoning.
6. Expert opinion
Within the vast realm of HF, different cardiac phenotypes may be distinguished with different likelihood to benefit from SNP infusion (). From the pure hemodynamic perspective, intravenous SNP is mostly beneficial in the setting of dilated LVs with heightened filling pressures and severe MR [Citation12], whereas a condition of elevated filling pressures but small LV chambers may not improve with SNP, which could rather cause rapid hypotension and hemodynamic deterioration. When translated to clinical scenarios, the former is mostly represented by acutely decompensated adv-HF, in which SNP-induced increase in cardiac output comes without the potential pro-arrhythmogenic side-effects of inotropic agents, whose benefit has been frequently questioned; the dose of SNP can be progressively up-titrated as long as organ perfusion pressure is maintained and systolic blood pressure is higher than approximately 90 mmHg in our experience [Citation22,Citation23]. Patients with adv-HF often exhibit diminished NO bioavailability due to the enhanced oxidative stress and may necessitate higher doses of SNP to achieve comparable hemodynamic effects observed in individuals with normal NO homeostasis; cyanide toxicity is an extremely rare event at the doses used for HF treatment (<3 mcg/kg/min), and the clinician should not refrain from administering SNP fearing this complication, as the benefits of this medication largely outweigh the risks [Citation12]. The latter scenario (i.e. high LV filling pressure with small LV chamber) is mostly defined by HFpEF, which is unlikely to improve significantly with SNP; apart from hypertensive crises, which would likely benefit from intravenous vasodilators with rapid onset and short half-life, SNP may seldom be used in HFpEF due to a higher chance of adverse events.
New-onset HFrEF is a heterogeneous condition with specific safety concerns. Patients most likely to respond to SNP are those with characteristics resembling those of adv-HF (i.e. dilated LV with severe MR), in whom SNP may be started with careful monitoring and progressive uptitration [Citation12,Citation14]. However, the risk of ‘coronary steal’ during acute ischemia or worsening LV outflow tract obstruction in Takotsubo syndrome shall be remembered. In acute myocardial infarction with high filling pressures, SNP infusion may be postponed until coronary revascularization is achieved [Citation16].
7. Conclusions
SNP is a powerful intravenous vasodilator with a wide range of efficacy and safety profiles; patients most likely to benefit from this medication are acutely decompensated adv-HF individuals with dilated LVs, reduced LV ejection fraction, and severe MR, while it should be used with caution in HFpEF subjects. The efficacy and safety of SNP in de novo HFrEF depends on the pathophysiological substrate of each individual patient.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Author contributions
P P Bocchino conceived the study. P P Bocchino, F Angelini, and S Frea wrote the first draft of the manuscript. All authors critically revised the manuscript and read and approved its final version.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Navaza A, Chevrier G, Alzari PM, et al. Single-crystal neutron diffraction structure of sodium pentacyanonitrosylferrate(2-) (sodium nitroprusside) dihydrate”. Acta Crystallogr C. 1989;45(6):839–841. doi: 10.1107/S0108270188013691
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986;78(1):1–5. doi: 10.1172/JCI112536
- Szadujkis-Szadurska K, Grzesk G, Szadujkis-Szadurski L, et al. Role of nitric oxide and cGMP in the modulation of vascular contraction induced by angiotensin II and bay K8644 during ischemia/reperfusion. Exp Ther Med. 2013;5(2):616–620. doi: 10.3892/etm.2012.846
- Grześk E, Malinowski E, Tejza B. Modulatory effect of sodium nitroprusside and 8Br-cGMP on mastoparan-7 induced contraction. Med Biol Sci. 2014;28(4):31–35. doi: 10.12775/MBS.2014.031
- Butler J, Usman MS, Anstrom KJ, et al. Soluble guanylate cyclase stimulators in patients with heart failure with reduced ejection fraction across the risk spectrum. Eur J Heart Fail. 2022;24(11):2029–2036. doi: 10.1002/ejhf.2720
- Page IH, Corcoran AC, Dustan HP, et al. Cardiovascular actions of sodium nitroprusside in animals and hypertensive patients. Circulation. 1955;11(2):188–198. doi: 10.1161/01.CIR.11.2.188
- Berkowitz C, McKeever L, Croke RP, et al. Comparative responses to dobutamine and nitroprusside in patients with chronic low output cardiac failure. Circulation. 1977;56(6):918–924. doi: 10.1161/01.CIR.56.6.918
- Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217–2226. doi: 10.1001/jama.296.18.2217
- Bastos MB, Burkhoff D, Maly J, et al. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41(12):1286–1297. doi: 10.1093/eurheartj/ehz552
- Ikonomidis I, Aboyans V, Blacher J, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and heart failure association. Eur J Heart Fail. 2019;21(4):402–424. doi: 10.1002/ejhf.1436
- Capomolla S, Pozzoli M, Opasich C, et al. Dobutamine and nitroprusside infusion in patients with severe congestive heart failure: hemodynamic improvement by discordant effects on mitral regurgitation, left atrial function, and ventricular function. Am Heart J. 1997;134(6):1089–1098. doi: 10.1016/S0002-8703(97)70030-9
- Garatti L, Frea S, Bocchino PP, et al. Sodium nitroprusside in acute heart failure: A multicenter historic cohort study. Int J Cardiol. 2022;369:37–44. doi: 10.1016/j.ijcard.2022.08.009
- Schwartzenberg S, Redfield MM, From AM, et al. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59(5):442–451. doi: 10.1016/j.jacc.2011.09.062
- Mullens W, Abrahams Z, Francis GS, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol. 2008;52(3):200–207. doi: 10.1016/j.jacc.2008.02.083
- Mann T, Cohn PF, Holman LB, et al. Effect of nitroprusside on regional myocardial blood flow in coronary artery disease. Results in 25 patients and comparison with nitroglycerin. Circulation. 1978;57(4):732–738. doi: 10.1161/01.CIR.57.4.732
- Cohn JN, Franciosa JA, Francis GS, et al. Effect of short-term infusion of sodium nitroprusside on mortality rate in acute myocardial infarction complicated by left ventricular failure: results of a Veterans Administration cooperative study. N Engl J Med. 1982;306(19):1129–1135. doi: 10.1056/NEJM198205133061902
- De Backer O, Debonnaire P, Gevaert S, et al. Prevalence, associated factors and management implications of left ventricular outflow tract obstruction in takotsubo cardiomyopathy: a two-year, two-center experience. BMC Cardiovasc Disord. 2014;14(1):147. doi: 10.1186/1471-2261-14-147
- Kapelios CJ, Shahim B, Lund LH, et al. Epidemiology, clinical characteristics and cause-specific outcomes in heart failure with preserved ejection fraction. Card Fail Rev. 2023;9:e14. doi: 10.15420/cfr.2023.03
- Ali D, Tran P, Ennis S, et al. Rising arterial stiffness with accumulating comorbidities associates with heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10(4):2487–2498. doi: 10.1002/ehf2.14422
- Tinker JH, Michenfelder JD. Sodium nitroprusside: pharmacology, toxicology and therapeutics. Anesthesiology. 1976;45(3):340–354. doi: 10.1097/00000542-197609000-00016
- Friederich JA, Butterworth JF. Sodium nitroprusside: twenty years and counting. Anesth Analg. 1995 4th;81(1):152–162. doi: 10.1097/00000539-199507000-00031
- Bocchino PP, Cingolani M, Frea S, et al. Organ perfusion pressure at admission and clinical outcomes in patients hospitalized for acute heart failure. Eur Heart J Acute Cardiovasc Care. 2023 Oct 26;44(Supplement_2):zuad133. doi: 10.1093/eurheartj/ehad655.1090
- Bocchino PP, Angelini F, Frea S. Intravenous vasodilators in acute heart failure patients with low basic blood pressure: may the benefit come from targeting perfusion rather than pressure? Eur Heart J. 2020;41(25):2409–2410. doi: 10.1093/eurheartj/ehaa372

